Abstract
Background
Epidemiological studies evaluating the association between folate intake and risk of pancreatic cancer have produced inconsistent results. The statistical power to examine this association has been limited in previous studies partly because of small sample size and limited range of folate intake in some studies.
Methods
We analyzed primary data from 14 prospective cohort studies that included 319 716 men and 542 948 women to assess the association between folate intake and risk of pancreatic cancer. Folate intake was assessed through a validated food-frequency questionnaire at baseline in each study. Study-specific relative risks (RRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards models and then pooled using a random effects model. All statistical tests were two-sided.
Results
During 7–20 years of follow-up across studies, 2195 pancreatic cancers were identified. No association was observed between folate intake and risk of pancreatic cancer in men and women (highest vs lowest quintile: dietary folate intake, pooled multivariable RR = 1.06, 95% CI = 0.90 to 1.25, Ptrend = .47; total folate intake [dietary folate and supplemental folic acid], pooled multivariable RR = 0.96, 95% CI = 0.80 to 1.16, Ptrend = .90). No between-study heterogeneity was observed (for dietary folate, Pheterogeneity = .15; for total folate, Pheterogeneity = .22).
Conclusion
Folate intake was not associated with overall risk of pancreatic cancer in this large pooled analysis.
CONTEXT AND CAVEATS
Prior knowledge
Because of extremely poor prognosis, prevention may be the only feasible way of reducing pancreatic cancer incidence. Previous studies have suggested that folate may be protective against pancreatic cancer, but the results were inconsistent.
Study design
Associations between dietary folate and total folate (dietary folate plus supplemental folic acid) intake and risk of pancreatic cancer were assessed in a pooled analysis of 14 prospective cohort studies.
Contribution
No association was observed between highest vs lowest consumption of dietary and total folate and risk of pancreatic cancer in men and women.
Implication
High folate intake is not associated with decreased risk of pancreatic cancer.
Limitations
Folate intake was measured at baseline and any changes during the follow-up period were not known. Folate intakes were self-reported and subject to measurement error.
From the Editors
Pancreatic cancer is among the most fatal malignancies worldwide; it ranks fourth for cancer deaths in the United States and sixth in Europe and Australia (1–3). The prognosis of pancreatic cancer remains extremely poor; only 7% of pancreatic cancers are diagnosed at an early stage when the disease is more treatable (4), and the overall 5-year survival rate is approximately 6% (1). Primary prevention remains the most feasible approach to reducing the incidence of pancreatic cancer, which makes the identification of modifiable risk factors essential.
Folate is a water-soluble B vitamin found naturally in foods such as leafy green vegetables and citrus fruits (5). It plays an important role in the formation of S-adenosylmethionine, the universal methyl donor, as well as in the formation of purine and thymidine for nucleotide synthesis (6). Given that dysregulation of DNA methylation and DNA synthesis are relevant in carcinogenesis, folate has attracted considerable attention in recent years with regard to cancer prevention. Pancreatic cancers exhibit aberrant patterns of DNA methylation (7–9) and many molecular–genetic alterations (10–12); thus, the availability of folate-derived methyl groups may plausibly influence the risk of pancreatic cancer through altered DNA methylation or mutation. Although folate is listed as probably protecting against pancreatic cancer by the 2009 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) report (13), prospective cohort studies evaluating folate intake in relation to pancreatic cancer risk have produced inconsistent results. High intake of dietary folate was associated with a statistically significant decrease in pancreatic cancer risk in the Alpha-Tocopherol Beta-Carotene (ATBC) Cancer Prevention Study (14) and the combined analysis of the Swedish Mammography Cohort and Cohort of Swedish Men (15) but not in the Netherlands Cohort Study (16) or the combined analysis of the Nurses’ Health Study (NHS) and Health Professional Follow-up Study (HPFS) (17); the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial cohort found an inverse association among women but not among men (18). In contrast, users of supplemental folic acid had a non-statistically significant elevation in risk in the ATBC cohort (14), whereas no association was found in the NHS and HPFS (17), the Swedish studies (15), or the PLCO Cancer Screening Trial (18). The Netherlands Cohort Study (16) did not evaluate supplemental folic acid intake. The association between folate intake and pancreatic cancer risk has also been evaluated in four case–control studies, and the results were inconsistent (19–22).
These conflicting results could be partly because of relatively small numbers of cancers and limited range of folate intake in some studies. To address these limitations, we analyzed the primary data from 14 prospective cohort studies conducted in North America, Europe, and Australia and then pooled the study-specific estimates to assess the association of folate intake with risk of pancreatic cancer. Our analyses included all of the prospective studies that had previously investigated the association between folate intake and pancreatic cancer risk (14–18), as well as seven prospective studies that have collected data on folate intake and pancreatic cancer (23–29) but have not analyzed the association yet.
Methods
Study Population
The analyses presented were conducted in the Pooling Project of Prospective Studies of Diet and Cancer (Pooling Project), an international consortium of prospective cohort studies, which was established in 1991 to summarize associations between diet and cancer risk (30). The Pooling Project developed a structured framework to search for relevant studies in the published literature and formulated inclusion criteria a priori. For the pancreatic cancer analyses, we identified 14 prospective cohort studies (14–18,23–29) that met the following criteria for inclusion in the Pooling Project: identification of at least 50 incident pancreatic cancers, assessment of long-term dietary intake, validation of the dietary assessment method or a closely related instrument, and at least one publication on a diet and cancer analysis. Because most studies included only one sex, studies that included both men and women were analyzed as two separate cohorts, resulting in 19 cohorts from these 14 studies (Table 1). Each of the studies included was reviewed and approved by the institutional review board of the institution at which the study was conducted.
Table 1.
Characteristics of the cohort studies included in the pooled analysis of folate intake and risk of pancreatic cancer*
| Study | Country | Follow-up period | Cohort, participants at baseline, No. | Age range at baseline, y | Pancreatic cancers, No. | Energy-adjusted median folate intake |
Multivitamin users, % | |
| (10th–90th percentile), μg/d | ||||||||
| Dietary folate | Total folate (dietary and supplemental) | |||||||
| Men | ||||||||
| ATBC Cancer Prevention Study | Finland | 1984–1999 | 26 987 | 50–69 | 204 | 256 (206–314) | 259 (207–329) | 8 |
| Cancer Prevention Study II Nutrition Cohort | United States | 1992–2001 | 66 165 | 50–74 | 210 | 310 (198–447) | 363 (212–777) | 33 |
| Cohort of Swedish Men | Sweden | 1998–2004 | 45 338 | 45–79 | 75 | 266 (201–354) | 278 (204–553) | 13 |
| Health Professional Follow-up Study | United States | 1986–2002 | 47 762 | 40–75 | 198 | 353 (242–514) | 404 (255–827) | 43 |
| Melbourne Collaborative Cohort Study | Australia | 1991–2003 | 14 908 | 40–69 | 28 | 280 (181–411) | NA | 13 |
| Netherlands Cohort Study | The Netherlands | 1986–1995 | 58 279† | 55–69 | 145 | 209 (158–294) | NA | 3 |
| New York State Cohort | United States | 1980–1987 | 30 363 | 15–107 | 90 | 409 (290–603) | 496 (307–874) | 38 |
| PLCO Cancer Screening Trial | United States | 1993–2004 | 29 914 | 55–74 | 90 | 436 (322–600) | 549 (345–1064) | 43 |
| Women | ||||||||
| Breast Cancer Detection Demonstration Project Follow-up Study | United States | 1987–1999 | 43 162 | 40–93 | 102 | 301 (183–503) | 382 (201–836) | 33 |
| Canadian National Breast Screening Study | Canada | 1980–2000 | 49 654† | 40–59 | 105 | 243 (168–343) | NA | NA |
| Cancer Prevention Study II Nutrition Cohort | United States | 1992–2001 | 74 138 | 50–74 | 164 | 271 (164–436) | 371 (181–779) | 42 |
| California Teachers Study | United States | 1995–2001 | 100 030 | 22–104 | 116 | 329 (237–458) | 487 (265–793) | 56 |
| Iowa Women’s Health Study | United States | 1986–2001 | 34 588 | 55–69 | 171 | 248 (169–364) | 281 (178–679) | 33 |
| Melbourne Collaborative Cohort Study | Australia | 1991–2003 | 22 830 | 40–69 | 35 | 260 (175–373) | NA | 19 |
| Netherlands Cohort Study | The Netherlands | 1986–1995 | 62 573† | 55–69 | 122 | 183 (135–260) | NA | 6 |
| New York State Cohort | United States | 1980–1987 | 22 550 | 15–107 | 48 | 378 (263–552) | 501 (289–861) | 49 |
| Nurses’ Health Study | United States | 1986–2002 | 68 478 | 40–65 | 178 | 274 (190–397) | 322 (202–709) | 43 |
| PLCO Cancer Screening Trial | United States | 1993–2004 | 28 315 | 55–74 | 60 | 366 (274–490) | 658 (308–1074) | 58 |
| Swedish Mammography Cohort | Sweden | 1987–2003 | 36 630 | 49–83 | 54 | 255 (184–356) | 278 (190–569) | 24 |
A total of 2195 incident pancreatic cancers (1040 men and 1155 women) were identified among 319 716 men and 542 948 women. Baseline cohort size and number of pancreatic cancers determined after applying study-specific exclusion criteria and excluding participants with previous cancer diagnosis other than non-melanoma skin cancer at baseline and natural log (loge)–transformed energy intake beyond three SDs from the study-specific loge-transformed mean energy intake of the population. ATBC = Alpha-Tocopherol Beta-Carotene; PLCO = Prostate, Lung, Colorectal, and Ovarian; NA = not applicable.
Canadian National Breast Screening Study and Netherlands Cohort Study are analyzed as case–cohort studies so the baseline cohort size does not reflect the above exclusions.
Ascertainment of Pancreatic Cancers
Incident pancreatic cancers were ascertained by linkage with a cancer registry (15,16,23,24,27–29), self-report with subsequent medical record review (17), or both (14,18,25,26). Some studies also had an additional linkage to mortality registries (14,15,17,23–28). The follow-up rate of each cohort generally exceeded 90% (30). Information on subtypes of pancreatic cancer was also collected in most studies.
Assessment of Folate Intake
Each study assessed usual intake of foods and nutrients with a baseline food-frequency questionnaire. The number of food items on the questionnaires ranged from 45 for the New York State Cohort (24) to 276 in the ATBC Cancer Prevention Study (14). The food data were converted into daily nutrient intakes according to the food composition database used in each study. Because most nutrients are associated with total energy intake and total energy intake is associated with pancreatic cancer risk, to minimize the confounding by total energy intake, nutrient intakes were energy adjusted using the residual method (31), in which nutrient intake was regressed against total energy intake and then standardized to energy intakes of 2100 kcal/d for men and 1600 kcal/d for women.
Estimates of folate intake from food only (dietary folate) were provided by all studies (Table 1). Data on folate intake from supplemental sources were not available in the Canadian National Breast Screening Study, the Melbourne Collaborative Cohort Study, and the Netherlands Cohort Study. In the Netherlands Cohort Study, the multivitamins that were used in the Netherlands when the study was initiated did not include folate; so the folate intake in this study is only from food sources. For the New York State Cohort, which only entered their supplement use data as user vs nonuser, supplemental folic acid intake was calculated for participants who reported using multivitamins by assuming that they took one multivitamin tablet per day and that each tablet contained 400 μg of folic acid (this is the amount of folic acid estimated for generic multivitamins for the 1980 food-frequency questionnaire in the NHS). Total folate intake was calculated as the sum of dietary folate plus supplemental folic acid for all studies with supplemental folic acid intake data. Although all studies used validated dietary assessment methods (14–18,23–29,32–36), only half the studies assessed specifically the validity of dietary folate intake (15,17,30,34,35,37). Among these studies, to determine how accurately the questionnaires estimated folate intake, folate intake from the food-frequency questionnaires used in the studies or a closely related questionnaire was compared with intake estimated by either multiple diet records or 24-hour recalls. The correlation coefficients comparing the two methods were generally greater than 0.40 (15,17,30,34,35,37).
Non-Dietary Covariates
Information on non-dietary factors was collected in each study at baseline. All the included studies provided information on age at enrollment, height, weight, smoking history, and alcohol consumption. Body mass index (kg/m2) was calculated using information on height and weight. Most studies also assessed education, diabetes status, and physical activity. The proportion of missing values was generally less than 5% for each covariate measured in a study (30).
Statistical Analysis
After applying the study-specific exclusion criteria, we further excluded participants with a previous cancer diagnosis other than non-melanoma skin cancer at baseline or who reported implausible energy intakes of more than three SDs from the study-specific natural log (loge)–transformed mean intake of total energy. Data analyses composed of two steps. First, we calculated study-specific relative risks (RRs) and 95% confidence intervals (CIs) using the Cox proportional hazards model. We tested the assumption of proportional hazards and observed no evidence of violation. Second, we pooled study-specific relative risks to calculate a pooled relative risk using the DerSimonian and Laird random effects model (38,39). Heterogeneity across studies was tested using the Q statistic (38,40).
Folate intake was analyzed in study-specific quintiles. For the Canadian National Breast Screening Study and the Netherlands Cohort Study, each of which used a case–cohort design (41), study-specific quintiles were based on the distributions in the subcohort; for the remaining studies, study-specific quintiles were based on the distributions in the entire cohort. In further analyses, folate intake was analyzed in study-specific quartiles to ensure that the results were not sensitive to the number of folate intake groups. In addition, folate intakes were also categorized by identical absolute cut points across studies. We used the lowest intake category as the reference category throughout the analyses, and the cut point for the referent category was chosen to ensure that the number of cancers in the referent category was large enough to generate stable relative risk estimates in each study. If no participants were diagnosed with pancreatic cancer in the highest intake category in a study, the participants in the highest category in that study were included in the second highest intake category. Linear trends were tested by the Wald test of a score variable set to the median values of the corresponding category of intake.
For all analyses of total folate intake, the Melbourne Collaborative Cohort Study and the Canadian National Breast Screening Study were not included because total folate intake data were not available in these two studies. Additionally, in the analysis of total folate intake using the study-specific quantile approach, the ATBC Cancer Prevention Study was excluded because folate intake in this study was primarily from diet: Less than 10% of the participants used supplements in this study. The Netherlands Cohort Study was also excluded from the study-specific quantile analyses of total folate intake because the multivitamins that were used in the Netherlands when the study was initiated did not include folate; so the folate intake in this study is only from food sources. However, the ATBC Cancer Prevention Study and the Netherlands Cohort Study were included in the analyses of total folate intake using categories defined by identical absolute intakes across studies.
Person-years of follow-up were calculated from the return date of the baseline questionnaire to pancreatic cancer diagnosis, death, loss to follow-up, if available, or administrative end of follow-up, whichever came first. In age-adjusted analyses, age at baseline (in years) and the year the baseline questionnaire was returned were used as stratification variables, thereby creating a time metric that simultaneously accounted for potential confounding by age and calendar time. In multivariable analyses, we additionally adjusted for body mass index (kg/m2, continuous), diabetes (yes, no), alcohol intake (0, 0.1–14.9, 15.0–29.9, ≥30 g/d), energy intake (kcal/d, continuous), and cigarette smoking (never, former [>0 to <15, ≥15 pack-years], current [>0 to <40, ≥40 pack-years]).
To evaluate whether the association between folate intake and risk of pancreatic cancer was log linear, we compared the model fit including linear and cubic spline terms selected by a stepwise regression procedure with the model fit with only the linear term using the likelihood ratio test (42). For this analysis, all studies were combined into a single dataset, and the models were stratified by study and adjusted for all the other covariates. Individuals reporting extremely high intakes of folate (top 1% of participants in each study) were excluded from the spline analysis to reduce the influence of extreme values. Additional analyses in which folate intake was modeled as a continuous variable were conducted if the nonparametric regression curves showed that the associations of pancreatic cancer risk with dietary and total folate intake were consistent with log-linear associations.
We further conducted subgroup analyses. Previous studies have suggested that the inverse association between folate intake and pancreatic cancer risk was restricted to individuals not taking multivitamins (14,15,17,43), a group with lower folate status than multivitamin users. We therefore examined the association between dietary folate intake and pancreatic cancer risk among individuals who did not take any supplements containing folic acid. Because the folate fortification of grain products was mandated by January 1998 in the United States and Canada, we repeated our analyses by excluding the follow-up after January 1998 for studies conducted in North America. In addition, we conducted a sensitivity analysis excluding the first 2 years of follow-up for all participants to rule out an effect of subclinical pancreatic cancer on folate intake. Because individuals with diabetes often change their diet after diagnosis, we further examined the association of interest among nondiabetics. Separate analyses were also conducted for the adenocarcinoma subtype of pancreatic cancer.
We then assessed whether the association between folate intake and pancreatic cancer risk varied by other factors. A meta-regression model (44) was used to test for effect modification by sex, study region (North America vs Europe; we mainly focused on North America and Europe because there is only one study in Australia), follow-up time (<5, ≥5 years), age at diagnosis (<69, ≥69 years), smoking status (never, ever), body mass index (<25, ≥25 kg/m2), methionine intake (tertiles), and alcohol consumption (0, >0 to < 15, ≥15 g/d).
To assess the influence of measurement error in folate intake, we conducted a measurement error correction analysis using the linear regression calibration method (45). We regressed the folate intake from the food-frequency questionnaires on the folate intake from the reference methods in the validation studies and then computed the corrected relative risks by dividing the uncorrected estimates by the obtained regression coefficients. The corrected relative risks were then pooled using a random effects model (38, 39).
All statistical tests were two-sided, and P values less than .05 were considered statistically significant. SAS statistical software (version 9.1; SAS Institute Inc, Cary, NC) was used for all analyses.
Results
During 7–20 years of follow-up in the 14 prospective cohort studies, 2195 incident pancreatic cancers (1040 men and 1155 women) were identified among 319 716 men and 542 948 women (Table 1). Total folate intake in men was lowest in the ATBC Cancer Prevention Study (energy-adjusted median = 259 μg/d, 10th–90th percentile = 207–329 μg/d) and highest in the PLCO Cancer Screening Trial (energy-adjusted median = 549 μg/d, 10th–90th percentile = 345–1064 μg/d). Total folate intake in women was lowest in the Swedish Mammography Cohort (energy-adjusted median = 278 μg/d, 10th–90th percentile = 190–569 μg/d) and highest in the PLCO Cancer Screening Trial (energy-adjusted median = 658 μg/d, 10th–90th percentile = 308–1074 μg/d). The prevalence of multivitamin use was less than 20% in the ATBC Cancer Prevention Study, Cohort of Swedish Men, Melbourne Collaborative Cohort Study, and the Netherlands Cohort Study.
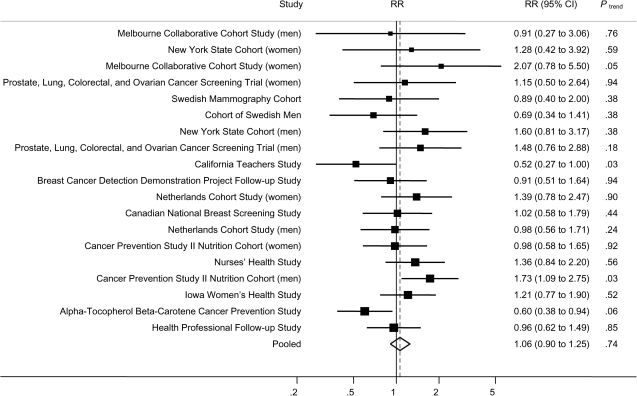
No overall association was observed between dietary folate intake and pancreatic cancer risk (Table 2). When we only adjusted for age in the models, no association between dietary folate and pancreatic cancer risk was observed (highest vs lowest quintile of dietary folate intake, pooled RR = 0.97, 95% CI = 0.84 to 1.13, Ptrend = .63). In the multivariable models, no association was observed (highest vs lowest quintile of dietary folate intake, pooled multivariable RR = 1.06, 95% CI = 0.90 to 1.25, Ptrend = .47), and the pooled relative risks did not differ greatly across quintiles. The study-specific multivariable relative risks for the highest vs the lowest quintile ranged from 0.52 to 2.07 across studies (Figure 1). Among these studies, statistically significant associations were observed only in the ATBC Cancer Prevention Study and the Cancer Prevention Study II Nutrition Cohort (men); a reduced risk was observed in the ATBC Cancer Prevention Study (highest vs lowest quintile of dietary folate intake, RR = 0.60, 95% CI = 0.38 to 0.94, Ptrend = .06), whereas in the Cancer Prevention Study II Nutrition Cohort, the risk estimate was in the opposite direction (RR = 1.73, 95% CI = 1.09 to 2.75, Ptrend = .03) (Figure 1). As shown in Table 2, there was no evidence of between-studies heterogeneity for women (Pheterogeneity = .50) or men and women combined (Pheterogeneity = .15), and the association did not differ between men and women (Pheterogeneity due to sex = .83). However, in the multivariable analysis, a statistically significant between-studies heterogeneity was observed for men (Pheterogeneity = .04). We further investigated whether the observed heterogeneity among men was because of differences by study region and found that the relative risk in men was higher for the North American studies than for the other studies (highest vs lowest quintile of dietary folate intake: for the North American studies, pooled multivariable RR = 1.35, 95% CI = 0.99 to 1.84; for the other studies, pooled multivariable RR = 0.72, 95% CI = 0.53 to 0.97) (data not shown in table or figure). The difference in the relative risks between these two regions was statistically significant (P = .003). In addition, among men, no between-studies heterogeneity was observed for North American studies (Pheterogeneity = .26) and the other studies (Pheterogeneity = .59).
Table 2.
Pooled relative risks of pancreatic cancer for dietary folate intake*
| Study population | RR (95% CI) |
Ptrend‡ | Pbetween-studies heterogeneity§ | Pbetween-studies heterogeneity due to sex║ | ||||
| Quintile of dietary folate intake† | ||||||||
| 1 | 2 | 3 | 4 | 5 | ||||
| Men | ||||||||
| No. of cancers | 210 | 198 | 203 | 205 | 224 | |||
| Age-adjusted | 1.00 (referent) | 0.90 (0.73 to 1.09) | 0.89 (0.67 to 1.17) | 0.89 (0.73 to 1.08) | 0.97 (0.76 to 1.24) | .83 | .17 | |
| Multivariable¶ | 1.00 (referent) | 0.92 (0.76 to 1.13) | 0.94 (0.70 to 1.25) | 0.95 (0.78 to 1.16) | 1.05 (0.77 to 1.42) | .47 | .04 | |
| Women | ||||||||
| No. of cancers | 246 | 230 | 222 | 202 | 255 | |||
| Age-adjusted | 1.00 (referent) | 0.89 (0.67 to 1.18) | 0.86 (0.70 to 1.06) | 0.78 (0.64 to 0.95) | 0.98 (0.81 to 1.19) | .52 | .35 | |
| Multivariable¶ | 1.00 (referent) | 0.96 (0.72 to 1.28) | 0.95 (0.78 to 1.15) | 0.87 (0.72 to 1.06) | 1.08 (0.90 to 1.30) | .84 | .50 | |
| Men and women | ||||||||
| No. of cancers | 456 | 428 | 425 | 407 | 479 | |||
| Age-adjusted | 1.00 (referent) | 0.90 (0.76 to 1.07) | 0.88 (0.75 to 1.03) | 0.83 (0.72 to 0.95) | 0.97 (0.84 to 1.13) | .63 | .26 | .95 |
| Multivariable¶ | 1.00 (referent) | 0.96 (0.80 to 1.13) | 0.95 (0.80 to 1.11) | 0.91 (0.79 to 1.04) | 1.06 (0.90 to 1.25) | .47 | .15 | .83 |
Folate intake was adjusted for energy intake. CI = confidence interval; RR = relative risk.
The quintiles were defined within each individual study.
P values were calculated using two-sided Wald test.
P values were for the highest quintile and were calculated using the Q statistic.
P values were for the highest quintile and were calculated using the two-sided Wald test.
Adjusted for body mass index (kg/m2, continuous), diabetes (yes, no), alcohol intake (g/d; 0, 0.1–14.9, 15.0–29.9, ≥30), energy intake (kcal/d, continuous), and cigarette smoking (never, former [<15, ≥15 pack-years], current [<40, ≥40 pack-years]). Age in years and year of questionnaire return were included as stratification variables.
Figure 1.
Study-specific and pooled multivariable relative risks (RRs) of pancreatic cancer according to highest vs lowest quintile of dietary folate intake. The solid squares and horizontal lines correspond to the study-specific multivariable RRs and 95% confidence intervals (CIs), respectively. The area of the solid square reflects the study-specific weight (inverse of the variance). The open diamond represents the pooled multivariable RR and 95% CI. The dashed vertical line indicates the pooled RR. The solid vertical line indicates a RR of 1.0.
The pooled multivariable relative risks of pancreatic cancer associated with dietary folate intake remained unchanged when we included only the studies with total folate intake data in our analysis, that is, when we excluded the studies in which total folate intake data were either unavailable (the Melbourne Collaborative Cohort Study and the Canadian National Breast Screening Study) or were primarily from diet (the ATBC Cancer Prevention Study and the Netherlands Cohort Study) (data not shown). When we restricted the analysis to individuals who did not use supplements containing folic acid, the estimates for dietary folate intake did not change materially except for the highest quintile that showed an increased risk (pooled multivariable RR = 1.20, 95% CI = 1.01 to 1.43, Ptrend = .08) compared with the lowest quintile of dietary folate intake (Table 3). There was no evidence of between-studies heterogeneity (Pheterogeneity = .42), and the association did not differ between men and women (Pheterogeneity due to sex = .36).
Table 3.
Pooled relative risks of pancreatic cancer for dietary folate intake among nonusers of supplements containing folic acid*
| RR (95% CI) |
Ptrend‡ | Pbetween-studies heterogeneity§ | Pbetween-studies heterogeneity due to sex║ | |||||
| Quintile of dietary folate intake† |
||||||||
| 1 | 2 | 3 | 4 | 5 | ||||
| No. of cancers | 272 | 291 | 256 | 252 | 297 | |||
| Age-adjusted | 1.00 (referent) | 1.06 (0.89 to 1.26) | 0.94 (0.77 to 1.13) | 0.93 (0.78 to 1.11) | 1.12 (0.95 to 1.33) | .43 | .73 | .41 |
| Multivariable¶ | 1.00 (referent) | 1.12 (0.94 to 1.33) | 1.01 (0.84 to 1.21) | 1.01 (0.84 to 1.22) | 1.20 (1.01 to 1.43) | .08 | .42 | .36 |
Folate intake was adjusted for energy intake. The Melbourne Collaborative Cohort Study and the Canadian National Breast Screening Study were not included because total folate data were not available in these two studies. CI = confidence interval; RR = relative risk.
The quintiles were defined within each individual study among nonusers of supplements containing folic acid.
P values were calculated using two-sided Wald test.
P values were for the highest quintile and were calculated using the Q statistic.
P values were for the highest quintile and were calculated using the two-sided Wald test.
Adjusted for body mass index (kg/m2, continuous), diabetes (yes, no), alcohol intake (g/d; 0, 0.1–14.9, 15.0–29.9, ≥30), energy intake (kcal/d, continuous), and cigarette smoking (never, former [<15, ≥15 pack-years], current [<40, ≥40 pack-years]). Age in years and year of questionnaire return were included as stratification variables.
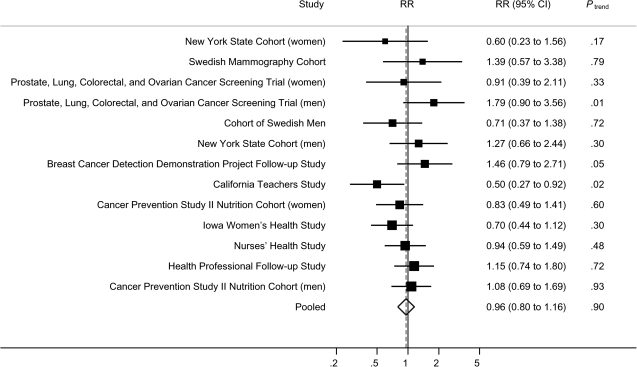
Next, we examined the association between total folate intake and pancreatic cancer risk. Total folate intake was not associated with overall risk of pancreatic cancer (for men and women combined, highest vs lowest quintile of total folate intake, pooled multivariable RR = 0.96, 95% CI = 0.80 to 1.16, Ptrend = .90) (Table 4), and the pooled relative risks did not vary much for quintiles 2 through 5. The study-specific multivariable relative risks for the highest vs the lowest quintile ranged from 0.50 to 1.79, with a statistically significant association being observed only in the California Teachers Study (Ptrend = .02) (Figure 2). There was no evidence of between-studies heterogeneity (Pheterogeneity = .22) (Table 4).
Table 4.
Pooled relative risks of pancreatic cancer for total folate intake from both food and supplements*
| Study population | RR (95% CI) |
Ptrend‡ | Pbetween-studies heterogeneity§ | Pbetween-studies heterogeneity due to sex║ | ||||
| Quintile of total folate intake† | ||||||||
| 1 | 2 | 3 | 4 | 5 | ||||
| Men | ||||||||
| No. of cancers | 125 | 119 | 139 | 133 | 147 | |||
| Age-adjusted | 1.00 (referent) | 0.88 (0.69 to 1.14) | 1.00 (0.78 to 1.27) | 0.97 (0.75 to 1.26) | 1.02 (0.80 to 1.30) | .32 | .56 | |
| Multivariable¶ | 1.00 (referent) | 0.93 (0.72 to 1.20) | 1.09 (0.85 to 1.39) | 1.06 (0.80 to 1.41) | 1.14 (0.89 to 1.45) | .11 | .44 | |
| Women | ||||||||
| No. of cancers | 198 | 177 | 160 | 194 | 164 | |||
| Age-adjusted | 1.00 (referent) | 0.87 (0.71 to 1.07) | 0.78 (0.63 to 0.97) | 0.90 (0.74 to 1.11) | 0.77 (0.61 to 0.96) | .17 | .34 | |
| Multivariable¶ | 1.00 (referent) | 0.93 (0.76 to 1.14) | 0.85 (0.69 to 1.05) | 0.98 (0.79 to 1.21) | 0.85 (0.67 to 1.07) | .39 | .30 | |
| Men and women | ||||||||
| No. of cancers | 323 | 296 | 299 | 327 | 311 | |||
| Age-adjusted | 1.00 (referent) | 0.88 (0.75 to 1.03) | 0.87 (0.74 to 1.02) | 0.93 (0.80 to 1.09) | 0.87 (0.73 to 1.03) | .53 | .29 | .07 |
| Multivariable¶ | 1.00 (referent) | 0.93 (0.79 to 1.09) | 0.94 (0.80 to 1.11) | 1.01 (0.86 to 1.19) | 0.96 (0.80 to 1.16) | .90 | .22 | .07 |
Folate intake was adjusted for energy intake. The Melbourne Collaborative Cohort Study and the Canadian National Breast Screening Study were not included because total folate data were not available in these two studies. CI = confidence interval; RR = relative risk.
The quintiles were defined within each individual study. The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study was excluded because folate intake in this study was primarily from diet; the Netherlands Cohort Study was also excluded because the folate intake in this study was only from food sources.
P values were calculated using two-sided Wald test.
P values were for the highest quintile and were calculated using the Q statistic.
P values were for the highest quintile and were calculated using the two-sided Wald test.
Adjusted for body mass index (kg/m2, continuous), diabetes (yes, no), alcohol intake (g/d; 0, 0.1–14.9, 15.0–29.9, ≥30), energy intake (kcal/d, continuous), and cigarette smoking (never, former [<15, ≥15 pack-years], current [<40, ≥40 pack-years]). Age in years and year of questionnaire return were included as stratification variables.
Figure 2.
Study-specific and pooled multivariable relative risks (RRs) of pancreatic cancer according to highest vs lowest quintile of total folate intake. The solid squares and horizontal lines correspond to the study-specific multivariable RRs and 95% confidence intervals (CIs), respectively. The area of the solid square reflects the study-specific weight (inverse of the variance). The open diamond represents the pooled multivariable RR and 95% CI. The dashed vertical line indicates the pooled RR. The solid vertical line indicates a RR of 1.0. The Melbourne Collaborative Cohort Study and the Canadian National Breast Screening Study were not included because total folate data were not available in these two studies; the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and the Netherlands Cohort Study were also excluded because folate intake in these two studies was primarily from or only from diet.
When folate intake was categorized as study-specific quartiles or using common intake cut points across cohorts (data not shown), the associations for dietary folate and total folate intake with risk of pancreatic cancer were similar to those observed when intakes were categorized as quintiles. To assess whether the association between folate intake and risk of pancreatic cancer was log linear, we conducted spline analyses (data not shown). The spline curves showed that the associations of pancreatic cancer risk with dietary and total folate intake were consistent with log-linear associations (Pnonlinear = .48 for dietary folate; Pnonlinear = .79 for total folate). Therefore, we conducted additional analyses in which folate intake was modeled as a continuous variable; no association was observed (for an increment of 100 μg of folate per day: for dietary folate intake, pooled multivariable RR = 1.01, 95% CI = 0.95 to 1.07, Pheterogeneity = .05; for total folate intake, pooled multivariable RR = 1.00, 95% CI = 0.97 to 1.02, Pheterogeneity = .10) (data not shown in table or figure).
When we repeated our analyses by excluding the follow-up time after the folate fortification (January 1998) for studies conducted in North America, the results did not materially change (highest vs lowest quintile: for dietary folate intake, pooled multivariable RR = 0.99, 95% CI = 0.84 to 1.16, Ptrend = .95; for total folate intake, pooled multivariable RR = 0.92, 95% CI = 0.75 to 1.13, Ptrend = .87). The results remained almost the same for analyses of only adenocarcinomas of the pancreas, when excluding individuals with diabetes, when excluding the first 2 years of follow-up, or after correction for measurement error in the assessment of folate intake (data not shown).
The association between folate intake and risk of pancreatic cancer was not modified by sex (all Pheterogeneity due to sex > .05) (Tables 2–4), study region (North America vs Europe and Australia) (Pinteraction = .19 for dietary folate intake; Pinteraction = .61 for total folate intake), length of follow-up (Pinteraction = .23 for dietary folate intake; Pinteraction = .28 for total folate intake), age at diagnosis (Pinteraction = .89 for dietary folate intake; Pinteraction = .06 for total folate intake), body mass index (Pinteraction = .89 for dietary folate intake; Pinteraction = .91 for total folate intake), or methionine intake (Pinteraction = .75 for dietary folate intake; Pinteraction = .89 for total folate intake) (data not shown).
We investigated whether the association between folate intake and pancreatic cancer risk varied by cigarette smoking (Table 5) and alcohol consumption (data not shown) because these may impair folate metabolism (15,46). Smoking modified the association with total folate intake (Pinteraction = .04) but not dietary folate intake (Pinteraction = .35). An inverse association between total folate intake and pancreatic cancer risk was observed among never smokers (highest vs lowest quartile of total folate intake, pooled multivariable RR = 0.77, 95% CI = 0.61 to 0.97, Ptrend = .08), but it must be noted that the trend for this association was only of borderline statistical significance (Table 5). No association was found among those who ever smoked. Among never smokers, the study-specific multivariable relative risks for the highest vs the lowest quartile of total folate intake ranged from 0.51 to 1.68 (RR > 1 in four cohorts and RR < 1 in nine cohorts; data not shown). The observed inverse association was not heavily influenced by a particular study and exclusion of any single study from the analysis had little impact on the finding. We found no statistically significant differences in the association between folate intake and pancreatic cancer risk by levels of alcohol consumption (Pinteraction = .28 for dietary folate intake; Pinteraction = .94 for total folate intake) (data not shown).
Table 5.
Pooled multivariable relative risks of pancreatic cancer for folate intake by smoking status*
| Folate intake by smoking strata | No. of cancers | RR (95% CI) |
Ptrend‡ | Pbetween-studies heterogeneity§ | Pinteraction║ | |||
| Quartile of folate intake† | ||||||||
| 1 | 2 | 3 | 4 | |||||
| Dietary folate | ||||||||
| Never¶ | 766 | 1.00 (referent) | 0.92 (0.75 to 1.14) | 0.92 (0.75 to 1.13) | 0.94 (0.76 to 1.16) | .56 | .82 | |
| Ever | 1385 | 1.00 (referent) | 0.96 (0.79 to 1.15) | 0.99 (0.84 to 1.15) | 1.05 (0.88 to 1.24) | .42 | .26 | .35 |
| Total folate# | ||||||||
| Never¶ | 631 | 1.00 (referent) | 0.90 (0.72 to 1.12) | 0.87 (0.70 to 1.09) | 0.77 (0.61 to 0.97) | .08 | .86 | |
| Ever | 903 | 1.00 (referent) | 0.90 (0.70 to 1.14) | 1.06 (0.79 to 1.43) | 1.09 (0.89 to 1.32) | .31 | .37 | .04 |
Folate intake was adjusted for energy intake. CI = confidence interval; RR = relative risk.
Adjusted for body mass index (kg/m2, continuous), diabetes (yes, no), alcohol intake (g/d; 0, 0.1–14.9, 15.0–29.9, ≥30), energy intake (kcal/d, continuous), and cigarette smoking (never, former [<15, ≥15 pack-years], current [<40, ≥40 pack-years]). Age in years and year of questionnaire return were included as stratification variables.
P values were calculated using two-sided Wald test.
P values were for the highest quartile and were calculated using the Q statistic.
P values for the highest quartile for the tests for interaction between smoking status and folate intake were calculated using the two-sided Wald test.
The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study was excluded from this stratum because the cohort included only current smokers.
The Melbourne Collaborative Cohort Study and the Canadian National Breast Screening Study were not included because total folate intake data were not available in these two studies. The Alpha-Tocopherol Beta-Carotene Cancer Prevention Study and the Netherlands Cohort Study were also excluded because total folate intake in these two studies was primarily or only from diet.
We also assessed the association of pancreatic cancer with supplemental folic acid intake only. Supplemental folic acid intake was not associated with pancreatic cancer risk (highest vs lowest tertile of supplemental folic acid intake, pooled multivariable RR = 0.94, 95% CI = 0.73 to 1.22, Ptrend = .76); statistically significant between-studies heterogeneity was observed (Pheterogeneity = .04). Further adjustment for dietary folate intake did not materially change the results for supplemental folic acid intake; similarly, the results for dietary folate intake were not materially changed when adjusted for supplemental folic acid intake (data not shown).
Discussion
In this large pooled analysis of 14 prospective cohort studies, dietary folate intake was not associated with overall risk of pancreatic cancer. This null association was largely unchanged when supplement users were excluded from the analysis. Similarly, no overall association was observed between total folate intake and risk of pancreatic cancer.
In contrast to the overall null results of this study, the 2009 WCRF/AICR report, which evaluated relevant studies up to 2006 [five cohort studies (14,15,17), two case–control studies (19,20), and one ecological study (47)], concluded that although the evidence available was limited, foods containing folate (but not folic acid supplements) probably protect against pancreatic cancer (13). Since 2006, four more studies [two cohort studies (16,18) and two case–control studies (21,22)] have examined the association between folate intake and pancreatic cancer risk, but the results have been inconsistent. Plasma folate levels were inversely associated with pancreatic cancer risk in a case–control study nested in the ATBC Cancer Prevention study (46), whereas no statistically significant association was found in a recent nested case–control study pooling data from the NHS, the HPFS, Physicians’ Health Study (PHS), and Women’s Health Initiative (WHI) (43). Genetic polymorphisms in folate-metabolizing genes, such as 5, 10-methylenetetrahydrofolate reductase (MTHFR) 677TT genotype, have also been inconsistently linked to pancreatic cancer risk (5).
In this study, we reduced differences in the studies by using harmonized exposure and covariate data, and by modeling variables similarly across studies. The null findings of the present pooled analysis may result from the dual effects of folate on carcinogenesis. Recent experiments have suggested that folate may prevent tumor development if administered before the existence of neoplastic lesions but may promote tumor progression once early lesions are established (48). The proposed mechanism for this dual role of folate relates to its essential role in DNA synthesis. As a source of single-carbon units that are necessary for DNA replication and repair, folate protects normal tissues from mutations and chromosomal damage; however, folate may enhance growth of already existing neoplastic lesions because rapidly proliferating tissues, such as tumors, have an increased demand for nucleotides. Therefore, the observed associations between folate intake and pancreatic cancer risk may reflect both these competing effects of folate, which could explain the diverse results from previous studies and the overall null findings of the pooled analysis.
The dual effects of folate could also explain the stronger protective effects of folate among never smokers observed in this pooled analysis. Compared with smokers, individuals who never smoke might be less likely to have harbored precursor lesions in the pancreas, which might allow the protective effect of folate to be detected. This is also consistent with the findings in a combined analysis of the NHS, HPFS, PHS, and WHI (43). In that analysis, the inverse association between plasma folate levels and pancreatic cancer risk was more apparent among never smokers than former or current smokers; moreover, in the analysis restricted to nonusers of multivitamins, a reduction in risk of pancreatic cancer was observed among never smokers (highest vs lowest quartile of plasma folate, odds ratio = 0.30, 95% CI = 0.08 to 1.04, Ptrend = .07), whereas no association was seen among former or current smokers (43). However, it should be noted that in the ATBC cohort of male smokers, an inverse association was observed between dietary folate intake (14) and plasma folate levels (46) and pancreatic cancer risk.
Folate fortification in the United States and Canada may have affected our results as well because baseline questionnaires for studies conducted in these countries might not reflect the actual folate levels after the fortification. We therefore repeated our analyses excluding follow-up after 1997 to examine the pre-folate fortification period for studies conducted in North America; the results remained unchanged. Although folate intake may also be influenced by preclinical pancreatic cancer, an analysis excluding the pancreatic cancers that were diagnosed during the first 2 years of follow-up demonstrated no changes in risk estimates.
Although previous studies have suggested that the benefit of dietary folate intake might be more apparent among nonusers of multivitamins (14,15,17,43), we did not observe a decreased risk with high levels of dietary folate intake among non-supplement users. On the contrary, an increased risk was observed in the highest category of dietary folate intake. However, there was no clear trend across categories; thus, these results should be interpreted with caution.
The strengths of this study include its large sample size, prospective design, and standardized categorizations of folate intake and other covariates across studies. The large number of pancreatic cancers allowed us to conduct subgroup analyses with greater statistical power than has been possible for any individual cohort. In addition, because the analyses were limited to prospective studies, recall and selection biases were minimized. Furthermore, by standardizing the categorizations of exposure and other covariates across studies, potential sources of heterogeneity between studies were minimized.
This study has some limitations. Dietary changes during the follow-up period cannot be addressed in our study because folate intake was measured only at baseline. In addition, if folate intake during childhood or early adulthood is more important for preventing pancreatic cancer, then our analysis of adult diet may not have captured the relevant exposure period. Confounding cannot be completely ruled out; however, it is probably of minor importance in this study as we adjusted established risk factors for pancreatic cancer in the models, and our age-adjusted and multivariable models yielded very similar results. Measurement error in folate intake in our study is inevitable as dietary intakes were self-reported. However, validation studies comparing food-frequency questionnaires with dietary records or 24-hour recalls showed that the food-frequency questionnaires used in the individual studies provided reasonably valid measurements of folate intake (15,17,30,37). In addition, folate intake calculated from the food-frequency questionnaire used in the HPFS was also highly associated with red cell folate level, which is considered as a good indicator of body stores of folate (17). When the estimates of relative risks in relation to folate intake were corrected for measurement error in folate intake, the risk estimates did not appreciably change.
In summary, folate intake was not associated with an overall decrease in risk of pancreatic cancer in this pooled analysis of 14 prospective cohort studies. Never smokers may be more sensitive to the preventive effect of total folate intake; however, this observation needs confirmation.
Funding
This study was supported by grants from the National Cancer Institute, National Institutes of Health, Bethesda, MD (CA55075 to W. C. Willett and CA124908 to C.S.F.).
Footnotes
The authors thank Shiaw-Shyuan Yaun for her assistance with data management and statistical support. The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the article; or the decision to submit the article for publication.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer. 2002;38(1):99–166. doi: 10.1016/s0959-8049(01)00350-1. [DOI] [PubMed] [Google Scholar]
- 3.AIHW (Australian Institute of Health and Welfare) and AACR (Australasian Association of Cancer Registries) Cancer in Australia: An Overview, 2008. Canberra, Australia: AIHW; 2008. [Google Scholar]
- 4.American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 5.Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006;131(4):1271–1283. doi: 10.1053/j.gastro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000;130(2):129–132. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 7.Sato N, Maitra A, Fukushima N, et al. Frequent hypomethylation of multiple genes overexpressed in pancreatic ductal adenocarcinoma. Cancer Res. 2003;63(14):4158–4166. [PubMed] [Google Scholar]
- 8.Ueki T, Toyota M, Skinner H, et al. Identification and characterization of differentially methylated CpG islands in pancreatic carcinoma. Cancer Res. 2001;61(23):8540–8546. [PubMed] [Google Scholar]
- 9.Ueki T, Toyota M, Sohn T, et al. Hypermethylation of multiple genes in pancreatic adenocarcinoma. Cancer Res. 2000;60(7):1835–1839. [PubMed] [Google Scholar]
- 10.Argani P, Rosty C, Reiter RE, et al. Discovery of new markers of cancer through serial analysis of gene expression: prostate stem cell antigen is overexpressed in pancreatic adenocarcinoma. Cancer Res. 2001;61(11):4320–4324. [PubMed] [Google Scholar]
- 11.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6(8):2969–2972. [PubMed] [Google Scholar]
- 12.Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7(12):4115–4121. [PubMed] [Google Scholar]
- 13.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2009. [Google Scholar]
- 14.Stolzenberg-Solomon RZ, Pietinen P, Barrett MJ, Taylor PR, Virtamo J, Albanes D. Dietary and other methyl-group availability factors and pancreatic cancer risk in a cohort of male smokers. Am J Epidemiol. 2001;153(7):680–687. doi: 10.1093/aje/153.7.680. [DOI] [PubMed] [Google Scholar]
- 15.Larsson SC, Hakansson N, Giovannucci E, Wolk A. Folate intake and pancreatic cancer incidence: a prospective study of Swedish women and men. J Natl Cancer Inst. 2006;98(6):407–413. doi: 10.1093/jnci/djj094. [DOI] [PubMed] [Google Scholar]
- 16.Keszei AP, Verhage BA, Heinen MM, Goldbohm RA, van den Brandt PA. Dietary folate and folate vitamers and the risk of pancreatic cancer in the Netherlands cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1785–1791. doi: 10.1158/1055-9965.EPI-08-1220. [DOI] [PubMed] [Google Scholar]
- 17.Skinner HG, Michaud DS, Giovannucci EL, et al. A prospective study of folate intake and the risk of pancreatic cancer in men and women. Am J Epidemiol. 2004;160(3):248–258. doi: 10.1093/aje/kwh214. [DOI] [PubMed] [Google Scholar]
- 18.Oaks BM, Dodd KW, Meinhold CL, Jiao L, Church TR, Stolzenberg-Solomon RZ. Folate intake, post-folic acid grain fortification, and pancreatic cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2010;91(2):449–455. doi: 10.3945/ajcn.2009.28433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baghurst PA, McMichael AJ, Slavotinek AH, Baghurst KI, Boyle P, Walker AM. A case-control study of diet and cancer of the pancreas. Am J Epidemiol. 1991;134(2):167–179. doi: 10.1093/oxfordjournals.aje.a116069. [DOI] [PubMed] [Google Scholar]
- 20.Silverman DT, Swanson CA, Gridley G, et al. Dietary and nutritional factors and pancreatic cancer: a case-control study based on direct interviews. J Natl Cancer Inst. 1998;90(22):1710–1719. doi: 10.1093/jnci/90.22.1710. [DOI] [PubMed] [Google Scholar]
- 21.Gong Z, Holly EA, Bracci PM. Intake of folate, vitamins B6, B12 and methionine and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control. 2009;20(8):1317–1325. doi: 10.1007/s10552-009-9352-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson LN, Cotterchio M, Gallinger S. Lifestyle, dietary, and medical history factors associated with pancreatic cancer risk in Ontario, Canada. Cancer Causes Control. 2009;20(6):825–834. doi: 10.1007/s10552-009-9303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- 24.Bandera EV, Freudenheim JL, Marshall JR, et al. Diet and alcohol consumption and lung cancer risk in the New York State Cohort (United States) Cancer Causes Control. 1997;8(6):828–840. doi: 10.1023/a:1018456127018. [DOI] [PubMed] [Google Scholar]
- 25.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(2):500–511. doi: 10.1002/cncr.10197. [DOI] [PubMed] [Google Scholar]
- 26.Flood A, Velie EM, Chaterjee N, et al. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. Am J Clin Nutr. 2002;75(5):936–943. doi: 10.1093/ajcn/75.5.936. [DOI] [PubMed] [Google Scholar]
- 27.Horn-Ross PL, Hoggatt KJ, West DW, et al. Recent diet and breast cancer risk: the California Teachers Study (USA) Cancer Causes Control. 2002;13(5):407–415. doi: 10.1023/a:1015786030864. [DOI] [PubMed] [Google Scholar]
- 28.Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women's Health Study. Am J Epidemiol. 1994;139(1):1–15. doi: 10.1093/oxfordjournals.aje.a116921. [DOI] [PubMed] [Google Scholar]
- 29.Terry P, Jain M, Miller AB, Howe GR, Rohan TE. Dietary intake of folic acid and colorectal cancer risk in a cohort of women. Int J Cancer. 2002;97(6):864–867. doi: 10.1002/ijc.10138. [DOI] [PubMed] [Google Scholar]
- 30.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163(11):1053–1064. doi: 10.1093/aje/kwj127. [DOI] [PubMed] [Google Scholar]
- 31.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 32.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 33.Horn-Ross PL, Lee VS, Collins CN, et al. Dietary assessment in the California Teachers Study: reproducibility and validity. Cancer Causes Control. 2008;19(6):595–603. doi: 10.1007/s10552-008-9124-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munger RG, Folsom AR, Kushi LH, Kaye SA, Sellers TA. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol. 1992;136(2):192–200. doi: 10.1093/oxfordjournals.aje.a116485. [DOI] [PubMed] [Google Scholar]
- 35.Flagg EW, Coates RJ, Calle EE, Potischman N, Thun MJ. Validation of the American Cancer Society Cancer Prevention Study II Nutrition Survey Cohort Food Frequency Questionnaire. Epidemiology. 2000;11(4):462–468. doi: 10.1097/00001648-200007000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Feskanich D, Marshall J, Rimm EB, Litin LB, Willett WC. Simulated validation of a brief food frequency questionnaire. Ann Epidemiol. 1994;4(3):181–187. doi: 10.1016/1047-2797(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 37.Glynn SA, Albanes D, Pietinen P, et al. Colorectal cancer and folate status: a nested case-control study among male smokers. Cancer Epidemiol Biomarkers Prev. 1996;5(7):487–494. [PubMed] [Google Scholar]
- 38.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 39.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 40.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 41.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. [Google Scholar]
- 42.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 43.Schernhammer E, Wolpin B, Rifai N, et al. Plasma folate, vitamin B6, vitamin B12, and homocysteine and pancreatic cancer risk in four large cohorts. Cancer Res. 2007;67(11):5553–5560. doi: 10.1158/0008-5472.CAN-06-4463. [DOI] [PubMed] [Google Scholar]
- 44.Stram DO. Meta-analysis of published data using a linear mixed-effects model. Biometrics. 1996;52(2):536–544. [PubMed] [Google Scholar]
- 45.Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med. 1989;8(9):1051–1069. doi: 10.1002/sim.4780080905. discussion 1071–1073. [DOI] [PubMed] [Google Scholar]
- 46.Stolzenberg-Solomon RZ, Albanes D, Nieto FJ, et al. Pancreatic cancer risk and nutrition-related methyl-group availability indicators in male smokers. J Natl Cancer Inst. 1999;91(6):535–541. doi: 10.1093/jnci/91.6.535. [DOI] [PubMed] [Google Scholar]
- 47.Corella Piquer D, Cortina Greus P, Coltell Simon O. [Nutritional factors and geographic differences in pancreatic cancer mortality in Spain] Rev Sanid Hig Publica (Madr). 1994;68(3):361–376. [PubMed] [Google Scholar]
- 48.Ulrich CM, Potter JD. Folate and cancer—timing is everything. JAMA. 2007;297(21):2408–2409. doi: 10.1001/jama.297.21.2408. [DOI] [PubMed] [Google Scholar]