Abstract
Oestrogen-stimulated preovulatory gonadotrophin surges are temporally regulated in a way which remains not fully understood. Mammalian ovulation requires surges of gonadotrophin-releasing hormone (GnRH), released from specialized neurones in the hypothalamus. Surge regulation is mediated by ovarian oestrogen (E2) feedback- acting as a negative signal until the early afternoon of the pro-oestrus phase, at which point it stimulates robust increases in GnRH release. Multiple lines of evidence suggest a role for the circadian clock in surge generation, but the presence of endogenous oscillators in several neuronal populations throughout the mediobasal hypothalamus complicates elucidating the underlying mechanisms of circadian regulation. In this study, we propose that endogenous oscillators within GnRH neurones are modulated by oestrogen to elicit GnRH surge secretion. One mechanism by which this may occur is through the upregulation of receptors of known stimulators of GnRH, such as kisspeptin’s cognate receptor, GPR54. Through analysis of mRNA and protein abundance patterns, we found that high levels of E2 elicit circadian expression profiles of GPR54 in vitro, and that disruption of endogenous GnRH oscillators of the clock dampens this effect. Additionally, while kisspeptin administration to GT1-7 cells does not result in surge-level secretion, we observed increased GnRH secretion from GT1-7 cells treated with positive feedback levels of E2. These results in this in vitro neuronal model system suggest a possible mechanism whereby receptor expression levels, and thus GnRH sensitivity to kisspeptin, may change dramatically over the pro-oestrus day. In this way, elevated ovarian E2 may increase kisspeptidergic tone while simultaneously increasing GnRH neuronal sensitivity to this neuropeptide for maximal surge release.
Introduction
The regulation of ovulation in female mammals requires precise temporal orchestration of neuronal cues, as positive feedback levels of ovarian E2 must be integrated with hypothalamic neural signals to elicit preovulatory surges of gonadotrophin-releasing hormone (GnRH) (1). Seen most strikingly in rodents, GnRH and subsequent LH surges are tightly temporally confined to the late afternoon of the pro-oestrus phase, such that pharmacological manipulations delay surges until the same time the following day (2, 3). Additionally, elevated exogenous E2 treatment following ovariectomy elicits LH surges, peaking around the initiation of darkness, for several consecutive days (4). Recently, the RFamide peptide kisspeptin has been shown to play an important role in neuroendocrine regulation of reproduction, including pubertal initiation and ovulation. While kisspeptin (also known as metastin) was initially identified for its anti-metastatic properties (5), further investigation implicates kisspeptin as a potent stimulator of GnRH neurones (6–11), making it a crucial component of the hypothalamus-pituitary-gonad axis. Kisspeptin knockout mice, as well as mice harboring a deletion of kisspeptin's cognate receptor, GPR54, are infertile (12, 13), exhibiting a phenotype similar to human idiopathic hypogonadal hypogonadism. Administration with exogenous GnRH can ameliorate this hypogonadism, suggesting that this neuropeptide exerts its effects on GnRH neurones (14). Kisspeptin neurones represent one likely integration site for ovarian E2, since nearly all kisspeptin neurones, both in the arcuate nuclei (ARC) and anteroventral periventricular nuclei (AVPV), express ERα (15). In most mammals, it is estimated that >85% of GnRH neurones in the median eminence (ME) express the kisspeptin receptor GPR54 (16), and 70–90% of GnRH perikarya are in close proximity to kisspeptinergic neuronal terminals (11). This, along with evidence that kisspeptin increases during the LH surge (17), antiserum of kisspeptin can block the LH surge (18), and infusion of novel GPR54 antagonists can inhibit GnRH and LH surge release in rodents and primates (19) lend credence to the model that kisspeptin is an essential mediator of the preovulatory GnRH surge.
Recent evidence additionally suggests that the temporal control of the neuroendocrine regulation of GnRH neurosecretion may involve dynamic changes in kisspeptin expression and release. In this model, kisspeptin-expressing neurones in the AVPV act as a cellular integrator of temporal neural signals originating from the proximally caudal suprachiasmatic nuclei (SCN), and hormonal cues in the form of elevated ovarian oestradiol levels from mature follicles (17) Consistent with this, recent studies have observed E2-dependent increases in vasopressinergic projections from the SCN (20), and that vasopressin can induce c-Fos expression in AVPV kisspeptidergic cells (21). Daily signals, as yet uncharacterized, from the SCN have long been thought to be required for LH surge generation, based on previous ablation studies (22, 23). In addition to a role of afferent signals from the SCN, more recent studies have demonstrated the presence of a peripheral circadian oscillator within GnRH neurones which is capable of functioning independently of the SCN (24–26). The immortalised GnRH-expressing and –secreting cell line, GT1-7, has been shown to possess functional endogenous circadian oscillators (24, 26), which may play a role in the intrinsic secretory mechanism in these neurones. While it remains unknown if endogenous GnRH cell-specific clocks are obligate for normal preovulatory surge generation, previous evidence in circadian clock mutant and knockout mice suggests a role for cellular clocks in the neuroendocrine regulation of normal reproductive function (24, 27–29).
In studies described below, we explored the possibility that E2 may mediate a portion of its surge-generation influence directly at the level of the GnRH neurone by modulating expression patterns of receptors of GnRH-stimulatory factors, namely kisspeptin. Temporal sensitization of GnRH neurones to the effects of kisspeptin could represent one of many redundant mechanisms designed to increase GnRH secretion in a time-gated fashion. Using the GT1-7 cell line as a homogenous neuronal model system in which to directly address these cellular changes, we have determined that exposure to positive feedback levels of oestradiol can induce or unmask transcriptional rhythmicity of GPR54 expression in these cells, and that protein abundance of GPR54 is also rhythmic under conditions of elevated E2, a phenomenon which appears to be mediated by intracellular ERβ receptors. Indeed, it is possible that multiple transcripts may exhibit circadian rhythms of expression following exposure to positive feedback levels of E2, such that this steroid hormone could sensitise GnRH neurones to multiple afferent stimuli, or increase native neuronal activity in a temporally-gated manner, which has been very recently explored (4).
Methods
Cell culture and treatments
GT1-7 cells were grown in DMEM (Mediatech, Herndon, VA) with 10% fetal bovine serum (Gemini Bio-Products, Sacramento, CA) and 1.0% Pen-Strep (penicillin; 100 U/ml), and streptomycin (0.1 mg/ml; Invitrogen, Carlsbad, CA). Cells were incubated at 37°C in 5% CO2/95% O2. To serum-synchronise cells, 10% FBS-DMEM media was replaced with a solution of 50% FBS and 50% serum-free (SF) DMEM for 2 hours. 50% serum-containing media was removed, and at this point, plates of GT1-7 cells were incubated with SF-DMEM containing either 100.0 pM 17-β-oestradiol (Sigma, St. Louis, MO) or ethanol (EtOH) vehicle. Separate plates of confluent GT1-7 cells were treated with 200.0 pM ERα agonist PPT (4,4',4"-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol, Tocris Bioscience, Ellisville, MO) or 200.0 pM of ERβ agonist DPN (2,3-bis(4-Hydroxyphenyl)-propionitrile, Sigma).
Derivation of sublcloned GT1-7ClkΔ19 cell line
Briefly, GT1-7 cells were stably transfected with the pTet-OFF regulator vector (Clontech, Mountain View, CA) using FuGene (Roche Molecular Diagnostics, Pleasanton, CA). After 24 h, the medium was changed and 900 µg/mL G418 (Invitrogen) added to select for transfected cells. G418-resistant cells were clonally selected by fluorescence activated cell sorting and clones were expanded. The mutant Clock-Δ19 gene was removed from a pcDNA3.1 construct (gift from J. Takahashi) with BamHI (New England BioLabs, Ipswitch, MA) and subcloned into doxycyline-controlled vector pTRE2hyg (Clontech) linearized with BamHI. A clone with the properly oriented gene inserted was identified by restriction digest analysis. ClockΔ19pTRE2hyg was subsequently transfected (using PolyJet; SignaGen Ijamsville, MD) into pTet-OFFGT1-7 cells. After 24 h, the medium was changed and 200 µg/mL hygromycin B (Invitrogen) added to select for transfected cells. Hygromycin-resistant cells were expanded and challenged with G418 to reconfirm the presence of pTet-OFF. Double-transfectant ClockΔ19pTre2hyg/pTet-OFFGT1-7 cells (hereafter referred to as GT1-7ClkΔ19 cells) were cultured either in the presence or absence of 10 µM doxycycline hyclate (Sigma-Aldrich). Verification of doxycycline-controlled Clock-Δ19 mRNA expression was verified by real time qPCR.
RNA isolation and protein extraction
Cells were harvested every 6 hours for a total of 72 hours for initial 17β-E2 vs. EtOH gene expression study (Figure 1), and for 48 hours for all experiments thereafter. Following removal of media, plates were washed with 1X PBS before Trizol (Invitrogen) reagent was added at a concentration of 2 × 106 cells per millilitre. RNA was isolated using a previously described protocol (30), followed by further purification using RNEasy mini kits (Qiagen). Protein extraction was performed in RIPA buffer with proteinase inhibitor cocktail added, and quantified using a bicinconchinic acid (BCA) assay (Pierce Biochemicals, Rockford, IL).
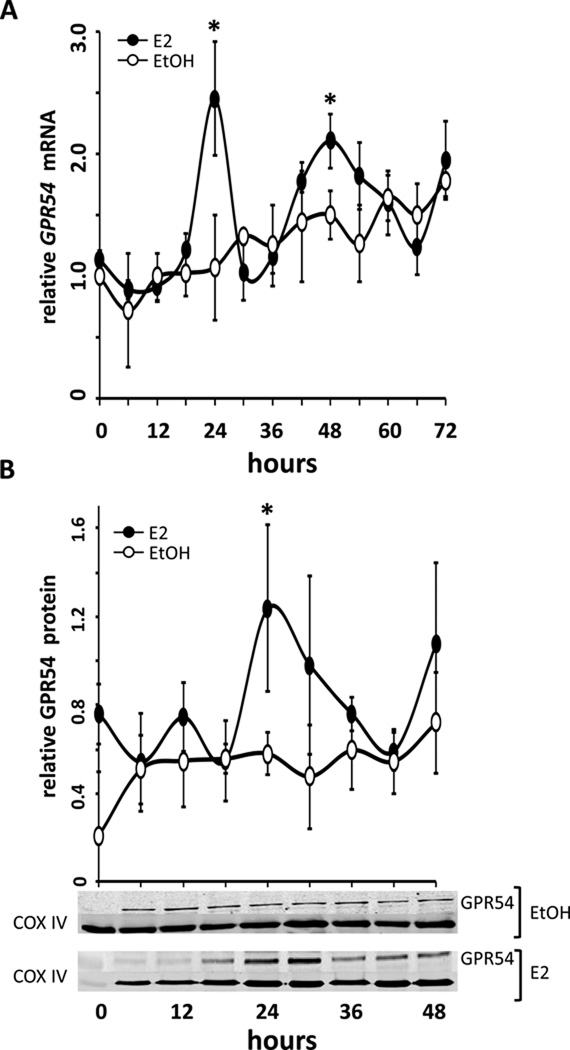
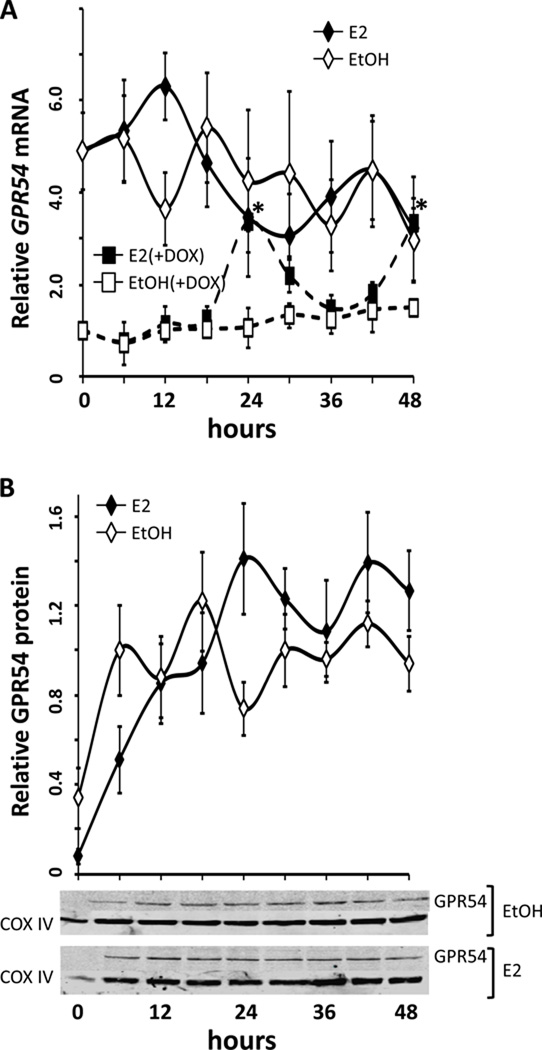
Figure 1.
GPR54 mRNA and protein expression in GT1-7 cells exhibits rhythmic expression only in the presence of oestradiol (E2). GT1-7 cells were treated with either 0.05% ethanol vehicle (EtOH, open circles) or 100.0 pM 17β-E2 (E2, filled circles) after serum synchronization. (A) GPR54 expression levels (mean ± SEM) are shown relative to the 0-hour, pre-treatment sample, using real time PCR. The changes in GPR54 expression occur with an approximate 24h periodicity, with E2-treated GT1-7 GPR54 abundance highest at 24 hours and rising again at 48 hours (*,P<0.05 vs. EtOH, n=4). (B) Protein abundance (mean ± SEM) of GPR54 in GT1-7 cells shows corresponding rhythmic expression patterns. In 17β-E2-treated cells, (E2, filled circles) GPR54 protein (normalized to COX IV) peaks at 24 hours (*,P<0.05 vs. EtOH) and 48 hours, although the latter did not reach statistical significance. Representative western blots show a ~45kDa band of GPR54 with peak levels at 24 and 48 hours, corresponding to average levels in (B), normalized to COXIV (~18kDa).
cDNA synthesis and real-time quantitative RT-PCR
2.0µg of total RNA from each sample was converted to cDNA using Sprint tubes (Clontech, Hercules, CA). 5.0 µg of cDNA was amplified using 15.0 µL of reverse transcriptase master mix containing SYBR-Green (Bio-rad). Sequence specific primers were used to amplify GPR54 (see supp. Table 1). Real-time qRT-PCR was performed and data analysis was done using Applied Biosystems 7300 RT-PCR system (Applied Biosystems, Foster City, CA). Negative controls were run without template cDNA. Relative expression levels were determined using the 2−ΔΔCt method. Primers were designed to specifically amplify Clock-Δ19, with the forward primer spanning the junction of exons 18 and 20. Forward primer: 5’ CAA GGC ATG TCA CAG ATG TT 3’ and reverse primer: 5’ GGA AAG TTG AAC AGA ACC AAA 3’. PCRs were carried out in a StepOnePlus Real-Time PCR System (Life Technologies Corporation, Carlsbad, CA) using Power SYBR master mix and the following thermal cycler profile: 95°C 10 m initial denaturation, followed by 40 cycle of 95°C 15 s and 60.5°C 30 s. Amplicon specificity was verified by melt-curve analysis using the StepOnePlus data analysis software.
Western blotting
Extracts from GT1-7s were obtained by protein extraction described above. Concentrations were determined using a BCA Assay (Pierce), and 20.0 µg of DNA was loaded into a 10% SDS-Polyacrylamide gel. After electrophoresis, proteins were transferred to a nitrocellulose membrane using the iBlot semi-dry transfer system (Invitrogen). Membranes were washed in a buffer containing 10% bovine serum albumin and 0.1% Tween-20 (Sigma), and probed with goat-anti GPR54 (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-COXIV (1:1,000) (Cell Signaling Technology, Danvers, MA). Visualization was performed using Li-Cor IRDye 800 rabbit-anti goat and goat-anti rabbit secondary antibodies, and quantified using a Li-Cor Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, Nebraska) Quantification of the bands was performed using Odyssey 2.1 software (Li-Cor).
Radioimmunoassay
Duplicate 100 µL media samples from 10cm plates of GT1-7 cells were incubated for 48h with EL-14 primary antibody directed against GnRH. Radiolabeled 125I-GnRH was then incubated with samples for an additional 48h. 3.0mL of 100% ethanol was added to samples (including standard curve tubes) and centrifuged at 2500rpm at 4°C for 15min. Pellets were loaded into a gamma-counter (Packard) and counted for 1 min/sample. Intra-assay CV and inter-assay CV were 3.2% and 6.8%, respectively.
Statistical Analysis
Comparisons of ethanol (EtOH) vs. 17β-oestradiol (E2) treatment groups across time were carried out using a two-way analysis of variance (ANOVA) with repeated measures, followed by Bonferroni’s post-hoc test. In all analyses, P<0.05 was considered significant, and demarcated as such.
Results
Experiment 1- Effects of positive feedback levels of E2 on transcript and protein abundance rhythms of GPR54 in GT1-7 cells
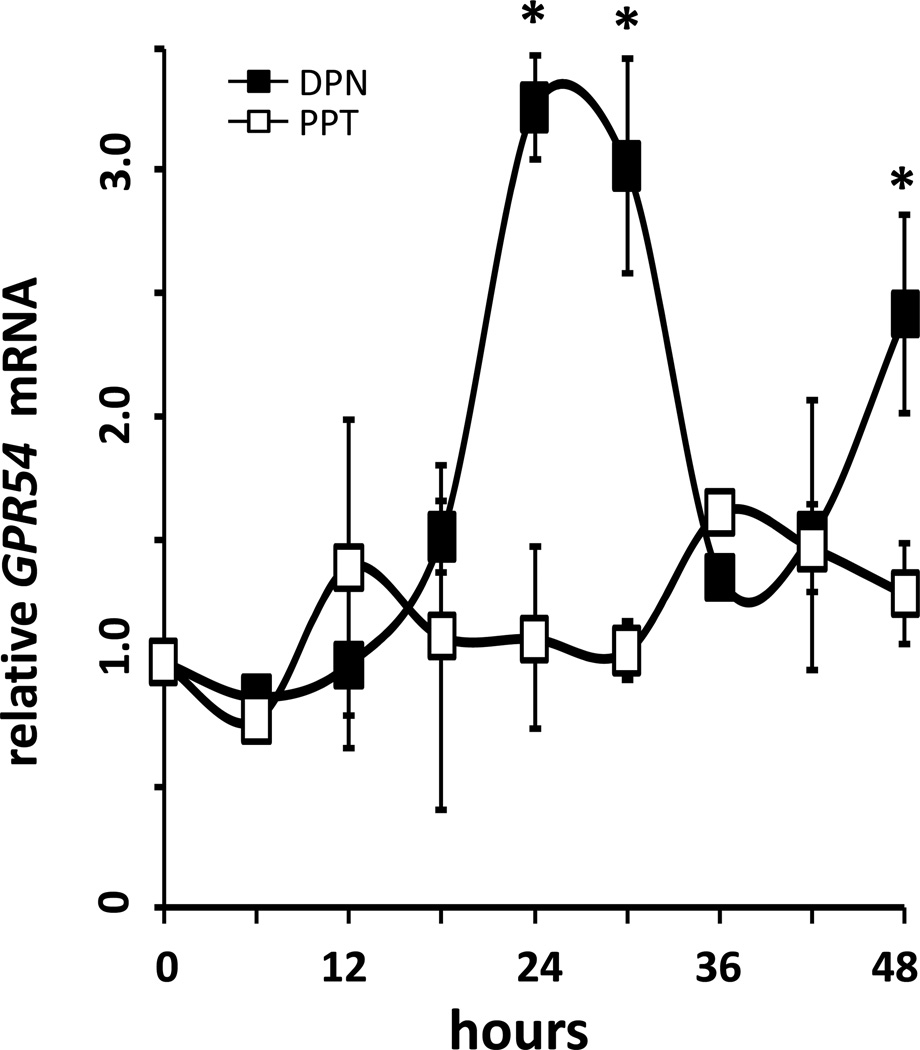
An earlier pilot study using expression microarrays revealed that several transcripts in GT1-7 cells exhibited rhythmic expression patterns only following treatment with 17β-oestradiol. Interestingly, we found that GPR54 expression levels appeared to exhibit significant rhythms following 24 hours of E2 exposure, in comparison to vehicle-treated controls. To confirm these preliminary results, we treated serum-synchronized GT1-7 cells with 100.0 pM 17β-oestradiol or 0.01% ethanol vehicle in serum-free and phenol red-free DMEM, and collected samples in TriZol every 6h for 72h. Relative expression results from real-time qRT-PCR revealed rhythmic increases in GPR54 mRNA levels only in E2-treated GT1-7 cells (Figure 1A), which exhibited significant peaks (*, P<0.05 vs. EtOH, n=4) at 24 hrs and again at 48 hours following E2 treatment. A third peak occurred at 72h following a nadir at 66h, but was not statistically different from levels observed in vehicle-treated cells, due to a steady rise in GPR54 mRNA in those cells over the sampling period. In agreement with this, levels of GPR54 protein were observed to show similar rhythmic peaks following treatment with 100.0 pM 17β-E2, also peaking at 24 (*, P<0.05 vs. EtOH, n=4) and 48 hrs following E2 treatment, although the difference at 48h did not reach statistical significance (Figure 1B). Additionally, since several previous studies have suggested that GnRH neurones express only oestrogen receptor beta (ERβ), but not ERα, we used specific agonists to explore if the above effect may be mediated via one of the classical genomic oestrogen receptors. In agreement with the observation that ERβ likely plays a larger role in GnRH neurones, we observed similarly robust rhythms of GPR54 transcript abundance following treatment with the ERβ-specific agonist DPN, but not with the ERβ-specific agonist PPT (Figure 2).
Figure 2.
GPR54 mRNA increases stimulated by 17β-E2 is mimicked by the ERβ agonist DPN, but not the ERα agonist PPT. GT1-7 cells were treated with 200.0pM PPT (PPT, open squares) or 200.0pM DPN (DPN, filled squares) after serum shock. GPR54 mRNA changes were quantified using real time PCR relative to the 0h time point, prior to treatment. GPR54 mRNA expression (mean ± SEM) showed a rhythmic pattern in DPN-treated cells, with peaks at 24-, 30- and 48 hours (P<0.05 vs PPT, n=4).
Experiment 2- Effects of estradiol treatment on kisspeptin-stimulated GnRH secretion
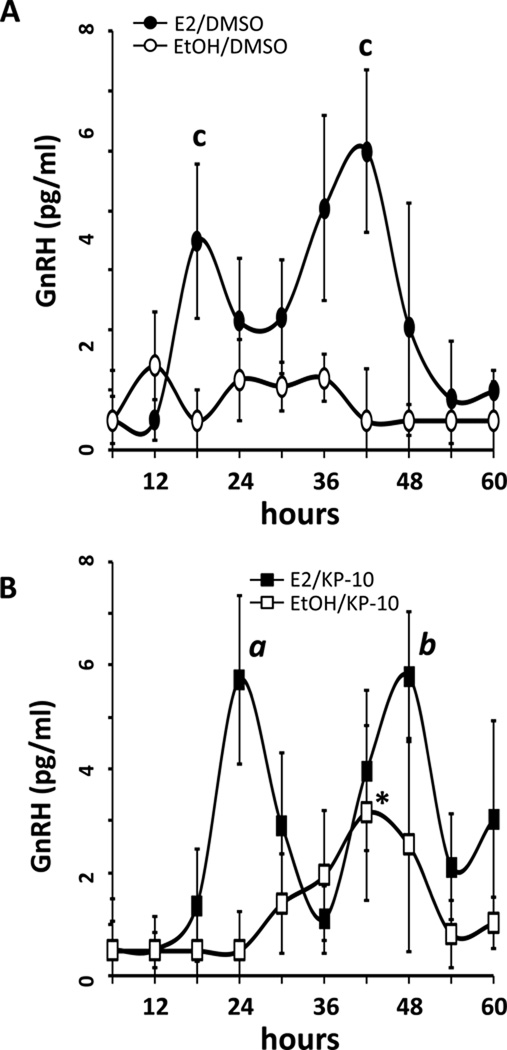
Following confirmation that exposure of GT1-7 cells to positive feedback levels of E2 resulted in rhythmic expression patterns of GPR54, we wanted to determine if temporal availability of this receptor resulted in commensurate sensitivity of these cells to kisspeptin stimulation, as measured by GnRH secretion into the culture medium. GT1-7 cells, either E2- or EtOH vehicle-treated, were exposed to 10.0 nM kisspeptin-10 for 45 minutes at 6 hr intervals, and GnRH secretion into the media was quantified by RIA. Kisspeptin-10 is comprised of amino acids 45–54 of the mature peptide, and is equally biologically active as the full protein (31). Similar to what was reported previously (32), kisspeptin-10 stimulated a minor increase in GnRH secretion from GT1-7 cells, but only between 30–48 hrs in serum-synchronized cells (Figure 3B, open squares; *, P<0.05 vs. EtOH/DMSO). Interestingly, GT1-7 cells exposed to E2 exhibited significant (a, P<0.05 vs. EtOH/KP-10; b, P<0.05 vs. EtOH) increases in GnRH secretion following Kiss-10 stimulation, with peaks in GnRH secretion occurring at 24 and 48 hours following E2 treatment (Figure 3B, filled squares), correlating temporally to elevated GPR54 abundance levels observed above in Figure 1. Of note, GnRH secretion from cells exposed to E2 and treated with DMSO vehicle instead of kisspeptin-10 was also both significantly elevated and rhythmic (c, P<0.05 vs. EtOH/DMSO), but exhibited slightly earlier secretory peaks at 18 and 42 hours, suggesting that positive feedback levels of E2 can increase GnRH secretion from GT1-7 cells even without stimulation by kisspeptin, confirming earlier observations using both short- and long-term oestradiol exposure (33).
Figure 3.
17β-E2 treatment potentiates Kisspeptin-induced GnRH secretion from GT1-7 cells. GT1-7 cells treated with 0.05% ethanol vehicle showed constitutively low GnRH release when stimulated with 0.01% DMSO (A; EtOH/DMSO, open circles), whereas GT1-7 cells exposed to 45 min treatments with 10.0 nM Kisspeptin-10 (B; EtOH/KP-10, open squares) produced modest but not significant GnRH release between 30 and 48 hours, except for 42h (*, P<0.05 vs. EtOH/DMSO, n=4). In contrast, GnRH release from GT1-7s treated with 100pM E2 and stimulated with DMSO (A; E2/DMSO, filled circles) showed circadian peaks in GnRH release at 18 and 42 hours after serum shock (c, P<0.05 vs EtOH, n=4). GnRH release from 100pM E2–treated cells (B; E2/KP-10, filled squares, n=4) also exhibited rhythmic release following kisspeptin stimulation, with peaks at 24 and 48 hours (a, P<0.05 vs EtOH/KP-10; b, P<0.05 vs EtOH/DMSO, n=4). Increases in GnRH secretion correspond with increased GPR54 mRNA and protein expression levels (Fig 1.) All values depicted are mean ± SEM.
Experiment 3- Determining the requirement of endogenous circadian clock activity for rhythmic abundance patterns of GPR54
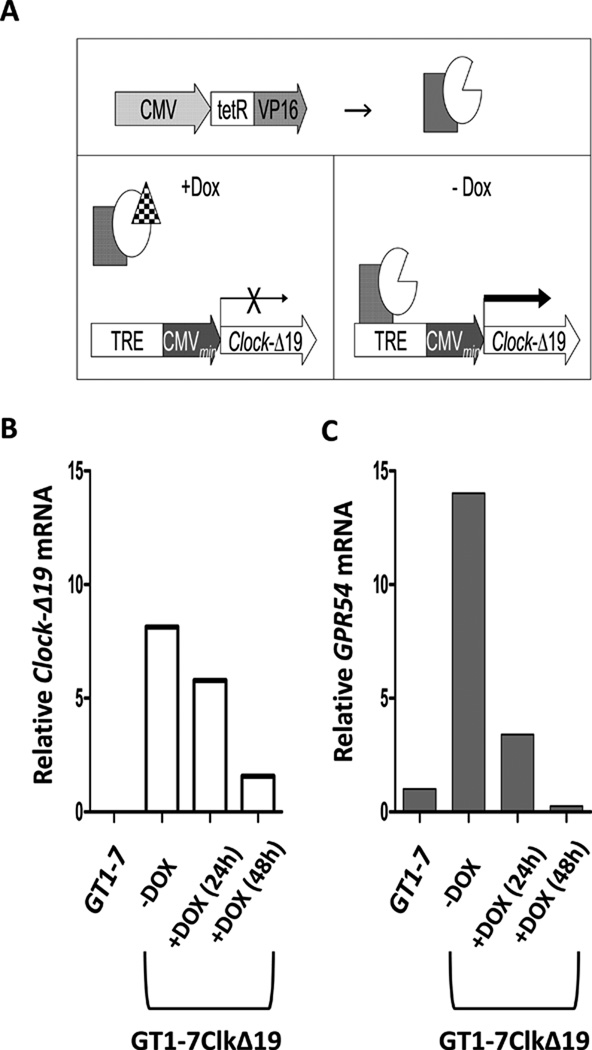
As a final step, we used a subcloned cell line derived from the GT1-7 cell line, GT1-7ClkΔ19 (Figure 4A), in which expression of the dominant negative CLOCK-Δ19 transcript is under the control of constitutively expressed TetR-VP16 operons, such that the mutant protein, driven by a tet-responsive element (TRE) can be conditionally repressed by exposure to the antibiotic doxycycline (DOX). Using this subcloned line, we can use DOX removal to initiate mutant protein expression, thus resulting in the rapid and robust disruption of endogenous circadian oscillators, an effect observed previously in this cell line using transient transfection (24). We thus used these cells to investigate the necessity of a functional circadian clock within GnRH neurones for the observed rhythmic abundance patterns of GPR54 following exposure to positive feedback levels of E2. As compared to GT1-7 cells, GT1-7ClkΔ19 cells express the mutant CLOCK-Δ19 protein at high levels, which are reduced over time by exposure to DOX (Figure 4B). Surprisingly, levels of GPR54 mRNA were also dramatically elevated in GT1-7ClkΔ19 cells, the levels of which returned to baseline following treatment with DOX (Figure 4C), suggesting that endogenous clock function may normally serve to keep kisspeptin receptor expression in check.
Figure 4.
The clock-disrupting CLOCK-Δ19 was stably transfected into GT1-7 cells using the tet-OFF in vitro system. (A) Expression of the dominant negative CLOCK-Δ19 occurs robustly in the absence of doxycycline (DOX, 10.0 µM) treatment. In the presence of DOX, CLOCK-Δ19 expression is repressed and the endogenous clock functions normally. (B) Clock-Δ19 mRNA is not present in wild-type GT1-7 cells, but is expressed in this subcloned line and is sensitive to DOX treatment. (C) Additionally, GPR54 mRNA is elevated in GT1-7Δ19 cells grown in the absence of DOX compared to wild-type GT1-7 cells or DOX-treated GT1-7Δ19 cells.
This increased expression was also observed over a longer temporal scale. As seen in Figure 5, GT1-7ClkΔ19 cells treated with either vehicle or 100.0 pM 17β-E2 display elevated and constitutive expression of GPR54 mRNA (Fig 5A) and GPR54 protein (Fig 5B), suggesting the requirement of a functional endogenous oscillator for E2-induced rhythmic expression patterns. Of note, GPR54 expression levels were constitutively higher in GT1-7ClkΔ19 cells in comparison to GT1-7 cells, both in the absence or presence of E2 exposure, suggesting that E2 is unable to modulate GPR54 expression levels in the absence of a functional circadian oscillator. 24h pretreatment of GT1-7ClkΔ19 cells with DOX decreased GPR54 mRNA levels in comparison to untreated cells, (Fig 4C and 5A), and 100.0 pM 17β-E2 treatment elicited a rhythm of GPR54 mRNA only in DOX-treated GT1-7Clk Δ19 cells (Fig 5A; filled squares/dashed lines).
Figure 5.
Induction of the dominant negative CLOCK-Δ19 in GT1-7 cells abolishes circadian variation in GPR54 mRNA and protein expression. (A) GPR54 mRNA expression in E2- (black diamonds, n=3) and EtOH- (white diamonds, n=3) treated GT1-7ClkΔ19 cells was elevated, but arrhythmic, in contrast to that observed in “clock-intact” DOX-pre-treated GT1-7 ClkΔ19 cells following E2 treatment (black squares, n=3; *, P<0.05 vs EtOH, white squares, n=3). (B) Average Western blot density (n=3) showing GPR54 protein expression in GT1-7ClkΔ19 cells treated with E2 or EtOH vehicle. No rhythms in GPR54 expression were detected in GT1-7ClkΔ19 cells. A representative western blot shows a ~45kDa band indicating GPR54, and COXIV loading controls (~18kDa). All values depicted are mean ± SEM.
Discussion
Although it is well established that elevated ovarian estradiol levels are required for LH and antecedent GnRH surge generation, it yet remains unclear how this steroid hormone can function as both a negative and positive feedback signal dependent upon concentration. Positive feedback levels of E2 do not correlate temporally with GnRH secretion above a particular threshold; circulating ovarian oestradiol levels are highest on the morning of pro-oestrus when GnRH secretion remains low (34). Noting the temporal confinement of the GnRH surge, it is thus conceivable that suprathreshold levels of oestradiol serve to initiate cellular processes which prime hypothalamic release patterns to elicit GnRH release governed by a circadian signal. While it is likely that synchronizing signals from the SCN and steroid hormone-sensitive AVPV play a role in surge generation, we chose to investigate possible direct effects of oestradiol on GnRH neurones themselves using the in vitro GT1-7 cell model. Recent studies have demonstrated that native GnRH neurones undergo alterations in sensitivity and neuronal activation with a circadian pattern only under the influence of positive feedback levels of oestradiol (4) thus we attempted to determine if one way E2 acts to elicit these changes in GnRH neuronal sensitivity is by affecting temporal patterns of gene expression of receptors for factors such as kisspeptin. Using real-time qRT-PCR and western blotting, we have demonstrated that kisspeptin’s cognate receptor, GPR54, exhibits circadian rhythms of expression in GT1-7 cells only following an appropriate duration of elevated oestradiol exposure. GPR54 protein abundance also exhibits a similar rhythm, interestingly without a noticeable lag between mRNA and protein, which may however be reflective of the temporal resolution of sampling, such that more frequent samples may reveal a lag of several hours.
Evidence suggests that E2 may sensitise GnRH neurones to multiple afferent signals, such as glutamate and GABA (35), as well as influencing temporal patterns of cation channel expression and abundance (36), such that increasing sensitivity to kisspeptin may represent one of several redundant mechanisms involved to insure GnRH surge generation. This is demonstrated in the current study in Figure 3, in which elevated and rhythmic GnRH secretion was observed from GT1-7 cells treated with E2 even without exogenous kisspeptin-10 stimulation. Our experiments suggest that E2 directly mediates effects on GT1-7 cell sensitivity and can stimulate GnRH secretion in vitro from these cells, and that these effects requirea functioning intracellular circadian oscillator. It should be noted, however, that these in vitro results have yet to be verified in GnRH neurones in vivo, and it remains unclear if GnRH-specific circadian oscillators are required for E2-stimulated GnRH surge generation in animal models. A previous study using GT1-7 cells showed that GPR54 mRNA exhibited low-amplitude circadian rhythms in the absence of E2 (26), while our current study observed more robust rhythms in the presence of E2. The current study does not elucidate how E2 treatment results in the rhythmic expression of this receptor; however, observation of differential transcript rhythms in the presence of steroid hormones is not unprecedented. Although still incompletely characterized, there is growing evidence that nuclear receptors can interact with core clock machinery (37), including recent evidence that glucocorticoids may act to repress rhythms of core clock and clock-controlled gene expression in both the brain and periphery (38, 39). Further studies will be required to determine more specifically how steroid hormones acting via either nuclear or membrane receptors might be interacting with endogenous oscillators.
Our results are also consistent with previous work using GT1-7 cells to investigate GPR54 expression. Specifically, another group noted that E2 stimulated GPR54 mRNA levels in GT1-7 cells at 24 hours, but did not explore transcript rhythms (40). Interestingly, the GT1-7ClkΔ19 cells, which harbour a disrupted circadian clock, exhibited constitutively elevated GPR54 levels, suggesting that in addition to an increase in GnRH neuronal sensitivity to kisspeptin on proestrus afternoon, the quiescence in GnRH secretion on the morning of pro-oestrus may also be mediated by an endogenous oscillator, keeping GnRH neuronal sensitivity low until the appropriate temporal window. Interestingly, preliminary results suggest that this observed elevation in GPR54 mRNA in GT1-7ClkΔ19 cells does not translate into increases in kisspeptin-stimulated GnRH secretion. Indeed, as observed in Figure 3, kisspeptin only stimulated GT1-7 cells at certain times, an effect that appears to have been potentiated by E2. Since GnRH neurones in vivo localize GPR54 to axonal terminals in the ME as well as in perikarya, it remains a possibility that GPR54 in GT1-7 cells are sub-functional due to a lack of this typical morphological configuration in vitro, resulting in diminished GnRH secretion following kisspeptin treatment. Another possibility underlying observed differences in kisspeptin’s ability to stimulate GnRH secretion in vivo vs. in vitro could be ascribed to the neurochemical context that is present in vivo only, supported by studies demonstrating modulation of GABA and glutamate release on to GnRH neurones in vivo (35).
In a very recent article, Robertson et al. demonstrated that kisspeptin expression levels in the AVPV increased concomitantly with the LH surge in E2-primed, ovariectomized (OVX) mice, and that the largest percentage of kisspeptin neurones expressing c-fos occurred at the same time, around circadian time (CT) 11-12 (17). This increase in hypothalamic kisspeptin expression and c-Fos co-localization appears to occur with a circadian period, but only under conditions of positive feedback levels of E2. Interestingly, a kisspeptin expression rhythm was not observed in unprimed OVX females, suggesting that E2 is required for inducing increases in kisspeptin expression. The necessity of E2 for circadian kisspeptin production further supports the presence of steroid-induced circadian rhythms in the hypothalamus. However, in line with the results presented above, a very recent study demonstrates that exogenously applied kisspeptin can only stimulate c-fos expression in GnRH neurones in vivo dependent on oestradiol exposure and time of day (21), further implicating changes in GnRH neuronal sensitivity gated by steroid milieu interacting with endogenous oscillators. There may also exist redundant mechanisms to ensure temporal control of GnRH surge generation, as earlier studies demonstrated that icv vasopressin injection can induce LH surges in both wild-type and Clock/Clock mutant mice, suggesting that increases in SCN-derived neuropeptides may be able to circumvent the absence of an endogenous GnRH oscillator. Interestingly, antagonism of the vasopressin receptor AVPR1A in wild-type mice was unable to prevent surges, raising the possibility that endogenously-timed kisspeptin release and reception may be sufficient for GnRH surge release in the absence of vasopressin signalling (41).
Additionally, our results suggest that this direct effect of E2 on kisspeptin receptor expression levels in GnRH neurones may be mediated by ERβ, the E2 receptor isoform which has been shown to be expressed in these cells (42, 43). ERβ activation in GnRH neurones is likely a functionally redundant mechanism to ensure optimal surge amplitude, however, since mice lacking ERα display a more severe reproductive phenotype than ERβ knockout mice (43). We can also not exclude the possibility that E2 and DPN could activate incompletely-characterized membrane-associated E2 receptors, which exert their effects via non-classical signalling mechanisms (44).
Future studies will investigate the signalling pathways by which E2 exerts its effects within GnRH neurones, and will explore transcriptional mechanisms underlying how E2 can alter patterns of transcriptional rhythmicity at the level of gene promoters, perhaps by the interaction of ERs with core circadian clock proteins, which has been observed to occur in cancer cell types (45). In addition to demonstrating an additional level of E2 modulation of neuroendocrine control of reproductions, our results suggest a novel form of transcriptional regulation, by which steroid hormones may alter the temporal nature of gene expression via interaction with endogenous cellular oscillators.
Abbreviations
- GnRH
gonadotrophin-releasing hormone
- OVX
ovariectomized
- E2
oestrogen
- LH
luteinising hormone
- ER
oestrogen receptor
- SCN
suprachiasmatic nuclei
- ARC
arcuate nuclei
- AVPV
antero-ventral periventricular nuclei
- MBH
medio-basal hypothalamus
- Kiss-1
kisspeptin-1
- icv
intracerebroventricular
References
- 1.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19(3):302–330. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 2.Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96(1):50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- 3.Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96(1):57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- 4.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A. 2005;102(43):15682–15687. doi: 10.1073/pnas.0504270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seminara SB. Metastin and its G protein-coupled receptor, GPR54: critical pathway modulating GnRH secretion. Front Neuroendocrinol. 2005;26(3–4):131–138. doi: 10.1016/j.yfrne.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102(6):2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuohung W, Kaiser UB. GPR54 and KiSS-1: role in the regulation of puberty and reproduction. Rev Endocr Metab Disord. 2006;7(4):257–263. doi: 10.1007/s11154-006-9020-2. [DOI] [PubMed] [Google Scholar]
- 8.Tena-Sempere M. GPR54 and kisspeptin in reproduction. Hum Reprod Update. 2006;12(5):631–639. doi: 10.1093/humupd/dml023. [DOI] [PubMed] [Google Scholar]
- 9.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30(10):504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J Neuroendocrinol. 2009;21(4):305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- 12.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27(44):12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano JM, Navarro VM, Fernandez-Fernandez R, Castano JP, Malagon MM, Aguilar E, Dieguez C, Magni P, Pinilla L, Tena-Sempere M. Ontogeny and mechanisms of action for the stimulatory effect of kisspeptin on gonadotropin-releasing hormone system of the rat. Mol Cell Endocrinol. 2006;257–258:75–83. doi: 10.1016/j.mce.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 16.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 17.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adachi S, Yamada S, Takatsu Y, Matsui H, Kinoshita M, Takase K, Sugiura H, Ohtaki T, Matsumoto H, Uenoyama Y, Tsukamura H, Inoue K, Maeda K. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J Reprod Dev. 2007;53(2):367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 19.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vida B, Deli L, Hrabovszky E, Kalamatianos T, Caraty A, Coen CW, Liposits Z, Kallo I. Evidence for suprachiasmatic vasopressin neurones innervating kisspeptin neurones in the rostral periventricular area of the mouse brain: regulation by oestrogen. J Neuroendocrinol. 22(9):1032–1039. doi: 10.1111/j.1365-2826.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams WP, 3rd, Jarjisian SG, Mikkelsen JD, Kriegsfeld LJ. Circadian control of kisspeptin and a gated GnRH response mediate the preovulatory luteinizing hormone surge. Endocrinology. 152(2):595–606. doi: 10.1210/en.2010-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31(2):147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 23.Mosko SS, Moore RY. Neonatal ablation of the suprachiasmatic nucleus. Effects on the development of the pituitary-gonadal axis in the female rat. Neuroendocrinology. 1979;29(5):350–361. doi: 10.1159/000122944. [DOI] [PubMed] [Google Scholar]
- 24.Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci. 2003;23(35):11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickok JR, Tischkau SA. In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology. 91(1):110–120. doi: 10.1159/000243163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao S, Kriegsfeld LJ. Daily changes in GT1-7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology. 2009;89(4):448–457. doi: 10.1159/000192370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennaway DJ. The role of circadian rhythmicity in reproduction. Hum Reprod Update. 2005;11(1):91–101. doi: 10.1093/humupd/dmh054. [DOI] [PubMed] [Google Scholar]
- 28.Kennaway DJ, Boden MJ, Voultsios A. Reproductive performance in female Clock Delta19 mutant mice. Reprod Fertil Dev. 2004;16(8):801–810. doi: 10.1071/rd04023. [DOI] [PubMed] [Google Scholar]
- 29.Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14(15):1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 31.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 32.Novaira HJ, Ng Y, Wolfe A, Radovick S. Kisspeptin increases GnRH mRNA expression and secretion in GnRH secreting neuronal cell lines. Mol Cell Endocrinol. 2009;311(1–2):126–134. doi: 10.1016/j.mce.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro CE, Abdul Saeed S, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3',5'-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol Endocrinol. 2003;17(9):1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- 34.Krey LC, Parsons B. Characterization of estrogen stimuli sufficient to initiate cyclic luteinizing hormone release in acutely ovariectomized rats. Neuroendocrinology. 1982;34(5):315–322. doi: 10.1159/000123320. [DOI] [PubMed] [Google Scholar]
- 35.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci. 2007;27(8):1913–1921. doi: 10.1523/JNEUROSCI.4738-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimura I, Ui-Tei K, Saigo K, Ishii H, Sakuma Y, Kato M. 17beta-estradiol at physiological concentrations augments Ca(2+) -activated K+ currents via estrogen receptor beta in the gonadotropin-releasing hormone neuronal cell line GT1-7. Endocrinology. 2008;149(2):774–782. doi: 10.1210/en.2007-0759. [DOI] [PubMed] [Google Scholar]
- 37.Grimaldi B, Bellet MM, Katada S, Astarita G, Hirayama J, Amin RH, Granneman JG, Piomelli D, Leff T, Sassone-Corsi P. PER2 controls lipid metabolism by direct regulation of PPARgamma. Cell Metab. 12(5):509–520. doi: 10.1016/j.cmet.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koyanagi S, Okazawa S, Kuramoto Y, Ushijima K, Shimeno H, Soeda S, Okamura H, Ohdo S. Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Mol Endocrinol. 2006;20(3):573–583. doi: 10.1210/me.2005-0165. [DOI] [PubMed] [Google Scholar]
- 39.Liu RY, Unmehopa UA, Zhou JN, Swaab DF. Glucocorticoids suppress vasopressin gene expression in human suprachiasmatic nucleus. J Steroid Biochem Mol Biol. 2006;98(4–5):248–253. doi: 10.1016/j.jsbmb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Jacobi JS, Martin C, Nava G, Jeziorski MC, Clapp C, Martinez de la Escalera G. 17-Beta-estradiol directly regulates the expression of adrenergic receptors and kisspeptin/GPR54 system in GT1-7 GnRH neurons. Neuroendocrinology. 2007;86(4):260–269. doi: 10.1159/000107770. [DOI] [PubMed] [Google Scholar]
- 41.Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod. 2006;75(5):778–784. doi: 10.1095/biolreprod.106.052845. [DOI] [PubMed] [Google Scholar]
- 42.Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23(13):5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73(9–10):985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gery S, Koeffler HP. Circadian rhythms and cancer. Cell Cycle. 9(6) doi: 10.4161/cc.9.6.11046. [DOI] [PMC free article] [PubMed] [Google Scholar]