Abstract
Cadmium is categorized as a human carcinogen especially involved in lung cancers. Angiogenesis is considered a fundamental requirement for tumorigenesis, but the mechanisms underlying the tumor angiogenesis induced by cadmium are poorly understood. Using in vitro and in vivo models, we investigated the angiogenic mechanisms of cadmium in human bronchial epithelial cells and tumor formation. Our results demonstrated that cadmium (CdCl2) activated extracellular signal-regulated kinases (ERK) and AKT signaling and elevated the expression of a key downstream proangiogenic molecule hypoxia-inducible factor-1 (HIF-1) in immortalized human lung epithelial BEAS-2B cells. Cadmium also induced reactive oxygen species (ROS) production, which could be inhibited by ROS scavengers, catalase and diphenyleneiodonium chloride. Inhibition of ROS generation also attenuated ERK, AKT, p70S6K1 activation, and HIF-1α expression. Similar results were obtained in normal human bronchial epithelial (NHBE) cells, showing that cadmium induced HIF-1 expression via ROS/ERK/AKT signaling pathway. Furthermore, cadmium induced vascular endothelial growth factor expression and transcriptional activation through ROS, ERK, and AKT pathways. Finally, cadmium transformed human bronchial epithelial cells in culture; the transformed cells induced tube formation in vitro, angiogenesis on chicken chorioallantoic membrane, and formed tumors in nude mice. Taken together, the results of this study provide explanation for the role and molecular mechanisms of cadmium in promoting angiogenesis in lung epithelial cells and malignant transformation and will be helpful for improved occupational protection, prevention, as well as chemotherapy of human lung cancers caused by heavy metal cadmium.
Keywords: cadmium, ROS, ERK, AKT, angiogenic factor, transformation
Cadmium and its compounds exist ubiquitously in the environment, food, drinking water, and particularly in the polluted occupational workplaces (Jarup et al., 1998). Heavy metals are toxic and have always presented an occupational and environmental concern for industrial personnel and populations living in the contaminated areas (Bernard, 2008; Sethi and Khandelwal, 2006). The International Agency for Research on Cancer has classified cadmium as a human carcinogen according to the evidence from both humans and experimental animal studies (IARC, 1993). Epidemiological and experimental studies have identified the target organs of carcinogenic cadmium compounds, including prostate (Achanzar et al., 2001; Benbrahim-Tallaa et al., 2007a,b; Goyer et al., 2004; Nakamura et al., 2002), breast (Barrett, 2009; Benbrahim-Tallaa et al., 2009; Brama et al., 2007; Siewit et al., 2010), urinary bladder (Kellen et al., 2007; Sens et al., 2004), kidney (Il'yasova and Schwartz, 2005), liver (Kawata et al., 2009; Qu et al., 2005), and lung (Beveridge et al., 2010; Cox, 2006), particularly in tobacco smokers. Tobacco consumption is an additional source of cadmium exposure (Satarug and Moore, 2004). Like many other plants, tobacco leaves selectively accumulate cadmium from soil. It has been estimated that one cigarette contains about 1–2 μg cadmium. When the exposure is through cigarette smoking, ∼10–50% of the cadmium content is absorbed in the lung, depending on the particle size and the solubility of cadmium compounds (Bernard, 2008; Jarup and Akesson, 2009; Sahmoun et al., 2005). After absorption, cadmium is transported by blood and stored in organs rich in a small protein, metallothionein, which exhibits high binding affinity for cadmium (Jin et al., 1998; Waalkes and Klaassen, 1985). Because cadmium has an exceptionally long (10–30 years) half-life in human body (Jarup et al., 1998), its carcinogenicity continues to be a major health concern.
Although the linkage between cadmium exposure and the incidence of tumor in some target organs is still elusive, cadmium is a proven cause of human lung cancers (Filipic et al., 2006; Joseph, 2009; Waalkes, 2003). Studies employing in vitro cell culture and in vivo animal models have revealed some of the mechanisms underlying cadmium carcinogenesis, including aberrant gene expression, DNA repair inhibition, apoptosis resistance, and oxidative stress induction; application of new techniques, such as differential gene and protein expression profiling, may provide further insights into the mechanisms involved in cadmium toxicity and carcinogenesis (Joseph, 2009). However, further studies are necessary to clarify the etiology of cadmium-induced carcinogenesis.
Angiogenesis is the process of growing new blood vessels from the preexisting ones (Woods et al., 2008). The blood vessel network mediates the delivery of oxygen and nutrients to tissues of the body and the removal of metabolites. Thus, angiogenesis is not only important for normal physiological functioning but also critical for many pathological processes such as tumor initiation, proliferation, and metastasis (Blagosklonny, 2004; Folkman, 2002; Prozialeck et al., 2006). It has been well established that angiogenesis is a fundamental requirement for tumor development. Studies have demonstrated that vascular endothelium could be a primary target of cadmium toxicity (Prozialeck et al., 2006; Woods et al., 2008). Some studies reported disruptive effects of cadmium on human endothelial cells (Helmestam et al., 2010; Kolluru et al., 2006; Majumder et al., 2008; Woods et al., 2008). Cadmium has dual effects: the low concentration of cadmium (5 and 10μM) increases vascular endothelial growth factor (VEGF) secretion, vascular endothelial growth factor receptor-2 (VEGFR2) activity, and the tube formation in human umbilical vein endothelial cells (HUVECs); whereas high concentration of cadmium causes cell damage and inhibits VEGF expression, VEGFR2 activity, and tube formation (Kim et al., 2011). A recent study has shown that cadmium impairs the capability of human breast cancer cells to induce angiogenesis (Pacini et al., 2009). However, little is known about the effects and molecular mechanisms of cadmium on the angiogenic potential of human lung epithelial cells.
To elucidate the role and mechanisms of cadmium in regulating angiogenesis, we investigated the effect of cadmium on reactive oxygen species (ROS), mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK), AKT, and p70S6K1 signaling pathways and the downstream proangiogenic factors hypoxia-inducible factor-1 (HIF-1) and VEGF in human lung epithelial cells. We report a novel mechanism that involves the activation of HIF-1 and VEGF through ROS, ERK, and AKT signaling pathways by cadmium that results in human lung epithelial cell malignant transformation. Clarifying the mechanisms of cadmium-induced angiogenesis, malignant transformation, and tumorigenesis would provide a rationale for occupational protection, prevention, and chemotherapy in cadmium-induced lung cancers.
MATERIALS AND METHODS
Reagents and antibodies.
Cadmium chloride hemi (CdCl2.21/2H2O), catalase, diphenyleneiodonium chloride (DPI), rapamycin, U0126, LY294002, dimethyl sulfoxide (DMSO), and anti–β-actin antibody were purchased from Sigma-Aldrich (St Louis, MO). CdCl2 was dissolved in H2O, with the pH of 5μM CdCl2 at 7.1; stocks of U0126, LY294002, and rapamycin were prepared in DMSO and stored at −20°C. Fetal bovine serum (FBS) was from Invitrogen/GIBCO (Grand Island, NY). Antibodies against phospho-ERK, phospho-AKT, AKT, and phospho-p70S6K1 were from Cell Signaling Technology (Beverly, MA). Antibodies against ERK and p70S6K1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against HIF-1α and HIF-1β were from BD Biosciences (San Jose, CA). 2′,7′-Dichlorofluorescein diacetate (DCFH-DA) was from Molecular Probe (Eugene, OR).
Adenoviruses.
The recombinant adenovirus, Ad-catalase, was purchased from the University of Iowa. Control virus carrying green fluorescent protein (Ad-GFP) was subcloned into adenoviral vector using Ad-Easy system (Stratagene, San Diego, CA). The viruses were amplified in Ad-293 cells, viral titers were determined using the Adeno-X Rapid Titer Kit from BD Biosciences. The cells were infected with adenoviruses at 25 multiplicities of infection (MOI).
Cell culture.
Human lung bronchial epithelial cells (BEAS-2B) and HUVECs were purchased from American Type Culture Collection (Manassas, VA). Normal human bronchial/tracheal epithelial (NHBE) cells were from Lonza (Walkersville, MD). BEAS-2B cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) medium (Cellgro, Manassas, VA) containing 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. HUVECs were cultured in Clonetics EBM-2 medium supplemented with EGM-2 MV SingleQuotes (Lonza). Primary NHBE cells were cultured in BEGF BulletKit (Lonza) containing bronchial epithelial growth medium and supplements. All the cells were cultured in humidified 5% CO2 incubator at 37°C. The time period of cadmium treatment is determined by the specific individual experiment.
Western blotting analysis.
After appropriate treatments, cells were washed with ice-cold PBS (140mM NaCl, 3mM KCl, 6mM Na2HPO4, and 1mM KH2PO4, pH 7.4), scraped off the dish gently, and harvested by centrifugation at 1200 × g for 5 min. The cell pellets were lysed for 30 min on ice using radioimmunoprecipitation assay buffer (150mM NaCl, 100mM Tris [pH 8.0], 1% Triton X-100, 1% deoxycholic acid, 0.1% SDS, 5mM EDTA, and 10mM NaF) containing 1mM phenylmethylsulfonyl fluoride, 2mM aprotinin, 2mM leupeptin, 2mM pepstatin A, 1mM sodium vanadate, and 1mM dithiothreitol. The cell lysates were centrifuged at 13,000 × g for 15 min, and the supernatants of the total protein extracts were collected. The concentrations of protein extracts were measured using Bio-Rad protein assay reagent (Richmond, CA) and a microplate reader from Berthold Technologies (Oak Ridge, TN). The protein samples were mixed with 2× loading buffer, heated at 100°C for 5 min, and subjected to SDS-polyacrylamide gel electrophoresis. The resolved proteins were transferred to nitrocellulose membranes and blocked with 5% nonfat milk in Tris buffer saline containing 0.05% Tween 20. The membranes were then incubated with appropriate first and horseradish peroxidase–conjugated secondary antibodies, and the protein blots were visualized by the incubation with enhanced chemiluminescence reagent from Pierce (Thermo Scientific, Rockford, IL). The luminescent signals were analyzed using an ImageQuant LAS 4000 Scanner of GE Healthcare (Piscataway, NJ).
Reverse transcription polymerase chain reaction.
Total RNAs of BEAS-2B cells treated with cadmium and appropriate inhibitors were isolated using TRIzol reagent from Invitrogen (Carlsbad, CA). RNA concentrations were measured using a NanoDrop spectrophotometer from Thermo Scientific (Wilmington, DE). One microgram of total RNAs was used as template to synthesize the first strand complementary DNA by Avian Myeloblastosis Virus reverse transcriptase and then amplified by GoTaq DNA Polymerase from Promega (Madison, WI). The primers used for PCR amplification are as follows: VEGF sense, 5′-TCGGGCCTCCGAAACCAT-3′; antisense, 5′-CCTGGTGAGAGATCTGGT-3′; glyceraldehyde-3-phosphate dehydrogenase sense, 5′-TGTTGCCATCAATGACCCCTT-3′; antisense, 5′-CTCCACGACGTACTCAGCG-3′. The PCR reaction was performed at 95°C for 5 min and 28 cycles with each at 95°C for 1 min, 59°C for 1 min, 72°C for 1 min, and with a 15-min extension at 72°C. PCR products were separated by 1% agarose gel and visualized by Phenix GelRed Nucleic Acid Stain (Candler, NC) under UV light. The fluorescent signals were analyzed using the ImageQuant LAS 4000 Scanner of GE Healthcare.
Luciferase (luc) reporter assay.
BEAS-2B cells were seeded in 12-well plates at a density of 2 × 105 cells/well and cultured overnight. The cells were transiently transfected using Lipofectamine (Invitrogen, Carlsbad, CA) with 0.4 μg reporter and 0.2 μg β-galactosidase (β-gal) plasmids. After changing with complete DMEM medium, the cells were either infected with 25 MOI adenoviruses for 48 h or exposed to appropriate inhibitors 12 h before harvest. The cells were treated with 2.5μM CdCl2 in the final 12 h of incubation. After washing with PBS, the cells were lysed with reporter lysis buffer from Promega. The luciferase activities of the cell extracts were measured using a fluorescent microplate reader (Berthold Technologies). β-Gal activities were determined in assay buffer containing 100mM phosphate [pH 7.5], 2mM MgCl2, 100mM β-mercaptoethanol, 1.33 mg/ml o-nitrophenyl β-D-galactopyranoside. The relative luciferase activities were normalized to the controls and calculated as the ratio of luc/β-gal.
Cell transformation and tumor formation assay.
BEAS-2B cells were exposed to CdCl2 for 6 months beginning with a concentration of 1μM. BEAS-2B cells passaged at the same time were used as the control. To test whether cadmium-exposed and control cells can grow independently of attachment, anchorage-independent assay was performed. An equal number of control BEAS-2B and cadmium-transformed (Cd-T) cells (3 × 103) were suspended in 2 ml DMEM (10% FBS) containing 0.5% SeaPlaque Agar (BioWhittaker Molecular Applications, Rockland, ME). Cell suspension was overlaid on 1 ml polymerized 0.3% SeaPlaque Agar in six-well plates; 1 ml 0.3% agar-DMEM (10% FBS) was overlaid on each well once a week. Colonies were counted and images were taken under microscope after 2 weeks. Experiments were performed in triplicate. To test whether the Cd-T cells can form tumors in nude mice, the Cd-T and control BEAS-2B cells were trypsinized and suspended in serum-free medium. An equal number of cells (1 × 106) in 20 μl were mixed with the same volume of Extracel Hydrogel (Glycosan Biosystems, Salt Lake City, UT) and injected subcutaneously into the flanks of female NU/NU-nuBR immunodeficient mice (Charles River Laboratories, Wilmington, MA). Two weeks later, the mice were sacrificed. The weight of tumor tissues was measured.
Intracellular H2O2 detection.
The specific fluorescent dye, DCFH-DA, for H2O2 is used to detect the intracellular H2O2 level. BEAS-2B cells were seeded in a six-well plate at 40–50% confluence and cultured overnight. The cells were treated without or with 1500 U/ml catalase or 5μM DPI for 30 min, followed by 5μM CdCl2 treatment for 2 h in serum-free medium. Then, 5μM DCFH-DA was added for 15 min. The cells were washed three times with PBS and fixed with 10% buffered formalin for 10 min. After washing, the fluorescent images were captured using a fluorescence microscope (Olympus, Miami, FL). Similar experiment was performed using BEAS-2B control and Cd-T cells stained by DCFH-DA.
Tube formation assay.
Control BEAS-2B and Cd-T cells were cultured to subconfluence. The cells were washed with 1 × PBS and incubated for 15 h in basic EBM-2 medium containing 1% FBS. HUVECs at subconfluence were starved overnight in EBM-2 basic medium containing 0.2% FBS. The 96-well plate was taken, and each well was precoated with 50 μl growth factor–reduced Matrigel. The plate was placed in a 37°C incubator to allow the gel to solidify. The starved HUVECs were trypsinized and counted in basic EBM-2 medium. The cells were mixed with 50 μl freshly collected conditioned media from control BEAS-2B and Cd-T cells and seeded to the 96-well plate at 1 × 104 cells/well. Tube formation was examined and photographed at 8 h under microscope. The lengths of the tubes were measured using Olympus cellSens Standard digital imaging software.
Tumor angiogenesis assay on chick chorioallantoic membrane.
Fertilized chicken eggs from Charles River Laboratories (North Franklin, CT) were incubated at 37°C. BEAS-2B cells were exposed to 5μM CdCl2 for 6 h on day 10. The cells were then washed with 1 × PBS, trypsinized, and suspended in serum-free DMEM medium at 1.5 × 107 cells/ml. Aliquots of 40 μl cell suspension were implanted onto the chick chorioallantoic membrane (CAM) of cultured chicken embryos. Four days after the implantation, the tumor tissues were harvested and photographed under a Nikon dissecting microscope. The branches of the newly formed blood vessels were counted and analyzed.
Statistics.
The values in the study were presented as mean ± SE. Differences were statistically analyzed by SigmaStat 3.1 (Systat Software, Inc.) using Student’s t-test. The difference is considered as significant at p < 0.05.
RESULTS
Cadmium Activated ERK, AKT, and P70S6K Signaling and Increased HIF-1α Expression in a Time-Dependent Manner
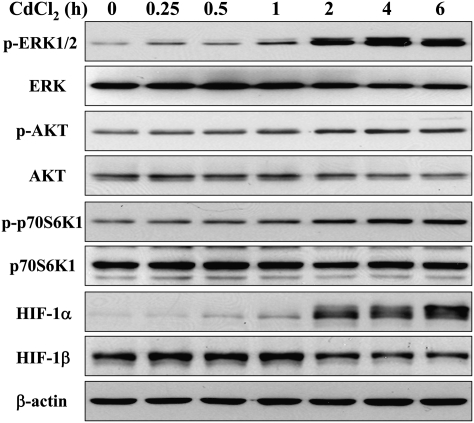
In order to determine the effects of cadmium on the signaling molecules in human lung epithelial cells, we first investigated whether cadmium treatment changes the expression of ERK, AKT, and p70S6K1, the important pathways regulating tumor angiogenesis through downstream signaling molecules HIF-1 and VEGF. Human airway epithelial BEAS-2B cells were exposed to 5μM CdCl2 for different periods of time. The effects on ERK, AKT, and p70S6K1 signaling were analyzed by immunoblotting. The phosphorylation/activation of ERK, AKT, and p70S6K1 and increased level of HIF-1α were induced by cadmium treatment in a time-dependent manner, whereas the total ERK, AKT, and p70S6K1 as well as HIF-1β were not elevated (Fig. 1).
FIG. 1.
Cadmium activates ERK and AKT signaling pathways in a time-dependent manner. BEAS-2B cells were exposed to 5μM CdCl2 for different periods of time. The total cellular lysates were analyzed by immunoblotting with antibodies against p-ERK, p-AKT, p-p70S6K1, HIF-1α, and β-actin; the membranes were stripped and reprobed for ERK, AKT, p70S6K1, and HIF-1β.
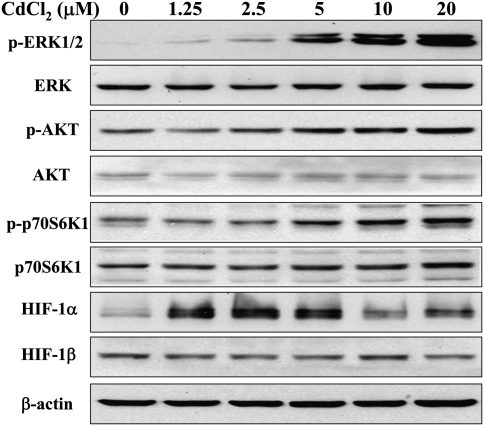
Cadmium Activated ERK, AKT, and P70S6K1 Signaling in a Dose-Dependent Manner and Increased HIF-1α Expression
BEAS-2B cells were treated for 4 h with different concentrations of CdCl2. The effects on ERK, AKT, and p70S6K1 signaling were analyzed by immunoblotting. The activations of ERK, AKT, and p70S6K1 were induced by 5, 10, and 20μM cadmium treatment in a dose-dependent manner, whereas the total ERK, AKT, and p70S6K1 as well as HIF-1β were not affected (Fig. 2). HIF-1α, a downstream target of ERK and AKT pathways, was remarkably induced by 1.25, 2.5, and 5μM cadmium treatment, then attenuated when treated with 10 and 20μM cadmium, indicating that other mechanism such as protein stability or toxicity due to treatment could also be involved in HIF-1α expression. Because exposure to 5μM of CdCl2 was sufficient to activate these pathways, the same treatment conditions were maintained in the following experiments.
FIG. 2.
Cadmium activates ERK and AKT signaling pathways in a concentration-dependent manner. BEAS-2B cells were treated for 4 h with indicated concentrations of CdCl2. The total cellular lysates were analyzed by immunoblotting with antibodies against p-ERK, p-p70S6K1, HIF-1α, and β-actin; the membranes were stripped and reprobed for ERK, p70S6K1, and HIF-1β.
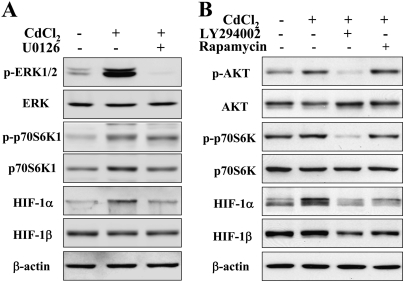
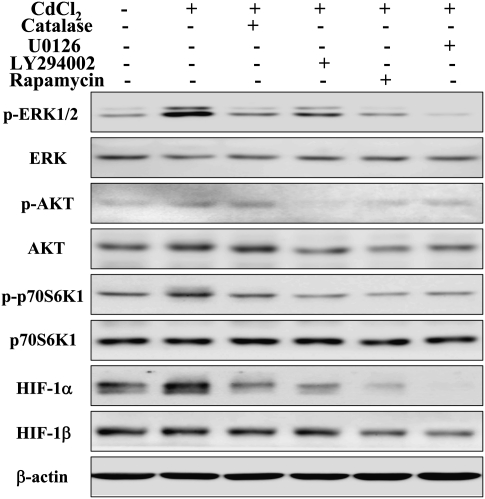
Inhibition of ERK and AKT Activation Suppressed Cadmium-Induced HIF-1α Expression
To further determine whether ERK and AKT pathways are necessary for cadmium-induced HIF-1α expression, BEAS-2B cells were pretreated with 20μM U0126, 15μM LY294002, or 5 nM rapamycin prior to 5μM CdCl2 treatment for 4 h. Western blotting results showed that U0126 (protein kinase-ERK kinase [MEK]/ERK inhibitor), LY294002 (phosphatidylinositol-3-kinase [PI3K]/AKT inhibitor) or rapamycin suppressed CdCl2-induced activation of p70S6K1 and expression of HIF-1α, as well as ERK or AKT activation, respectively (Fig. 3). The results indicate that ERK and AKT activation were required for cadmium-induced HIF-1α expression through p70S6K1 activation.
FIG. 3.
Cadmium-induced activation of ERK signaling was suppressed by the specific inhibitors U0126 (A), LY294002 and rapamycin (B). BEAS-2B cells were incubated with 20μM U0126, 15uM LY294002 or 5nM rapamycin for 30 min, followed by exposure to 5μM CdCl2 for 4 h. The total cellular lysates were analyzed by immunoblotting with antibodies against p-ERK, p-p70S6K1, HIF-1α, and β-actin. The membranes were stripped and reprobed for ERK, AKT, p70S6K1, and HIF-1β.
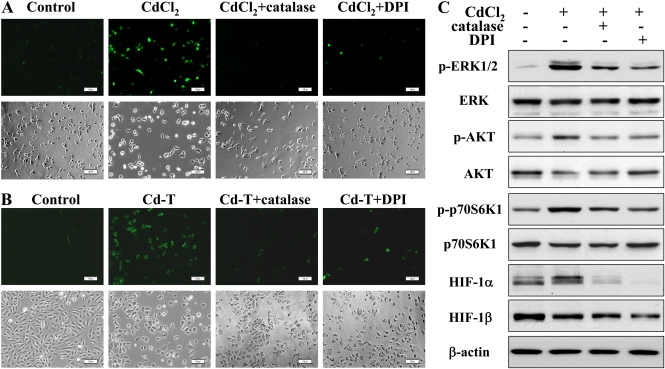
Cadmium-Induced ROS Production and ROS Are Upstream of ERK and AKT Signaling Pathways
ROS, such as H2O2, are commonly induced by carcinogens and involved in elevated expression of HIF-1α and VEGF (Gao et al., 2004). To test the effect of cadmium on ROS production, the level of H2O2 was determined by DCFH-DA staining. Cadmium induced H2O2 generation in the CdCl2-treated and Cd-T BEAS-2B cells, and the induction was remarkably suppressed by the ROS inhibitors, catalase and DPI (Figs. 4A and 4B), suggesting that cadmium induces ROS generation. Furthermore, catalase and DPI also inhibited cadmium-induced expression of HIF-1α through ERK, AKT, and p70S6K1 signaling (Fig. 4C). These results indicate that cadmium-induced ROS increase the expression of HIF-1α through ERK and AKT activation.
FIG. 4.
ROS inhibitors suppress cadmium-induced ROS generation and the activation of ERK signaling. (A) BEAS-2B cells were incubated with 1500 U/ml catalase and 5μM DPI for 30 min in serum-free medium, followed by addition of 5μM of cadmium for 2 h. The cells were fixed and stained by DCFH-DA; fluorescent images were captured using a fluorescent microscope (upper panel). The corresponding phase micrographs are shown in the bottom panel. Bar, 100 μm. (B) Catalase- and DPI-treated control and Cd-T BEAS-2B cells were fixed and stained, and the images were captured as described above. Bar, 100 μm. (C) BEAS-2B cells were exposed to 5μM CdCl2 for 4 h after 30 min of incubation with 1500 U/ml catalase and 5μM DPI. The total cellular lysates were analyzed by immunoblotting with antibodies against p-ERK, p-p70S6K1, HIF-1α, and β-actin. The membranes were stripped and reprobed for ERK, p70S6K1, and HIF-1β.
Cadmium -Induced HIF-1α Expression through ROS Generation and Activation of ERK and AKT Pathways in Normal Human Bronchial Epithelial (NHBE) Cells
In order to validate the results found in BEAS-2B cells, we did similar experiments with cadmium treatment and tested the levels of signaling molecules as above in NHBE cells. Primary NHBE cells were incubated with the inhibitors as indicated for 30 min, followed by exposure to 5μM CdCl2 for 4 h. Similarly as in BEAS-2B cells, cadmium induced activation of ERK, AKT, p70S6K1, and expression of HIF-1α, and their inductions were suppressed by catalase, U0126, LY294002, and rapamycin (mammalian target of rapamycin [mTOR]/p70S6K1 inhibitor) (Fig. 5). These results further confirmed the ability of cadmium to induce HIF-1α expression through ROS generation and ERK and AKT signaling in human lung epithelial cells.
FIG. 5.
Cadmium-induced activation of ERK and AKT signaling was suppressed by the specific inhibitors catalase, U0126, LY294002, and rapamycin in normal human bronchial epithelial (NHBE) cells. NHBE cells were incubated with 1500 U/ml catalase, 20μM U0126, 15μM LY294002, and 5nM rapamycin for 30 min, followed by exposure to 5μM CdCl2 for 4 h. The total cellular lysates were analyzed by immunoblotting with antibodies against p-ERK, p-AKT, p-p70S6K1, HIF-1α, and β-actin. The membranes were stripped and reprobed for ERK, AKT p70S6K1, and HIF-1β.
Cadmium-Induced ROS Initiated a Signaling Cascade that Includes ERK and AKT Signaling and Led to VEGF Production
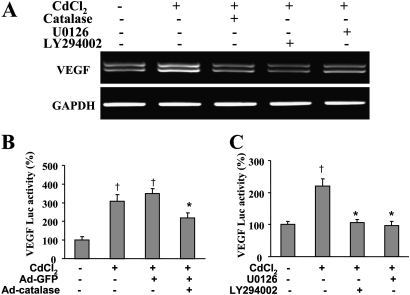
VEGF is considered a key growth factor that promotes tumor angiogenesis. It has been well known that HIF-1α can regulate VEGF transcriptional activation through binding to its promoter (Forsythe et al., 1996). To investigate the potential role of cadmium on critical VEGF production, the expression and transcriptional activation of VEGF in cadmium-treated cells was analyzed by reverse transcription polymerase chain reaction (RT-PCR) and luciferase reporter assay without or with the pretreatment of catalase, U0126, or LY294002. As shown in Figure 6A, cadmium remarkably induced the expression of VEGF messenger RNA (mRNA), and the induction could be inhibited by catalase, LY294002, and U0126. Consistent with mRNA levels of VEGF, similar results were obtained for VEGF luciferase activity (Figs. 6B and 6C). These results demonstrate that cadmium has the potential to stimulate tumor angiogenesis in lung epithelial cells via VEGF expression, which is dependent on ROS production, ERK, AKT, and HIF-1 signaling.
FIG. 6.
Cadmium-induced VEGF expression is suppressed by inhibitors catalase, U0126, and LY294002. (A) BEAS-2B cells were incubated with 1500 U/ml catalase, 20μM U0126, and 15μM LY294002 for 30 min, followed by treatment with 5μM CdCl2 for 12 h. Total cellular RNAs were purified with TRIzol. The VEGF mRNA expression was determined by RT-PCR. (B) BEAS-2B cells were seeded in 12-well plates at a density of 2 × 105 cells/well and transfected using Lipofectamine with 0.4 μg reporter and 0.2 μg β-gal plasmids. Fresh DMEM medium was then changed, and the cells were infected with 25 MOI adenoviruses for 36 h and exposed to 5μM CdCl2 for 12 h. The cells were lysed with reporter lysis buffer; cellular extracts were used to determine luciferase activity and β-gal expression. The relative luciferase activities were normalized to the controls and calculated as the ratio of luc/β-gal. † indicates significant difference compared with the BEAS-2B control. * indicates significant difference compared with the control of BEAS-2B exposed to CdCl2 or infected with Ad-GFP. (C) BEAS-2B cells, seeded in 12-well plates, were transfected with 0.4 μg reporter and 0.2 μg β-gal plasmids. After 36 h, cells were incubated with 20μM U0126 and 15μM LY294002 for 30 min, followed by treatment with 5μM CdCl2 for 12 h. The cells were lysed with reporter lysis buffer, and the luciferase activity and β-gal expression were measured and presented as described above.
Chronic Cadmium Exposure Caused Malignant Transformation of Cells and Resulted in Tumor-Forming Ability in Nude Mice
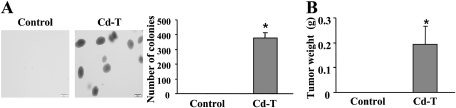
To test whether chronic exposure to cadmium leads to malignant transformation and tumorigenesis, BEAS-2B control and Cd-T cells were used to perform colony assay, which represents the capacity of anchorage-independent growth in vitro. As shown in Figure 7A, control BEAS-2B cells cultured in the absence of cadmium for more than 6 months could not form colonies, whereas Cd-T cells formed significant colonies, showing a characteristic of malignant cells. Meanwhile, to confirm whether Cd-T cells can form tumors in vivo, control BEAS-2B and Cd-T cells were injected subcutaneously into the flanks of nude mice, and tumor growth was monitored 2 weeks later. As anticipated, Cd-T cells formed large tumor xenografts in nude mice, whereas control BEAS-2B cells had no tumor-forming ability (Fig. 7B).
FIG. 7.
Cadmium-induced malignant transformation in soft agar tumor formation in nude mice. (A) Equal number of BEAS-2B and Cd-T cells (3 × 103) were suspended in 2 ml DMEM (10% FBS) containing 0.5% SeaPlaque Agar. Cell suspension was overlaid on 1 ml polymerized 0.3% agar solution in six-well plates; 1 ml 0.3% DMEM-agar solution was overlaid on each well once a week. Colonies were counted and images were taken under microscope after 2 weeks. The data are presented as mean ± SE (n = 3). * indicates significant difference compared with the control. Pictures are representatives of each treatment. (B) The Cd-T BEAS-2B cells were suspended in serum-free medium and mixed with the same volume of Extracel Hydrogel. Aliquots of 1 × 106 cells were injected subcutaneously into the flanks of nude mice. After 2 weeks, tumor tissues were collected and measured in weight. The data are presented as mean ± SE (n = 8). * indicates significant difference compared with the control.
Cadmium Induces Tube Formation In Vitro and Tumor Angiogenesis In Vivo
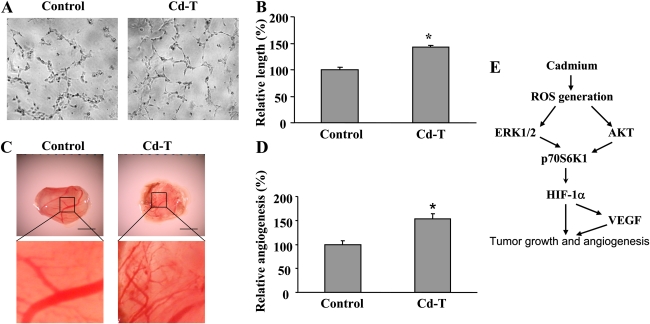
To determine the proangiogenic ability of cadmium, we performed tube formation assay with human endothelial cells in vitro and CAM assay in vivo. HUVECs in the conditioned medium prepared from control BEAS-2B cells could not form tubes at 8 h, but tube formation was significantly induced by the conditioned medium made from Cd-T cells (Figs. 8A and 8B), indicating that some proangiogenic factors may be secreted from the Cd-T cells. To confirm the tumor angiogenic ability of cadmium in vivo, we implanted control and cadmium-treated BEAS-2B cells on the CAM. Tumor tissues formed by the cadmium-treated BEAS-2B cells developed significantly more blood vessels compared with the tissues in control group (Figs. 8C and 8D). These results suggest that cadmium has the ability of inducing angiogenesis in vivo. The signaling mechanism found in this study is summarized in Figure 8E.
FIG. 8.
Cadmium induces endothelial tube formation in vitro and tumor angiogenesis in vivo. (A) BEAS-2B and Cd-T cells cultured to subconfluence were washed with 1 × PBS and incubated for 15 h in basic EBM-2 medium containing 1% FBS. HUVECs at subconfluence were starved overnight in EBM-2 basic medium containing 0.2% FBS. The starved HUVECs were trypsinized and counted in basic EBM-2 medium. The cells were mixed with 50 μl equal volume of freshly collected conditioned media from BEAS-2B and Cd-T cells and seeded to the Matrigel-precoated 96-well plate at 1 × 104 cells/well. Represented tube formation was examined and photographed at 8 h under microscope. (B) The lengths of the tubes were measured using Olympus cellSens Standard digital imaging software. The results were presented as mean ± SE (n = 6). * indicates significant difference compared with the control. (C) Fertilized chicken eggs were incubated at 37°C for 9 days. Cd-T cells at 4×106 were used for angiogenesis assay on the CAM. The CAM tissues were harvested and photographed 4 days after implantation. Pictures are representatives of each treatment. (D) The branches of newly formed blood vessels were counted and analyzed as mean ± SE. * indicates significant difference compared with the control. (E) The outline of the findings in this study.
DISCUSSION
Cadmium and its compounds were classified as human carcinogens based on the evidence from both humans and experimental animals (IARC, 1993). Angiogenesis is a fundamental requirement for tumorigenesis and tumor development (Blagosklonny, 2004; Folkman, 2002). The carcinogenic mechanisms underlying cadmium toxicity have been investigated (Filipic et al., 2006; Joseph, 2009). However, our understanding of the mechanisms of tumor angiogenesis induced by cadmium is very limited (Prozialeck et al., 2006; Woods et al., 2008), and results are sometimes paradoxical. Early studies carried out in the absence of serum and utilizing relatively high concentrations of cadmium demonstrated direct toxicity of cadmium on endothelial cells (Kishimoto et al., 1991, 1994, 1996a,b). On the other hand, it has also been reported that endothelial cells are rather resistant to the acute cytotoxicity of cadmium even at concentrations up to 1mM (Woods et al., 2008). Studies investigating angiogenic effects of cadmium revealed dysfunctional and antiangiogenic actions of cadmium on human vascular endothelial cells (Kolluru et al., 2006; Woods et al., 2008). The ability of cadmium to directly inhibit the process of angiogenesis in endothelial cells implicated that cadmium could inhibit the growth of some types of cancer (Prozialeck et al., 2006). These findings may explain the elusive carcinogenic effects of cadmium in certain target organs but cannot explain the angiogenic role of cadmium or the accumulated evidence that cadmium is an unequivocal cause of lung cancers. Cadmium may cause various damages to different organs depending on the dose, route, ways, and duration of exposure. The present study investigated the role of cadmium in the critical pathways regulating tumor angiogenesis and their downstream key signaling molecules, HIF-1 and VEGF, in human bronchial epithelial cells.
ROS, including superoxide, hydrogen peroxide, and hydroxyl group, can be induced by particular heavy metals including cadmium (Chen et al., 2011; Yang et al., 2009). PI3K/AKT and MAPK/ERK have been considered as the crucial regulatory pathways in carcinogenesis and tumor angiogenesis (Jiang and Liu, 2009). Here, we investigated the role of cadmium on ROS, ERK, and AKT signaling pathways in human bronchial epithelial cells. Our results indicate that cadmium induces activation of ERK and AKT, as well as their downstream molecules p70S6K1 in a time- and concentration-dependent manner in immortalized human lung epithelial BEAS-2B cells. Acute cadmium treatment induces ROS generation, which can be inhibited by catalase and DPI. To understand the cellular mechanisms by which cadmium promotes cell transformation, lung bronchial epithelial cells BEAS-2B were exposed to cadmium for 26 weeks. These Cd-T cells were characterized by the increase of proliferation rate and anchor-independent growth. Similar to acute exposure to cadmium, chronic exposure to cadmium also induces ROS production, and ROS inhibitors attenuate the endogenous ROS level in Cd-T cells. Furthermore, treatment with ROS inhibitors, catalase or DPI, suppresses cadmium-induced activation of ERK and AKT and its downstream molecule p70S6K1 in BEAS-2B cells. Similar results were obtained in primary normal human bronchial epithelial cells. These results indicate that cadmium may activate ERK and AKT signaling pathways through ROS generation, thus activating p70S6K1 signaling. The mechanism of cadmium in inducing ROS generation is not known yet. The activation of nicotinamide adenine dinucleotide phosphate-oxidase is likely involved in the ROS induction. However, the precise mechanisms how cadmium leads to ROS generation remain to be interesting topics addressed in future studies. Previous studies showed that ERK and AKT pathways can be activated by growth factors or hypoxia and can promote tumor angiogenesis through expression of HIF-1 and VEGF (Trisciuoglio et al., 2005). HIF-1 is a transcription factor that activates transcription of many genes including VEGF (Semenza, 2003); moreover, VEGF is the key growth factor that stimulates angiogenesis (Ferrara, 1999). Our previous studies also demonstrate that some growth factors such as epidermal growth factor and insulin can induce ROS generation, increase HIF-1 and VEGF expression, and promote tumor angiogenesis in ovarian and prostate cancers (Liu et al., 2006; Zhou et al., 2007). Tumor angiogenesis is mainly regulated by VEGF, the key angiogenic endothelial growth factor. VEGF and its receptors are required for tumor growth, invasion, and metastasis (McMahon, 2000; Verheul and Pinedo, 2000) and have been found to be expressed in various human lung cancers (Ilhan et al., 2004). Application of VEGF inhibitors has been considered as a therapeutic approach for lung tumors (Kerbel, 2004). In the present study, we found that HIF-1α is induced by cadmium through ROS, AKT/ERK, and p70S6K1 signaling pathways in both BEAS-2B and NHBE cells. HIF-1 is the most important regulator of VEGF expression by binding to the hypoxia response element in the VEGF promoter (Forsythe et al., 1996; Semenza, 2003). It is composed of HIF-1α and HIF-1β subunits. HIF-1α is induced by hypoxia, growth factors, and oncogenes and is often upregulated in many human cancers. Here, we show that cadmium remarkably induces expression of VEGF at transcriptional level in BEAS-2B cells, suggesting its high potential to stimulate angiogenesis. The cadmium-induced VEGF transcription is significantly inhibited by catalase, ERK inhibitor U0126, and PI3K/AKT inhibitor LY294002. These results further confirmed that cadmium induces VEGF expression through ROS induction, ERK, and AKT activation.
Anchorage-independent growth is one of the characteristics of malignant cells. We found that chronic exposure to cadmium at 5μM for 26 weeks renders malignant transformation because the Cd-T cells form colonies. To further establish full transformation of Cd-T cells in vivo, Cd-T cells were tested for the ability of tumor formation in nude mice. Two weeks after implantation, the Cd-T BEAS-2B cells formed significantly larger tumors compared with the control cells; this result is consistent with the fact that cadmium has the ability of inducing tumors (Filipic et al., 2006; Joseph, 2009; Waalkes, 2003). These results further confirm that chronic cadmium exposure has a strong potential of inducing cancers in lung epithelial cells.
Because control BEAS-2B cells could not form tumor in nude mice, we employed tube formation and CAM assay to test the proangiogenic ability of cadmium in vitro and in vivo. Conditioned medium prepared from Cd-T cells significantly induced tube formation using human endothelial cells, indicating the secretion of proangiogenic factors in the conditioned medium made from Cd-T cells. We further demonstrated that cadmium-treated BEAS-2B cells significantly increased angiogenesis responses in vivo than the control BEAS-2B cells. These results suggest that cadmium has the ability of inducing angiogenesis.
Taken together, the results of this study demonstrate that cadmium induces ROS production, activates ERK and AKT pathways, and upregulates key proangiogenic molecules HIF-1 and VEGF expression in human airway epithelial cells (Fig. 8E). The activation of ERK and AKT signaling is mediated by ROS. Furthermore, cadmium also induces malignant transformation, angiogenesis in vivo, and tumorigenesis in nude mice, suggesting that cadmium is a carcinogen, and the underlying angiogenic mechanisms may involve ROS production, ERK, and AKT signaling activation. Clarifying the angiogenic mechanisms induced by cadmium, especially in the most prevalent lung cancer, would enable us to have a more complete understanding of the carcinogenicity of cadmium and also provide a novel rationale to improve the occupational health protection, prevention, and chemotherapy in lung cancers caused by cadmium.
FUNDING
National Institute of Environmental Health Sciences (NIH R21ES017237); National Cancer Institute (NIH R01CA109460); National Heart, Lung, and Blood Institute (NIH R01HL091456).
References
- Achanzar WE, Diwan BA, Liu J, Quader ST, Webber MM, Waalkes MP. Cadmium-induced malignant transformation of human prostate epithelial cells. Cancer Res. 2001;61:455–458. [PubMed] [Google Scholar]
- Barrett JR. Cadmium and breast cancer: Exposure associated with basal-like phenotype. Environ. Health Perspect. 2009;117:A552. doi: 10.1289/ehp.117-a552b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Liu J, Webber MM, Waalkes MP. Estrogen signaling and disruption of androgen metabolism in acquired androgen-independence during cadmium carcinogenesis in human prostate epithelial cells. Prostate. 2007a;67:135–145. doi: 10.1002/pros.20479. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Tokar EJ, Diwan BA, Dill AL, Coppin JF, Waalkes MP. Cadmium malignantly transforms normal human breast epithelial cells into a basal-like phenotype. Environ. Health Perspect. 2009;117:1847–1852. doi: 10.1289/ehp.0900999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waterland RA, Dill AL, Webber MM, Waalkes MP. Tumor suppressor gene inactivation during cadmium-induced malignant transformation of human prostate cells correlates with overexpression of de novo DNA methyltransferase. Environ. Health Perspect. 2007b;115:1454–1459. doi: 10.1289/ehp.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A. Cadmium & its adverse effects on human health. Indian J. Med. Res. 2008;128:557–564. [PubMed] [Google Scholar]
- Beveridge R, Pintos J, Parent ME, Asselin J, Siemiatycki J. Lung cancer risk associated with occupational exposure to nickel, chromium VI, and cadmium in two population-based case-control studies in Montreal. Am. J. Ind. Med. 2010;53:476–485. doi: 10.1002/ajim.20801. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Antiangiogenic therapy and tumor progression. Cancer Cell. 2004;5:13–17. doi: 10.1016/s1535-6108(03)00336-2. [DOI] [PubMed] [Google Scholar]
- Brama M, Gnessi L, Basciani S, Cerulli N, Politi L, Spera G, Mariani S, Cherubini S, d'Abusco AS, Scandurra R, et al. Cadmium induces mitogenic signaling in breast cancer cell by an ERalpha-dependent mechanism. Mol. Cell Endocrinol. 2007;264:102–108. doi: 10.1016/j.mce.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen W, Shen T, Han X, Kontos CD, Huang S. Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic. Biol. Med. 2011;50:624–632. doi: 10.1016/j.freeradbiomed.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LA., Jr Quantifying potential health impacts of cadmium in cigarettes on smoker risk of lung cancer: A portfolio-of-mechanisms approach. Risk Anal. 2006;26:1581–1599. doi: 10.1111/j.1539-6924.2006.00848.x. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999;56:794–814. doi: 10.1046/j.1523-1755.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- Filipic M, Fatur T, Vudrag M. Molecular mechanisms of cadmium induced mutagenicity. Hum. Exp. Toxicol. 2006;25:67–77. doi: 10.1191/0960327106ht590oa. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao N, Shen L, Zhang Z, Leonard SS, He H, Zhang XG, Shi X, Jiang BH. Arsenite induces HIF-1alpha and VEGF through PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol. Cell Biochem. 2004;255:33–45. doi: 10.1023/b:mcbi.0000007259.65742.16. [DOI] [PubMed] [Google Scholar]
- Goyer RA, Liu J, Waalkes MP. Cadmium and cancer of prostate and testis. Biometals. 2004;17:555–558. doi: 10.1023/b:biom.0000045738.59708.20. [DOI] [PubMed] [Google Scholar]
- Helmestam M, Stavreus-Evers A, Olovsson M. Cadmium chloride alters mRNA levels of angiogenesis related genes in primary human endometrial endothelial cells grown in vitro. Reprod. Toxicol. 2010;30:370–376. doi: 10.1016/j.reprotox.2010.05.003. [DOI] [PubMed] [Google Scholar]
- IARC. Beryllium, Cadmium, Mercury and Exposures in the Glass Manufacturing Industry. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans. 1993;Vol. 58 International Agency for Research on Cancer, Lyon, France, pp. 444. Available at: http://monographs.iarc.fr/ENG/Monographs/vol58/volume58.pdf. Accessed August 22, 1997. [PMC free article] [PubMed] [Google Scholar]
- Ilhan N, Ilhan N, Deveci F. Functional significance of vascular endothelial growth factor and its receptor (receptor-1) in various lung cancer types. Clin. Biochem. 2004;37:840–845. doi: 10.1016/j.clinbiochem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Il'yasova D, Schwartz GG. Cadmium and renal cancer. Toxicol. Appl. Pharmacol. 2005;207:179–186. doi: 10.1016/j.taap.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Jarup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure—a review of the literature and a risk estimate. Scand. J. Work Environ. Health. 1998;24(Suppl. 1):1–51. [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology. 1998;19:529–535. [PubMed] [Google Scholar]
- Joseph P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. 2009;238:272–279. doi: 10.1016/j.taap.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Kawata K, Shimazaki R, Okabe S. Comparison of gene expression profiles in HepG2 cells exposed to arsenic, cadmium, nickel, and three model carcinogens for investigating the mechanisms of metal carcinogenesis. Environ. Mol. Mutagen. 2009;50:46–59. doi: 10.1002/em.20438. [DOI] [PubMed] [Google Scholar]
- Kellen E, Zeegers MP, Hond ED, Buntinx F. Blood cadmium may be associated with bladder carcinogenesis: The Belgian case-control study on bladder cancer. Cancer Detect. Prev. 2007;31:77–82. doi: 10.1016/j.cdp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Kerbel RS. Antiangiogenic drugs and current strategies for the treatment of lung cancer. Semin. Oncol. 2004;31:54–60. doi: 10.1053/j.seminoncol.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Kim J, Lim W, Ko Y, Kwon H, Kim S, Kim O, Park G, Choi H, Kim O. The effects of cadmium on VEGF-mediated angiogenesis in HUVECs. J. Appl. Toxicol. 2011 doi: 10.1002/jat.1677. . Advance Access published on March 21, 2011. doi:10.1002/jat.1677. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Fukuzawa Y, Abe M, Isobe M, Hashimoto M, Tada M. Cadmium injury of cultured human vascular endothelial cells. Hum. Cell. 1991;4:329–334. [PubMed] [Google Scholar]
- Kishimoto T, Oguri T, Ohno M, Matsubara K, Yamamoto K, Tada M. Effect of cadmium (CdCl2) on cell proliferation and production of EDRF (endothelium-derived relaxing factor) by cultured human umbilical arterial endothelial cells. Arch. Toxicol. 1994;68:555–559. doi: 10.1007/s002040050113. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Oguri T, Yamabe S, Tada M. Effect of cadmium injury on growth and migration of cultured human vascular endothelial cells. Hum. Cell. 1996a;9:43–48. [PubMed] [Google Scholar]
- Kishimoto T, Ueda D, Isobe M, Tada M. Cadmium injures tube formation by cultured human vascular endothelial cells. Hum. Cell. 1996b;9:244–250. [PubMed] [Google Scholar]
- Kolluru GK, Tamilarasan KP, Geetha PS, Durgha NP, Chatterjee S. Cadmium induced endothelial dysfunction: Consequence of defective migratory pattern of endothelial cells in association with poor nitric oxide availability under cadmium challenge. Cell Biol. Int. 2006;30:427–438. doi: 10.1016/j.cellbi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Liu LZ, Hu XW, Xia C, He J, Zhou Q, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic. Biol. Med. 2006;41:1521–1533. doi: 10.1016/j.freeradbiomed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Majumder S, Muley A, Kolluru GK, Saurabh S, Tamilarasan KP, Chandrasekhar S, Reddy HB, Purohit S, Chatterjee S. Cadmium reduces nitric oxide production by impairing phosphorylation of endothelial nitric oxide synthase. Biochem. Cell Biol. 2008;86:1–10. doi: 10.1139/o07-146. [DOI] [PubMed] [Google Scholar]
- McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5(Suppl. 1):3–10. doi: 10.1634/theoncologist.5-suppl_1-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yasunaga Y, Ko D, Xu LL, Moul JW, Peehl DM, Srivastava S, Rhim JS. Cadmium-induced neoplastic transformation of human prostate epithelial cells. Int. J. Oncol. 2002;20:543–547. [PubMed] [Google Scholar]
- Pacini S, Punzi T, Morucci G, Gulisano M, Ruggiero M. A paradox of cadmium: A carcinogen that impairs the capability of human breast cancer cells to induce angiogenesis. J. Environ. Pathol. Toxicol. Oncol. 2009;28:85–88. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i1.90. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR, Woods JM. The vascular endothelium as a target of cadmium toxicity. Life Sci. 2006;79:1493–1506. doi: 10.1016/j.lfs.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Qu W, Diwan BA, Reece JM, Bortner CD, Pi J, Liu J, Waalkes MP. Cadmium-induced malignant transformation in rat liver cells: Role of aberrant oncogene expression and minimal role of oxidative stress. Int. J. Cancer. 2005;114:346–355. doi: 10.1002/ijc.20736. [DOI] [PubMed] [Google Scholar]
- Sahmoun AE, Case LD, Jackson SA, Schwartz GG. Cadmium and prostate cancer: A critical epidemiologic analysis. Cancer Invest. 2005;23:256–263. doi: 10.1081/cnv-200055968. [DOI] [PubMed] [Google Scholar]
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ. Health Perspect. 2004;112:1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Sens DA, Park S, Gurel V, Sens MA, Garrett SH, Somji S. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol. Sci. 2004;79:56–63. doi: 10.1093/toxsci/kfh086. [DOI] [PubMed] [Google Scholar]
- Sethi PK, Khandelwal D. Cadmium exposure: Health hazards of silver cottage industry in developing countries. J. Med. Toxicol. 2006;2:14–15. doi: 10.1007/BF03161007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewit CL, Gengler B, Vegas E, Puckett R, Louie MC. Cadmium promotes breast cancer cell proliferation by potentiating the interaction between ERalpha and c-Jun. Mol. Endocrinol. 2010;24:981–992. doi: 10.1210/me.2009-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisciuoglio D, Iervolino A, Zupi G, Del Bufalo D. Involvement of PI3K and MAPK signaling in bcl-2-induced vascular endothelial growth factor expression in melanoma cells. Mol. Biol. Cell. 2005;16:4153–4162. doi: 10.1091/mbc.E04-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul HM, Pinedo HM. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin. Breast Cancer. 2000;1(Suppl. 1):S80–S84. doi: 10.3816/cbc.2000.s.015. [DOI] [PubMed] [Google Scholar]
- Waalkes MP. Cadmium carcinogenesis. Mutat. Res. 2003;533:107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Klaassen CD. Concentration of metallothionein in major organs of rats after administration of various metals. Fundam. Appl. Toxicol. 1985;5:473–477. doi: 10.1016/0272-0590(85)90094-6. [DOI] [PubMed] [Google Scholar]
- Woods JM, Leone M, Klosowska K, Lamar PC, Shaknovsky TJ, Prozialeck WC. Direct antiangiogenic actions of cadmium on human vascular endothelial cells. Toxicol. In Vitro. 2008;22:643–651. doi: 10.1016/j.tiv.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LY, Wu KH, Chiu WT, Wang SH, Shih CM. The cadmium-induced death of mesangial cells results in nephrotoxicity. Autophagy. 2009;5:571–572. doi: 10.4161/auto.5.4.8311. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Liu LZ, Fu B, Hu X, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate insulin-induced VEGF and HIF-1alpha expression through the activation of p70S6K11 in human prostate cancer cells. Carcinogenesis. 2007;28:28–37. doi: 10.1093/carcin/bgl085. [DOI] [PubMed] [Google Scholar]