Abstract
Mercury is a widespread environmental contaminant with neurotoxic impacts that have been observed over a range of exposures. In addition, there is increasing evidence that inorganic mercury (iHg) and organic mercury (including methyl mercury) have a range of immunotoxic effects, including immune suppression and induction of autoimmunity. In this study, we investigated the effect of iHg on a model of autoimmune heart disease in mice induced by infection with coxsackievirus B3 (CVB3). We examined the role of timing of iHg exposure on disease; in some experiments, mice were pretreated with iHg (200 μg/kg, every other day for 15 days) before disease induction with virus inoculation, and in others, they were treated with iHg after the acute (viral) phase of disease but before the development of dilated cardiomyopathy (DCM). iHg alone had no effect on heart pathology. Pretreatment with iHg before CVB3 infection significantly increased the severity of chronic myocarditis and DCM compared with control animals receiving vehicle alone. In contrast, treatment with iHg after acute myocarditis did not affect the severity of chronic disease. The increased chronic myocarditis, fibrosis, and DCM induced by iHg pretreatment were not due to increased viral replication in the heart, which was unaltered by iHg treatment. iHg pretreatment induced a macrophage infiltrate and mixed cytokine response in the heart during acute myocarditis, including significantly increased interleukin (IL)-12, IL-17, interferon-γ, and tumor necrosis factor-α levels. IL-17 levels were also significantly increased in the spleen during chronic disease. Thus, we show for the first time that low-dose Hg exposure increases chronic myocarditis and DCM in a murine model.
Keywords: mercury, autoimmune, coxsackievirus, cytokine, myocarditis, dilated cardiomyopathy
Mercury (Hg) is a heavy metal with well-defined neurotoxicity (National Research Council [U.S.] Committee on the Toxicological Effects of Methylmercury, 2000) and increasing evidence for immunotoxic effects (Schiraldi and Monestier, 2009; Silbergeld et al., 2005). Hg is of global public health importance because many populations worldwide are exposed at levels that exceed recommended guidelines of safe levels (Mahaffey et al., 2004; WHO, 2010). Exposure to Hg compounds can occur through occupation, diet, and consumption of contaminated fish. Exposures to inorganic mercury (iHg) are associated with the manufacture of fluorescent light bulbs, pressure gauges, use of Hg-containing dental amalgams (Gochfeld, 2003), and artisanal gold mining (Silbergeld et al., 2005). An association has been reported between self-reported occupational exposure to Hg (primarily in those working with dental amalgams) and diagnosis of systemic lupus erythematosus (SLE; Cooper et al., 2004). We and others have reported increases in serum biomarkers of immune activation and autoantibodies in individuals exposed to Hg through artisanal gold mining and/or consumption of contaminated fish (Alves et al., 2006; Gardner et al., 2010; Nyland et al., 2011, forthcoming; Silva et al., 2004). Many studies in rodents have demonstrated that Hg exposure activates the immune response in certain inbred strains resulting in lupus-like autoimmune disease, as well as accelerating pathology and death in the graft-versus-host model of SLE (Hultman and Nielsen, 2001; Monestier et al., 1994; Nielsen and Hultman, 2002; Via et al., 2003).
The mechanism(s) by which Hg induces autoimmune disease is still being elucidated. In rodent models, Hg treatment either in vivo or ex vivo induces elevated interleukin (IL)-4 production from spleen cells resulting in a Th2 response and proliferation of B cells and the Th2-associated antibodies IgG1 and IgE (Bagenstose et al., 1999; Schiraldi and Monestier, 2009). These animal model findings are consistent with the type of immune response known to be induced by Hg exposure in humans of increased autoantibodies and immune complex deposition (Gardner et al., 2010; Nyland et al., 2011; Schiraldi and Monestier, 2009). Although Hg increases a Th2 response, in reality, Hg activates a “mixed” Th1 and Th2 response with increased interferon (IFN)-γ production as well (Bagenstose et al., 1999; Fournie et al., 2002).
Myocarditis, or inflammation of the myocardium, leads to around half of all dilated cardiomyopathy (DCM) cases in the United States (Roger et al., 2011). DCM is the most common form of cardiomyopathy requiring a heart transplant due to heart failure (Drory et al., 1991; Feldman and McNamara, 2000; Roger et al., 2011). Viral infection, particularly by coxsackievirus B3 (CVB3), is the most frequent cause of myocarditis in developed countries (Blauwet and Cooper, 2010; Guarner et al., 2007). CVB3 infection is believed to induce autoimmunity by viral-induced damage resulting in exposure of the immune system to the intracellular protein cardiac myosin and by activation of Toll-like receptors (TLRs) during the innate immune response similar to an adjuvant (Cihakova et al., 2004; Fairweather and Rose, 2007b; Zhang et al., 2009). In a hybrid model where susceptible BALB/c mice are inoculated with heart-passaged CVB3 (i.e., infectious CVB3 and heart proteins), mice develop disease that is similar to that observed in humans, with the development of acute inflammatory myocarditis from day 7 to 14 postinfection (pi) followed by progression to chronic myocarditis and DCM that persists from day 35 to at least day 90 pi (Fairweather et al., 2001, Fairweather and Rose, 2007a). In this model, inflammation is driven by the TLR-4–derived proinflammatory cytokines IL-1β and IL-18, and their levels are directly correlated with the extent of inflammation in the heart (Fairweather et al., 2003, 2005; Lane et al., 1992). Further, TLR-4 and IL-12 receptor β1 signaling are critical for progression of disease in susceptible male BALB/c mice (Frisancho-Kiss et al., 2006, 2007).
To investigate whether low-dose Hg exposure could alter acute or chronic myocarditis/DCM in this model (similar to its effects on other models of autoimmune disease), we treated mice with iHg prior to inoculation with CVB3 or in the interval between inoculation and the development of chronic myocarditis/DCM. Disease status was evaluated during the acute and chronic stages of the disease.
MATERIALS AND METHODS
Treatment protocol.
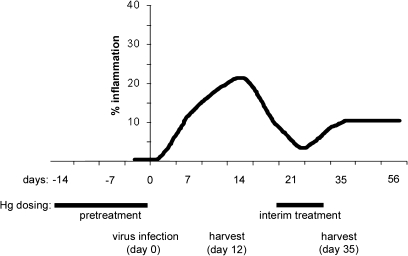
Male BALB/cJ (BALB/c) mice (6–8 weeks old; The Jackson Laboratory) were used in this study because myocarditis and DCM are more prevalent in men (Roger et al., 2011) and male BALB/c mice develop more severe myocarditis and DCM (Frisancho-Kiss et al., 2006, 2007). Mice were pretreated with low-dose iHg (HgCl2, 200 μg/kg body weight sc, or vehicle [PBS] every other day for 2 weeks, 100 μl per dose per mouse) before infection with CVB3 and heart proteins on day 0 according to Fairweather and Rose (2007a). For “interim” treatment, mice were injected sc with iHg (200 μg/kg body weight every other day for 1 week, 4 doses in 100 μl per mouse) beginning on day 20 pi following resolution of the acute phase of CVB3-induced myocarditis. We chose to use iHg and sc dosing because this method is frequently found in the literature (Gilbert et al., 2011; Pollard et al., 2001; Santarelli et al., 2006) allowing for better comparison of our findings with those of others in addition to providing more controlled levels of exposure. The model time line and dosing is illustrated in Figure 1. In a separate study, we examined the effect of iHg treatment alone (in the absence of CVB3 inoculation) on cardiac pathology.
FIG. 1.
Dosing and model time line. Male BALB/cJ mice received either pretreatment with iHg every other day (day −14 to day 0) or interim treatment (day 20 to 27). Disease was initiated with CVB3 infection on day 0 with 103 PFU of heart-passaged virus containing cardiac proteins ip. Myocarditis/DCM was examined during the acute (day 12 pi) or chronic (day 35 pi) phase.
Induction of myocarditis and DCM.
Mice were inoculated ip with 103 plaque-forming units (PFU) of a heart-passaged stock of CVB3 that contained cardiac proteins (Nancy strain; American Type Culture Collection). Mice were maintained under pathogen-free conditions in the animal facility at the Johns Hopkins University School of Medicine or University of South Carolina School of Medicine, with prior approval obtained from the Animal Care and Use Committees of the Johns Hopkins Bloomberg School of Public Health or University of South Carolina for all procedures. Individual experiments were conducted at least three times with 8–10 mice per treatment group.
Evaluation of myocarditis and fibrosis.
Mice were euthanized at day 12 pi (acute myocarditis) or day 35 pi (chronic myocarditis/DCM), and hearts were cut longitudinally, fixed in 10% phosphate-buffered formalin, and embedded in paraffin. Sections were cut 5 μm thick at various depths in the tissue section and stained with hematoxylin and eosin (H&E) to determine the level of inflammation and DCM or Masson’s trichrome to determine collagen deposition. Sections were examined by two independent investigators in a blinded manner. Myocarditis and fibrosis were assessed as the percentage of the heart section with inflammation or fibrosis (collagen deposition) compared with the overall size of the heart section, with the aid of a microscope eyepiece grid, as described previously (Fairweather et al., 2003). The development of DCM was assessed by gross observation of histology sections at low magnification, as described previously (Fairweather et al., 2004). Characterization of the immune infiltrate was assessed in a blinded manner by morphological evaluation of 15 adjacent fields of view at ×60 magnification (image area of ∼105 mm2) per heart by a pathologist in the University of South Carolina School of Medicine Instrument Resource Core Facility.
Cytokine measurement.
The spleen was also removed for cytokine analysis. The whole spleen and right half of each heart were frozen in dry ice immediately after harvest and stored at −80°C until homogenized. Tissues were thawed and homogenized at 10% wt/vol in 2% fetal bovine serum/minimal essential medium, and supernatants were stored at −80°C until used in cytokine assays. Cytokines were measured in tissue supernatants using bead-based multiplex cytokine kits (Bio-Plex; Bio-Rad), according to manufacturer’s instructions. The limits of detection for the cytokines were as follows: IL-1α, 1.32 pg/ml; IL-1β, 1.70 pg/ml; IL-2, 1.98 pg/ml; IL-3, 1.32 pg/ml; IL-4, 2.43 pg/ml; IL-5, 1.69 pg/ml; IL-6, 1.02 pg/ml; IL-9, 1.36 pg/ml; IL-10, 1.04 pg/ml; IL-12/23 p40, 1.15 pg/ml; IL-12 p70, 1.20 pg/ml; IL-13, 1.57 pg/ml; IL-17a, 1.44 pg/ml; IFN-γ, 1.30 pg/ml; eotaxin, 1.70 pg/ml; granulocyte colony-stimulating factor (G-CSF), 1.69 pg/ml; granulocyte-macrophage–colony stimulating factor (GM-CSF), 1.58 pg/ml; monocyte chemoattractant protein (MCP-1), 1.71 pg/ml; macrophage inflammatory protein (MIP)-1α, 1.57 pg/ml; MIP-1β, 1.20 pg/ml; regulated upon activation normal T-cell expressed and presumably secreted (RANTES), 0.95 pg/ml; tumor necrosis factor (TNF)-α, 1.73 pg/ml. Cytokines were expressed as pg/g of tissue ±SEM.
Viral titers.
The level of infectious virus was determined in individual homogenates by plaque assay according to standard procedures (Fairweather and Rose, 2007a). Samples were processed in the same manner as for cytokine analysis. Dilutions of tissue homogenate supernatants were incubated on Vero cell monolayers (ATCC) for 1 h at 37°C and 5% CO2 to allow viral attachment and then incubated for 3 days to allow plaque formation. Virus titers were expressed as the mean PFU/g of tissue ±SEM. The limit of detection was 10 PFU/g tissue.
Statistical analysis.
Data comparing two groups were analyzed by the Student’s t-test for normally distributed data or the Mann-Whitney U-test for nonparametric data (significance of observed differences between treatment groups for severity of myocarditis, cellular infiltrate characterization, and cytokine levels). Fisher’s exact test was used to evaluate treatment differences in frequency data (significance of observed differences between treatment groups for frequency of DCM). Multiple comparisons were analyzed by ANOVA with a Bonferroni correction. A value of p < 0.05 was considered significant.
RESULTS
Hg Pretreatment Does Not Alter Acute Myocarditis or Viral Replication
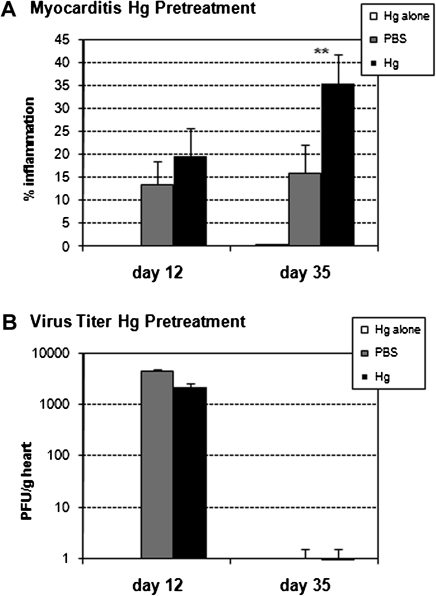
Treatment with low-dose iHg for 2 weeks prior to disease induction (pretreatment) (Fig. 1) did not significantly alter acute myocarditis at day 12 pi (Fig. 2a). Treatment of mice with iHg alone (Fig. 2a) or uninfected heart homogenate (data not shown) did not result in the development of acute myocarditis. Our results confirm a previous study using a virus-only model of CVB3 myocarditis where methyl mercury (MeHg) pretreatment of female BALB/c mice for 12 weeks prior to CVB3 infection had no significant effect on acute myocarditis at day 7 pi (Ilback et al., 1996). There was a trend for Hg to increase acute myocarditis in that study, similar to our findings (Fig. 2a), but the increase was not significant (Ilback et al., 1996; Schiraldi and Monestier, 2009). Although CVB3 infection has been shown to alter Hg uptake in various organs (not described for the heart), Hg treatment has not been reported to alter viral replication in the heart (Frisk et al., 2008; Ilback et al., 1996). iHg pretreatment did not alter viral replication in the heart at day 12 pi (Fig. 2b). No viral replication was detected in the heart of iHg-treated mice, which were not inoculated but age-matched to day 12 pi (data not shown).
FIG. 2.
Hg pretreatment exacerbated CVB3-induced autoimmune myocarditis at day 35 pi. PBS-treated (vehicle control) and Hg-treated mice were compared for the percent inflammation in the heart at day 12 and 35 pi (A). For pretreatment, mice received 100 μl HgCl2 at 200 μg/kg in PBS or vehicle alone every other day for 2 weeks. The group listed as “Hg alone” did not receive CVB3. Data shown are the mean ± SEM of 8–10 mice per treatment group. Data are from one representative experiment of four. (B) Hearts were collected on day 12 and 35 pi, homogenized, and homogenates analyzed by plaque assay for viral replication. Data are presented as the mean PFU/g heart tissue ±SEM of 8–10 mice per treatment group from one representative experiment of three. * indicates p < 0.01.
Hg Pretreatment Increases Chronic CVB3-Induced Autoimmune Myocarditis
iHg pretreatment significantly increased chronic CVB3-induced myocarditis at day 35 pi (Fig. 2a). Treatment of mice with iHg alone (Fig. 2a) or uninfected heart homogenate (data not shown) did not result in the development of chronic myocarditis/DCM. We previously reported that infectious CVB3 is cleared from the heart by day 14 pi (Fairweather et al., 2001), and so as expected no infectious virus was detected in the heart at day 35 pi (Fig. 2b). Thus, the mechanism for iHg-induced exasperated chronic disease does not appear to be mediated by increasing viral replication in the heart.
Increased Mixed Cytokine Response in the Heart with Elevated Macrophages During Acute Myocarditis with Hg Pretreatment
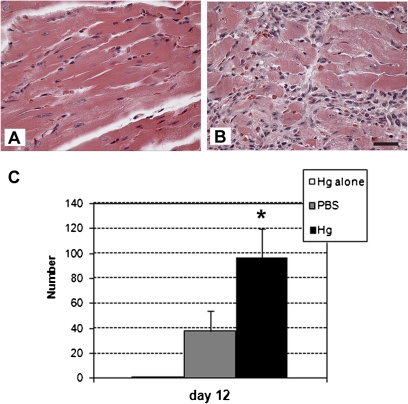
iHg pretreatment significantly increased the number of macrophages in the heart (Fig. 3) but not eosinophils or mast cells, although there was no significant change in the overall level of inflammation in the heart (Fig. 2a). However, iHg pretreatment did not alter mast cell or eosinophil numbers in the heart (data not shown).
FIG. 3.
Hg pretreatment increased macrophage infiltrate in the heart during acute myocarditis. (A) PBS-pretreated (vehicle control) and (B) Hg-pretreated mice were compared for the number of macrophages in the heart on day 12 pi. Fifteen adjacent fields of view were counted at ×60 magnification (bar represents 50 μ) beginning at a randomly selected location in the left ventricle of each heart. Data are from one representative experiment of two. (C) The total number of macrophages identified by morphological identification in all fields of view is shown. Data are presented as the mean total number per heart ±SEM of 5–10 mice per treatment group from one representative experiment of two. * indicates p < 0.05.
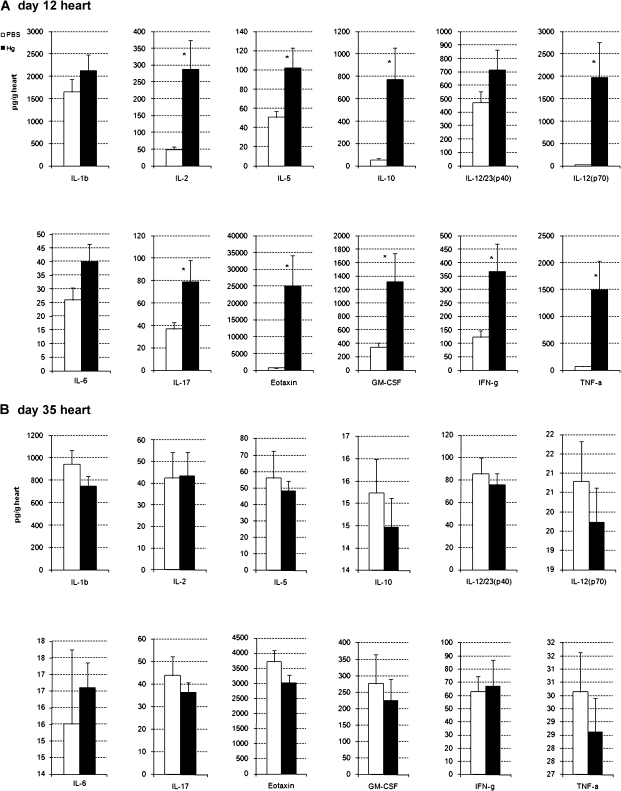
Because Hg has been shown to increase Th1 and Th2 cytokine responses in rodent models of Hg-induced autoimmune disease (Schiraldi and Monestier, 2009), we examined a wide panel of cytokines in the heart at day 12 pi (Fig. 4a) and day 35 pi (Fig. 4b) using multiplex kits. iHg pretreatment significantly altered cytokine levels in the heart only during acute myocarditis but not during chronic disease (Fig. 4). Similar to other Hg-induced autoimmune models, we found that iHg exposure significantly increased a number of proinflammatory (e.g., IL-1α, IL-2, IL-12 (p70), IL-17a, G-CSF, GM-CSF, IFN-γ, MIP-1α, and TNF-α) and Th2-associated (e.g., IL-5, IL-10, eotaxin) cytokines in the heart during acute myocarditis (Fig. 4a). No significant difference was observed in the level of IL-1β, IL-3, IL-4, IL-6, IL-9, IL-13, IL-12/23 (p40), MCP-1, MIP-1β, or RANTES in the heart during acute myocarditis in iHg-pretreated mice compared with PBS controls (Fig. 4a or data not shown).
FIG. 4.
Hg pretreatment increases cytokines in the heart during acute myocarditis. PBS-pretreated (vehicle control) and Hg-pretreated mice were compared for the level of cytokines in the heart on (A) day 12 and (B) 35 pi. Hearts were collected, homogenized, and homogenates analyzed for cytokine level by Bio-Plex. Data are expressed as mean cytokine level (pg/g tissue) ±SEM of 8–10 mice per treatment group. Data are shown from one representative experiment of four. * indicates p < 0.01.
Hg Pretreatment Increases Fibrosis and Prevalence of DCM During Chronic Myocarditis
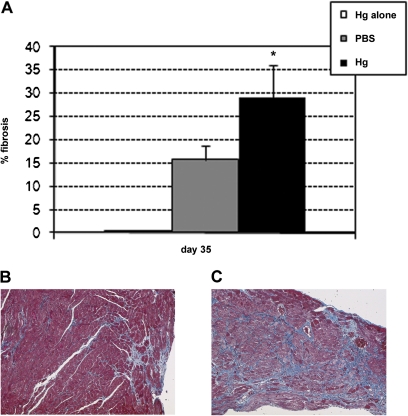
We examined the effect of iHg exposure prior to inoculation and in the interval between the acute and chronic phases of myocarditis. iHg pretreatment did not induce fibrosis at day 12 pi, and hearts were similar to those of untreated BALB/c mice (data not shown). In contrast, at day 35 pi, iHg pretreatment significantly exacerbated fibrosis in the heart (Fig. 5). iHg pretreatment also significantly increased the incidence of DCM at day 35 pi compared with PBS-inoculated controls (Fig. 6). Pretreatment of mice with iHg alone did not induce fibrosis or DCM in age-matched mice (Fig. 5a).
FIG. 5.
Hg pretreatment increases fibrosis in the heart. PBS-treated (vehicle control) and Hg-treated mice were compared for the percent of the heart section with fibrosis on day 35 pi. Mice received 100 μl HgCl2 at 200 μg/kg in PBS or vehicle alone every other day for 2 weeks followed by 103 PFU of CVB3 ip on day 0. The group listed as “Hg alone” did not receive CVB3. On day 35 pi, (B) PBS and (C) Hg hearts were collected and stained with Masson’s trichrome to detect collagen deposition. (A) Data are presented as the mean percent of the heart section staining positive for fibrosis ±SEM of 8–10 mice per treatment group from one representative independent experiment of three. * indicates p < 0.01.
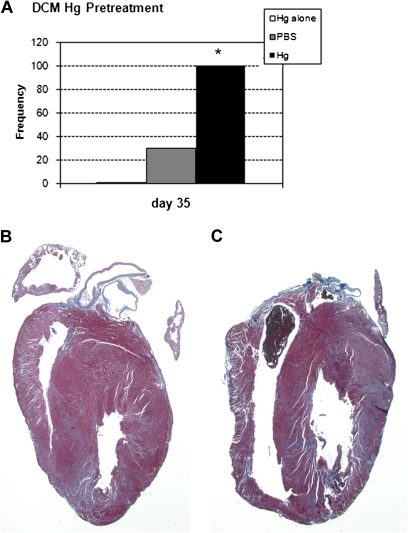
FIG. 6.
Hg pretreatment increases the prevalence of DCM at day 35 pi. PBS-pretreated (vehicle control) and Hg-pretreated mice were compared for the prevalence of dilated phenotype on day 35 pi. (A) Data are expressed as the frequency of hearts per group with dilatation. H&E-stained sections from (B) PBS-pretreated and (C) Hg-pretreated mice, shown at ×1.25. * indicates significantly different by Fisher’s exact test.
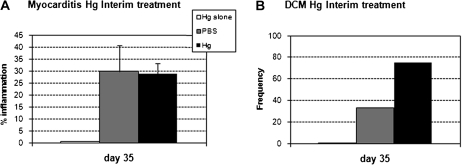
Interim Hg Treatment Does Not Alter Chronic Myocarditis
We explored whether the timing of iHg treatment relative to disease induction impacted the exacerbation of inflammation and prevalence of DCM during the chronic phase of the disease. Interim iHg treatment (following acute myocarditis and before the development of the chronic phase; see Fig. 1) had no significant effect on inflammation at day 35 pi (Fig. 7a) or DCM prevalence (Fig. 7b).
FIG. 7.
Interim Hg treatment does not change disease severity. PBS-treated (vehicle control) and Hg-treated mice were compared for the (A) percent inflammation in the heart at day 35 pi. For interim treatment, mice received 100 μl HgCl2 at 200 μg/kg in PBS or vehicle every other day for 1 week beginning on day 20 pi. The group listed as “Hg alone” did not receive CVB3. Data shown are the mean ± SEM of 8–10 mice per treatment group. Data are from one representative independent experiment of four. (B) Mice were compared for the prevalence of dilated phenotype on day 35 pi. Data are expressed as the frequency of hearts per group with the dilated phenotype.
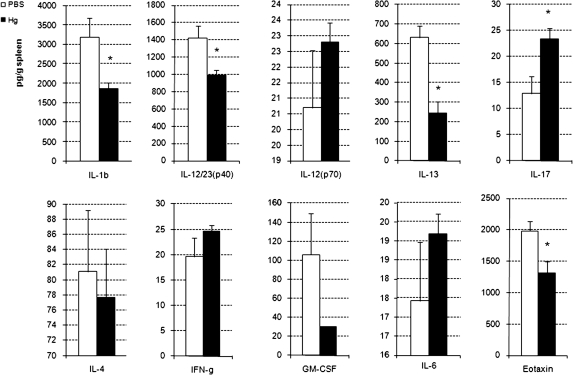
Hg Pretreatment Alters Splenic Cytokines During Chronic Myocarditis
Previously, Ilback et al. (1996) found that MeHg significantly increased splenocyte proliferation in response to concanavalin A following CVB3 infection. Because cytokines were unchanged in the heart during chronic myocarditis, we investigated whether splenic cytokines were altered during chronic CVB3 autoimmune myocarditis. We found that iHg pretreatment induced significant changes in several splenic cytokines at day 35 pi (Fig. 8). IL-1β, IL-23, IL-13, and eotaxin levels in the spleen were significantly decreased with iHg pretreatment. Conversely, splenic IL-17 levels were significantly increased with iHg pretreatment.
FIG. 8.
Hg pretreatment modulates cytokine levels in the spleen. PBS-pretreated (vehicle control) and Hg-pretreated mice were compared for the level of cytokines in the spleen on day 35 pi. Spleens were collected, homogenized, and homogenates analyzed for cytokine level by Bio-Plex. Data are expressed as mean cytokine level (pg/g tissue) ±SEM of 8–10 mice per treatment group. Data are shown from one representative independent experiment of four. * indicates p < 0.01.
DISCUSSION
In this study, we report for the first time that low-dose iHg pretreatment significantly increases chronic CVB3-induced autoimmune myocarditis, fibrosis, and DCM. iHg pretreatment significantly altered cytokine levels in the heart during acute myocarditis. Although, there was no overall change in the severity of acute inflammation, the number of infiltrating macrophages (but not eosinophils or mast cells) was significantly increased in the heart during acute myocarditis. Thus, iHg pretreatment shifts in immune cell subsets during acute myocarditis without changing overall inflammation levels, as we demonstrated previously (Fairweather et al., 2005). Future studies will need to determine whether these shifts in cardiac immune cell populations during acute myocarditis were accompanied by changes in functional or activation states. Shifts in immune cell populations and activation levels could explain the significant changes in cytokines observed in the heart during acute myocarditis in Hg-treated mice.
It is interesting that IL-17a was significantly increased in the heart of Hg-treated compared with PBS-treated mice during acute myocarditis (Fig. 4a) and in the spleen during chronic myocarditis (Fig. 8). However, the increase in IL-17 was not accompanied by changes in other cytokines in the IL-17 regulatory axis including IL-23 and IL-6. Recently, IL-17 was found to not alter the severity of acute myocarditis in the experimental autoimmune myocarditis (EAM) model but to be necessary for chronic fibrosis and progression to DCM (Baldeviano et al., 2010). Our data show that Hg increases cardiac and splenic IL-17 levels providing a potential mechanism for how Hg pretreatment could increase chronic fibrosis and DCM in this study without altering the severity of acute myocarditis. These data are consistent with other studies of inflammatory heart disease leading to DCM in which both IFN-γ and IL-17, along with other cytokines, were increased in a mixed cytokine milieu prior to development of DCM (Kania et al., 2009). Although IL-17 and IFN-γ inhibit each other in vitro, this has not been observed in vivo, potentially because other cytokines and factors influence the relationship as well.
Elevated IL-5, IL-10, and eotaxin in iHg-pretreated mice suggest that Hg pretreatment may have also increased immune responses associated with Th2 immunity. IL-5 and eotaxin are known to increase the number and activation of mast cells and eosinophils, although that was not observed in these studies. Mast cells have been proposed to skew immune responses to a more Th2 response following Hg treatment in other models of Hg-induced autoimmunity (Schiraldi and Monestier, 2009). Mast cells have also been found to play a role in exacerbating myocarditis, fibrosis, and chronic DCM in our CVB3 model (Fairweather et al., 2004; Frisancho-Kiss et al., 2007). Increased levels of IL-10 (and Th2 cytokines) in the heart during acute myocarditis may regulate the proinflammatory effects of elevated TNF-α, IL-12, IL-17, and IFN-γ (Fig. 4a; Roether et al., 2002). Overall, our findings in this study clearly demonstrate that low doses of iHg are able to affect the immune response in the heart resulting in significantly worse autoimmune heart disease.
The temporal relationship between iHg exposure and induction of disease appears to be critical for modulation of disease because interim iHg exposure did not significantly alter chronic disease. These results are consistent with other studies that examined the effect of iHg pretreatment on EAM in mice (Silbergeld et al., 2005). These results are also similar to our studies in the graft-versus-host disease model of lupus nephritis in which pretreatment with iHg exacerbated glomerulonephritis and hastened death (Via et al., 2003). Importantly, our findings in this study are consistent with those of Ilback et al. (1996, 2000) who found that pretreatment of mice with a high dose of MeHg (3.9 mg/kg) for 12 weeks prior to CVB3 infection did not significantly alter acute myocarditis. Hg was found to increase proliferation of splenocytes to concanavalin A ex vivo. However, cytokine levels were not examined in the heart and viral replication in the heart was not quantitated in those studies. Although sera TNF-α and IFN-γ levels were significantly increased following CVB3 infection, they were not altered in Hg+CVB3 compared with CVB3-treated mice (Ilback et al., 1996). We are the first to examine the effect of iHg treatment on the chronic phase of myocarditis and DCM.
Recent findings have revealed that genes necessary for fibrosis induction are upregulated during acute CVB3 myocarditis resulting in the gradual remodeling that produces fibrosis during the chronic phase of disease (Fairweather, unpublished data). Thus, we only observe fibrosis in this model of CVB3-induced autoimmune myocarditis by day 35 pi in susceptible mice (Fairweather and Rose, 2007a), which is exasperated with iHg pretreatment. These findings are consistent with studies in mice and humans linking Hg exposure and increased antifibrillarin antibodies in scleroderma, an autoimmune disease with a significant induction of fibrosis (Arnett et al., 2000; Hultman et al., 1989). Interestingly, cardiac fibrosis associated with scleroderma tends to be patchy and distributed throughout the myocardium (Champion, 2008), as we find in the CVB3-induced model of autoimmune myocarditis.
Hg may also contribute to the progression to DCM by mechanisms other than a direct effect on the immune response. In 1999, a study was published that examined trace elements in the heart of 13 patients with idiopathic DCM and found that Hg levels in the myocardium were 22,000 times higher than controls (Frustaci et al., 1999). The mechanism to generate these elevated Hg levels or even the sequence of events (infection vs. exposure) is unknown, but Hg may compete with selenium, decreasing the activity of selenium-dependent enzymes. Selenium is a trace element with both structural and enzymatic roles that are essential for normal cardiac physiology (Cooper et al., 2007). Exposure to elevated levels of Hg may reduce selenium’s bioavailability (Hg has a high binding affinity for selenium [Gailer et al., 2000], which is higher than that for sulfur), negatively impacting cardiac function. Selenium deficiency has been shown to convert an “amyocarditic” strain of CVB3 (one that induces high levels of viral replication in the heart but low or no inflammation) to a myocarditic strain (one that induces high levels of viral replication in the heart and myocarditis; Beck et al., 1995, 2003a,b). However, the effect of selenium deficiency on autoimmune myocarditis has not been established, and we did not examine cardiac Hg or selenium levels in this study. In an epidemiologic study of 1833 men with heart disease, men with elevated Hg levels (≥2 μg/g) in hair samples were at a 2.9-fold increased risk of cardiovascular death compared with those with lower hair Hg levels (Salonen et al., 1995). Although that study did not specifically examine myocarditis/DCM patients, their findings are consistent with these experimental findings that Hg exposures can increase cardiovascular disease.
In this study, we present data that show that low-dose iHg exposure can induce significant changes in the immune response resulting in increased autoimmune heart disease. The interaction with viral infection events, related to cytokine production and iHg-induced alterations in the immune response in this model, is demonstrated by the importance of the timing of iHg exposure.
FUNDING
National Institute of Environmental Health Sciences at the National Institutes of Health (T32 ES007141 and K99/R00 ES015426 to J.F.N.); Heinz Family Foundation.
Acknowledgments
The authors would like to thank Dr John Fuseler of the University of South Carolina School of Medicine Instrument Resource Core Facility for his morphological evaluation of the heart slides. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
References
- Alves MF, Fraiji NA, Barbosa AC, De Lima DS, Souza JR, Dórea JG, Cordeiro GW. Fish consumption, mercury exposure and serum antinuclear antibody in Amazonians. Int. J. Environ. Health Res. 2006;16:255–262. doi: 10.1080/09603120600734147. [DOI] [PubMed] [Google Scholar]
- Arnett FC, Fritzler MJ, Ahn C, Holian A. Urinary mercury levels in patients with autoantibodies to U3-RNP (fibrillarin) J. Rheumatol. 2000;27:405–410. [PubMed] [Google Scholar]
- Bagenstose LM, Salgame P, Monestier M. Cytokine regulation of a rodent model of mercuric chloride-induced autoimmunity. Environ. Health Perspect. 1999;107(Suppl. 5):807–810. doi: 10.1289/ehp.99107s5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J. Nutr. 2003a;133:1463S–1467S. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- Beck MA, Shi Q, Morris VC, Levander OA. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat. Med. 1995;1:433–436. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- Beck MA, Williams-Toone D, Levander OA. Coxsackievirus B3-resistant mice become susceptible in Se/vitamin E deficiency. Free Radic. Biol. Med. 2003b;34:1263–1270. doi: 10.1016/s0891-5849(03)00101-1. [DOI] [PubMed] [Google Scholar]
- Blauwet LA, Cooper LT. Myocarditis. Prog. Cardiovasc. Dis. 2010;52:274–288. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion HC. The heart in scleroderma. Rheum. Dis. Clin. North Am. 2008;34:181–190. doi: 10.1016/j.rdc.2007.12.002. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cihakova D, Sharma RB, Fairweather D, Afanasyeva M, Rose NR. Animal models for autoimmune myocarditis and autoimmune thyroiditis. Methods Mol. Med. 2004;102:175–193. doi: 10.1385/1-59259-805-6:175. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Occupational risk factors for the development of systemic lupus erythematosus. J. Rheumatol. 2004;31:1928–1933. [PubMed] [Google Scholar]
- Cooper LT, Rader V, Ralston NV. The roles of selenium and mercury in the pathogenesis of viral cardiomyopathy. Congest. Heart Fail. 2007;13:193–199. doi: 10.1111/j.1527-5299.2007.06410.x. [DOI] [PubMed] [Google Scholar]
- Drory Y, Turetz Y, Hiss Y, Lev B, Fisman EZ, Pines A, Kramer MR. Sudden unexpected death in persons less than 40 years of age. Am. J. Cardiol. 1991;68:1388–1392. doi: 10.1016/0002-9149(91)90251-f. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Gatewood SJ, Njoku DB, Rose NR. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am. J. Pathol. 2004;165:1883–1894. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Yusung SA, Barrett MA, Davis SE, Steele RA, Gatewood SJ, Rose NR. IL-12 protects against coxsackievirus B3-induced myocarditis by increasing IFN-gamma and macrophage and neutrophil populations in the heart. J. Immunol. 2005;174:261–269. doi: 10.4049/jimmunol.174.1.261. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. From infection to autoimmunity. J. Autoimmun. 2001;16:175–186. doi: 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: A model of autoimmune disease for studying immunotoxicity. Methods. 2007a;41:118–122. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairweather D, Rose NR. 2007b. Immunopathogenesis of autoimmune disease. In Immunotoxicology and Immunopharmacology, 3rd ed. (R. Luebke, R. House, and I. Kimber, Eds.), pp. 423–436. CRC Press, Boca Raton, FL. [Google Scholar]
- Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, Rose NR. IL-12 receptor beta1 and toll-like receptor 4 increase IL-1beta- and IL-18-associated myocarditis and coxsackievirus replication. J. Immunol. 2003;170:4731–4737. doi: 10.4049/jimmunol.170.9.4731. [DOI] [PubMed] [Google Scholar]
- Feldman AM, McNamara D. Myocarditis. N. Engl. J. Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- Fournie GJ, Saoudi A, Druet P, Pelletier L. Th2-type immunopathological manifestations induced by mercury chloride or gold salts in the rat: Signal transduction pathways, cellular mechanisms and genetic control. Autoimmun. Rev. 2002;1:205–212. doi: 10.1016/s1568-9972(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, Rose NR, Fairweather D. Cutting edge: Cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- Frisancho-Kiss S, Nyland JF, Davis SE, Frisancho JA, Barrett MA, Rose NR, Fairweather D. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain Res. 2006;1126:139–147. doi: 10.1016/j.brainres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Frisk P, Molin Y, Ilback NG. Tissue uptake of mercury is changed during the course of a common viral infection in mice. Environ. Res. 2008;106:178–184. doi: 10.1016/j.envres.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Magnavita N, Chimenti C, Caldarulo M, Sabbioni E, Pietra R, Cellini C, Possati GF, Maseri A. Marked elevation of myocardial trace elements in idiopathic dilated cardiomyopathy compared with secondary cardiac dysfunction. J. Am. Coll. Cardiol. 1999;33:1578–1583. doi: 10.1016/s0735-1097(99)00062-5. [DOI] [PubMed] [Google Scholar]
- Gailer J, George GN, Pickering IJ, Madden S, Prince RC, Yu EY, Denton MB, Younis HS, Aposhian HV. Structural basis of the antagonism between inorganic mercury and selenium in mammals. Chem. Res. Toxicol. 2000;13:1135–1142. doi: 10.1021/tx000050h. [DOI] [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brasil: A cross-sectional study. Environ. Res. 2010;110:345–354. doi: 10.1016/j.envres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert KM, Rowley B, Gomez-Acevedo H, Blossom SJ. Coexposure to mercury increases immunotoxicity of trichloroethylene. Toxicol. Sci. 2011;119:281–292. doi: 10.1093/toxsci/kfq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gochfeld M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Saf. 2003;56:174–179. doi: 10.1016/s0147-6513(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Guarner J, Bhatnagar J, Shieh WJ, Nolte KB, Klein D, Gookin MS, Penaranda S, Oberste MS, Jones T, Smith C, et al. Histopathologic, immunohistochemical, and polymerase chain reaction assays in the study of cases with fatal sporadic myocarditis. Hum. Pathol. 2007;38:1412–1419. doi: 10.1016/j.humpath.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Hultman P, Eneström S, Pollard KM, Tan EM. Anti-fibrillarin autoantibodies in mercury-treated mice. Clin. Exp. Immunol. 1989;78:470–477. [PMC free article] [PubMed] [Google Scholar]
- Hultman P, Nielsen JB. The effect of dose, gender, and non-H-2 genes in murine mercury-induced autoimmunity. J. Autoimmun. 2001;17:27–37. doi: 10.1006/jaut.2001.0521. [DOI] [PubMed] [Google Scholar]
- Ilback NG, Lindh U, Wesslen L, Fohlman J, Friman G. Trace element distribution in heart tissue sections studied by nuclear microscopy is changed in Coxsackie virus B3 myocarditis in methyl mercury-exposed mice. Biol. Trace Elem. Res. 2000;78:131–147. doi: 10.1385/BTER:78:1-3:131. [DOI] [PubMed] [Google Scholar]
- Ilback NG, Wesslen L, Fohlman J, Friman G. Effects of methyl mercury on cytokines, inflammation and virus clearance in a common infection (coxsackie B3 myocarditis) Toxicol. Lett. 1996;89:19–28. doi: 10.1016/s0378-4274(96)03777-0. [DOI] [PubMed] [Google Scholar]
- Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc. Med. 2009;19:247–252. doi: 10.1016/j.tcm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Lane JR, Neumann DA, Lafond-Walker A, Herskowitz A, Rose NR. Interleukin 1 or tumor necrosis factor can promote Coxsackie B3-induced myocarditis in resistant B10.A mice. J. Exp. Med. 1992;175:1123–1129. doi: 10.1084/jem.175.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ. Health Perspect. 2004;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monestier M, Losman MJ, Novick KE, Aris JP. Molecular analysis of mercury-induced antinucleolar antibodies in H-2S mice. J. Immunol. 1994;152:667–675. [PubMed] [Google Scholar]
- National Research Council (U.S.) Committee on the Toxicological Effects of Methylmercury. Toxicological Effects of Methylmercury. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Nielsen JB, Hultman P. Mercury-induced autoimmunity in mice. Environ. Health Perspect. 2002;110(Suppl. 5):877–881. doi: 10.1289/ehp.02110s5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyland JF, Fillion M, Barbosa F, Jr, Shirley DL, Chine C, Lemire M, Mergler D, Silbergeld EK. Biomarkers of methyl mercury exposure immunotoxicity among fish consumers in Amazonian Brazil. Environ. Health Perspect. 2011 doi: 10.1289/ehp.1103741. doi:10.1289/ehp.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyland JF, Wang SB, Shirley DL, Santos EO, Ventura AM, de Souza JM, Silbergeld EK. Fetal and maternal immune responses to methylmercury exposure: A cross-sectional study. Environ. Res. 2011;111:584–589. doi: 10.1016/j.envres.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM, Pearson DL, Hultman P, Deane TN, Lindh U, Kono DH. Xenobiotic acceleration of idiopathic systemic autoimmunity in lupus-prone bxsb mice. Environ. Health Perspect. 2001;109:27–33. doi: 10.1289/ehp.0110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roether S, Rabbani H, Mellstedt H, Abedi-Valugerdi M. Spontaneous downregulation of antibody/autoantibody synthesis in susceptible mice upon chronic exposure to mercuric chloride is not owing to a general immunosuppression. Scand. J. Immunol. 2002;55:493–502. doi: 10.1046/j.1365-3083.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart disease and stroke statistics—2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen JT, Seppänen K, NNyyssönen K, Korpela H, Kauhanen J, Kantola M, Tuomilehto J, Esterbauer H, Tatzber F, Salonen R. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation. 1995;91:645–655. doi: 10.1161/01.cir.91.3.645. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Bracci M, Mocchegiani E. In vitro and in vivo effects of mercuric chloride on thymic endocrine activity, NK and NKT cell cytotoxicity, cytokine profiles (IL-2, IFN-gamma, IL-6): Role of the nitric oxide-L-arginine pathway. Int. Immunopharmacol. 2006;6:376–389. doi: 10.1016/j.intimp.2005.08.028. [DOI] [PubMed] [Google Scholar]
- Schiraldi M, Monestier M. How can a chemical element elicit complex immunopathology? Lessons from mercury-induced autoimmunity. Trends Immunol. 2009;30:502–509. doi: 10.1016/j.it.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Silva IA, Nyland JF. Mercury and autoimmunity: Implications for occupational and environmental health. Toxicol. Appl. Pharmacol. 2005;207:282–292. doi: 10.1016/j.taap.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Silva IA, Nyland JF, Gorman A, Perisse A, Ventura AM, Santos EC, de Souza JM, Burek CL, Rose NR, Silbergeld EK. Mercury exposure, malaria, and serum antinuclear/antinucleolar antibodies in Amazon populations in Brazil: A cross-sectional study. Environ. Health. 2004;3:11–22. doi: 10.1186/1476-069X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via CS, Nguyen P, Niculescu F, Papadimitriou J, Hoover D, Silbergeld EK. Low-dose exposure to inorganic mercury accelerates disease and mortality in acquired murine lupus. Environ. Health Perspect. 2003;111:1273–1277. doi: 10.1289/ehp.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Children's Exposure to Mercury Compounds. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- Zhang P, Cox CJ, Alvarez KM, Cunningham MW. Cutting edge: Cardiac myosin activates innate immune responses through TLRs. J. Immunol. 2009;183:27–31. doi: 10.4049/jimmunol.0800861. [DOI] [PMC free article] [PubMed] [Google Scholar]