Abstract
Previously, we reported that perfluorooctanoic acid (PFOA) promotes liver cancer in a manner similar to that of 17β-estradiol (E2) in rainbow trout. Also, other perfluoroalkyl acids (PFAAs) are weakly estrogenic in trout and bind the trout liver estrogen receptor. The primary objective of this study was to determine whether multiple PFAAs enhance hepatic tumorigenesis in trout, an animal model that represents human insensitivity to peroxisome proliferation. A two-stage chemical carcinogenesis model was employed in trout to evaluate PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluorooctane sulfonate (PFOS), and 8:2 fluorotelomer alcohol (8:2FtOH) as complete carcinogens or promoters of aflatoxin B1 (AFB1)- and/or N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced liver cancer. A custom trout DNA microarray was used to assess hepatic transcriptional response to these dietary treatments in comparison with E2 and the classic peroxisome proliferator, clofibrate (CLOF). Incidence, multiplicity, and size of liver tumors in trout fed diets containing E2, PFOA, PFNA, and PFDA were significantly higher compared with AFB1-initiated animals fed control diet, whereas PFOS caused a minor increase in liver tumor incidence. E2 and PFOA also enhanced MNNG-initiated hepatocarcinogenesis. Pearson correlation analyses, unsupervised hierarchical clustering, and principal components analyses showed that the hepatic gene expression profiles for E2 and PFOA, PFNA, PFDA, and PFOS were overall highly similar, though distinct patterns of gene expression were evident for each treatment, particularly for PFNA. Overall, these data suggest that multiple PFAAs can promote liver cancer and that the mechanism of promotion may be similar to that of E2.
Keywords: estradiol, hepatocarcinogenesis, perfluoroalkyl acid, perfluorooctanoic acid, perfluorooctane sulfonate, tumor promotion, microarray, transcript profiling
Polyfluorinated chemicals (PFCs) have been manufactured by either electrochemical fluorination to produce mixtures of branched eight-carbon isomers or telomerization to synthesize linear fluorotelomers. Perfluoroalkyl acids (PFAAs) are intermediates or by-products formed during the production or breakdown of these fluoropolymers, widely used as surfactants, surface protectors, paper and textile coatings, polishes, and fire-retardant foams (Fromme et al., 2009). Biotransformation of fluorotelomers, such as polyfluoroalkyl phosphate esters, used to coat paper packaging that comes into contact with food, may also be a significant source of human exposure to PFAAs (D'eon and Mabury, 2011). Perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) are members of the broader class of PFAAs, which are structurally characterized by a hydrophobic fluorinated carbon chain of varying length with either a carboxylic or sulfonic acid end group (Supplementary figure 1). Blood levels of PFOA and PFOS in U.S. residents are estimated to be about 4 and 20 ppb, respectively, though these levels have declined in recent years (Calafat et al., 2007; Olsen et al., 2003). Other PFAAs have also been detected in humans and wildlife worldwide, including perfluorononanoic acid (PFNA) and perfluorodecanoic acid (PFDA) (Calafat et al., 2007; Kannan et al., 2004; Martin et al., 2004). The residence time of PFOA varies among species, ranging from hours in the female rat to days in canine and rainbow trout (Hanhijarvi et al., 1988; Martin et al., 2003b). In contrast, humans have very limited capacity for elimination of PFAAs, as the estimated half-lives of PFOA and PFOS are 3.8 and 5.4 years, respectively (Olsen et al., 2007).
PFOA and other PFAAs are peroxisome proliferators (PPs), a class of chemicals that also includes some plasticizers, hypolipidemic drugs, herbicides, solvents, and certain long-chain fatty acids. Many biological responses to PPs are mediated by interaction with the PP-activated receptor α (PPARα), which is highly expressed in the liver (Holden and Tugwood, 1999). PFOA and other PPs are nongenotoxic hepatocarcinogens or promoters of hepatocarcinogenesis in rodents (reviewed in Abdellatif et al., 1991; Lai, 2004), though differences in susceptibility have been observed among species. Mice and rats are highly susceptible to liver toxicity and cancer caused by peroxisome proliferating chemicals, whereas humans and nonhuman primates are insensitive or nonresponsive (Holden and Tugwood, 1999; Lai, 2004). The weak response of humans to PPs has been attributed to the low level of PPARα expression in human liver (Palmer et al., 1998). New evidence showing that the environmental PPARα agonist di(2-ethylhexyl) phthalate (DEHP) significantly increased liver cancer incidence in PPARα null mice (Ito et al., 2007) suggests that some PPs may act via PPARα-independent modes of action to increase risk of hepatocarcinogenesis.
Recently, our laboratory utilized the rainbow trout (Oncorhynchus mykiss), as an animal model that mimics human insensitivity to peroxisome proliferation, to investigate alternative mechanisms of action for PFAAs. Chronic dietary exposure to PFOA enhanced liver cancer in trout and elicited changes in hepatic gene expression indicative of estrogen exposure, whereas the classic PP, clofibrate (CLOF), was ineffective (Tilton et al., 2008). Thus, we deduced that the cancer-enhancing effects of PFOA in trout were due to novel mechanisms related to estrogen signaling, rather than the typical PP response observed for this chemical in rodent models. Subsequently, we reported that multiple PFAAs, including PFOA, PFNA, PFDA, and PFOS, are weakly estrogenic in rainbow trout based upon induction of the estrogen-sensitive biomarker plasma protein vitellogenin (Vtg) and evidence for direct interaction of these compounds with the trout liver estrogen receptor (ER) (Benninghoff et al., 2011). Moreover, none of these compounds elicited a typical PP response in trout liver. The estrogen-like action of these compounds is likely not restricted to trout, as multiple PFAAs increase activity of a human ERα gene reporter, and were demonstrated to dock effectively in silico to the ligand-binding domain of the human and mouse ERα (Benninghoff et al., 2011).
The objective of the present study was to determine the impact of multiple PFAAs with reported estrogen-like activity on hepatic tumorigenesis in rainbow trout, a well-established model used for chemically induced liver cancer in humans (Bailey et al., 1996). A two-stage chemical carcinogenesis model was employed to evaluate PFOA, PFNA, PFDA, PFOS, and 8:2 fluorotelomer alcohol (8:2FtOH) as potential complete carcinogens and promoters of aflatoxin B1 (AFB1)- and/or N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced liver cancer. A toxicogenomics approach was utilized to evaluate mechanisms of chemical hepatocarcinogenesis in PFAA-exposed trout compared with 17β-estradiol (E2) and the classic PP, CLOF. We hypothesized that PFAAs, identified previously as weak xenoestrogens, would enhance liver carcinogenesis and produce a hepatic gene expression profile indicative of an estrogen-like transcriptional response.
MATERIALS AND METHODS
Materials.
Analytical grade AFB1, E2, PFOA, PFNA, PFDA, and 8:2FtOH were obtained from Sigma-Aldrich (St Louis, MO). PFOS and CLOF were purchased from Fluka Chemical Corp. (St Louis, MO). MNNG was obtained from ChemService (West Chester, PA). All other reagents were purchased from Sigma-Aldrich or other general laboratory suppliers and were of the highest purity available. Chemical structures for compounds tested as tumor promoters are provided in Supplementary figure 1.
Animals.
Mount Shasta strain rainbow trout were hatched and reared at the Sinnhuber Aquatic Research Laboratory at Oregon State University in Corvallis, Oregon. Fish were maintained in flow-through 375-l tanks at 12°C with activated carbon water filtration on a 12:12 h light:dark cycle. All procedures for treatment, handling, maintenance, and euthanasia of animals used in this study were approved by the Oregon State University Institutional Animal Care and Use Committee.
Tumor study, necropsy, and histopathology.
An overview of the study design is provided in Supplementary figure 2. Approximately 3500 fry were initiated at 10 weeks postspawn with an aqueous exposure to 10 ppb AFB1 or 0.01% EtOH (noninitiated sham controls) for 30 min; a second cohort of about 1000 fry was AFB1 or sham initiated at 15 weeks of age. To determine whether the expected tumor-promoting effects of PFOA and related compounds are carcinogen or target organ dependent, a third cohort of about 1000 fry was initiated at 10 weeks postspawn with a 30-min aqueous exposure to 35 ppm MNNG, a multiorgan carcinogen in trout (Hendricks et al., 1995), or 0.01% dimethyl sulfoxide (noninitiated sham control). After initiation, fry were fed Oregon Test Diet (OTD), a semipurified casein-based diet, for 1 month (Lee et al., 1991). Then, within each initiation cohort, trout were randomly distributed into dietary treatment groups with 125 animals assigned to duplicate tanks (250 fish/treatment) (Supplementary figure 2). In the first cohort, fish were fed experimental diets containing 5 ppm E2, 2000 ppm PFOA (approximately 50 mg/kg body weight/day), 2000 ppm FtOH or 2000 ppm CLOF ad libitum (2.8–5.6% of body weight) 5 days per week for 6 months. PFNA and PFDA experimental diets were initially administered at 2000 ppm based upon prior testing of PFOA without significant mortality (Tilton et al., 2008; unpublished observations). Due to an unexpected number of mortalities in the PFNA and PFDA treatment groups early in the study, diet concentrations were reduced to 200 ppm PFDA (5 mg/kg/day) or 1000 ppm PFNA (25 mg/kg/day) for the remainder of the exposure period. In the second cohort (AFB1 at 15 weeks), trout were fed 100 ppm PFOS (2.5 mg/kg/day); this lower test concentration of PFOS was selected based upon observed lethal toxicity at the 2000 ppm diet level (unpublished data). Finally, MNNG-initiated trout were fed 5 ppm E2 or 2000 ppm PFOA. All experimental diets were prepared monthly, stored frozen at −20°C and then thawed to 4°C a few days prior to feeding. Most test compounds were added directly to the oil portion of the OTD, though 8:2FtOH was incorporated into the diet via an oil-in-water emulsification. At conclusion of the 6-month promotion diet period, animals were once again fed standard OTD for the remainder of the study.
At 12.5 months postspawn, juvenile trout were euthanized with an overdose (250 ppm) of tricane methanesulfonate (MS-222) and necropsied over a 1-week period. Livers, kidneys, stomachs, and swim bladders were preserved in Bouin’s solution for up to 7 days for histological examination of tumors by hematoxylin and eosin staining. Neoplasms were classified according to the criteria described by Hendricks et al. (1984). The effect of experimental diets on tumor incidence was modeled by logistic regression (LOGISTIC procedure, SAS version 9.2; SAS Institute, Cary, NC); analyses included diet treatment, sex, body weight, and replicate tank as experimental factors. Firth’s bias correction was used as the likelihood penalty when a maximum likelihood estimate was not obtained. Some fish in this study showed symptoms of a liver disease of unknown origin, which was characterized by pale or jaundiced livers. To determine whether this idiopathic disease impacted the study outcome, logistic regression analyses were performed using two data sets: all subjects included all experimental subjects, males and females, regardless of disease symptoms; final subjects excluded any fish that showed symptoms of idiopathic liver disease. Data, statistical analyses, and conclusions presented in this article are for the final subjects data set, unless noted otherwise, whereas information and analysis of the all subjects data set is available in the Supplementary materials. Tumor multiplicity (number of tumors per tumor-bearing animal) and size data were analyzed by the Kruskal-Wallis test with Dunnett’s with post hoc test for multiple comparisons (GraphPad Prism 5, La Jolla, CA).
Microarray experiment.
Two weeks after the start of experimental diets, 24 fry (sex undetermined) from each of the sham-exposed treatment groups were removed from the study (12 fish/duplicate tank), euthanized by MS-222 and randomly distributed to create three pools of eight livers (n= 3). Total hepatic RNA was extracted from pooled whole liver samples using TRIzol reagent (Sigma-Aldrich), purified using the RNeasy Mini Kit (Qiagen, Valencia, CA) and evaluated for quality using the Bioanalyzer 2100 (Agilent, Palo Alto, CA). A reference RNA pool was made by combining equal amounts of RNA from all control RNA samples. Because PFOS trout were treated at a later age, a separate time-matched reference RNA pool was prepared for competitive hybridization of PFOS samples.
Details on the development, manufacture, and quality control assessment of the OSUrbt version 5.0 microarray have been provided previously (Benninghoff and Williams, 2008; Tilton et al., 2005) (Gene Expression Omnibus [GEO] platform accession ID: GPL5478). For detection of gene expression on the OSUrbt-v5 array, the Genisphere 3DNA Array 900 kit (Hatfield, PA) was used according to the supplier’s protocol in a standard dye-swap reference sample design as previously described (Benninghoff and Williams, 2008). Note that the RNA reference for competitive hybridization of PFOS samples was a separate time-matched pool of RNA obtained from sham-initiated control-fed trout at 15 weeks. Each reverse transcription reaction also included spiked-in messenger RNA (mRNA) corresponding to SpotReport Alien Oligo control features (Stratagene, La Jolla, CA). Hybridization of complementary DNA and capture reagents to the OSUrbt arrays was performed using the Hybex Microarray Incubation system (SciGene Corp., Sunnyvale, CA) as described previously (Benninghoff and Williams, 2008). Within 24 h of hybridization, array images at a resolution of 5μm were obtained using the Axon GenePix Pro 4200A scanner (Molecular Devices Corp., Sunnyvale, CA) at 543 nm and 633 nm excitation wavelengths for Cy3 and Cy5, respectively, with saturation tolerance set at 1% and laser power set at 90%.
Array image files were processed with ratio centering, and spot intensities were quantified using GenePix Pro software (Molecular Devices). Protocols for the maintenance, processing, and filtering of raw data sets (technical replication and fold-change criteria) were detailed previously (Benninghoff and Williams, 2008). All data files associated with this experiment are available at the GEO online data repository (Accession ID: GSE31085). Statistical analyses of gene expression were performed using the normalized geometric mean expression values for each biological replicate to compare each individual experimental treatment to the control (MultiExperiment Viewer [MeV]) (Saeed et al., 2003); a statistically significant change in gene expression was inferred when p < 0.05 (Welch’s t-test, between subjects and assuming unequal variances). Unsupervised, bidirectional hierarchical clustering and principal components analyses were performed using MeV. Normalized data were also exported to Prism 5 for pairwise Pearson correlation analyses of gene expression profiles.
Gene annotation and ontology analysis.
Manual annotation of differentially regulated array features was performed as previously described (Benninghoff and Williams, 2008). For the proteins encoded by the putative trout homolog mRNAs, functional information was inferred from annotations in the Gene Ontology (GO), Online Mendelian Inheritance in Man (OMIM), and SwissProt Protein Knowledgebase databases. Automatic annotation of the entire OSUrbt-v5 array was performed using traditional basic local alignment search tool (BLAST) in a two-step process, as follows. First, the array 70mer oligo sequences were queried against the NCBI expressed sequence tag (EST) databases for rainbow trout, salmon (Salmo salar) and zebrafish (Danio rerio). Of the 1676 features on the OSUrbt-v5 array, 1384 EST matches were obtained. The resulting top EST hit (E < 10−4) for each array feature was then used for a translated BLASTx search against the NCBI nonredundant protein sequence (nr) database. The resulting top hit (E < 10−6), excluding hypothetical proteins, was considered the best match for array feature identification; 1103 gene matches were obtained from the NCBI nr database. NCBI accession numbers for the top hits were used to obtain gene symbols for each array feature using BioThesaurus (Liu et al., 2006).
Gene ontology enrichment analysis was performed using High-Throughput GoMiner (Zeeberg et al., 2005). For each treatment, the list of differentially regulated genes (Supplementary table 5) was compared with an auto-generated list derived from gene ontologies for rainbow trout (NCBI taxonomy ID 8022), zebrafish (ID:7095), and human (ID:9606). Because the OSUrbt-v5 array is a medium-sized array (about 1450 genes) with probes focused on processes involved in carcinogenesis, reproduction, toxicological response, and stress physiology, it was necessary to automatically generate a global list of genes to avoid potential pathway bias inherent in a targeted array. All available database resources were searched, and all evidence levels were included in the analysis. A minimum of two genes per category was set for generation of category statistics, and 100 randomizations were used for the enrichment analysis. A significant effect of dietary treatment on GO term category (biological process) enrichment was inferred when p < 0.05, as determined by a one-sided Fisher’s exact test after false discovery rate (FDR) correction. Cluster Image Maps (CIM) for biological processes over- and underrepresented in treatment gene lists were generated using CIMminer (Weinstein, 2004) with GO categories clustered by Euclidian distance method with average linkage. To visualize and compare relationships among differentially regulated GO categories associated with dietary E2 and PFNA, differentially regulated gene lists were subjected to analysis in AgriGO (Du et al., 2010) using the singular enrichment analysis tool against the zebrafish gene ontology database.
Quantitative real-time PCR.
To validate changes in gene expression detected on the OSUrbt array, mRNA levels of select genes were evaluated by the quantitative real-time PCR (qRT-PCR) as described previously (Benninghoff and Williams, 2008), with a few modifications. Total RNA (1 μg) was reverse transcribed (Superscript II; Invitrogen, Carlsbad, CA) according to the supplier’s protocol with oligo d(T)18 primer and a final reaction volume of 50 μl. Primer sequences are provided in Supplementary table 1, and qRT-PCR was performed using the PerfeCta SYBR Green FastMix (Quanta Biosciences, Gaithersburg, MD) on a Mastercycler ep Realplex (Eppendorf, Hauppauge, NY). PCR standards for each target gene were prepared by gel purification of PCR products (QIAX II; Qiagen), quantified using the PicoGreen dsDNA Quantification Kit (Molecular Probes, Eugene, OR) and serially diluted for final concentrations ranging from 0.001 to 100 ng DNA. All qRT-PCR expression values were normalized by the geometric mean fold change of four housekeeping genes (actb, gapdh, top2a, and atp5b). Then, for comparison to microarray expression values, log2 fold change ratios were calculated for treated samples compared with the same reference pool that was utilized in the microarray study. qRT-PCR data were analyzed by one-way ANOVA with Dunnett’s post hoc test for multiple comparisons, and a significant change in gene expression was inferred when p < 0.05.
RESULTS
Promotion of AFB1- or MNNG-initiated Hepatocarcinogenesis by PFAAs
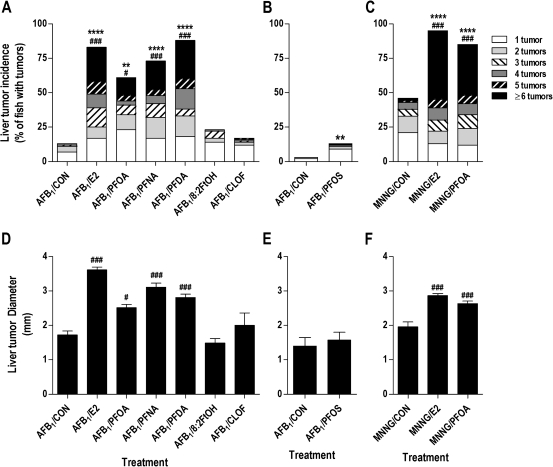
Initiation with 10 ppb AFB1 resulted in a moderate rate of liver tumor incidence (13%) in 12-month-old trout (Table 1 and Fig. 1A), whereas no tumors were observed in sham-initiated animals. The 5 ppm E2 promotion diet markedly enhanced liver tumor incidence to 83% (p < 0.0001), increased liver tumor multiplicity (p < 0.001), and doubled the average liver tumor size (p < 0.001) (Figs. 1A and 1D). Postinitiation exposure to experimental diets containing PFOA, PFNA, or PFDA resulted in a hepatic tumor response similar to that of E2, and PFDA was the most potent promoting agent tested in this study. Interestingly, 200 ppm PFDA increased liver tumor incidence to a greater extent (26% higher) than did a 10-fold higher diet concentration of PFOA. Dietary PFOA, PFNA, and PFDA also significantly increased tumor multiplicity and size in a manner similar to that of E2 (Fig. 1D). In contrast, postinitiation dietary exposure to 8:2FtOH or the classic PP compound CLOF did not change liver tumor incidence, burden, or size. Liver tumor incidence in trout initiated with AFB1 at 15 weeks was only substantially lower at 1% (Table 1); dietary PFOS increased the liver cancer rate to 13% (p = 0.0014), though tumor burden and multiplicity remained unchanged compared with time-matched controls (Figs. 1B and 1E). Logistic regression analyses for the E2, PFOA, PFNA, PFDA, and PFOS treatment groups showed that the experimental diet was the primary factor driving tumor response (p-values ranging from 0.0014 to <0.0001); reduced body weight was a minor factor associated with tumor outcome, whereas fish sex, replicate tank, or idiopathic liver disease did not impact tumor outcome (Supplementary table 2 and fig. 3). Dietary treatment with E2, PFOA, PFNA, PFDA, or PFOS significantly increased relative liver weight, though this observation could be partially attributed to lower body weight in some of these treatment groups (Supplementary figure 4).
TABLE 1.
Impact of Dietary PFCs on AFB1-Induced Liver Carcinogenesis
| Treatmenta | Tumor class (%) |
||||||
| Incidence (%) | HCA | HCC | MA | MC | CCA | CCC | |
| Initiated at 10 weeks | |||||||
| Sham/CON | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sham/E2 | 7* | 0 | 58 | 0 | 33 | 8 | 0 |
| Sham/PFOA | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sham/PFNA | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sham/PFDA | 5 | 0 | 0 | 0 | 100 | 0 | 0 |
| Sham/FtOH | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sham/CLOF | 1 | 0 | 0 | 0 | 100 | 0 | 0 |
| AFB1/CON | 13 | 26 | 23 | 2 | 47 | 0 | 2 |
| AFB1/E2 | 83#### | 6 | 22 | 4 | 65 | 1 | 2 |
| AFB1/PFOA | 62## | 10 | 27 | 1 | 54 | 4 | 5 |
| AFB1/PFNA | 72#### | 5 | 17 | 0 | 68 | 3 | 8 |
| AFB1/PFDA | 88#### | 7 | 24 | 1 | 63 | 1 | 4 |
| AFB1/FtOH | 23 | 12 | 29 | 3 | 52 | 2 | 2 |
| AFB1/CLOF | 15 | 11 | 29 | 6 | 41 | 5 | 8 |
| Initiated at 15 weeks | |||||||
| Sham/CON | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sham/PFOS | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AFB1/CON | 1 | 0 | 29 | 0 | 71 | 0 | 0 |
| AFB1/PFOS | 13†† | 5 | 10 | 5 | 68 | 3 | 10 |
Note. CCA, cholangiocellular adenoma; CCC, cholangiocellular carcinoma; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; MA, mixed adenoma; MC, mixed carcinoma.
Treatment groups are indicated as initiation/diet (see “Materials and Methods” section for complete details.
p < 0.05 compared with Sham/CON; ##p < 0.01; ####p < 0.0001 compared with AFB1/CON; ††p < 0.01 compared with AFB1/CON (15 weeks) as determined by logistic regression analysis.
FIG. 1.
Perfluoroalkyls increase liver tumor incidence, multiplicity and size in AFB1- and MNNG-initiated trout. (A–C) Liver tumor incidence and multiplicity (males and females). (D–F) Average liver tumor size ± SE. Trout were initiated with 10 ppm AFB1 at 10 (A, D) or 15 weeks of age (B, E) or with 35 ppm MNNG at 10 weeks (C, F). Details on experimental diets are provided in “Materials and Methods.” **p < 0.01 and ****p < 0.0001, significant difference in tumor incidence compared with CON diet (within each initiation group) as determined by logistic regression analysis (complete results in Supplementary tables 2 and 3). #p < 0.05 and ###p < 0.001, significant difference in tumor multiplicity or size compared with CON diet (within each initiation group) as determined by the Kruskal-Wallis test with Dunnett’s post hoc test for multiple comparisons. A color version of this figure is available in the online version of the article.
A third cohort of trout was initiated with 35 ppm MNNG to determine whether the tumor-promoting effects of dietary PFOA was specific to hepatocarcinogenesis or dependent upon the initiating carcinogen. Initiation with the multiorgan carcinogen MNNG resulted in tumorigenesis of the liver, kidney, stomach, and swim bladder (Table 2). Dietary exposure to 5 ppm E2 and 2000 ppm PFOA significantly increased liver tumor incidence (p < 0.0001), multiplicity (p <0.001), and size (p <0.001) compared with control diet (Figs. 1C and 1F). Kidney and stomach carcinogenesis were not significantly affected by E2 or PFOA (Table 2), and the apparent impact of these compounds on swim bladder tumor incidence was confounded by significant overdispersion among the replicate tanks (Supplementary fig. 5). Logistic regression analyses for MNNG-initiated groups showed that experimental diet was the primary factor impacting liver tumor outcome (p < 0.0001), and there was not a significant effect of fish sex, replicate tank, or idiopathic liver disease on liver carcinogenesis (Supplementary table 3).
TABLE 2.
Impact of Dietary PFCs on MNNG-Induced Multiorgan Carcinogenesis
| Treatmenta | Incidence (%) |
Liver tumor class |
||||||||
| Stomach | Kidney | SB | Liver | HCA | HCC | MA | MCC | CCA | CCC | |
| Sham/CON | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MNNG/CON | 99 | 37 | 45 | 51 | 25 | 28 | 3 | 39 | 2 | 3 |
| MNNG/E2 | 99 | 49 | 51 | 97* | 33 | 13 | 1 | 51 | 1 | 1 |
| MNNG/PFOA | 99 | 29 | 34 | 86* | 26 | 11 | 4 | 55 | 3 | 1 |
Note. CCA, cholangiocellular adenoma; CCC, cholangiocellular carcinoma; HCA, hepatocellular adenoma; HCC, hepatocellular carcinoma; MA, mixed adenoma; MC, mixed carcinoma; SB, swimbladder.
Treatment groups are indicated as initiation/diet (see “Materials and Methods” section for complete details
*p < 0.0001 compared with MNNG.
Histological evaluation of tumors in 12.5-month-old trout confirmed previous observations from our laboratory that the predominant liver tumor type in AFB1- or MNNG-initiated animals was mixed carcinoma with hepatocellular adenoma, and hepatocellular carcinoma as secondary tumor types (Tables 1 and 2). Tumor type profiles were not noticeably different among the various tumor promotion diets, though cholangiocellular tumors (adenoma and carcinoma) were more common in AFB1-initiated trout fed E2 or PFAA promotion diets.
Perfluoroalkyl Modulation of Hepatic Gene Expression
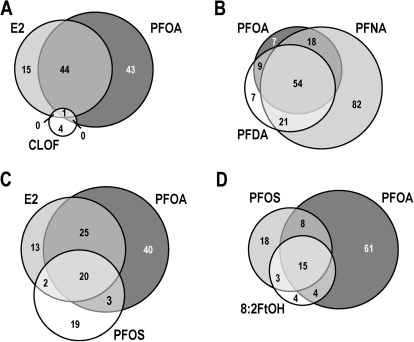
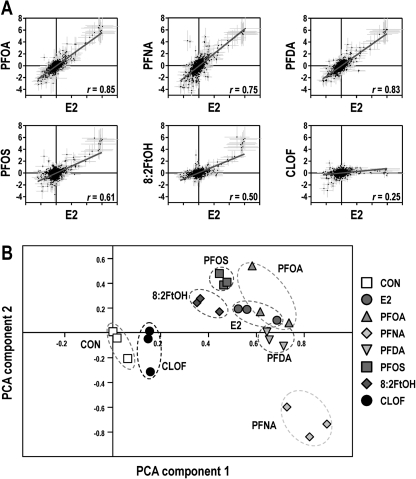
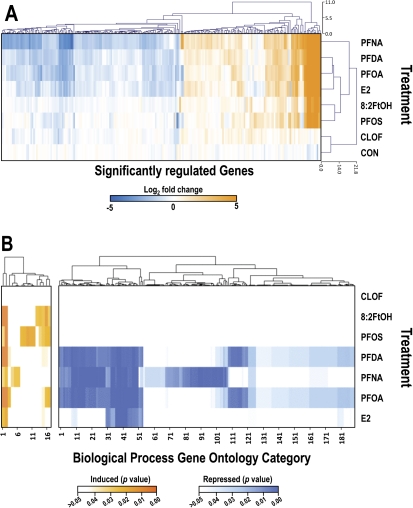
In the present study, we used the trout OSUrbt-v5 microarray to examine hepatic transcriptional responses to several structurally related polyfluorinated compounds in comparison with E2 and CLOF (GEO accession GSE31085). Quality control analysis of array data showed that intra- and interarray variability was generally low and that hybridization was consistent and reproducible (Supplementary figure 6). Multiple criteria were used to reduce the original raw data sets to a subset of array features considered significantly regulated by any one of the experimental treatments (Supplementary table 4). Average expression values, accession numbers, and gene annotations for select array features that passed all stringency criteria are shown in Supplementary table 5. The impact of E2, PFOA, and CLOF on hepatic gene expression was very similar to prior observations in our laboratory (Fig. 2) (Tilton et al., 2008). Dietary PFOA, PFNA, and PFDA commonly altered expression of 54 genes, of which many were shared with the E2 group. Genes regulated by PFOS and FtOH were somewhat similar to E2 and the PFAAs, whereas CLOF had very little effect on liver gene expression in trout. Several analytical approaches were utilized to compare PFAA gene expression profiles to E2, a model estrogen, and CLOF, a classic PP. Pairwise Pearson correlation analyses for significantly regulated genes revealed strong correlations among E2, PFOA, PFNA, PFDA treatments (r ≥ 0.84), whereas the E2, PFOS, and FtOH groups were modestly similar (r values from 0.66 to 0.83) (Fig. 3A; Supplementary table 6). Principal components analysis (PCA) was employed to reduce the dimensionality of the data set so that general relationships between the promotion diets could be discerned more easily (Fig. 3B). Transcript profiles for E2, PFOA, and PFDA treatments were highly similar, indicated by close proximity in the PCA plot, whereas PFOS and FtOH were moderately similar (within the same quadrant); all treatments were distinct from CLOF and CON groups. Also, the expression profile for PFNA was sufficiently unique to form a separate cluster distant from all other treatment groups. Bidirectional clustering of genes differentially regulated by at least one of the experimental diets showed distinct patterns of expression corresponding to two primary nodes in the sample tree, with one node encompassing all PFCs and E2 and the second node including CLOF and control groups (Fig. 4A). Distinct patterns of gene expression were evident for each experimental condition, particularly for PFNA, which formed a separate subnode within the estrogen group. These patterns remained consistent when this analysis was applied to the entire array data set (Supplementary figure 7).
FIG. 2.
Venn diagrams depicting overlap of differentially regulated genes among experimental treatments. The total number of genes differentially regulated induced by the experimental treatment is indicated for each intersection. A color version of this figure is available in the online version of the article.
FIG. 3.
Dietary exposure to PFAAs induces an estrogen-like hepatic gene expression profile in trout. (A) Pairwise correlation of hepatic gene expression profiles. Values shown are the log2 geometric mean of fold change for each array feature ± SE (n = 3). Pearson correlation coefficients (r) are indicated for each comparison, and overlay lines indicate results of least-squares linear regression analysis. A color version of this figure is available in the online version of this article. (B) PCA on experimental condition. PC1 and PC2 are shown and account for 57.9 and 9.6% of experiment variance, respectively. Symbols represent biological replicates (n = 3), and dashed circles represent overlap, or lack thereof, among treatment groups. A color version of this figure is available in the online version of the article.
FIG. 4.
Bi-directional hierarchical clustering of gene expression data and CIM showing impact of treatment diet on enrichment of biological process GO terms. (A) Unsupervised bi-directional hierarchical cluster analysis. The heat map shows expression data (geometric mean of log2 values, n = 3) for genes differentially regulated twofold up or down (p < 0.05 by Welch’s t-test) in at least one treatment group clustered by array feature (top tree) and treatment (left tree). (B) Gene ontology enrichment analysis was performed using GoMiner, and unsupervised cluster analyses of GO categories were performed using CIMminer as described above. Scale bars represent the range of FDR-corrected p values: orange for biological process categories induced by experimental diets, blue for those repressed and white for unchanged. The indicated numbers for GO term categories correspond to rows in Supplementary tables 7 and 8. A significant effect of dietary treatment on enrichment of the GO term category (biological process) was inferred p < 0.05 as determined by a one-sided Fisher’s exact test after FDR correction.
Transcripts differentially regulated by the estrogen-like treatments, including E2, PFOA, PFNA, and PFDA, represent biological processes involved in cell proliferation; apoptosis; signal transduction; transcription; protein translation, modification and transport; phase I and II metabolism; redox regulation; and adaptive immune response (Supplementary tables 5, 7 and 8 and figs. 8 and 9). Overall, the estrogenic transcriptional profile observed in this study is highly similar to previous trout experiments in our laboratory, as a similar set of estrogen biomarker genes were differentially regulated, including vtg, ctds, esr1, rtn9-a1, sec61ab, vhsv4, and ikk1, among others (Benninghoff and Williams, 2008; Tilton et al., 2008). Moreover, typical gene markers indicative of a typical transcriptional response to PPs, such as crot and acat1, were not significantly regulated by E2, the polyfluorinated compounds tested in this study or the classic PPAR agonist CLOF; however, catalase expression was significantly repressed by PFNA (Supplementary table 5). Also of note, dietary exposure to all of the fluorochemicals tested caused significant enrichment of GO categories response to estradiol stimulus and ER signaling pathway. Though the transcriptional profiles for E2 and the PFCs examined in this study were broadly similar, some distinctions were evident (Fig. 4B; Supplementary tables 7 and 8). In particular, the perfluoroalkyl carboxylic acids significantly suppressed expression of several genes involved in regulation of the blood coagulation cascade and the complement pathway; E2 similarly repressed genes in these pathways, though to a lesser extent. Additionally, several genes associated with phase I and II metabolism (gstp1, cyp3a27, mgst1 and cbr1) were differentially regulated by dietary PFOS and/or FtOH, but not E2 or the perfluoroalkyl carboxylic acids.
Expression of select genes differentially induced or repressed was verified by qRT-PCR, including a2m, ctsd, cyp1a, cyp2k5, hpx, pgds, tcpbp, trx, and vtg. Generally, qRT-PCR values followed a pattern similar to that acquired using the microarray (Supplementary figure 10). However, the magnitude of change in gene expression detected by qRT-PCR was occasionally greater compared with the microarray data (e.g., vtg) due to saturation beyond the linear range of detection on the array. Overall, results of these analyses confirm that our strategy for identification of differentially regulated genes from the OSUrbt-v5 data set resulted in the detection of meaningful changes in gene expression.
DISCUSSION
We report for the first time that multiple PFAAs enhance hepatocarcinogenesis via an estrogen-like mechanism in rainbow trout, an animal model that recapitulates human insensitivity to peroxisome proliferation. Previously, we demonstrated that dietary exposure to the ubiquitous environmental contaminant PFOA enhanced AFB1-initiated liver tumorigenesis in trout (Tilton et al., 2008). Subsequent in vitro and in vivo experiments showed that several perfluoroalkyl carboxylic acids and sulfonates have weak estrogen activity, likely via direct interaction with the ER (Benninghoff et al., 2011); moreover, in this animal model, PFAAs did not elicit the typical PP response expected for PPARα ligands. In the present study, we tested the hypothesis that PFAAs structurally related to PFOA would similarly impact liver tumorigenesis. We determined that chronic exposure to three different PFAAs via the diet, including PFOA, PFNA, and PFDA, markedly increased hepatocarcinogenesis in trout in a manner similar to the prototypical estrogen, E2. Also, tumor promotion by PFOA was restricted to the liver but not dependent upon the initiating carcinogen. Dietary exposure to PFOS caused a modest increase in liver tumor incidence, possibly due to the lower diet concentration selected for this compound or the slightly older age of these fish at initiation and start of dietary treatment.
Although the diet concentrations of PFAAs tested in this study (100–2000 ppm, or 2.5–50 mg/kg body weight/day) are typical for PP cancer studies in rodents, these levels were substantially greater than would be expected from a typical human environmental exposure (Fromme et al., 2009). Extrapolation from a 2-week dietary dose-response study in trout with PFOA and PFDA (Benninghoff et al., 2011) suggests that the diet concentrations employed in this tumor promotion study result in blood levels in the micromolar range, considerably higher than the nanomolar range reported for these compounds in human blood (Calafat et al., 2007; Olsen et al., 2003). Evidence from a previous limited dose-response tumor study with PFOA in trout suggested that a lower dietary exposure to PFAAs might not substantially increase liver cancer risk in animals that are insensitive to peroxisome proliferation (Tilton et al., 2008). However, the observation from the present study that 200 ppm PFDA increased tumor incidence to an even greater extent than 2000 ppm PFOA (88 and 62% incidence, respectively) points to the need for further studies utilizing a comprehensive dose-response approach with individual PFAAs to appropriately assess cancer risk for these compounds. Moreover, because multiple members of this chemical class are often detected in blood and tissue samples (Calafat et al., 2007; Lau et al., 2007), the potential for additive or synergistic effects of PFAA mixtures in promoting liver carcinogenesis should not be ignored.
The liver gene expression profiles obtained by the trout custom DNA microarray were highly similar among E2 and PFAA treatments, suggesting that these compounds likely act via a common mechanism of action to promote hepatocarcinogenesis in trout. Previously, we identified a set of 17 hepatic genes as biomarkers of estrogen exposure (Benninghoff and Williams, 2008), of which 13 were differentially regulated by PFAAs in trout. Although the specific mechanism for promotion of liver cancer by estrogens in trout is not known, results of this and previous gene expression profiling experiments (Benninghoff and Williams, 2008; Tilton et al., 2006, 2008) point to the involvement of genes associated with cell growth, apoptosis, cell signaling, regulation of transcription, protein stability, and transport and immune response. For example, E2- or PFAA-dependent promotion of hepatocarcinogenesis may involve disruption of the nuclear factor kappa B signaling pathway (e.g., nfkb1, ikk1, ikbe) or suppression of innate immune response (e.g., C-3, C-9, mbl) (Sun and Karin, 2008; Vainer et al., 2008). Interestingly, the gene expression profiles for PFAAs obtained from the trout microarray are generally similar to profiles reported by Wei et al. (2007, 2009) following aqueous exposures of PFOA, PFOS, and various mixtures of PFAAs in rare minnow (Gobiocypris rarus). In rat liver, the transcriptional response to an oral gavage of PFOA or PFOS was dominated by genes associated with lipid metabolism and transport, including genes in the peroxisomal fatty acid oxidation pathway (e.g., Acat1) (Guruge et al., 2006; Hu et al., 2005). However, few transcripts associated with the metabolism and transport of lipids and cholesterol were significantly altered by PFAA exposure in trout (<3% of all regulated features), and several of these were also regulated by E2. These observations, along with the recent discovery that PFOA, PFNA, PFDA, and PFOS competitively bind to the trout ER (Benninghoff et al., 2011), provide further evidence that PFAAs promote hepatic cancer in this species via an estrogen-like mechanism involving activation of the ER, rather than via interaction with PPARα and induction of peroxisomal proliferation.
At the time liver tissues were collected for the microarray study, all three perfluoroalkyl carboxylic acids had been administered at the same diet concentration (2000 ppm) for 2 weeks. Thus, apparent distinctions in transcriptional profiles among PFOA, PFNA, and PFDA may reflect chemical-specific responses, differences in the strength of interaction with molecular targets mediating the transcription response or possible differences in uptake, distribution or elimination of these chemicals in vivo. Martin et al. (2003a; 2003b) reported that values for bioconcentration and residence time of PFAAs in trout liver generally increased with increasing length of the fluorinated carbon chain (half-life of 5 days for PFOA compared with 14 days for PFDA). However, the high similarity in transcriptional response to PFOA and PFDA observed in this study did not reflect these apparent differences in chemical pharmacokinetics, most likely due to the daily dietary exposure protocol employed. Dietary PFNA altered hepatic expression of 175 transcripts (65 induced, 110 repressed), nearly twice the number for PFOA and PFDA; however, many of these array features were similarly induced or repressed by all three carboxylic acids and E2, though to differing extent. A case in point is dysregulation of the blood coagulation pathway induced by PFNA, a reported side effect of pharmacological estrogen exposure (Sherif, 1999).
Only a few definitive chemical-specific gene targets were identified in this study, most notably st2s2 and cyp3a7 for PFOS and gstp1 for 8:2FtOH. Additionally, the modest transcriptional response to PFOS as compared with the carboxylic acids tested should be considered in the context of the lower dietary exposure (200 ppm). Dietary 8:2FtOH (2000 ppm) modified relatively few transcripts, most of which were highly sensitive estrogen biomarker genes (e.g., vtg, zrp, esr1). Previously, we determined that 8:2FtOH was not overtly estrogenic in trout and does not interact with the ER (Benninghoff et al., 2011); it is possible that the transcriptional activity of this chemical observed in this study may be due to in vivo metabolism of 8:2FtOH to PFOA or other estrogenic derivative (Brandsma et al., 2011). Other laboratories have also reported estrogen-like activity of PFAAs and some fluorotelomers, although inconsistencies among these reports suggest that some species are more responsive to one compound class than the other (Ishibashi et al., 2008; Liu et al., 2007; Maras et al., 2006).
In conclusion, we report the important finding that multiple PFAAs, including PFOA, PFNA, PFDA, and PFOS, enhance liver tumorigenesis in trout, an animal model that is not responsive to peroxisome proliferation. Evidence from gene expression profiling suggests that the mechanism of action for PFAA-dependent promotion of hepatocarcinogenesis likely involves interaction with the hepatic ER. Finally, this study highlights the use of an alternative animal model to reveal novel estrogen-like action of multiple PFAAs in modulating chemical carcinogenesis.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work was supported in part by the National Institute of Environmental Health Sciences (P30 ES03850, T32 ES07060, P30 ES00210, P42 ES016465, and R01 ES013534) and the Utah Agricultural Experiment Station.
Supplementary Material
Acknowledgments
The authors wish to acknowledge the assistance of Eric Johnson and Greg Gonnerman at the Sinnhuber Aquatic Research Laboratory (SARL) for care of the animals used in this study. The technical assistance of Marilyn Henderson, Lisbeth Siddens, Trevor Fish and Brittany Packard is also gratefully acknowledged. During this study, A.D.B. served as a member of the external peer review panel for the Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services to review the Draft Toxicological Profile for Perfluoroalkyl Acids. To our knowledge, none of the other authors has any professional or financial affiliation that may be perceived as having biased the presentation.
References
- Abdellatif A, Préat V, Taper H, Roberfroid M. The modulation of rat liver carcinogenesis by perfluorooctanoic acid, a peroxisome proliferator. Toxicol. Appl. Pharmacol. 1991;111:530–537. doi: 10.1016/0041-008x(91)90257-f. [DOI] [PubMed] [Google Scholar]
- Bailey GS, Williams DE, Hendricks JD. Fish models for environmental carcinogenesis: The rainbow trout. Environ. Health Perspect. 1996;104(Suppl. 1):5–21. doi: 10.1289/ehp.96104s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff AD, Bisson WH, Koch DC, Ehresman DJ, Kolluri SK, Williams DE. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors. in vitro. Toxicol. Sci. 2011;120:42–58. doi: 10.1093/toxsci/kfq379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff AD, Williams DE. Identification of a transcriptional fingerprint of estrogen exposure in rainbow trout liver. Toxicol. Sci. 2008;101:65–80. doi: 10.1093/toxsci/kfm238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma SH, Smithwick M, Solomon K, Small J, de Boer J, Muir DC. Dietary exposure of rainbow trout to 8:2 and 10:2 fluorotelomer alcohols and perfluorooctanesulfonamide: Uptake transformation and elimination. Chemosphere. 2011;82:253–258. doi: 10.1016/j.chemosphere.2010.09.050. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ. Health Perspect. 2007;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’eon JC, Mabury SA. Exploring indirect sources of human exposure to perfluoroalkyl carboxylates (PFCAs): Evaluating uptake, elimination, and biotransformation of polyfluoroalkyl phosphate esters (PAPs) in the rat. Environ. Health Perspect. 2011;119:344–350. doi: 10.1289/ehp.1002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38:W64–W70. doi: 10.1093/nar/gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W, Wilhelm M, Twardella D. Perfluorinated compounds–exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health. 2009;212:239–270. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Guruge KS, Yeung LW, Yamanaka N, Miyazaki S, Lam PK, Giesy JP, Jones PD, Yamashita N. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA) Toxicol. Sci. 2006;89:93–107. doi: 10.1093/toxsci/kfj011. [DOI] [PubMed] [Google Scholar]
- Hanhijarvi H, Ylinen M, Haaranen T, Nevalainen T. A proposed species difference in the renal excretion of perfluorooctanoic acid in the beagle dog and rat. In: Beynen AC, Solleveld HA, editors. New Development of Biosciences: Their Implications for Laboratory Animal Science. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1988. [Google Scholar]
- Hendricks JD, Meyers TR, Shelton DW. Histological progression of hepatic neoplasia in rainbow trout (Salmo gairdneri) Natl. Cancer Inst. Monogr. 1984;65:321–336. [PubMed] [Google Scholar]
- Hendricks JD, Shelton DW, Loveland PM, Pereira CB, Bailey GS. Carcinogenicity of dietary dimethylnitrosomorpholine, N-methyl-N′-nitro-N-nitrosoguanidine, and dibromoethane in rainbow trout. Toxicol. Pathol. 1995;23:447–457. doi: 10.1177/019262339502300402. [DOI] [PubMed] [Google Scholar]
- Holden PR, Tugwood JD. Peroxisome proliferator-activated receptor alpha: Role in rodent liver cancer and species differences. J. Mol. Endocrinol. 1999;22:1–8. doi: 10.1677/jme.0.0220001. [DOI] [PubMed] [Google Scholar]
- Hu W, Jones PD, Celius T, Giesy JP. Identification of genes responsive to PFOS using gene expression profiling. Environ. Toxicol. Pharmacol. 2005;19:57–70. doi: 10.1016/j.etap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Yamauchi R, Matsuoka M, Kim JW, Hirano M, Yamaguchi A, Tominaga N, Arizono K. Fluorotelomer alcohols induce hepatic vitellogenin through activation of the estrogen receptor in male medaka (Oryzias latipes) Chemosphere. 2008;71:1853–1859. doi: 10.1016/j.chemosphere.2008.01.065. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yamanoshita O, Asaeda N, Tagawa Y, Lee CH, Aoyama T, Ichihara G, Furuhashi K, Kamijima M, Gonzalez FJ, et al. Di(2-ethylhexyl)phthalate induces hepatic tumorigenesis through a peroxisome proliferator-activated receptor alpha-independent pathway. J. Occup. Health. 2007;49:172–182. doi: 10.1539/joh.49.172. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang JH, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Lai DY. Rodent carcinogenicity of peroxisome proliferators and issues on human relevance. J. Environ. Sci. Health, Part C. 2004;22:37–55. doi: 10.1081/GNC-120038005. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lee BC, Hendricks JD, Bailey GS. Toxicity of mycotoxins in the feed of fish. In: Smith JE, editor. Mycotoxins and Animal Feedstuff: Natural Occurrence, Toxicity and Control. Boca Raton, FL: CRC Press; 1991. pp. 607–626. [Google Scholar]
- Liu C, Du Y, Zhou B. Evaluation of estrogenic activities and mechanism of action of perfluorinated chemicals determined by vitellogenin induction in primary cultured tilapia hepatocytes. Aquat. Toxicol. 2007;85:267–277. doi: 10.1016/j.aquatox.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu ZZ, Zhang J, Wu C. BioThesaurus: A web-based thesaurus of protein and gene names. Bioinformatics. 2006;22:103–105. doi: 10.1093/bioinformatics/bti749. [DOI] [PubMed] [Google Scholar]
- Maras M, Vanparys C, Muylle F, Robbens J, Berger U, Barber JL, Blust R, De Coen W. Estrogen-like properties of fluorotelomer alcohols as revealed by mcf-7 breast cancer cell proliferation. Environ. Health Perspect. 2006;114:100–105. doi: 10.1289/ehp.8149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JW, Mabury SA, Solomon KR, Muir DC. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss) Environ. Toxicol. Chem. 2003a;22:196–204. [PubMed] [Google Scholar]
- Martin JW, Mabury SA, Solomon KR, Muir DC. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss) Environ. Toxicol. Chem. 2003b;22:189–195. [PubMed] [Google Scholar]
- Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DC, Mabury SA. Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ. Sci. Technol. 2004;38:373–380. doi: 10.1021/es034727+. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, Armitage JB, Herron RM, Medhdizadehkashi Z, Nobiletti JB, et al. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ. Health Perspect. 2003;111:1892–1901. doi: 10.1289/ehp.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CN, Hsu MH, Griffin KJ, Raucy JL, Johnson EF. Peroxisome proliferator activated receptor-alpha expression in human liver. Mol. Pharmacol. 1998;53:14–22. [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- Sherif K. Benefits and risks of oral contraceptives. Am. J. Obstet. Gynecol. 1999;180:S343–S348. doi: 10.1016/s0002-9378(99)70694-0. [DOI] [PubMed] [Google Scholar]
- Sun B, Karin M. NF-kappaB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228–6244. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- Tilton S, Gerwick L, Hendricks J, Rosato C, Corley-Smith G, Givan S, Bailey G, Bayne C, Wililams D. Use of a rainbow trout oligonucleotide microarray to determine transcriptional patterns in aflatoxin B1-induced hepatocellular carcinoma compared to adjacent liver. Toxicol. Sci. 2005;88:319–330. doi: 10.1093/toxsci/kfi309. [DOI] [PubMed] [Google Scholar]
- Tilton SC, Givan SA, Pereira CB, Bailey GS, Williams DE. Toxicogenomic profiling of the hepatic tumor promoters indole-3-carbinol, 17β-estradiol and β-naphthoflavone in rainbow trout. Toxicol. Sci. 2006;90:61–72. doi: 10.1093/toxsci/kfi341. [DOI] [PubMed] [Google Scholar]
- Tilton SC, Orner GA, Benninghoff AD, Carpenter HM, Hendricks JD, Pereira CB, Williams DE. Genomic profiling reveals an alternate mechanism for hepatic tumor promotion by perfluorooctanoic acid in rainbow trout. Environ. Health Perspect. 2008;116:1047–1055. doi: 10.1289/ehp.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainer GW, Pikarsky E, Ben-Neriah Y. Contradictory functions of NF-kappaB in liver physiology and cancer. Cancer Lett. 2008;267:182–188. doi: 10.1016/j.canlet.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Wei Y, Dai J, Liu M, Wang J, Xu M, Zha J, Wang Z. Estrogen-like properties of perfluorooctanoic acid as revealed by expressing hepatic estrogen-responsive genes in rare minnows (Gobiocypris rarus) Environ. Toxicol. Chem. 2007;26:2440–2447. doi: 10.1897/07-008R1.1. [DOI] [PubMed] [Google Scholar]
- Wei Y, Shi X, Zhang H, Wang J, Zhou B, Dai J. Combined effects of polyfluorinated and perfluorinated compounds on primary cultured hepatocytes from rare minnow (Gobiocypris rarus) using toxicogenomic analysis. Aquat. Toxicol. 2009;95:27–36. doi: 10.1016/j.aquatox.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Weinstein JN. Integromic analysis of the NCI-60 cancer cell lines. Breast Dis. 2004;19:11–22. doi: 10.3233/bd-2004-19103. [DOI] [PubMed] [Google Scholar]
- Zeeberg BR, Qin H, Narasimhan S, Sunshine M, Cao H, Kane DW, Reimers M, Stephens RM, Bryant D, Burt SK, et al. High-Throughput GoMiner, an ‘industrial-strength’ integrative gene ontology tool for interpretation of multiple-microarray experiments, with application to studies of Common Variable Immune Deficiency (CVID) BMC Bioinformatics. 2005;6:168. doi: 10.1186/1471-2105-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.