Abstract
Exposure to high concentrations of hexavalent chromium (Cr[VI]) in drinking water is reported to induce oral mucosa tumors in F344 rats and intestinal tumors in B6C3F1 mice. To investigate the modes of action underlying these tumors, 90-day drinking water studies (with interim necropsy at day 8) were conducted with concentrations of 0.1–182 mg/l Cr(VI), administered as 0.3–520 mg/l sodium dichromate dihydrate. Blood and tissue samples were analyzed for chromium content, oxidative stress, iron levels, and gross and microscopic lesions. Results for the F344 rats are described herein and compared with results from B6C3F1 mice published previously. After 90 days of exposure, total chromium concentrations in the rat and mouse oral mucosae were comparable, yet significant dose-dependent decreases in the reduced-to-oxidized glutathione ratio (GSH/GSSG) were observed only in rats. In the duodenum, changes in GSH/GSSG were only observed in mice. Levels of 8-hydroxydeoxyguanosine were not increased in the oral or duodenal mucosae of either species. Glutathione levels were increased in the duodenum but decreased in the jejunum of both species, indicating potential differential responses in the intestinal segments. Histiocytic infiltration was observed in the duodenum of both species, yet duodenal cytokines were repressed in mice but increased in rats. Serum and bone marrow iron levels were more decreased in rats than mice. Collectively, these data suggest that Cr(VI)-induced carcinogenesis in the rodent alimentary canal involves oxidative stress; however, differences in histopathology, cytokines, and iron status suggest potential contributions from other factors as well.
Keywords: drinking water, oxidative stress, carcinogenesis, Cr(VI), MOA
Chronic ingestion of hexavalent chromium (Cr(VI)), in the form of sodium dichromate dihydrate (Na2Cr2O7•2H2O or SDD), in drinking water has been found to induce oral mucosa tumors in rats at concentrations ≥ 172 mg SDD/l, and intestinal tumors in mice at concentrations ≥ 57.3 mg SDD/l (National Toxicology Program [NTP], 2008; Stout et al., 2009). Villous cytotoxicity and crypt hyperplasia were observed in the mouse small intestine, whereas no obvious non-neoplastic lesions were observed in the oral mucosae of rats (NTP, 2008; Stout et al., 2009). The different lesions suggest that the tumors in the two sites may have arisen through different carcinogenic modes of action (MOAs). Although the NTP (2008) 2-year bioassay demonstrated that Cr(VI) was carcinogenic in rodents following chronic exposure to high concentrations in drinking water, the study did not provide adequate data for understanding how the tumors arose or why different tumors were observed in each species. Because mice and rats developed tumors in different tissues of the alimentary canal, comparisons of species-specific pathology and biochemistry in the target tissues (i.e., oral and intestinal mucosae) are expected to inform the MOAs for carcinogenicity in each tissue.
Several authors have proposed or discussed possible MOAs for alimentary cancers in rodents (McCarroll et al., 2010; Stern, 2010; Thompson et al., 2011a). However, currently available data are insufficient to conclusively support any of the hypothesized MOAs. This is especially true for oral squamous cell carcinomas originating from the rat palate after 2 years of exposure to SDD, where no non-neoplastic lesions were reported (NTP, 2007, 2008; Stout et al., 2009). The number of oral cavity tumors was significantly elevated as compared to current and historical controls in the highest dose group (516 mg/l) in both males (6/49) and females (11/50), as well as historical controls in females in the 172 mg/l treatment group (2/50) (NTP, 2008; Stout et al., 2009). Stout et al. (2009) noted that 21 chemicals have been shown to cause oral cavity tumors in rats, but no chemicals have been shown to cause oral cavity tumors in male mice, and only one caused oral cavity tumors in female mice. These findings may suggest an inherent susceptibility of rats to oral cancers.
In addition to oral tumors, rats also exhibited a dose-dependent decrease in mean red blood cell (RBC) volume, mean cell hemoglobin (Hb) and hematocrit, indicating microcytic hypochromic anemia (NTP, 2008). Earlier studies reported similar anemic effects in rats exposed to Cr(VI), regardless of whether administered in feed or water (NTP, 1996, 1997, 2007). Although anemic effects were also observed in mice in the NTP (2008) study, effects in mice were milder. Interestingly, one study has shown that anemia can increase the risk of oral cancer in rats (Prime et al., 1983); thus, it is conceivable that the anemic effects in rats play a role in the MOA of the oral tumors.
To better understand the MOAs for the alimentary cancers observed in the NTP 2-year bioassay (NTP, 2008), Thompson et al. (2011a) conducted a comprehensive review of available literature to develop a plausible MOA for the intestinal tumors in mice, identified key data gaps in that MOA, and designed a 90-day drinking water study to address those data gaps. Although a formal MOA analysis was not conducted for the oral cavity tumors observed in rats, data necessary to evaluate possible key events in the MOAs were collected from the target tissues of both species. These biochemical, toxicogenomic, and toxicokinetic data should inform the MOAs for Cr(VI) in the mouse intestine as well as in the rat oral mucosa. For the reasons described above, endpoints related to tissue and serum iron levels were collected. The overall study design is summarized in Table 1 and is similar to that of the 90-day NTP study (NTP, 2007). Herein we report results from the 90-day rat study including descriptions of the histopathological findings, biochemical analyses related to oxidative status, and the chromium content in target tissues. Moreover, these results are compared with those previously reported for mice (Thompson et al., 2011b) to provide additional insight into key events in the MOAs. These data will provide phenotypic anchoring for future toxicogenomic results, which will be published separately upon completion.
TABLE 1.
Treatment Groups
| Number of female F344 rats |
||||||||
| Aa |
B |
C |
D |
E |
||||
| Toxicology and histopathology |
Biochemical evaluations |
Gene expression analysis |
Mutation analysis |
Toxicokinetic analyses |
||||
| SDD (mg/l) | Day 8 | Day 91 | Day 8 | Day 91 | Day 8 | Day 91 | Day 91 |
Day 91 |
| 0 | 5 | 10 | 10 | 15 | 10 | 10 | 10 | 5 |
| 0.3 | 5 | 10 | 10 | 15 | 10 | 10 | 10 | 5 |
| 4 | 5 | 10 | 10 | 15 | 10 | 10 | 10 | 5 |
| 60 | 5 | 10 | 10 | 15 | 10 | 10 | 10 | 5 |
| 170 | 5 | 10 | 10 | 15 | 10 | 10 | 10 | 5 |
| 520 | 5 | 10 | 10 | 15 | 10 | 10 | 10 | 5 |
Cohort subgroups for assays.
MATERIALS AND METHODS
Test substance.
SDD (CAS 7789-12-0) (99.95% pure) was obtained from Sigma-Aldrich, Inc. (Milwaukee, WI) and stored at room temperature and protected from light. The dose formulations were prepared at concentrations of 0.3, 4, 60, 170, and 520 mg/l SDD in tap water, which is equivalent to 0.1, 1.4, 4.9, 20.9, 59.3, and 181 mg/l Cr(VI) (the Cr(VI) concentration is equivalent to ∼35% of the SDD concentration). On the first, third, fifth, and seventh (final) batch preparations, samples of formulations for each dose group, including the control, were collected and shipped to Brooks Rand Laboratories (Seattle, WA) for analysis of Cr(VI) content. The first batch was also analyzed for total chromium. Samples were prepared and analyzed in accordance with EPA Method SW-7196A to confirm the Cr(VI) concentrations of the administered drinking water. In Method 7196A, Cr(VI) is complexed with diphenylcarbazide in an acidic solution and absorbance is measured at 540 nm. Batches found to differ from the target concentration by ±10% were not used. Prior to use, dose formulations of SDD were stored in sealed Nalgene carboys at room temperature protected from light, which has been shown to be stable for 42 days in dosed water formulations at a concentration of 41.8 mg/l when stored under these conditions (NTP, 2008).
Animals and husbandry.
The in-life portion of the study was conducted at Southern Research Institute (Birmingham, AL), the same research facility that conducted both the NTP 13-week and 2-year bioassays (NTP, 2007, 2008). The same strain of female rats (Fischer 344/N) was used in the current study as in the NTP studies. Rats in the current study were obtained from Charles River Laboratories International, Inc. (Stone Ridge, NY), whereas the rats in the NTP studies were obtained from Taconic Farms (Germantown, NY). The rats were ∼4 weeks of age when they arrived and were allowed to acclimate for ∼2 weeks. At the start of the study, the rats weighed between 83.1 and 126.4 g. Animals were allowed ad libitum access to the same chow as in the NTP studies, namely, irradiated NTP-2000 Wafers (Zeigler Bros., Gardners, PA). Water (dosed or control) was supplied in amber glass water bottles. Teflon-lined lids with stainless steel, double-balled sipper tubes were used. Water bottles were changed twice weekly or as needed. Analysis of tap water from the animal facility prior to the study indicated that no contaminants were present that would be expected to interfere with or affect the outcome of the study.
Rats were group-housed (five per cage) in solid bottom, polycarbonate cages on a stainless steel rack in a room maintained at a temperature of 60.7°F–84.1°F and relative humidity of 28.4–100%. Excursions outside the desired temperature (69°F–75°F) and humidity (35–65%) ranges were brief in duration and did not adversely affect the health of the animals or outcome of the study. Fluorescent lighting provided illumination ∼12 h/day. Irradiated hardwood bedding chips (Sani-Chips; P.J. Murphy Forest Products Corp., Montville, NJ) were used as bedding material. No known contaminants were present in the bedding that would have been expected to interfere with or affect the outcome of the study. Cage size and animal care conformed to the Guide for the Care and Use of Laboratory Animals, the U.S. Department of Agriculture through the Animal Welfare Act (Public Law 99-198), and to the applicable Standard Operating Procedures of Southern Research Institute.
Study design.
Male and female F344 rats responded similarly to Cr(VI) in the previous 2-year bioassay (NTP, 2008); therefore, only female rats were used in this present study. Animals were assigned to treatment groups as indicated in Table 1 and were provided with food and drinking water ad libitum until study termination at days 8 or 91 (Table 1). Water and food consumption were measured weekly for each cage of animals throughout the study, and values were reported as an average daily consumption (milliliters/animal or grams/animal, respectively). During treatment, animals were weighed on day 1, weekly thereafter, and prior to scheduled euthanasia. All animals were observed at least twice daily during the prestudy and study periods for signs of mortality and moribundity. Each animal was removed from its cage and examined for clinical signs of toxicity (e.g., alopecia, eye discharge, piloerection, hyperexcitability) on day 1 and weekly thereafter.
Pathology and histopathology.
Pathology and histopathology were assessed in five animals from each treatment group on day 8 and 10 animals per treatment group on day 91 (subgroup A, Table 1). The animals were euthanized by CO2 asphyxiation and subjected to a postmortem examination that included, but was not limited to, examination of the external surfaces of the body, all orifices of the body, and the cranial, thoracic, abdominal, and pelvic cavities and their contents. Based on findings of the NTP 2-year bioassay (2008), the oral cavity, duodenum, jejunum, and any gross lesions were collected from each rodent and saved in 10% neutral buffered formalin for histopathologic evaluation. Tissue sections (5 μm) were stained with hematoxylin and eosin for microscopic examination and evaluated by a veterinary pathologist for evaluation and diagnosis. Tissues were diagnosed and categorized using standardized nomenclature with lesions ranked for severity.
GSH and GSSG analyses.
Reduced glutathione (GSH) and oxidized glutathione (GSSG) parameters were measured in five animals from each treatment group (subgroup B, Table 1). GSH and GSSG were measured in plasma, as well as in oral mucosal tissue (from hard and soft palate) and duodenal and proximal jejunal mucosae (scraped) on days 8 and 91. Samples were collected, processed, and stored as previously described (Thompson et al., 2011b). GSH and GSSG were determined fluorometrically using the o-phthalaldehyde procedure as previously described (Senft et al., 2000). The calculation for redox potential (ΔE) is described in detail elsewhere (Dalton et al., 2004) and is as follows: ΔE = −240 mV − (61.5 mV/2) × log([GSH]2/[GSSG]).
8-Isoprostane and 8-hydroxydeoxyguanosine analyses.
Lipid oxidation was measured in five animals from each treatment group (subgroup B, Table 1). One sample of oral cavity and one sample of duodenum from each rat were snap-frozen and stored at −80°C until homogenization. Homogenization buffer solution was prepared as previously described (Thompson et al., 2011b). Tissues were then homogenized in 0.3 ml using an Omni THQ homogenizer (Omni International, Kennesaw, GA) and a disposable hard tissue tip. After homogenization, samples were snap-frozen and stored at −80°C until assayed. 8-Isoprostane (8-isoprostane is also known as 8-iso-prostaglandin F2α, 8-epi-PGF2α, and 15-isoprostane F2t)2 was analyzed via OxiSelect 8-iso-Prostaglandin F2α ELISA Kit obtained from Cell Biolabs (San Diego, CA) and normalized to protein content via BCA assay (Pierce, Rockford, IL). Oxidative DNA damage was measured in five animals from each treatment group (subgroup B, Table 1); these samples were prepared as described in Thompson et al. (2011b).
Cytokine and chemokine analyses.
Cytokines and chemokines were measured in five animals from each treatment group (subgroup B, Table 1). One sample of oral cavity and one sample of duodenum were prepared as described above (for 8-isoprostane). For serum collection, each animal was anesthetized with CO2/O2, and blood samples were collected from the retro-orbital sinus into serum separator tubes containing no anticoagulant. The contents of the tubes were centrifuged to separate serum. The tissue and sera samples for rats were analyzed for 22 cytokines/chemokines via a Milliplex Rat Cytokine Kit (Millipore, Billerica, MA) on a Luminex 200 (Austin, TX). The cytokines/chemokines included in this kit are shown in Supplementary table S1. Cytokine levels were normalized to protein content.
Iron status.
Serum iron levels were measured in 10 animals from each treatment group (5 each from subgroups A and D, Table 1). Iron status was evaluated in half of the animals designated for macroscopic and microscopic pathological evaluation on day 91. Animals were anesthetized using CO2/O2, and blood samples (∼0.4 ml) were collected from the retro-orbital plexus into tubes containing no anticoagulant. The contents of the tubes were centrifuged to separate serum. One aliquot of serum was used for measurement of serum iron, and another was snap-frozen and stored at −80°C for potential future analysis. Serum iron was measured using the Cobas c501 Clinical Chemistry Analyzer (Version 04-02; Roche Diagnostics, Indianapolis, IN). Blood ferritin and transferrin were measured using commercial ELISA kits purchased from ALPCO (Salem, NH). The iron content in bone marrow smears was also assessed in the same five animals from the “Mutation analysis” group (Table 1). In each animal, one bone marrow smear was prepared and stained with Prussian blue to assess the presence of iron in different treatment groups.
Measurement of total chromium and iron in tissues.
Tissue levels of total chromium and iron were measured in five animals (subgroup E, Table 1). Samples of the oral cavity, glandular stomach, duodenum, jejunum, ileum, liver, plasma, and RBCs were collected for evaluation of total chromium (Cr) and iron (Fe) content from animals exposed for 90 days. Animals were anesthetized using CO2, and tissues were removed, flushed of contents, snap frozen, and stored at approximately −80°C. Samples were shipped frozen to Brooks Rand Laboratories where ∼100 mg of tissue was digested in nitric acid in a controlled microwave digestion program. Samples were then brought to a final volume of 8 ml with deionized water. Analysis was performed using EPA Draft Method 1638 (modified) using inductively coupled plasma-mass spectrometry with Dynamic Reaction Cell (DRC) technology. Digested samples were analyzed utilizing internal standardization with rhodium. This method incorporates ionization of the sample in an inductively coupled radio frequency plasma, with detection of the resulting ions by mass spectrometer on the basis of their mass-to-charge ratio. The limit of detection was 0.02 μg Cr/g tissue. Iron levels were simultaneously measured in these tissue samples, and the limit of detection was 0.2 μg Fe/g tissue.
Data management and statistical evaluation.
During the in-life phase of the study, Provantis (Version 7, Instem Life Sciences Systems, Ltd, Staffordshire, U.K.) was used for the direct on-line capture of most in-life and pathology data. In addition, Provantis interfaced with the Cobas c501 Clinical Chemistry Analyzer (Version 04-02, Roche Diagnostics) for capture of serum iron data. Environmental monitoring of animal rooms (i.e., temperature/humidity and light/dark cycles) was performed using the Edstrom Watchdog System (Version 5.13; Edstrom Industries, Inc., Waterford, WI). The remainder of the data was collected manually.
For consistency with NTP practices, biochemical and clinical endpoints were first tested for dose-related trends using Jonckheere's test (Jonckheere, 1954). Biochemical data sets with a significant trend were then analyzed by Williams' (parametric) or Shirley's tests (nonparametric) (Shirley, 1977), whereas Dunnett's (parametric) or Dunn's (nonparametric) tests were run if there was not a monotone trend. Water consumption and body weight data were analyzed by one-way ANOVA followed by Dunnett's tests. Food intake was found to be non-normally distributed and was analyzed by Kruskall-Wallis test, followed by Wilcoxon-Mann-Whitney test with Bonferroni adjustment. Statistical packages used included R (http://www.R-project.org), Prism 5 for Mac (GraphPad Software, San Diego, CA, www.graphpad.com), and Provantis. Microscopic lesion data were analyzed by Fisher's exact test (one-sided) using Prism 5 for Mac.
RESULTS
Gross and Histopathological Findings
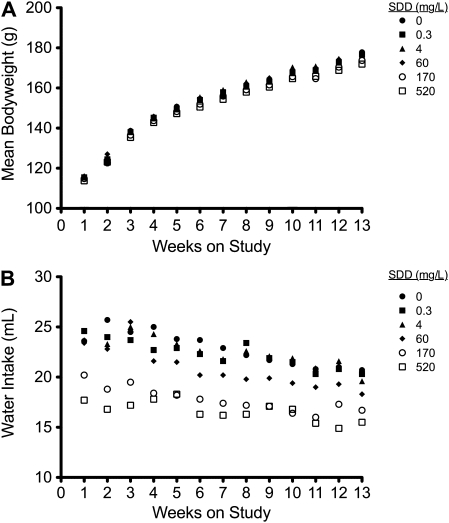
Exposure of rats to SDD for 90 days had no effect on survival, nor were there any clinical signs of treatment-related toxicity. The mean body weight in the 520 mg/l SDD treatment group was generally lower than control animals (Fig. 1A), but only significantly on weeks 5, 6, 12, and 13. Administration of SDD had no effect on food consumption in any treatment group. Water consumption was significantly lower than control animals every week at 170 and 520 mg/l SDD and for most weeks at 60 mg/l (Fig. 1B). The average daily doses of SDD to rats at day 8 and day 91 of the study are shown in Table 2. The average daily doses of SDD in the companion 90-day mouse study are also provided for comparison (Thompson et al., 2011b).
FIG. 1.
Weekly mean body weight (A) and water intake (B) for rats during the 13-week study.
TABLE 2.
Average Daily Dose of Ingested SDD
| SDD (mg/kg) |
||||||
| SDD, mg/l (nominal) | 0.3 | 4 | 14 | 60 | 170 | 520 |
| Rat | ||||||
| Average daily dose (day 8) | 0.06 | 0.8 | ND | 12.1 | 29.9 | 80.9 |
| Average daily dose (day 91) | 0.05 | 0.6 | ND | 8.3 | 20.4 | 58.6 |
| Mousea | ||||||
| Average daily dose (day 8) | 0.08 | 1.1 | 3.3 | 14.0 | 37.4 | 86.8 |
| Average daily dose (day 91) | 0.07 | 0.9 | 3.1 | 13.2 | 33.0 | 88.7 |
Note. ND, not done.
Data for mouse body weight, intake, and dose are taken from Thompson et al. (2011b).
No treatment-related gross lesions were observed in the oral cavity or small intestine on day 8, nor were any microscopic lesions observed in the oral cavity. In the duodenum, villous atrophy, apoptosis, crypt cell hyperplasia, and histiocytic infiltration were observed at ≥ 170 mg/l SDD (Table 3, Supplementary figure S1). Apoptosis and crypt cell hyperplasia were observed at 60 mg/l, but only in one of five animals. No microscopic lesions were observed at ≤ 4 mg/l SDD. With the exception of histiocytic infiltration, similar lesions were observed in the jejunum in the 170 and 520 mg/l treatment groups. These results are summarized in Table 3.
TABLE 3.
Summary of Histopathology in the Rat Duodenum and Jejunum
| Pathology | 0 | 0.3 | 4 | 60 | 170 | 520 |
| Day 8 | ||||||
| Duodenum | ||||||
| Villous atrophy | –– | –– | –– | –– | 3/5a (1), 2/5a (2) | 2/5 (1) |
| Apoptosis | –– | –– | –– | 1/5 (1) | 3/5a (1), 1/5a (2) | 3/5 (1) |
| Crypt cell hyperplasia | –– | –– | –– | 1/5 (1) | 3/5a (2), 2/5a (3) | 1/5a (1), 4/5a (2) |
| Histiocytic infiltration in the villous | –– | –– | –– | –– | 2/5 (1), 1/5 (2) | 4/5a (1) |
| Jejunum | ||||||
| Villous atrophy | –– | –– | –– | –– | 4/5a (1) | 3/5 (1) |
| Apoptosis | –– | –– | –– | –– | 3/5 (1) | 2/5 (1) |
| Crypt cell hyperplasia | –– | –– | –– | –– | 3/5a (1), 2/5a (2) | 2/5a (1), 3/5a (2) |
| Day 91 | ||||||
| Duodenum | ||||||
| Villous atrophy | –– | –– | –– | –– | 1/10 (2) | –– |
| Apoptosis | –– | –– | –– | 3/10 (1) | 3/10a (1), 1/10a (2) | 6/10a (1), 1/10a (2) |
| Crypt cell hyperplasia | –– | –– | –– | –– | 5/10a (1) | 4/10a (1) |
| Histiocytic infiltration in the villous | –– | –– | –– | 2/10a(1), 7/10a(2) | 1/10a(2), 6/10a(3), 3/10a(4) | 1/10a(1), 1/10a(2), 2/10a(3),6/10a(4) |
| Jejunum | ||||||
| Villous atrophy | –– | –– | 1/10 (3) | –– | –– | –– |
| Apoptosis | –– | –– | 1/10 (1) | –– | 2/10 (1) | –– |
| Crypt cell hyperplasia | –– | –– | 2/10 (1) | –– | 3/10 (1) | 1/10 (1) |
| Histiocytic infiltration in the villous | –– | –– | –– | 3/10 (1) | 6/10a (1), 1/10a (2) | 5/10a (1), 4/10a (2) |
Note. Values in parentheses are severity scores for the lesions (1, minimal; 2, mild; 3, moderate; 4, marked). Histopathological grading—villous atrophy: minimal = < 25% decrease in villous height; mild = ≥ 25 and ≤ 50% decrease in villous height; moderate = ≥ 50 and ≤ 75% decrease in villous height; marked = ≥ 75% decrease in villous height; apoptosis: minimal = < 5 apoptotic cells per 40× or high power field (HPF); mild = approximately ≥ 5 and ≤ 10 apoptotic cells per HPF; moderate = ≥ 10 and ≤ 20 apoptotic cells per HPF; marked = ≥ 20 apoptotic cells per HPF; crypt cell hyperplasia: minimal = ≥ 1 and ≤ 2 times the normal crypt depth; mild = ≥ 2 and ≤ 3 times the normal crypt depth; moderate = ≥ 3 and ≤ 4 times the normal crypt depth; marked = ≥ 4 times the normal crypt depth; and histiocytic infiltration in the villous lamina propria: minimal = a few macrophages in fewer than half of the villi; mild = macrophages in the villi which were readily discernible at 10× and present in ≥ 50 and < 75% of the villi.
Significantly different (p ≤ 0.05) from control by Fisher's exact test (severity scores were combined for statistical tests).
Consistent with findings from a previous 13-week drinking water study conducted by the NTP (NTP, 2007), no treatment-related gross lesions were observed after 90 days of exposure to SDD. Also, there were no microscopic lesions observed in the rat oral cavity. In the duodenum, apoptosis was observed at ≥ 60 mg/l SDD, and crypt cell hyperplasia at ≥ 170 mg/l (Table 3). Histiocytic infiltration was present in almost all animals at ≥ 60 mg/l (Supplementary fig. S2). Apoptosis, crypt cell hyperplasia, and villous atrophy were observed in the jejunum at concentrations as low as 4 mg/l; however, the incidences were not statistically different from control animals in any treatment group, and in many instances the lesions were not observed at higher concentrations (Table 3). In contrast, there were concentration-dependent increases in histiocytic infiltration beginning at 60 mg/l. Notably, histiocytic infiltration in the duodenum was the only SDD-induced histopathological lesion previously reported in the rat small intestine (NTP, 2007, 2008).
Cytokine and Chemokine Analyses
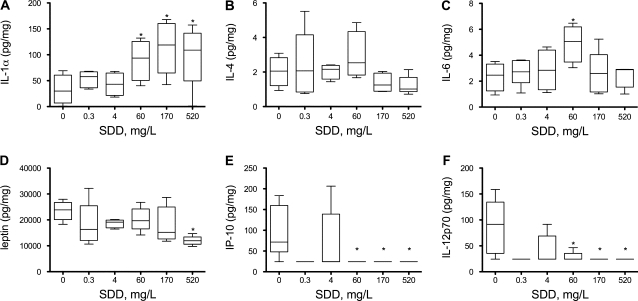
Histiocytic infiltration was observed in the rat duodenum in the previous NTP studies (NTP, 2007, 2008) as well as this current study; therefore, several cytokines and chemokines (listed in Supplementary table S1) were measured in the duodenum, oral mucosa, and plasma on day 91. In these tissues, the data were not normally distributed and exhibited inhomogeneous variance. None of the cytokines/chemokines exhibited significant concentration-dependent monotone trends in the oral mucosa. In the duodenum, only two of the examined proteins (IL-1α and IL-4) exhibited significant monotone trends. IL-1α was significantly elevated at ≥ 60 mg/l (Fig. 2A). Interleukin-4 exhibited a significant monotone trend that was inversely proportional to dose (Fig. 2B); however, no treatment groups were significantly different from control animals. Among the cytokines/chemokines that did not show a significant trend, only IL-6 had a treatment group that differed significantly from control, namely, the 60 mg/l SDD treatment group (Fig. 2C). In serum, only three of the examined proteins exhibited significant monotone trends; none of the other cytokines/chemokines examined were significantly altered in any treatment group. As shown in Figure 2D, leptin appeared to be reduced by SDD exposure. Both IL-12p70 and IP-10 also appeared to be reduced as a result of SDD exposure (Figs. 2E–F).
FIG. 2.
Measures of cytokines/chemokines in the rat duodenum (A–C) and serum (D–F) on day 91. All plots (except C) were significant for trend; *p < 0.05 compared to control group by Shirley's test. In plot (C), statistically different from control by Dunn's test (p < 0.05). Groups without boxes were mostly comprised of nondetect values.
Tissue and Serum Iron Levels
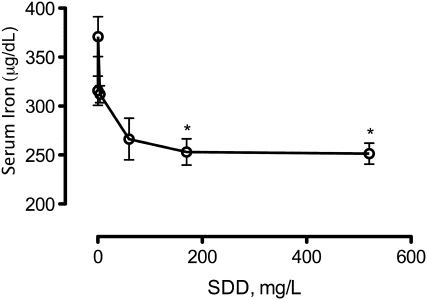
Exposure to SDD was previously shown to induce mild microcytic hypochromic anemia in rats (NTP, 2008). Therefore, iron status was assessed after 90 days of exposure to SDD. Bone marrow smears stained for iron content with Prussian blue indicated lower levels in rats exposed to 170 and 520 mg/l SDD (Table 4). Serum iron levels were significantly decreased at ≥ 170 mg/l (Fig. 3). Ferritin and transferrin levels exhibited no clear treatment-related effects (Supplementary table S2).
TABLE 4.
Scores of Iron Content in Rat Bone Marrow Smears
| SDD mg/l | Moderate | Moderate/slight | Slight |
| 0 | 4/5 | 1/5 | 0/5 |
| 0.3 | 5/5 | 0/5 | 0/5 |
| 4 | 5/5 | 0/5 | 0/5 |
| 60 | 4/5 | 1/5 | 0/5 |
| 170 | 2/5 | 3/5 | 0/5 |
| 520 | 0/5 | 0/5 | 5/5 |
Note. Scores are based on the grading scheme of Gale et al. (1963).
FIG. 3.
Serum iron levels in rats on day 91. *p < 0.05 compared to control by ANOVA followed by Dunnett's test.
Effects of SDD on Oxidative Status
The ratio of GSH/GSSG is a key indicator of cellular redox status (Meister and Anderson, 1983; Moriarty-Craige and Jones, 2004; Schafer and Buettner, 2001) and was therefore measured in several portions of the alimentary canal in order to examine whether SDD exposure induced changes in redox status. Table 5 presents the effects of SDD on the levels of GSH and GSSG in the rat oral, duodenal, and jejunal epithelia, as well as in the plasma on day 8. Although statistically significant effects were observed for GSSG, GSH/GSSG ratio, and ΔE in the rat oral mucosa at the 0.3 mg/l SDD, there were no effects at any other concentration, as such, this effect may not be treatment related. In both the duodenum and jejunum, there were significant increases in GSH at ≥ 170 mg/l and GSSG at ≥ 60 mg/l. However, there were no changes in the GSH/GSSG ratio or ΔE in the duodenum in any treatment group. In contrast, the GSH/GSSG ratio was significantly decreased at 520 mg/l in the jejunum. In the plasma, there were significant increases in GSH (≥ 60 mg/l), GSSG (≥ 170 mg/l), and redox potential (≥ 60 mg/l).
TABLE 5.
Redox Values in Rat Oral and Intestinal Epithelia and Plasma on Day 8
| SDD (mg/l) | GSH (nmol/mg) |
GSSG (nmol/mg) |
GSH/GSSG |
ΔE GSSG/2GSH (mV)a |
||||
| Average | SEM | Average | SEM | Average | SEM | Average | SEM | |
| Oral cavity | ||||||||
| 0 | 36.86 | 2.297 | 3.16 | 0.308 | 11.81 | 0.457 | −207.26 | 0.505 |
| 0.3 | 35.35 | 2.328 | 4.42b | 0.135 | 7.97c | 0.297 | −201.43c | 1.411 |
| 4 | 33.38 | 1.297 | 3.39 | 0.175 | 9.98 | 0.665 | −203.66 | 1.311 |
| 60 | 32.42 | 2.112 | 3.06 | 0.205 | 10.65 | 0.542 | −204.12 | 1.310 |
| 170 | 35.30 | 3.020 | 3.39 | 0.361 | 10.51 | 0.364 | −205.03 | 1.209 |
| 520 | 33.47 | 3.583 | 3.19 | 0.125 | 10.39 | 0.818 | −203.89 | 2.634 |
| Duodenum | ||||||||
| 0 | 13.12 | 0.352 | 0.24 | 0.008 | 55.97 | 2.031 | −214.33 | 0.731 |
| 0.3 | 14.34 | 0.795 | 0.27 | 0.021 | 54.76 | 1.830 | −215.17 | 0.459 |
| 4 | 14.62 | 1.174 | 0.26 | 0.011 | 55.29 | 2.853 | −215.43 | 1.694 |
| 60 | 15.01 | 1.380 | 0.27d | 0.012 | 54.57 | 2.931 | −215.55 | 1.879 |
| 170 | 15.52d | 0.712 | 0.31e | 0.016 | 50.62 | 2.516 | −215.16 | 1.125 |
| 520 | 15.57d | 0.860 | 0.28d | 0.005 | 54.93 | 2.600 | −216.28 | 1.319 |
| Jejunum | ||||||||
| 0 | 7.82 | 0.610 | 0.17 | 0.008 | 48.02 | 4.817 | −204.97 | 2.394 |
| 0.3 | 7.90 | 0.382 | 0.18 | 0.013 | 45.29 | 3.523 | −204.54 | 1.529 |
| 4 | 8.23 | 0.333 | 0.19 | 0.011 | 43.24 | 1.020 | −204.66 | 0.382 |
| 60 | 8.41 | 0.481 | 0.24d | 0.004 | 35.35 | 2.156 | −202.12 | 1.581 |
| 170 | 9.96d | 0.422 | 0.26e | 0.021 | 38.84 | 3.799 | −205.52 | 1.813 |
| 520 | 11.07e | 0.581 | 0.36e | 0.008 | 30.74e | 1.543 | −203.99 | 1.353 |
| Plasma | GSH (μM) | GSSG (μM) | ||||||
| 0 | 15.85 | 0.215 | 5.76 | 0.103 | 2.75 | 0.032 | −118.22 | 0.24 |
| 0.3 | 15.99 | 0.487 | 5.76 | 0.173 | 2.78 | 0.080 | −118.43 | 0.70 |
| 4 | 16.58 | 0.257 | 5.66 | 0.157 | 2.93 | 0.046 | −119.66 | 0.17 |
| 60 | 18.99d | 0.532 | 7.06 | 0.128 | 2.69 | 0.074 | −120.30d | 0.71 |
| 170 | 21.54e | 0.491 | 7.66e | 0.160 | 2.81 | 0.024 | −122.60e | 0.37 |
| 520 | 23.93e | 0.316 | 8.47e | 0.112 | 2.83 | 0.074 | −124.06e | 0.52 |
See “Materials and Methods” section for calculation.
Statistically significant from control by Dunn's test (p < 0.05).
Statistically significant from control by Dunn's test (p < 0.01).
Statistically significant from control by Shirley's tests (p < 0.05).
Statistically significant from control by Shirley's tests (p < 0.01).
Changes in GSH, GSSG, and redox potential after 90 days of exposure to SDD in drinking water are shown in Table 6. In the oral mucosa, significant decreases in GSH and significant increases in GSSG were observed at ≥ 170 mg/l SDD. Significant decreases in the GSH/GSSG ratio and redox potential occurred at ≥ 60 mg/l. In the duodenum, statistically significant increases in GSH and GSSG occurred at ≥ 170 mg/l; however, there was no apparent change in the GSH/GSSG ratio or redox potential. In the jejunum, a significant decrease in GSH, the GSH/GSSG ratio, and redox potential (i.e., increased ΔE) occurred at ≥ 60 mg/l SDD, while GSSG levels were significantly elevated at ≥ 170 mg/l. In the plasma, significant increases in GSH and GSSG were observed at ≥ 60 mg/l, and a significant decrease in the GSH/GSSG ratio started at 170 mg/l SDD (Table 6).
TABLE 6.
Redox Values in Rat Oral and Intestinal Epithelia and Plasma on Day 91
| GSH (nmol/mg) |
GSSG (nmol/mg) |
GSH/GSSG |
ΔE GSSG/2GSH (mV)a |
|||||
| SDD (mg/l) | Average | SEM | Average | SEM | Average | SEM | Average | SEM |
| Oral cavity | ||||||||
| 0 | 38.30 | 0.973 | 3.04 | 0.126 | 12.66 | 0.383 | −208.80 | 0.497 |
| 0.3 | 39.22 | 0.992 | 2.93 | 0.110 | 13.43 | 0.266 | −209.92 | 0.351 |
| 4 | 36.30 | 1.006 | 2.97 | 0.124 | 12.28 | 0.501 | −207.65 | 0.743 |
| 60 | 34.42 | 1.403 | 3.64 | 0.241 | 9.64b | 0.796 | −203.55b | 1.487 |
| 170 | 31.17b | 2.032 | 3.82b | 0.253 | 8.36c | 0.915 | −200.11c | 2.255 |
| 520 | 28.23c | 1.336 | 3.70b | 0.103 | 7.67c | 0.503 | −197.89c | 1.569 |
| Duodenum | ||||||||
| 0 | 18.90 | 1.058 | 0.25 | 0.011 | 75.08 | 4.620 | −223.00 | 1.461 |
| 0.3 | 17.60 | 0.592 | 0.24 | 0.005 | 72.59 | 2.461 | −221.72 | 0.886 |
| 4 | 18.50 | 0.844 | 0.25 | 0.017 | 75.06 | 5.518 | −222.69 | 1.409 |
| 60 | 20.51 | 0.757 | 0.29 | 0.017 | 71.13 | 4.092 | −223.43 | 0.976 |
| 170 | 22.73b | 1.129 | 0.33b | 0.013 | 70.27 | 5.631 | −224.53 | 1.632 |
| 520 | 25.15c | 0.933 | 0.38c | 0.020 | 67.31 | 5.019 | −225.35 | 1.385 |
| Jejunum | ||||||||
| 0 | 16.43 | 0.858 | 0.30 | 0.011 | 54.64 | 3.469 | −216.87 | 1.560 |
| 0.3 | 16.14 | 0.556 | 0.30 | 0.021 | 55.31 | 4.830 | −216.75 | 1.582 |
| 4 | 14.61 | 0.395 | 0.33 | 0.016 | 44.48 | 2.795 | −212.63 | 1.061 |
| 60 | 13.43b | 0.731 | 0.35 | 0.012 | 38.19b | 1.599 | −209.46b | 1.235 |
| 170 | 11.53c | 0.515 | 0.45c | 0.032 | 26.09c | 1.992 | −202.25c | 1.425 |
| 520 | 10.82c | 0.796 | 0.48c | 0.022 | 22.32c | 0.891 | −199.35c | 1.416 |
| Plasma | GSH (μM) | GSSG (μM) | ||||||
| 0 | 32.27 | 1.950 | 4.12 | 0.141 | 7.85 | 0.434 | −141.53 | 1.52 |
| 0.3 | 31.45 | 0.892 | 3.90 | 0.191 | 8.15 | 0.443 | −141.76 | 1.06 |
| 4 | 35.85 | 1.475 | 4.81 | 0.151 | 7.46 | 0.299 | −142.35 | 1.01 |
| 60 | 44.75b | 1.505 | 7.09b | 0.469 | 6.38 | 0.292 | −143.22 | 0.60 |
| 170 | 50.81c | 1.946 | 9.20c | 0.642 | 5.70b | 0.626 | −143.15 | 1.88 |
| 520 | 52.09c | 2.470 | 14.10c | 0.730 | 3.75c | 0.315 | −137.99 | 1.76 |
See “Materials and Methods” section for calculation.
Statistically significant from control by Shirley's tests (p < 0.05).
Statistically significant from control by Shirley's tests (p < 0.01).
In addition to GSH and GSSG parameters, lipid oxidation and DNA oxidation were assessed in the duodenum and oral mucosa at day 91. Relative to controls, there were no statistically significant changes in 8-isoprostane, a measure of lipid oxidation, in the duodenum or oral mucosa at any exposure concentration (data not shown). Levels of 8-hydroxydeoxyguanosine (8-OHdG), a measure oxidative DNA damage, were not statistically altered in the duodenum or oral mucosa of rats at any SDD concentration (Supplementary fig. S3).
Tissue Concentrations of Total Chromium
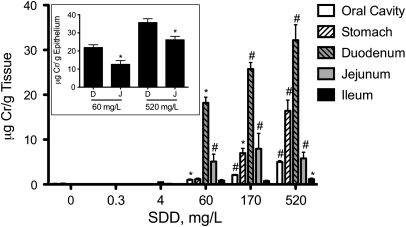
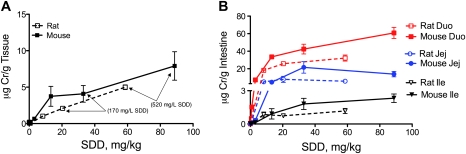
To better understand chromium disposition in the rat alimentary canal, total tissue chromium (Crt) levels were measured in the oral cavity, stomach, duodenum, jejunum, and ileum following 90 days of exposure to SDD in drinking water (Fig. 4). With the exception of the glandular stomach and ileum, statistically significant increases occurred at ≥ 60 mg/l SDD. Significant increases in the glandular stomach and ileum occurred at ≥ 170 mg/l and 520 mg/l, respectively. Within the intestinal segments, the Crt levels were higher in the more proximal portions (duodenum) than distal portions (ileum). However, measurement of chromium in scraped samples of the duodenum and proximal 6 cm of the jejunum indicate that chromium levels are generally comparable between the duodenum and proximal jejunum (Fig. 4, inset). These data suggest that the Crt levels in the jejunum (∼65 cm in length) likely decrease in a proximal-to-distal fashion. Chromium levels in the oral cavity, the tissue that developed tumors in rats in the NTP 2-year bioassay (NTP, 2008), were much lower than in the duodenum.
FIG. 4.
Concentration of total Cr (Crt) in the rat alimentary canal on day 91. Data plotted are mean and SEM. *, Significantly different from control by Shirley's test (p < 0.05); #, Significantly different from control by Shirley's test (p < 0.01). The inset shows a comparison of Cr in scraped epithelial cells from the duodenum (D) and proximal 6 cm of the jejunum (J) in the 60 and 520 mg/l SDD treatment groups. Data represent mean and SEM. **, Significantly different by t-test.
Comparisons of Gastrointestinal Tissue Dosimetry and Oxidative Stress in Rats and Mice
Comparison of the Crt levels in the oral mucosae of rats and mice indicates slightly lower tissue levels in rats on a milligram per kilogram basis of SDD dose (Fig. 5A). It is apparent, however, that Crt levels in the oral mucosae were higher in mice than rats in their respective 520 mg/l SDD treatment groups. In the small intestine, Crt levels were higher in mice than rats within each of the three intestinal segments (Fig. 5B).
FIG. 5.
Concentration of total Cr (Crt) in the oral (A) and intestinal mucosae (B) of rats and mice on day 91. The two highest SDD drinking water concentrations are shown in (A) for reference. Duo, duodenum; Jej, jejunum; and Ile, ileum.
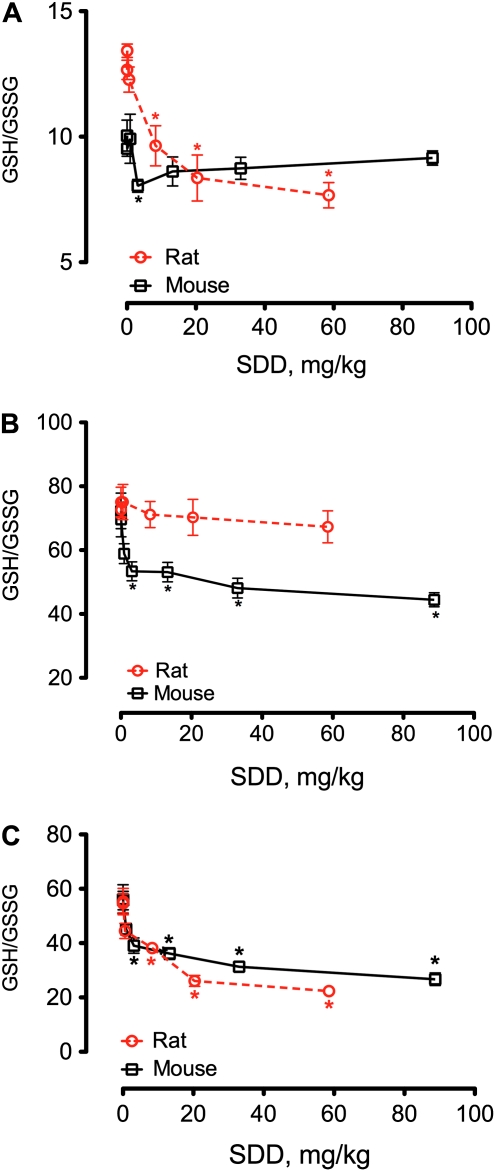
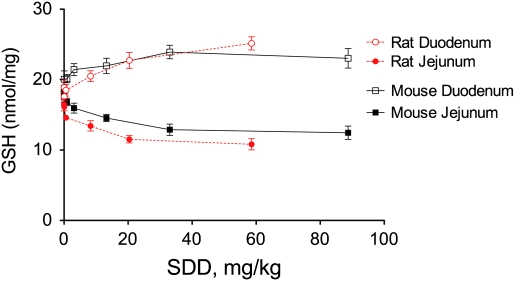
The effects of SDD on the GSH/GSSG ratios in both species are plotted in Figure 6 on a milligram per kilogram body weight basis for comparison. In the oral mucosae, SDD elicited significant decreases in the GSH/GSSG ratio in rats at concentrations ≥ 8 mg/kg but had essentially no effect on the GSH/GSSG ratio in mice (Fig. 6A). In the duodenum, SDD significantly reduced the GSH/GSSG ratio in mice at ≥ 3 mg/kg but had no similar effect in rats (Fig. 6B). In the jejunum, SDD reduced the GSH/GSSG ratio in both species (Fig. 6C). GSH levels were increased in the duodenal mucosae of both species at day 91 but were decreased in the jejunal mucosae (Fig. 7), perhaps suggesting tissue-specific differences in response to SDD.
FIG. 6.
Measures of GSH/GSSG ratios in the oral mucosa (A), duodenum (B), and jejunum (C) of rats and mice on day 91. Data for rats are from Tables 5 and 6, whereas data for mice are from Thompson et al. (2011b). *, Statistically different from respective control group by Shirley's test.
FIG. 7.
Effects of SDD on intestinal GSH levels in rats and mice at day 91.
DISCUSSION
The purpose of this drinking water study, and the companion study by Thompson et al. (2011b), was to investigate the carcinogenic MOAs of Cr(VI) in the target tissue of rats and mice by investigating possible key events that may precede tumor formation. Consistent with the earlier NTP 90-day drinking water study (NTP, 2007), no obvious gross lesions were observed in any tissues of rats exposed to SDD. Likewise, there were no histopathological lesions in the oral mucosa at day 8 or day 91. With the exception of histiocytic infiltration, many of the intestinal lesions described in Table 3 were not previously reported. One explanation for the differences in histopathology is that the water intake in this current study, while consistent with published intake values (U.S. EPA, 1988), was higher than in the NTP studies (NTP, 2007, 2008). For example, the mean water consumption in the 520 mg/l group at 13 weeks in this study was 15.5 g/day, whereas the values for female rats in the 500 mg/l group at 13 weeks in the NTP 90-day study was 9.1 g/day, and 8.4 g/day in the 516 mg/l group in the NTP 2-year bioassay (NTP, 2007, 2008). The reason for these differences is unclear, but the data imply that the mg/kg body weight doses in the current study were higher than in the NTP study at comparable milligram per liter SDD concentrations.
The histopathological findings in the rat small intestine were generally similar to those reported for mice (Thompson et al., 2011b), with one notable exception. In mice, cytoplasmic vacuolization was observed at both day 8 and day 91 starting at 170 and 60 mg/l SDD, respectively. At both time points, vacuolization occurred at lower SDD concentrations than villous atrophy and crypt cell hyperplasia. Vacuolization can be a sign of injury and thus suggests that damage to the villous epithelium resulted in crypt epithelial hyperplasia in mice. There are many potential causes of vacuolization including altered lipid metabolism, sequestration of absorbed material, autophagy, endoplasmic reticulum stress, and proteasome dysfunction (Franco and Cidlowski, 2009; Henics and Wheatley, 1999; Mimnaugh et al., 2006). The apparent difference in treatment-related vacuolization in rats and mice may indicate subtle (but important) differences in response to Cr(VI) in the two species.
Cr(VI) readily reacts with reductants such as GSH and ascorbate, which can generate intermediate chromium species, reactive oxygen species, and changes in redox status (Zhitkovich, 2005). Table 7 provides a comparison of the redox effects of Cr(VI) in target tissues of rats and mice at day 91 with the tumor outcomes reported in the NTP 2-year bioassay (NTP, 2008). For all tissues that developed tumors in the 2-year bioassay, oxidative stress was present at day 91. In the oral mucosae, oxidative stress and tumors occurred in rats but not mice, despite the fact that oral mucosae Crt levels were slightly higher in mice.
TABLE 7.
Summary of Study Findings
| Oral mucosa |
Duodenum |
Jejunum |
|||||||
| Species | Oxidative stress | Cell proliferation | Tumora | Oxidative stress | Cell proliferation | Tumora | Oxidative stress | Cell proliferation | Tumora |
| Rat | Yes | No | Yes | No | Yesb | No | Yes | Noc | No |
| Mouse | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
Tumor results from NTP (2008) 2-year cancer bioassay.
Although crypt hyperplasia was evident, it was more severe at day 8 than day 91 and may have continued to resolve with time.
Cell proliferation was not dose-dependent or statistically significant (see Table 3).
Comparison of the effects in the duodenum of both species also suggests involvement of oxidative stress (Table 7). Tumors were only observed in the mouse duodenum in NTP (2008), and oxidative stress was apparent only in the duodenal epithelium of mice at day 91. It is apparent, however, that GSH and GSSG levels increased in the rat duodenum as a function of dose (Table 6)—suggesting that an adaptive response may have prevented significant changes in the GSH/GSSG ratio or the GSSG/2GSH redox potential (i.e., ΔE). Although lesions were observed in the rat duodenum at day 91 in the absence of oxidative stress, this observation might simply reflect that at the time of euthanasia, the cells had adapted to oxidative stress but that the tissue was still undergoing repair. In this regard, the incidences for atrophy and crypt hyperplasia at day 91 were lower than day 8 (Table 3, Supplementary fig. S4)—implying that the lesions might have resolved if the study duration were longer, which would be consistent with the negative tumor findings in the 2-year bioassay (NTP, 2008). Notably, the mg/kg SDD dose decreased in rats with increased duration (Table 2).
In the jejunum, oxidative stress was again observed in a tissue that developed tumors; however, it was also present in the rat jejunum, which did not develop tumors (Table 7). As was observed in the duodenum, the incidences for atrophy and crypt hyperplasia at day 91 were lower or even absent when compared with day 8 (Table 3, Supplementary fig. S4), and thus these tissues may have recovered if the study duration were longer. Notably, the apparent discrepancy in redox status and histopathology in the jejunum might be explained by the anatomical location of the analyses. Jejunal redox measures were taken from the proximal 6 cm, whereas histopathology was assessed about midway through the jejunum (i.e., at ∼32 cm). Dosimetry data herein suggest that Crt levels decrease distally within the intestinal tract; thus, redox changes in the mid portion of the jejunum (where histopathology was assessed) were likely milder than in the proximal jejunum. Given the higher water intake in this study relative to the NTP studies, it cannot be ruled out that the proximal jejunum of rats in this current study would have experienced prolonged oxidative stress and injury if the study had continued.
Taken together, this study and that by Thompson et al. (2011b) suggest that the rodent duodenum and jejunum respond differently to SDD. Within each species, the chromium levels in scraped duodenal and proximal jejunal samples were similar, yet the redox responses differed. In both species, oxidative stress was more severe in the proximal jejunum than duodenum. In rats, SDD did not significantly alter the GSH/GSSG ratio or redox potential in the duodenum but significantly decreased both parameters in the jejunum. In mice, SDD significantly decreased the GSH/GSSG ratio in the duodenum and jejunum but only significantly decreased redox potential in the jejunum (Thompson et al., 2011b). In both species, GSH levels increased in the duodenum and decreased in the jejunum as a function of SDD (Fig. 7). Interestingly, previous studies using consecutive 10-cm sections of the rat small intestine indicated that duodenal segments could reduce oxidants infused into the lumen more efficiently than jejunal segments via release of cysteine (Dahm and Jones, 2000). These data suggest that the duodenum and jejunum regulate oxidative stress differently and underscore the importance of understanding both dosimetry and MOA in predicting the toxicity of ingested Cr(VI).
Despite signs of oxidative stress in the rat oral mucosa and mouse duodenum, no increases in 8-isoprostane or 8-OHdG were observed in either species. Possible explanations for these negative findings are that the assays lacked sufficient sensitivity to detect changes or that repeated exposure to SDD resulted in the induction of homeostatic processes. Considering that oxidative stress and inflammation are interrelated (Kruidenier and Verspaget, 2002; Rahman and MacNee, 2000; Roberts et al., 2009) and that histiocytic infiltration was observed in the small intestines of rats and mice (NTP, 2007, 2008; Stout et al., 2009), we expected to see increased cytokine levels in both species following SDD exposure (Thompson et al., 2011a). Contrary to expectations, duodenal levels of the pro-inflammatory cytokines IL-1β and TNFα were decreased in mice (Thompson et al., 2011b). In contrast to mice, the pro-inflammatory cytokine IL-1α was elevated in the rat duodenum. Interestingly, the proteasome is required for pro-inflammatory signaling, and proteasome inhibitors are anti-inflammatory and can induce vacuolization (Elliott et al., 2003; Mimnaugh et al., 2006; Neurath et al., 1998; Reinstein, 2004). Whether the differences in vacuolization in the two species relate to differences in cytokine signaling is unknown; however, toxicogenomic data from the animals in these studies are expected to provide additional information regarding immune and oxidative responses.
Previous SDD drinking water studies have indicated anemic effects in both species, with greater severity in rats (NTP, 2007, 2008; Stout et al., 2009). Similar findings were observed in this current study and our companion study in mice (Thompson et al., 2011b); specifically, iron levels in serum and bone smears were lower in rats. In regard to the specificity of SDD-induced oral tumors in rats, iron deficiency has been linked to the etiology of oral cavity cancers (Lucenteforte et al., 2009; Prime et al., 1983; Richie et al., 2008). For example, Prime et al. (1983) reported that anemia increases the risk of oral cancer in rats. In humans, Richie et al. (2008) reported that mild iron deficiency and low GSH levels increased the risk of oral cavity cancer. Lucenteforte et al. (2009) evaluated data from 46 human studies investigating the risk of oral and pharyngeal cancer and reported possible associations between iron deficiency and excess oral cancer risk.
Mechanistic explanations for the association between anemia and oral cancers are unknown. However, chromium can compete with iron for Hb and transferrin binding (Ani and Moshtaghie, 1992; Gray and Sterling, 1950; Hopkins and Schwarz, 1964); such competition could result in a reduction in the rate of iron-consuming pathways, including Hb synthesis, and the consequent appearance of anemia. Chromium might also alter protein function via cofactor competition. For example, Cr(VI) has been hypothesized to deplete ascorbate levels, thereby decreasing the ascorbate-dependent activity of the histone demethylase JHDM2A (Sun et al., 2009). Similarly, iron-dependent histone demethylases can be inhibited via competition with nickel ions (Chen et al., 2006, 2010). Thus, oxidative stress, iron depletion and/or competition, and depletion of reductants like GSH and ascorbate might alter critical enzymes involved in DNA structure and repair.
In summary, the data herein, together with that in Thompson et al. (2011b), support that oxidative stress may be a key event in the MOA for Cr(VI)-induced carcinogenesis. Additional analyses currently underway with tissues collected from the rat and mouse 90-day studies should further inform the key events in the MOAs. Moreover, the pharmacokinetic data collected in these studies will improve our understanding of the species difference in Cr(VI) disposition and will be used to develop physiologically based pharmacokinetic models for extrapolation between species and from the very high concentrations of Cr(VI) that cause cancer in rodents to environmentally relevant human exposures.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Cr(VI) Panel of the American Chemistry Counsel.
Supplementary Material
Acknowledgments
The authors thank Drs Michael Dourson, Kirk Kitchin, David Gaylor, and Xianglin Shi for review of an earlier version of the manuscript. The authors also thank Andrew Tachovsky, Kenneth Burkhalter, and Elizabeth Kuriakose for assistance with statistical analyses; Dr Rich May for his assistance with the ELISA assays; and Dr Michael Carakostas for insights regarding clinical findings. We are also grateful for the contributions provided by the Toxicology Excellence for Risk Assessment Expert Panel overseeing the Cr(VI) Research Program (a panel report is available at http://www.tera.org/Peer/Chromium/Chromium.htm).
References
- Ani M, Moshtaghie AA. The effect of chromium on parameters related to iron metabolism. Biol. Trace Elem. Res. 1992;32:57–64. doi: 10.1007/BF02784588. [DOI] [PubMed] [Google Scholar]
- Chen H, Giri NC, Zhang R, Yamane K, Zhang Y, Maroney M, Costa M. Nickel ions inhibit histone demethylase JMJD1A and DNA repair enzyme ABH2 by replacing the ferrous iron in the catalytic centers. J. Biol. Chem. 2010;285:7374–7383. doi: 10.1074/jbc.M109.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ke Q, Kluz T, Yan Y, Costa M. Nickel ions increase histone H3 lysine 9 dimethylation and induce transgene silencing. Mol. Cell. Biol. 2006;26:3728–3737. doi: 10.1128/MCB.26.10.3728-3737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm LJ, Jones DP. Rat jejunum controls luminal thiol-disulfide redox. J. Nutr. 2000;130:2739–2745. doi: 10.1093/jn/130.11.2739. [DOI] [PubMed] [Google Scholar]
- Dalton TP, Chen Y, Schneider SN, Nebert DW, Shertzer HG. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic. Biol. Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Zollner TM, Boehncke WH. Proteasome inhibition: A new anti-inflammatory strategy. J. Mol. Med. 2003;81:235–245. doi: 10.1007/s00109-003-0422-2. [DOI] [PubMed] [Google Scholar]
- Franco R, Cidlowski JA. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- Gale E, Torrance J, Bothwell T. The quantitative estimation of total iron stores in human bone marrow. J. Clin. Invest. 1963;42:1076–1082. doi: 10.1172/JCI104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Sterling K. The tagging of red cells and plasma proteins with radioactive chromium. J. Clin. Invest. 1950;29:1604–1613. doi: 10.1172/JCI102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henics T, Wheatley DN. Cytoplasmic vacuolation, adaptation and cell death: A view on new perspectives and features. Biol. Cell. 1999;91:485–498. doi: 10.1016/s0248-4900(00)88205-2. [DOI] [PubMed] [Google Scholar]
- Hopkins LL, Jr, Schwarz K. Chromium (3) binding to serum proteins, specifically siderophilin. Biochim. Biophys. Acta. 1964;90:484–491. doi: 10.1016/0304-4165(64)90228-4. [DOI] [PubMed] [Google Scholar]
- Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- Kruidenier L, Verspaget HW. Review article: Oxidative stress as a pathogenic factor in inflammatory bowel disease—Radicals or ridiculous? Aliment. Pharmacol. Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- Lucenteforte E, Garavello W, Bosetti C, La Vecchia C. Dietary factors and oral and pharyngeal cancer risk. Oral Oncol. 2009;45:461–467. doi: 10.1016/j.oraloncology.2008.09.002. [DOI] [PubMed] [Google Scholar]
- McCarroll N, Keshava N, Chen J, Akerman G, Kligerman A, Rinde E. An evaluation of the mode of action framework for mutagenic carcinogens case study II: Chromium (VI) Environ. Mol. Mutagen. 2010;51:89–111. doi: 10.1002/em.20525. [DOI] [PubMed] [Google Scholar]
- Meister, A., and Anderson, M. E. (1983). Glutathione. Annu. Rev. Biochem.52, 711–760. [DOI] [PubMed]
- Mimnaugh EG, Xu W, Vos M, Yuan X, Neckers L. Endoplasmic reticulum vacuolization and valosin-containing protein relocalization result from simultaneous hsp90 inhibition by geldanamycin and proteasome inhibition by velcade. Mol. Cancer Res. 2006;4:667–681. doi: 10.1158/1541-7786.MCR-06-0019. [DOI] [PubMed] [Google Scholar]
- Moriarty-Craige, S. E., and Jones, D. P. (2004). Extracellular thiols and thiol/disulfide redox in metabolism. Annu. Rev. Nutr.24, 481–509. [DOI] [PubMed]
- Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut. 1998;43:856–860. doi: 10.1136/gut.43.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program (NTP) Final Report. Potassium Dichromate (Hexavalent): The Effects of Potassium Dichromate in Sprague-Dawley Rats Administered in Diet. 1996. National Toxicology Program PB97125355. National Institute of Environmental Health Sciences. [Google Scholar]
- NTP. Final Report on the Reproductive Toxicity of Potassium Dichromate (CAS No. 7778-50-9) Administered in Diet to BALB/c Mice. 1997. NTIS No. PB97-144919. National Toxicology Program PB97144919. National Institute of Environmental Health Sciences. [Google Scholar]
- NTP. NTP Technical Report on the Toxicity Studies of Sodium Dichromate Dihydrate (CAS No. 7789-12-0) Administered in Drinking Water to Male and Female F344/N Rats and B6C3F1 Mice and Male BALB/c and am3-C57BL/6 Mice. 2007. NTP Toxicity Report Series Number 72, National Institute of Environmental Health Science Publication No. 07-5964. [PubMed] [Google Scholar]
- NTP. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Sodium Dichromate Dihydrate (CAS No. 7789-12-0) in F344/N Rats and B6C3F1 Mice (Drinking Water Studies), NTP TR 546. 2008. National Institute of Environmental Health Science Publication No. 08-5887. [PubMed] [Google Scholar]
- Prime SS, MacDonald DG, Rennie JS. The effect of iron deficiency on experimental oral carcinogenesis in the rat. Br. J. Cancer. 1983;47:413–418. doi: 10.1038/bjc.1983.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000;16:534–554. doi: 10.1034/j.1399-3003.2000.016003534.x. [DOI] [PubMed] [Google Scholar]
- Reinstein E. Immunologic aspects of protein degradation by the ubiquitin-proteasome system. Isr. Med. Assoc. J. 2004;6:420–424. [PubMed] [Google Scholar]
- Richie JP, Jr, Kleinman W, Marina P, Abraham P, Wynder EL, Muscat JE. Blood iron, glutathione, and micronutrient levels and the risk of oral cancer. Nutr. Cancer. 2008;60:474–482. doi: 10.1080/01635580801956477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RA, Laskin DL, Smith CV, Robertson FM, Allen EM, Doorn JA, Slikker W., Jr Nitrative and oxidative stress in toxicology and disease. Toxicol. Sci. 2009;112:4–16. doi: 10.1093/toxsci/kfp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, F. Q., and Buettner, G. R. (2001). Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med.30, 1191–1212. [DOI] [PubMed]
- Senft AP, Dalton TP, Shertzer HG. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal. Biochem. 2000;280:80–86. doi: 10.1006/abio.2000.4498. [DOI] [PubMed] [Google Scholar]
- Shirley E. A non-parametric equivalent of Williams' test for contrasting increasing dose levels of a treatment. Biometrics. 1977;33:386–389. [PubMed] [Google Scholar]
- Stern AH. A quantitative assessment of the carcinogenicity of hexavalent chromium by the oral route and its relevance to human exposure. Environ. Res. 2010;110:798–807. doi: 10.1016/j.envres.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Stout MD, Herbert RA, Kissling GE, Collins BJ, Travlos GS, Witt KL, Melnick RL, Abdo KM, Malarkey DE, Hooth MJ. Hexavalent chromium is carcinogenic to F344/N rats and B6C3F1 mice after chronic oral exposure. Environ. Health Perspect. 2009;117:716–722. doi: 10.1289/ehp.0800208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zhou X, Chen H, Li Q, Costa M. Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol. Appl. Pharmacol. 2009;237:258–266. doi: 10.1016/j.taap.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CM, Haws LC, Harris MA, Gatto NM, Proctor DM. Application of the U.S. EPA mode of action framework for purposes of guiding future research: A case study involving the oral carcinogenicity of hexavalent chromium. Toxicol. Sci. 2011a;119:20–40. doi: 10.1093/toxsci/kfq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CM, Proctor DM, Haws LC, Hébert CD, Grimes SD, Shertzer HG, Kopec AK, Hixon JG, Zacharewski TR, Harris MA. Investigation of the mode of action underlying the tumorigenic response induced in B6C3F1 mice exposed orally to hexavalent chromium. Toxicol. Sci. 2011b;123:58–70. doi: 10.1093/toxsci/kfr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA. Recommendations for and Documentation of Biological Values for Use in Risk Assessment. Cincinnati, OH: U.S. Environmental Protection Agency; 1988. [Google Scholar]
- Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem. Res. Toxicol. 2005;18:3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.