Abstract
A large body of evidence had indicated that the basal forebrain cholinergic neurons (BFCN) are selectively vulnerable to early degeneration in Alzheimer’s disease (AD). However, recent studies have demonstrated reductions in cortical activity of the cholinergic enzyme choline acetyltransferase (ChAT) only in late disease. To addresses this controversy, we compared abnormatlities in the BFCN and their axons in non-demented young, non-demented old, pathologically mild and pathologically severe AD. Cholinergic axonal abnormalities in the form of thickened fibers and ballooned terminals emerged in middle age, displayed an increase in non-demented old cases and were reduced in density in severe AD, indicating that loss of crotical cholinergic axons in AD occurs preferrentially in fibers containing abnormalities. PHF1 immunoreactive pre-tangles and tangles were observed as early as the third decade of life prior to their appearance in entorhinal/perirhinal cortex and increased progressively in number in pathologically mild and severe AD. Amyloid-β-positive basal forebrain plaques were of the diffuse type and emerged in old individuals and displayed a progressive increase in AD. These results indicate that BFCN neuronal pathology emerges very early in the course of aging and AD. ChAT-containing cortical axonal abnormalities may explain stable ChAT activity in early AD.
Introduction
Among the many cortical neurochemical changes in Alzheimer’s disease (AD), the most consistent and widespread is a marked loss of cortical cholinergic markers (see(Geula and Mesulam, 1999) for review). Virtually all cortical areas in the AD brain display a depletion of their cholinergic axons (Geula and Mesulam, 1996) and a resultant reduction in the cholinergic synthetic enzyme choline acetyltransferase (ChAT) and the hydrolytic enzyme acetylcholinesterase (AChE) (Perry and others, 1977; Davies, 1979; Rossor and others, 1982). In addition, the number of the basal forebrain cholinergic neurons (BFCN), from which cortical cholinergic innervation emanates displays a significant decrease (Arendt and others, 1985; Mufson and others, 1989; Lehericy and others, 1993). The fact that basal forebrain cholinergic system is involved in the cognitive processing of memory and attention (Muir and others, 1992; Voytko and others, 1994; Leanza and others, 1996; Fine and others, 1997), processes which are also deficient in AD, and that the loss of cortical cholinergic innervation in AD shows a significant relationship with the severity of dementia (DeKosky and others, 1992; Lehericy and others, 1993; Bierer and others, 1995), gave rise to the suggestion that the cholinergic loss in AD contributes, at least in part, to the dementia observed in this disorder. They also form the basis of cholinergic therapy in AD.
A large body of early evidence indicated that cortical cholinergic denervation occurs early in the course of the disease process in AD. For example, in their study of neurochemical changes in temporal neocortex, Perry and coworkers (Perry and others, 1981) divided AD cases into neuropathologically mild, moderate and advanced groups on the basis of cortical plaque counts. In the mild group, only ChAT activity was significantly reduced but other markers studied were normal. In the moderate group, in addition to ChAT, losses in dopamine β-hydroxylase and glutamic acid decarboxylase and an increase in substance P were found. In the severe group, an additional decrease was observed in cholecystokinin. Biopsy samples of the frontal lobe obtained within a year of the appearance of clinical symptoms have been shown to display an up to 95% reduction of ChAT activity (Bowen and others, 1982; Bowen and others, 1983). Brains of patients in whom biopsy samples at mild to moderately severe stages of AD had shown a significant reduction in cortical ChAT activity, displayed a further significant reduction of this marker at autopsy (DeKosky and others, 1992). In addition, nearly all comparisons have indicated that the loss of cortical cholinergic innervation in AD is more widespread and substantial when compared with the loss of a number of other neurotransmitter systems (Geula and Mesulam, 1999). These observations were interpreted as an indication of the selective vulnerability of the basal forebrain cholinergic system to early degeneration in AD.
Recent studies of mild cognitive impairment (MCI), the potential prodromal stage of AD, have cast doubt on the early vulnerability of cortical cholinergic innervation in this disorder. Two studies have demonstrated that the levels of cortical ChAT activity show no decrement in MCI and mild AD, and show a deficit only in moderate to severe AD cases (Davis and others, 1999; DeKosky and others, 2002). One possible interpretation of these findings is that a deficit in the basal forebrain cholinergic system does not occur early in AD and is only a feature of more advanced disease. This interpretation has gained support by the finding that the number of BFCN remains unchanged in MCI when compared with cognitively normal elderly (Gilmor and others, 1999).
Contrary to the above interpretation, Mesulam et al. (Mesulam and others, 2004) have shown that the BFCN display considerable accumulation of abnormal epitopes of tau in neurofibrillary tangles and pre-tangles in cognitively normal elderly and in individuals with MCI. The present report extends this finding to earlier stages of life by analyzing the cortical cholinergic system in non-demented young, non-demented old, pathologically mild AD and pathologically severe AD. The findings demonstrate that abnormalities in cortical cholinergic axons and tauopathy within the BFCN occur very early in the course of life (as early as the third decade) and progress in frequency in old age and AD.
Methods
Case Information and Tissue Processing
Brains from 13 normal individuals without signs of neurological or psychiatric disorders ranging in age between 26 – 93 years and 10 brains from individuals with clinical and pathological diagnosis of AD were used in these experiments. Acquisition of postmortem human tissue was carried out according to protocols approved by an institutional review board. Clinical records were available for every subject. In some of the young control cases where the clinical record did not contain enough information to allow determination of cognitive function, such information was obtained from the next of kin. Brains of non-demented cases were obtained from autopsies at various hospitals. Brains of AD cases were obtained from a brain bank or from hospital autopsy services. Consecutive brains received at our laboratory from non-demented and demented individuals were used in the present experiments. All AD cases had been clinically diagnosed by a community neurologist. None of the non-demented and demented cases had undergone extensive neuropsychological examination. Age, sex, postmortem interval (PMI), and cause of death for each subject are indicated in Table 1. The PMI in the various groups of subjects were not significantly different (p>0.05).
Table 1.
Characteristics of the Cases Used
| Case # | Age/Years | Sex | Postmortem Interval/h | Cause of Death | Braak Stage | Diagnosis |
|---|---|---|---|---|---|---|
| 1 | 26 | M | 8 | Lung Failure Following Transplant | 0 | Normal Young |
| *2 | 27 | M | 11 | Gunshot Wound to Heart | 0 | Normal Young |
| 3 | 45 | M | 13 | Non-Hodgkin’s Lymphoma | I | Normal Young |
| 4 | 50 | F | 48 | Myocardial Infarction | I | Normal Young |
| 5 | 57 | F | 6 | Pneumonia | I | Normal Young |
| 6 | 61 | F | 22 | Metastatic Carcinoma | II | Normal Young |
| 7 | 72 | M | 17 | Pulmonary Embolism | I | Normal Old |
| 8 | 73 | M | 16 | Lung Cancer | II | Normal Old |
| 9 | 77 | F | 15 | Myocardial Infarction | II | Normal Old |
| 10 | 82 | M | 24 | Pneumonia | III | Normal Old |
| 11 | 83 | F | 23 | Myocardial Infarction | III | Normal Old |
| *12 | 87 | F | 12 | Cardiopulmonary Failure | II | Normal Old |
| 13 | 93 | M | 22 | Cerebellar Hemorrhage | III | Normal Old |
| 14 | 77 | F | 12 | Cardiopulmonary Failure | IV | AD, Pathol. Mild |
| 15 | 80 | M | 26 | Pneumonia | IV | AD, Pathol. Mild |
| 16 | 81 | F | 28 | Myocardial Infarction | V | AD, Pathol. Mild |
| 17 | 89 | M | 22 | Myocardial Infarction | IV | AD, Pathol. Mild |
| 18 | 89 | F | 21 | Pneumonia | V | AD, Pathol. Mild |
| 19 | 57 | M | 4 | Cardiopulmonary Failure | VI | AD, Pathol. Severe |
| 20 | 72 | M | 7 | Cardiac Arrest | V | AD, Pathol. Severe |
| 21 | 75 | M | 23 | Pneumonia | VI | AD, Pathol. Severe |
| 22 | 87 | M | 23 | Cardiac Arrest | VI | AD, Pathol. Severe |
| 23 | 88 | F | 5 | Pneumonia | VI | AD, Pathol. Severe |
M – Male, F – Female, h – Hour, AD – Alzheimer’s disease, Pathol. – Pathologically.
These cases did not have complete material for some of the staining methods used and therefore they were excluded from the quantitative analysis. They were used only for qualitative analysis.
One hemisphere of each brain was cut into 2–3 cm thick blocks and placed in 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4) for 36–40 hours at 4° C and then taken through sucrose gradients for cryoprotection (10–40% in phosphate buffer). Blocks containing the regions of interest were sectioned at 40 μm and stored in 0.1 M phosphate buffer at 4° C until used. Reports of neuropathologists from whom brains were obtained indicated no abnormalities in any of the control brains used except age-appropriate changes (Khachaturian, 1985) (e.g. small to moderate numbers of diffuse amyloid deposits in some elderly cases). The neuropathological diagnosis of AD was made by the neuropathologists from whom brains were obtained according to the criteria suggested by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (Mirra and others, 1991).
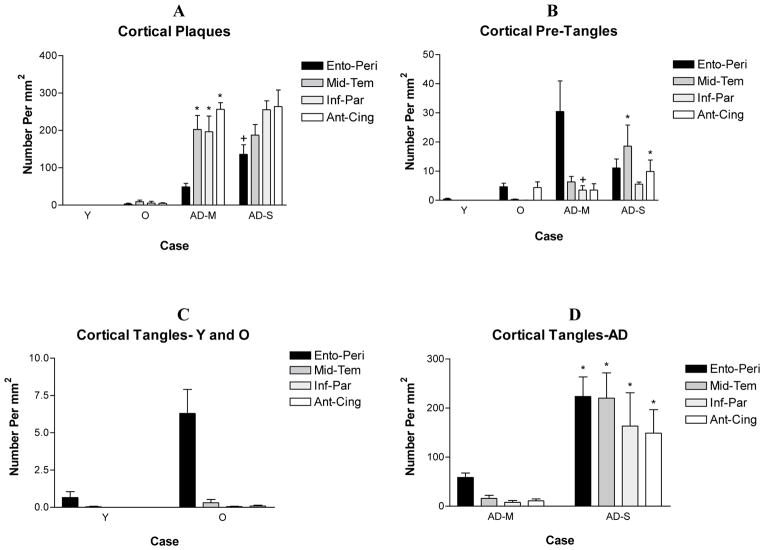
Brains from non-demented individuals below 65 years were designated as young and those from individuals 65 years and above were designated as old. Based on the density of cortical plaques and tangles as well as the Braak staging of tangle progression (Braak and Braak, 1991), AD brains were divided into pathologically mild and pathologically severe groups (see results, Fig. 1 and Table 1).
Figure 1.
The density of cortical plaques and tangles in the four groups of subjects. The only pathology observed in young cases (<65 years) consisted of a small number of pre-tangles and tangles in the entorhinal/peri-rhinal cortex. The density of plaques, tangles and pre-tangles displayed a progressive decrease in non-demented old and AD cases. Based on the relative density of cortical plaques and tangles and the Braak staging of tangle formation (Table 1), AD cases were divided into pathologically mild and pathologically severe cases. The density of tangles in all cortical areas and the density of plaques in the entorhinal cortex were statistically significantly greater in pathologically severe AD when compared with pathologically mild AD. A. * p<0.001 compared to normal old, +p<0.01 compared with pathologically mild. B. * and + p<0.05 compared with non-demented old. D. * p<0.001 compared with non-demented old, p<0.01 compared with pathologically mild. Analysis was carried out using all cases in Table 1 except those identified by an asterisk. Y – Non-Demented Young, O – Non-Demented Old, AD-M, Pathologically Mild AD, AD-S – Pathologically Severe AD.
Acetylcholinesterase Histochemistry
AChE activity within cortical axons was visualized in a representative series of sections from each brain with the help of a highly sensitive histochemical method. The principles of this method (incubation in a dilute Karnovsky-Roots medium followed by metal ion-diaminobenzidine intensification) have been described by Hanker et al. (Hanker and others, 1973) and Tago et al. (Tago and others, 1986). We have introduced a number of changes in this method as described elsewhere (Geula and Mesulam, 1989; Geula and Mesulam, 1996). We have demonstrated that AChE and ChAT, a specific cholinergic enzyme, visualize an identical population of cholinergic axons in normal and AD brains (Mesulam and Geula, 1992; Geula and Mesulam, 1996). Thus in the present study, histochemically visualized AChE activity in cortical fibers identifies cholinergic axons.
To ensure that the reaction product obtained was specific, the butyrylcholinesterase inhibitor ethopropazine (2×10−5 M) was routinely added to the incubation medium. Specificity of the AChE reaction was further ascertained by using 10−4 M of the specific AChE inhibitor BW284C51.
Immunohistochemistry
Series of sections from each brain were processed immunohistochemically using the avidin-biotin-peroxidase (ABC) method as described elsewhere (Geula and others, 1998), utilizing the Vectastain Elite ABC kit (Vector Laboratories, Berlingame, CA). The antibodies used were a specific polyclonal antibody against ChAT (generous gift of Dr. Louis Hersh, University of Kentucky Medical School, 1/300 – 1/500), a monoclonal antibody against abnormally phosphorylated tau (PHF-1, recognizes tau phosphorylated at Ser 396/404; generous gift of Dr. Peter Davies) and a polyclonal antibody against Aβ (generous gift of Dr. Dennis Selkoe). We have successfully used these antibodies in the past for visualizing each antigen (Wu and others, 1997; Geula and others, 1998; Geula and others, 2003b). To control for non-specific staining, sections stained using the above antibodies were compared with sections stained in the absence of primary antibodies or in the presence of non-specific IgG in place of primary antibodies.
For visualization of ChAT within axons, sections were stained immunohistochemically and then underwent silver intensification according to a method described elsewhere (Mesulam and others, 1992).
Quantitative Analysis of Matched Sections
Matching sections stained for Aβ, PHF1 and AChE were available in all cases. Matching sections through the basal forebrain stained for ChAT were also available in all cases. However, optimum ChAT staining for visualization of cortical cholinergic axons was available for two cases in each group. Therefore, the latter was used to qualitatively ascertain the results obtained with AChE histochemistry.
The density of cholinergic fiber abnormalities, Aβ immunoreactive plaques and PHF1 immunoreactive tangles and pre-tangles within the cerebral cortex were determined using a counting box with known dimensions (500 μm ×500 μm) at 200X magnification. The counting box was randomly placed at the cortical surface in one field in at least three sections each within the cortical areas of interest. The box was systematically moved from the cortical surface to white matter and the number of objects of interest in each box counted. Counts were obtained from the entorhinal/peri-rhinal cortex (Brodmann area 28), middle temporal gyrus (area 21), inferior parietal lobule (area 39–40) and anterior cingulate cortex (area 24). These areas were chosen because they have been shown by us and others to be vulnerable to AD pathology (Arnold and others, 1991; Arriagada and others, 1992; Geula and others, 1997). The counts obtained from the three cortical columns in each case were expressed as numbers per mm2.
All PHF1-positive magnocellular neurons and tangles in 3 sections spanning the anterior, intermediate and posterior sectors of the nucleus basalis of Meynert (nbM) – Ch4 (Ch4a, Ch4i and Ch4p) were counted. We have shown that virtually all magnocellular nbM neurons are ChAT-positive and are therefore cholinergic (Mesulam and Geula, 1988). Adjacent sections stained for ChAT were used to ascertain that counts are obtained from the cholinergic neurons only. Aβ immunoreactive plaques were also counted within the nbM region. Counting was carried out at 100X magnification using a counting box placed in the eyepiece of a compound microscope. The data were compiled as the number of immunoreactive profiles per section.
Within the cerebral cortex and nbM, the number of tangles and pre-tangles were determined separately. Pre-tangles were defined as normal neurons with PHF1 immunoreactivity but without the presence of characteristic morphological features of tangles according to criteria described elsewhere (Lauckner and others, 2003).
In three non-demented cases, systematically sampled sections (1 in 25) through each structure were available. In all such cases, structures of interest were counted in all available sections and were expressed as number of immunoreactive profiles per section.
Statistical Analysis
Data for each portion of the study were subjected to a test for normality. All data were normally distributed and therefore parametric statistical analysis was used. Analysis of variance followed by appropriate post hoc tests was used to detect significant group differences. Relationships were determined using Pearson correlations. The probability for accepting a significant difference was set at p<0.05.
Results
The immunohistochemical and histochemical methods used resulted in visualization of AChE- and ChAT-positive cholinergic axons, PHF1 immunoreactive pre-tangles and tangles and Aβ-positive plaques in the cerebral cortex and basal forebrain. Cholinergic neurons of the basal forebrain also displayed robust ChAT immunoreactivity.
Cortical Plaques and Tangles
Aβ immunoreactive plaques were absent in the cerebral cortex of young brains. A subpopulation of old brains contained a low to moderate density of Aβ deposits, particularly prominent in the anterior cingulate cortex and middle temporal gyrus. The density of plaques displayed a substantial increase in AD brains.
Consistent with the susceptibility of the entorhinal/peri-rhinal cortex as a site of the formation of the first cortical tangles, a low density of PHF1-positive pre-tangles were seen in this region in the majority of young brains. The density of pre-tangles displayed an increase in the entorhinal/peri-rhinal cortex of old brains, and pre-tangles were also observed in neocortical areas in this group. An increased density of pre-tangles was observed in all cortical areas in AD. The distribution and density of tangles was similar to that of pre-tangles, except the density of tangles was higher, particularly in AD.
Quantitative analysis confirmed the distribution pattern of cortical plaques, pre-tangles and tangles. Based on the counts of cortical plaques and tangles (Fig. 1) and the Braak staging of tangle formation, the AD group was divided into a neuropathologically mild (Braak stage IV–V) and a neuropathologically severe (Braak stage V–VI) group (Table 1).
With the exception of entorhinal/peri-rhinal cortex, the density of cortical Aβ deposits displayed a dramatic increase in pathologically mild AD when compared with normal controls (p<0.001). In pathologically severe AD cases, there was an additional slight but non-significant increase in the density of Aβ deposits (p>0.05). In the entorhinal cortex, a significant increase in Aβ deposits was observed in pathologically severe AD when compared with pathologically mild AD (p<0.01).
In the cingulate cortex and middle temporal gyrus, pre-tangles displayed a significant increase only in pathologically severe AD when compared with the normal control groups (p<0.05). In the inferior parietal lobule, pre-tangles also displayed a significant increase in pathologically mild AD over controls (p<0.05). In the entorhinal/peri-rhinal cortex, the density of pre-tangles showed a progressive but non-significant increase from young control to pathologically mild AD groups (p>0.05). In all cortical areas examined, tangles displayed a dramatic increase in pathologically severe AD over the controls (p<0.001) and over pathologically mild AD (p<0.01).
Cortical Cholinergic Axonal Abnormalities
In the youngest cases (Case # 1 and 2, Table 1), AChE and ChAT staining revealed a dense plexus of cholinergic axons. Stained axons were thin and relatively homogeneous in diameter. In ChAT immunostained sections, axons displayed small, fine varicosities. In all other cases, the cholinergic axons stained with the above markers displayed several abnormalities. These included (1) considerably thicker axons with reduced or no branching when compared with adjacent normal axons, (2) swollen, ballooned terminals at axonal endings and (3) collections of terminal swellings in a chandelier arrangement.
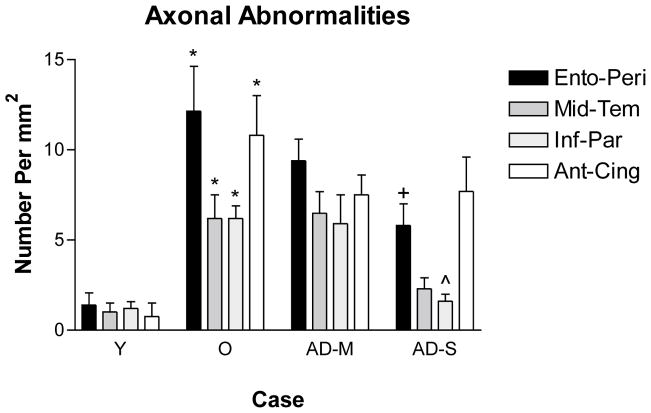
Excluding the youngest cases, non-demented young brains displayed a small number of the above abnormalities in cortical cholinergic fibers. In every cortical area examined, the group of non-demented old individuals displayed significantly increased density of cholinergic fiber abnormalities when compared with the young (p<0.05). The numbers of cholinergic axonal abnormalities in the non-demented group of subjects showed a highly significant correlation with age (Table 2).
Table 2.
Correlations Between Cholinergic Abnormalities and Age in Non-Demented Cases
| Type of Abnormality and Brain Region | Correlation | p Value |
|---|---|---|
|
| ||
| Cholinergic Axonal Abnormalities | ||
| Entorhinal/Perirhinal Cortex | 0.8744 | <0.001 |
| Middle Temporal Gyrus | 0.8425 | <0.003 |
| Inferior Parietal Cortex | 0.8821 | <0.0008 |
| Anterior Cingulate Cortex | 0.8279 | <0.004 |
|
| ||
| Basal Forebrain Pre-Tangles | 0.8032 | <0.003 |
|
| ||
| Basal Forebrain Tangles | 0.5656 | =0.07 |
The numbers of cholinergic axonal abnormalities remained constant in pathologically mild AD (p>0.05). In the entorhinal/peri-rhinal and inferior parietal cortices of pathologically severe AD, the numbers of cholinergic axonal abnormalities displayed a significant decrease (p<0.05), suggesting selective degeneration of cholinergic axons containing abnormalities. In the middle temporal gyrus, this decrease was not statistically significant (p>0.05) and in the anterior cingulate cortex of pathologically severe AD, the number of cholinergic abnormalities remained constant.
Qualitative observations demonstrated loss of cortical cholinergic axons in all cortical regions of AD brains in a pattern consistent with our previous observations(Geula and Mesulam, 1996).
Basal Forebrain Plaques and Tangles
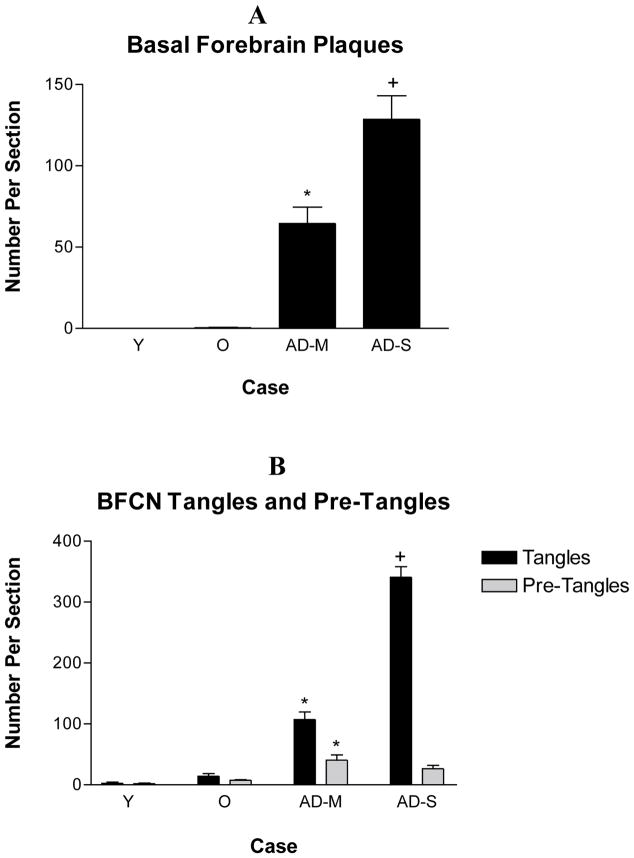
The area of basal forebrain in which the BFCN are located was completely devoid of Aβ deposits in young cases. In non-demented old cases, this region contained sparse Aβ deposits. In pathologically mild AD, the number of basal forebrain Aβ-positive plaques showed a significant increase when compared with the normal old (p<0.001). A further significant increase in the number of Aβ deposits was observed in the basal forebrain of pathologically severe AD cases (p<0.001). Nearly all Aβ-positive plaques in the basal forebrain were of the diffuse type. Rarely were compact plaques seen in this region (see {} for review).
As was the case in the entorhinal/peri-rhinal cortex, pre-tangles were detected in a subpopulation of the nbM-Ch4 neurons in young cases. Slightly higher numbers of pre-tangles were seen in these neurons in old cases. In pathologically mild AD, the number of nbM-Ch4 pre-tangles displayed a significant increase (p<0.001), and these numbers remained stable in pathologically severe AD (p>0.05). In the group of non-demented cases, the numbers of nbM-Ch4 tangles displayed a significant correlation with age (Table 2).
Tangles were also present in the BFCN of a subpopulation of young brains and in higher numbers in old brains. Significantly larger numbers of tangles were seen within the nbM-Ch4 in pathologically mild AD (p<0.001) with a further significant increase in pathologically severe AD (p<0.001). The correlation between the number of nbM-Ch4 tangles and age in non-demented individuals displayed a trend towards significance (p = 0.07; Table 2).
Of particular relevance to the vulnerability of the BFCN, the brain of case # 1 (26 years old, Table 1) which contained no pre-tangles or tangles within the entorhinal/peri-rhinal or any other cortical region examined, contained pre-tangles in the BFCN. Furthermore, of the 8 cases with the earliest stages of tangle formation (Braak stage I & II, Table 1) with cortical pre-tangles and tangles only within the entorhinal/peri-rhinal cortex, all contained BFCN pre-tangles and tangles.
Discussion
Early Abnormalities in Cortical Cholinergic Axons
The results of the present set of experiments clearly demonstrate early vulnerability of the basal forebrain cholinergic system to pathology in the course of aging and AD. Abnormalities in cortical cholinergic axons were first observed in non-demented young individuals, and their frequency displayed an increase in the non-demented aged. It could be argued that the thickening of cortical cholinergic axons and ballooning of terminals described here may represent normal variations in axonal morphology rather than abnormalities. However, two observations argue against this possibility. First, in the youngest cases, all cholinergic axons and terminals were consistently thin and of the same diameter without any of the variations described above. Second, the density of axonal changes displayed a significant decrease within most cortical areas in pathologically severe AD. Given that cortical cholinergic axons display a major depletion in AD(Geula and Mesulam, 1996), the above finding suggests that this loss occurs preferentially in axons which display the abnormalities we have observed. This line of reasoning is consistent with the qualitative observation that cortical cholinergic axons display similar abnormalities to those described in this report in rats with lesions of the BFCN (Arendash and others, 1987).
Some studies of cortical ChAT activity have shown upregulation of this enzyme in the hippocampus and frontal cortex in individuals with MCI and mild AD(DeKosky and others, 2002; Ikonomovic and others, 2003). It is likely that the emergence of cholinergic fiber abnormalities in the form of thickened axons and ballooned terminals, which we found to contain ChAT immunoreactivity, is responsible, at least in part, for the increased ChAT activity in MCI and the reported preservation of ChAT activity in mild AD. The above observations suggest that cortical ChAT activity alone cannot give an accurate indication of the health of the basal forebrain cholinergic system. Rather, direct visualization of cholinergic neurons and axons is needed to detect the earliest pathology in this system in the course of normal aging and AD.
Early Pathology in Basal Forebrain Cholinergic Neurons
The BFCN contained pre-tangles and/or tangles very early in the course of normal aging, starting in the third decade of life. The numbers of BFCN tangles and pre-tangles displayed a progressive and significant increase in pathologically mild AD and a further significant increase in pathologically severe AD. These observations are consistent with recent findings in individuals with MCI(Mesulam and others, 2004) and with qualitative observations of the basal forebrain during normal aging and AD (Sassin and others, 2000).
Of great interest, BFCN pre-tangles and tangles were observed prior to such pathology in the entorhinal/peri-rhinal cortex, and the density of this pathology in the BFCN increased concurrent with the appearance of pathology in the above cortical area. Given the established observation that cortical tangles and pre-tangles first appear in the entorhinal/peri-rhinal cortex in the course of aging and then spread progressively to other cortical areas (Hyman and others, 1984; Braak and Braak, 1991), our observations suggest very early vulnerability of the BFCN to the process of tangle formation. Our findings further suggest that the BFCN, rather than the entorhinal/peri-rhinal cortex may be the site at which tau pathology appears first.
The general pattern of the appearance of Aβ-positive plaques in the basal forebrain followed that of the cerebral cortex. Furthermore, consistent with our earlier observations{}, compact Aβ deposits, which contain activated glia and dystrophic neurites and are considered the pathologic plaque variant (Geula, 2000) were conspicuously absent from the basal forebrain. Therefore, it appears that the basal forebrain region within which the BFCN are located does not display selective vulnerability to plaque formation. It remains to be determined whether intraneuronal accumulation of soluble Aβ oligomers, which have been shown to exert detrimental and toxic effects on neurons (Lambert and others, 1998; Hartley and others, 1999; Zhang and others, 2002), occurs early within the BFCN.
Functional Implications of Early Cholinergic Pathology
The early pathology observed in the cholinergic system of the basal forebrain and the involvement of this system in the cognitive processing of memory and attention supports the current attempts in cholinergic therapy in AD. Cholinesterase inhibitors are among a handful of therapeutic agents in AD and have been shown to result in modest improvements in cognition, behavior and daily activities, primarily by inhibiting the cholinergic hydrolytic enzyme AChE, thereby increasing the available pool of the neurotransmitter acetylcholine (Farlow, 2002; Bullock and others, 2005). Our observations indicate that current experimental therapeutic approaches, particularly those targeting tau and its abnormal phosphorylation, are likely to enhance the function of the basal forebrain cholinergic system early in the course of dementia and thereby to result in cognitive improvement.
In addition to tauopathy and axonal abnormalities, two other early alterations have been described in the BFCN. First, these neurons have been shown to display a significant loss of high affinity (Trk-A) and low affinity (p75NTR) neurotrophin receptors concomitant with the appearance of cognitive abnormalities in MCI (Mufson and others, 2000; Mufson and others, 2002). Given the dependence of the BFCN on NGF for protection against damage (Tuszynski and others, 1990; Koliatsos and others, 1994), it is likely that the loss of neurotrophin receptors in MCI contributes to the loss of the BFCN in AD. In addition, we have observed a substantial and selective loss of the calcium binding protein calbindin-D28K from the BFCN in the course of aging in the human and non-human primates (Wu and others, 2003; Geula and others, 2003a). We have suggested that the age-related loss of calbindin is likely to deprive the BFCN from the capacity to buffer excess intracellular calcium and leave them vulnerable to neurodegenerative insults with the potential to lead to excessive rises in intracellular calcium and lead to the degeneration of the BFCN due to calcium toxicity. The relationship between the early BFCN tau pathology and alterations in NGF receptors and calbindin remain unexplored. It is likely that the above alterations will also be used for development of novel therapeutic approaches aimed at improving the function of the cortical cholinergic system.
Conclusions
The findings of the present experiments, along with the reported loss of neurotrophin receptors from the BFCN in MCI and the age-related loss of calbindin from these neurons, are clear indications of the selective and early vulnerability of the basal forebrain cholinergic system to damage and loss in the course of aging and AD. They also justify continued therapeutic efforts aimed at ameliorating the early cholinergic deficit in AD. These observations, along with the vulnerability of this system in other neurodegenerative disorders, suggest that the basal forebrain cholinergic system can serve as a model for the investigation of the causes of selective neuronal vulnerability in neurodegenerative disorders.
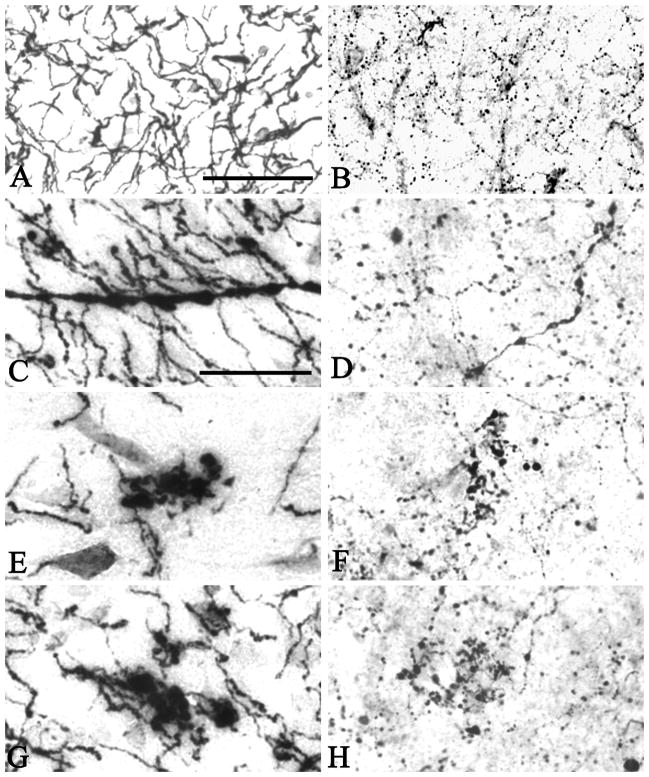
Figure 2.
In the youngest case investigated (Case #1), AChE activity (A) and ChAT immunoreactivity (B) were present within cholinergic axons homogenously thin in diameter. Immunohistochemistry for ChAT visualized small varicosities within axons. In brains from older cases and AD brains, one or more of a number of abnormalities were seen in both AChE (C, E and G) and ChAT (D, F, H) stained material. These included thickened axons (C and D) and swollen and ballooned varicosities at axonal endings (E–H), which often occurred in a chandelier arrangement. Scale bar in A is 100 μm and also applies to B, and the scale bar in C is 50 μm and also applies to D–H.
Figure 3.
The density of cortical cholinergic axonal abnormalities in the four groups of subjects. No such abnormalities were present in the youngest cases (Case # 1 and 2). A small number of such abnormalities were present in the rest of the cases below 64 years and the density of such abnormalities displayed a significant increase in non-demented old cases compared with the young (p<0.05). The density of axonal abnormalities remained constant in pathologically mild AD and was reduced in pathologically severe AD and significantly so in the entorhinal cortex and the inferior parietal lobule. Quantitative analysis was conducted on all of the cases in Table 1 except those identified by an asterisk. Y – Non-Demented Young, O – Non-Demented Old, AD-M, Pathologically Mild AD, AD-S – Pathologically Severe AD.
Figure 4.
The number of basal forebrain plaques, tangles and pre-tangles within cholinergic neurons in each section. Plaques were absent from the basal forebrain of young individuals. They were seen in old individuals in moderate numbers. A significant increase in the number of plaques was seen in pathologically mild AD and another significant increase in pathologically severe AD. The density of pre-tangles was higher in pathologically mild AD when compared with non-demented old cases. The density of tangles showed a further significant increase in the cholinergic neurons in pathologically severe AD. Quantitative analysis was carried out using all of the cases in Table 1 except those identified by an asterisk. A. *p<0.001 compared with non-demented old cases, +p<0.001 compared with pathologically mild cases. B. *p<0.001 compared with non-demented old cases, +p<0.001 compared with pathologically mild cases. Y – Non-Demented Young, O – Non-Demented Old, AD-M, Pathologically Mild AD, AD-S – Pathologically Severe AD.
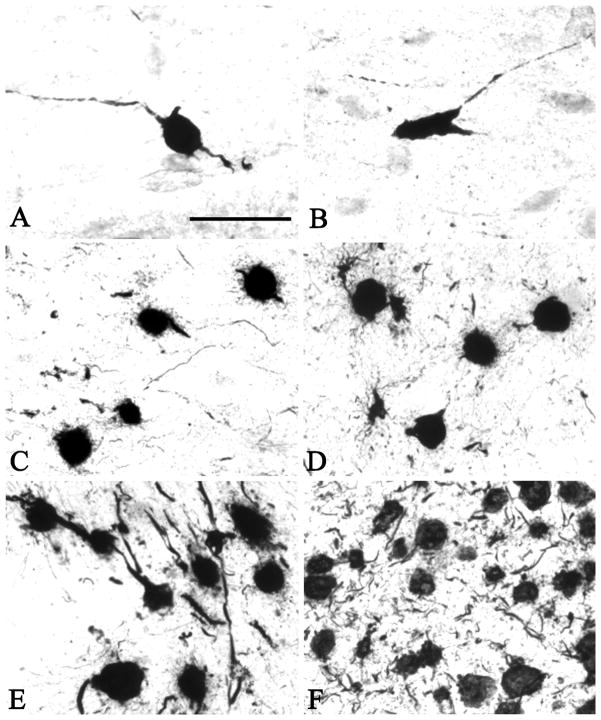
Figure 5.
PHF1-positive pre-tangles and tangles in the basal forebrain of young (A and B, Case #1), middle aged (C, Case #), old (D, Case #), pathologically mild (E, Case #) and pathologically severe AD (F, Case #). Pre-tangles were present in the cholinergic neurons in the 26 year-old (Case 1, A and B) in whose brain no tangles and pre-tangles were found in the entorhinal/peri-rhinal cortex. Thereafter, the numbers of tangles and pre-tangles within the basal forebrain cholinergic neurons displayed a progressive increase. Scale bar in A is 100 μm and also applies to B-F.
Acknowledgments
Sources of Support: This work was supported in part by grants from the National Institute on Aging (AG14706 [C.G.] and T32 AG23480 [A.N.]).
Part of the tissue used in these experiments was provided by the Harvard Brain Tissue Resource Center at McLean Hospital, Belmont, MA, which is supported by PHS (MH/NS 31862).
References
- Arendash GW, Millard WJ, Dunn AJ, Meyer EM. Long-term neuropathological and neurochemical effects of nucleus basalis lesions in the rat. Science. 1987;238:952–6. doi: 10.1126/science.2890210. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bigl V, Tennstedt A, Arndt A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer’s disease. Neurosci. 1985;14:1–14. doi: 10.1016/0306-4522(85)90160-5. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cerebral Cortex. 1991;1:103–16. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–8. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- Bierer LM, Haroutunian V, Gabriel S, Knott PJ, Carlin LS, Purohit DP, Schmeidler J, Kanof P, Davis KL. Neurochemical correlates of dementia severity in Alzheimer’s disease: relative importance of the cholinergic deficits. J Neurochem. 1995;64:749–50. doi: 10.1046/j.1471-4159.1995.64020749.x. [DOI] [PubMed] [Google Scholar]
- Bowen DM, Allen SJ, Benton JS, Goodhardt MJ, Haan EA, Palmer AM, Sims NR, Smith CT, Spillane JA, Esiri MM, Neary D, Snowdon JS, Wilcock GK, Davison AN. Biochemical assessment of serotonergic and cholinergic dysfunction and cerebral atrophy in Alzheimer’s disease. J Neurochem. 1983;41:266–72. doi: 10.1111/j.1471-4159.1983.tb11838.x. [DOI] [PubMed] [Google Scholar]
- Bowen DM, Benton JS, Spillane JA, Smith CCT, Allen SJ. Choline acetyltransferase activity and histopathology of frontal neocortex from biopsies of demented patients. J Neurol Sci. 1982;57:191–202. doi: 10.1016/0022-510x(82)90026-0. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer’s disease. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Bullock R, Touchon J, Bergman H, Gambina G, He Y, Rapatz G, Nagel J, Lane R. Rivastigmine and donepezil treatment in moderate to moderately-severe Alzheimer’s disease over a 2-year period. Curr Med Res Opin. 2005;21(8):1317–27. doi: 10.1185/030079905X56565. [DOI] [PubMed] [Google Scholar]
- Davies P. Neurotransmitter-related enzymes in senile dementia of the Alzheimer type. Brain Res. 1979;171:319–27. doi: 10.1016/0006-8993(79)90336-6. [DOI] [PubMed] [Google Scholar]
- Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V. Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA. 1999;281:1401–6. doi: 10.1001/jama.281.15.1401. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Harbaugh RE, Schmitt FA, Bakay RAE, Chui HC, Knopman DS, Reeder TM, Shetter AG, Senter HJ, Markesbery WR. Cortical biopsy in Alzheimer’s disease: diagnostic accuracy and neurochemical, neuropathological, and cognitive correlates. Ann Neurol. 1992;32:625–32. doi: 10.1002/ana.410320505. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett D, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–55. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- Farlow M. A clinical overview of cholinesterase inhibitors in Alzheimer’s disease. Int Psychogeriatr. 2002;14(Suppl 1):93–126. doi: 10.1017/s1041610203008688. [DOI] [PubMed] [Google Scholar]
- Fine A, Hoyle C, Maclean CJ, Levatte TL, Baker HF, Ridley RM. Learning impairments following injection of a selective cholinergic immunotoxin, ME20.4 IgG-saporin, into the basal nucleus of Meynert in monkeys. Neurosci. 1997;81:331–43. doi: 10.1016/s0306-4522(97)00208-x. [DOI] [PubMed] [Google Scholar]
- Geula C. Pathological diagnosis of Alzheimer’s disease. In: Scinto LFM, Daffner KR, editors. Early Diagnosis of Alzheimer’s Disease. Totowa: Humana Press; 2000. pp. 65–81. [Google Scholar]
- Geula C, Bu J, Nagykery N, Scinto LF, Chan J, Joseph J, Parker R, Wu CK. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol. 2003a;455(2):249–59. doi: 10.1002/cne.10475. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam M-M. Cortical cholinergic fibers in aging and Alzheimer’s disease: a morphometric study. Neurosci. 1989;33:469–81. doi: 10.1016/0306-4522(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam M-M. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cerebral Cort. 1996;6:165–77. doi: 10.1093/cercor/6.2.165. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam M-M. Cholinergic systems in Alzheimer disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. 2. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 269–92. [Google Scholar]
- Geula C, Mesulam M-M, Saroff DM, Wu C-K. Relationship between plaques, tangles and loss of cortical cholinergic fibers in Alzheimer’s disease. J Neuropathol Exp Neurol. 1997;57:63–75. doi: 10.1097/00005072-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam M-M, Saroff DM, Wu C-K. Relationship between plaques, tangles, and loss of cortical cholinergic fibers in Alzheimer’s disease. J Neuropathol Exp Neurol. 1998;57:63–75. doi: 10.1097/00005072-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Geula C, Nagykery N, Wu CK, Bu J. Loss of calbindin-D28K from aging human cholinergic basal forebrain: relation to plaques and tangles. J Neuropathol Exp Neurol. 2003b;62(6):605–16. doi: 10.1093/jnen/62.6.605. [DOI] [PubMed] [Google Scholar]
- Gilmor ML, Erickson JD, Varoqui H, Hersh LB, Bennett DA, Cochran EJ, Mufson EJ, Levey AI. Preservation of nucleus basalis neurons containing choline acetyltransferase and the vesicular acetylcholine transporter in the elderly with mild cognitive impairment and early Alzheimer’s disease. J Comp Neurol. 1999;411:693–704. [PubMed] [Google Scholar]
- Hanker JS, Thornburg LP, Yates PE, Moore HG. The demonstration of cholinesterases by the formation of osmium blacks at sites of Hatchett’s brown. Histochemie. 1973;37:223–42. doi: 10.1007/BF00304184. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19(20):8876–84. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–70. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Ikonomovic MD, Mufson EJ, Wuu J, Cochran EJ, Bennett DA, DeKosky ST. Cholinergic plasticity in hippocampus of individuals with mild cognitive impairment: correlation with Alzheimer’s neuropathology. J Alzheimers Dis. 2003;5(1):39–48. doi: 10.3233/jad-2003-5106. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Price DL, Gouras GK, Cayouette MH, Burton LE, Winslow JW. Highly selective effects of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 on intact and injured basal forebrain magnocellular neurons. J Comp Neurol. 1994;343:247–62. doi: 10.1002/cne.903430206. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95(11):6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner J, Frey P, Geula C. Comparative distribution of tau phosphorylated at Ser262 in pre-tangles and tangles. Neurobiol Aging. 2003;24(6):767–76. doi: 10.1016/s0197-4580(02)00228-2. [DOI] [PubMed] [Google Scholar]
- Leanza G, Muir J, Nilsson OG, Wiley RG, Dunnett SB, Bjorklund A. Selective immunolesioning of the basal forebrain cholinergic system disrupts short-term memory in rats. Eur J Neurosci. 1996;8:1535–44. doi: 10.1111/j.1460-9568.1996.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Hirsch EC, Cervera-Pierot P, Hersh LB, Bakchine S, Piette F, Duyckaerts C, Hauw J-J, Javoy-Agid F, Agid Y. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. J Comp Neurol. 1993;330:15–31. doi: 10.1002/cne.903300103. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Shaw P, Mash D, Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol. 2004;55(6):815–28. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol. 1988;275:216–40. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Geula C. Overlap between acetylcholinesterase-rich and choline acetyltransferase-positive (cholinergic) axons in human cerebral cortex. Brain Res. 1992;577:112–20. doi: 10.1016/0006-8993(92)90543-i. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Hersh LB, Mash DC, Geula C. Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. J Comp Neurol. 1992;318:316–28. doi: 10.1002/cne.903180308. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Bothwell M, Kordower JH. Loss of nerve growth factor receptor-containing neurons in Alzheimer’s disease: a quantitative analysis across subregions of the basal forebrain. Exp Neurol. 1989;105:221–32. doi: 10.1016/0014-4886(89)90124-6. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ma SY, Cochran EJ, Bennett DA, Beckett LA, Jaffar S, Saragovi HU, Kordower JH. Loss of nucleus basalis neurons containing trkA immunoreactivity in individuals with mild cognitive impairment and early Alzheimer’s disease. J Comp Neurol. 2000;427:19–30. doi: 10.1002/1096-9861(20001106)427:1<19::aid-cne2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ma SY, Dills J, Cochran EJ, Leugrans S, Wuu J, Bennett DA, Jaffar S, Gilmor ML, Levey AI, Kordower JH. Loss of basal forebrain p75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer’s disease. J Comp Neurol. 2002;443:136–53. doi: 10.1002/cne.10122. [DOI] [PubMed] [Google Scholar]
- Muir JL, Dunnett SB, Robbins TW, Everitt BJ. Attentional functions of the forebrain cholinergic systems: effects of intraventricular hemicholinium, physostigmine, basal forebrain lesions and intracortical grafts on a multiple-choice serial reaction time task. Exp Brain Res. 1992;89:611–22. doi: 10.1007/BF00229886. [DOI] [PubMed] [Google Scholar]
- Perry EK, Blessed G, Tomlinson BE, Perry TH, Crow TJ, Cross AJ, Dockray GJ, Dimaline R, Arragui A. Neurochemical activities in human temporal lobe related to aging and Alzheimer-type changes. Neurobiol Aging. 1981;2:251–6. doi: 10.1016/0197-4580(81)90032-4. [DOI] [PubMed] [Google Scholar]
- Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE. Neurotransmitter enzyme abnormalities in senile dementia. J Neurol Sci. 1977;34:247–65. doi: 10.1016/0022-510x(77)90073-9. [DOI] [PubMed] [Google Scholar]
- Rossor MN, Garrett NJ, Johnson AL, Mountjoy CQ, Roth M, Iversen LL. A post-mortem study of the cholinergic and GABA systems in senile dementia. Brain. 1982;105:313–30. doi: 10.1093/brain/105.2.313. [DOI] [PubMed] [Google Scholar]
- Sassin I, Schultz C, Thal DR, Rub U, Arai K, Braak E, Braak H. Evolution of Alzheimer’s disease-related cytoskeletal changes in the basal nucleus of Meynert. Acta Neuropathol (Berl) 2000;100(3):259–69. doi: 10.1007/s004019900178. [DOI] [PubMed] [Google Scholar]
- Tago H, Kimura H, Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical staining. J Histochem Cytochem. 1986;34:1431–8. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, UHS, Amaral DG, Gage FH. Nerve growth factor infusion in the primate brain reduces lesion- induced cholinergic neuronal degeneration. J Neurosci. 1990;10:3604–14. doi: 10.1523/JNEUROSCI.10-11-03604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994;14:167–86. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CK, Nagykery N, Hersh LB, Scinto LF, Geula C. Selective age-related loss of calbindin-D28k from basal forebrain cholinergic neurons in the common marmoset (Callithrix jacchus) Neuroscience. 2003;120(1):249–59. doi: 10.1016/s0306-4522(03)00248-3. [DOI] [PubMed] [Google Scholar]
- Wu C-K, Mesulam M-M, Geula C. Age-related loss of calbindin from human basal forebrain cholinergic neurons. Neuroreport. 1997;8:2209–13. doi: 10.1097/00001756-199707070-00024. [DOI] [PubMed] [Google Scholar]
- Zhang Y, McLaughlin R, Goodyer C, LeBlanc A. Selective cytotoxicity of intracellular amyloid beta peptide1-42 through p53 and Bax in cultured primary human neurons. J Cell Biol. 2002;156(3):519–29. doi: 10.1083/jcb.200110119. [DOI] [PMC free article] [PubMed] [Google Scholar]