Abstract
Background
Stress, alcohol cues and dysregulated stress responses increase alcohol craving and relapse susceptibility, but few pharmacologic agents are known to decrease stress and cue-induced alcohol craving and associated stress dysregulation in humans. Here we report findings from a preliminary efficacy study of the alpha1 receptor antagonist, prazosin, in modulating these relapse-relevant factors in alcohol dependent (AD) individuals.
Methods
Seventeen early abstinent, treatment-seeking alcohol dependent individuals (12 Males /5 Females) were randomly assigned to receive either placebo or 16 mg daily prazosin in a double-blind, placebo controlled manner over four weeks. During week 4, all patients participated in a 3-day laboratory experiment involving 5-min guided imagery exposure to stress, alcohol cue and neutral-relaxing/control conditions, one exposure per day, on consecutive days in a random, counterbalanced order. Alcohol craving, anxiety and negative emotion, cardiovascular measures, plasma hypothalamic-pituitary-adrenal (HPA; cortisol, ACTH) were assessed repeatedly in each session.
Results
The prazosin group (n=9) versus the placebo group (n=8) showed significantly lower alcohol craving, anxiety and negative emotion following stress exposure. The placebo group also showed significantly increased stress and cue-induced alcohol craving, anxiety, negative emotion and blood pressure as well as a blunted HPA response relative to the neutral condition, while the prazosin group showed no such increases in craving, anxiety, negative emotion and blood pressure, and no blunted HPA response to stress and alcohol cue exposure.
Conclusions
Prazosin appears efficacious in decreasing stress- and cue-induced alcohol craving and may normalize the stress dysregulation associated with early recovery from alcoholism. Further research to assess the efficacy of prazosin in reducing alcohol craving and stress-related relapse risk is warranted.
Keywords: Prazosin, Alcohol Dependence, Stress, Alcohol Craving, HPA axis
Introduction
Approximately one third of Americans consume enough alcohol to be considered at risk for alcohol dependence and the societal health costs of alcohol misuse are projected to be at almost $200 billion per annum (The Surgeon General's Call to Action, 2007; Williams, 2006). Despite this, there are currently only three medications approved by the US Food and Drug Administration (FDA) for the treatment of alcohol use disorders– acamprosate (Campral), naltrexone (Trexan) and disulfiram (Antabuse) (Ross and Peselow, 2009). Disulfiram has shown limited efficacy in terms of abstinence rates and craving reduction due to adverse effects and compliance issues (Hughs and Cook, 1997; Miller et al., 200). Acamprosate and naltrexone show only moderate efficacy (Ducci and Goldman, 2008; Zimmerman et al., 2004) and craving and relapse rates remain high (Brandon et al., 2007). Alcohol-related allostatic adaptations in stress and reward pathways are associated with compulsive alcohol seeking (Koob, 2009; Koob and Kreek, 2007; Sinha, 2001), and the currently approved medications do not specifically target stress-induced craving and related adaptations. The development of pharmacotherapies that target stress system dysregulation offers a promising new approach.
Alcohol dependence and excessive drinking are associated with increased anxiety related co-morbidity (Grant et al., 2004), persistent and enhanced central nervous system (CNS) noradrenergic activity, sympatho-adrenal medullary activation, and increased noradrenergic signaling both during early withdrawal and following protracted abstinence (Fox et al., 2007; Koob and Le Moal, 1997; Kushner et al., 1999; Rasmussen et al., 2006, 2009; Sinha, 2001; Sinha et al., 2009). The period of early abstinence from alcohol is also associated with increased negative emotion and alcohol craving, most commonly in the face of stress and alcohol cues which can increase the risk of relapse (Brady et al., 2006; Cooney et al., 1997; Fox et al., 2007; Litt & Cooney, 1999; Sinha, 2007; Sinha et al., 2011). Additionally, dysregulated stress responses marked by elevated resting blood pressure and heart rate, and increased resting cortisol and ACTH (Adinoff et al., 2005; Sinha et al., 2009, 2011), and suppressed HPA response to stress and alcohol cues (Adinoff et al., 2005; Ehrenreich et al., 1997; Fox et al., 2007; Patkar et al., 2003, 2004; Sinha et al., 2009; 2011) have also been documented during this early alcohol abstinence period. Furthermore, high basal cortisol and suppressed stress-induced cortisol responses are associated with alcohol relapse outcomes (Adinoff et al., 2005; Brady et al., 2006; Junghanns et al., 2003; Sinha et al., 2011).
Recent evidence from animal studies have shown that noradrenergic agents, such as prazosin, could be of benefit in decreasing chronic alcohol related anxiety, alcohol self administration and stress-induced reinstatement of alcohol seeking. Rats selectively bred for alcohol preference showed a reduction in alcohol intake for up to 5 consecutive days following prazosin administration (Rasmussen et al., 2009) as well as suppressed responding for ethanol during acute withdrawal (Walker et al., 2008). A new report has also found prazosin to significantly decrease stress-induced reinstatement of yohimbine-induced and footshock-induced alcohol seeking in laboratory animals (Le et al., 2011). In addition, the drug has been used successfully to treat nightmares in individuals with PTSD, a group that shows some of the same alterations in sympatho-adrenal medullary and HPA axis function as those observed in alcohol dependent individuals (Cukor et al., 2009; Raskind et al., 2007; Taylor et al., 2008). A recent pilot study of prazosin in 24 alcohol dependent individuals engaged in out-patient treatment also showed it to be a potentially effective pharmacologic treatment for alcohol dependence (Simpson et al., 2009), with the prazosin group reporting fewer drinking days per week and fewer drinks per week. To date, however, a systematic investigation regarding the efficacy of this alpha1-antagonist in reducing stress-induced and alcohol cue-induced craving, anxiety, and stress responsivity has not been conducted. The current study aims to assess the efficacy of prazosin, a centrally and peripherally acting alpha(1)-adrenergic antagonist (Boynton et al., 2009; Simpson et al., 2009; Thompson et al. 2008), in decreasing stress and alcohol cue related craving, anxiety and dysfunctional stress responses in early abstinent alcohol dependent patients.
Materials and Methods
Participants
Seventeen treatment-seeking alcohol dependent individuals (12M/5F) who responded to local advertisements around the New Haven area participated in the study. Current alcohol dependence was determined using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders IV (SCID IV - First et al., 1997) as well as positive urine toxicology screens for alcohol metabolite (EtG – ethyl glucothionide) collected upon entry into inpatient treatment. Exclusion criteria included DSM-IV dependence for any drug other than alcohol or nicotine. Participants using prescribed medications for any psychiatric or medical disorders were also excluded, and all individuals underwent stringent medical assessments including electrocardiography and laboratory tests of renal, hepatic, pancreatic, hematopoietic and thyroid function. Written and verbal consent were obtained from all participants and the Human Investigation Committee of the Yale University School of Medicine approved the study.
Design
A placebo-controlled, double-blind mixed experimental design was used in the current study. All subjects were exposed to three personalized guided imagery conditions (stress, alcohol cue, neutral/relaxing) across three consecutive days, one imagery condition per day, in a randomized and counterbalanced order. Medication Group, (placebo Vs prazosin) was the between-subjects factor; Imagery Condition (stress, alcohol cue, neutral) and Time-points (6 repeated assessments in each laboratory session) were within-subjects factors. Such a design allowed us to assess inter and intra-individual differences in subjects between groups in response to stress and cue provocation relative to a neutral – control imagery condition within each subject, and to model the interaction of these factors on response to medication.
General Procedures
All participants were admitted to the Clinical Neuroscience Research Unit (CNRU) of the Connecticut Mental Health Center (CMHC) for five weeks of inpatient treatment and study participation. The CNRU is a locked inpatient treatment research facility with no access to alcohol or drugs and very limited access to visitors. Alcohol and drug testing was conducted regularly to ensure drug abstinence. As subjects were treatment seeking, they participated in four weeks of group counseling treatment for alcohol dependence using the standard 12-Step based alcohol and drug counseling manual as a guide (Project Match 12-Step Manual, 1994; Mercer et al., 1992). During the first week of inpatient stay, alcohol dependent participants were administered structured baseline assessments measuring psychiatric and substance use history. In the second week, scripts for the guided imagery induction were developed as described in previous studies (Sinha et al., 2003). A laboratory experiment involving participation in 3 testing sessions over 3 days was conducted approximately 21 days after admission to allow for steady state levels of study medication. Research staff was blind to which imagery condition was presented on what day. Subjects also remained blind until imagery presentation.
Prazosin Dosing Schedule
All prazosin pills (marketed by Watson Pharmaceuticals) were purchased through the pharmacy located at the CMHC and the research pharmacist ensured that both active and placebo capsules used for medication administration appeared identical. All placebo pills contained lactose. Subjects were randomly assigned to placebo or prazosin, and five days after inpatient admission were initiated on a 2 week dose escalation schedule similar to previous studies (Raskind et al, 2007; Simpson et al., 2009). Briefly, dosing started at 1 mg taken at bed time on days1-2, followed by 3 mg (1 mg tid) on days 3-4, 6 mg (2-2-2 mg) on days 5-7, 10 mg (2-2-6mg dosing) on days 8-10, 14 mg on days 10-13 (4-4-6) and full dose of 16 mg (4 - 4 -8 mg) on day 14. Study medications were administered at 7:30 am, 3 pm and 10 pm daily. Experimental sessions were conducted in week 4, and medication dose was tapered over 5 days following the laboratory sessions. Randomization procedures were conducted by the CMHC research pharmacist, experienced in Urn randomization procedures (Stout et al., 1994). In the current study, nine subjects were randomized into the placebo group and eight into the prazosin group.
Safety and Side Effects Measures
These comprised: a) weekly sitting and standing vital signs (HR/BP) taken every 15 minutes for one hour, three times weekly; b) assessment of laboratory chemistry at study admission and discharge, including electrocardiography, renal, hepatic, and pancreatic tests, and c) assessment of any changes in physical and mental functioning three times weekly in order to evaluate any potential adverse events or side effects.
Imagery Script Development Procedures
Imagery script development was conducted in a session prior to the laboratory procedures, approximately 14 days following inpatient admission. Procedures are based on methods developed by Lang and his colleagues (Lang et al. 1980, 1983; Miller et al. 1987), and further adapted in our previous studies (Fox et al., 2005; Sinha, 2009; Sinha et al. 1992, 2000, 2003). Briefly, the stress imagery script was based on subjects' descriptions of a recent personal stressful event that made them “sad, mad or upset”, that they were not able to control in the moment, and that was experienced as “most stressful”. “Most stressful” was determined by having the subjects rate their perceived stress on a 10-point Likert scale where 1= not at all stressful and 10= the most stress they felt recently in their life. Only situations rated as 8 or above were accepted as appropriate for script development (e.g. being fired from their job, marital conflict situation). The alcohol-related cues scripts were developed by having subjects identify a recent situation that included alcohol-related stimuli and resulted in subsequent alcohol use (e.g. walking by their favorite bar; watching others drink alcohol). Alcohol-related situations that were associated with negative affect or psychological distress were not allowed. A relaxing, non-physiologically arousing and non-alcohol related script was developed from the subjects' description of a personal, relaxing situation (e.g., being at the beach; fall afternoon reading at the park).
In addition to the script development, on the day prior to the laboratory sessions, subjects were brought into the testing room in order to acclimatize them to specific aspects of the study procedures including the subjective rating forms and training in relaxation and imagery procedures, as previously described in Sinha et al. (2003).
Laboratory Sessions (conducted across three consecutive days)
Subjects were brought into the testing room at 7:45 AM. All subjects were allowed an initial smoke break at 7:30 AM in order to reduce potential nicotine withdrawal during the session. After settling in a sitting position on a hospital bed, a heparin-treated catheter was inserted by the research nurse in the antecubital region of the subject's non-preferred arm, in order to periodically obtain blood samples. A blood pressure cuff was placed on the subject's preferred arm and a pulse sensor was placed on the subject's forefinger. Self–reports of craving, anxiety and emotion were completed immediately after set-up. This was followed by a one-hour adaptation period during which the subjects were instructed to practice relaxation. At 9:00 AM, subjects were provided headphones and given the following instructions for the 5-minute imagery procedure: “Close your eyes and imagine the situation being described, ‘as if’ it were happening right now. Let your body and mind get completely involved in the situation, doing what you would do in the real situation”.
Alcohol craving, anxiety and negative emotion ratings, heart rate (HR), blood pressure (BP) and blood samples for cortisol and ACTH, were collected at baseline (- 20 minutes prior to imagery), immediately following imagery presentation (0) and every 15 minutes after the imagery period, up to 75 minutes (+15, +30, +45, +60, +75). Plasma norepinephrine levels were also collected at baseline. At the end of each session, relaxation instructions were provided to ameliorate any residual effects of imagery exposure. Subjects were then free to leave the testing room and eat breakfast.
Laboratory Assessments
Subjective Measures
Alcohol Craving: The desire for using alcohol was assessed using a 10-point visual analog scale (VAS) in which 0=“not at all” and 10=“extremely high”. Anxiety and Negative Emotion: (Differential Emotion Scale – DES; Izard, 1972). The scale comprises 30 adjectives (or items) and participants were required to rate on a 5-point Likert scale the extent to which each word described the way s/he felt at the current time. The scale for each item ranged from 1 to 5 in which 1 = “very slightly or not at all” and 5 = “very strongly”. Data were loaded onto the following negative emotion sub-scales: anxiety, anger, fear, sadness. The subscales of anger, fear and sadness were subsequently collapsed to form a general negative emotion subscale.
Cardiovascular Measures
A Critikon Dinamap 120 Patient Monitor (GE Medical Systems, Tampa, FL) was used to assess blood pressure and a pulse sensor was attached to the subject's finger to provide a continuous measure of pulse.
Blood Samples
ACTH and cortisol samples were obtained in heparinized tubes, and blood samples for basal NE determination were collected in tubes containing EGTA and reduced glutathione. All tubes were placed on ice immediately after drawing, and then aliquoted after being centrifuged at 4C within 30 minutes of collection. Blood samples for HPA axis measures were stored at -70C and processed at the Yale Center for Clinical Investigation Core Laboratories using standard radioimmuno-assay procedures. Basal norepinephrine levels were analyzed in Dr. Anderson's laboratory at Yale University. Samples were alumina-extracted from 1.0 ml plasma, separated by reverse phase ion-pair high performance liquid chromatography (HPLC) and detected using coulometry (Coulochem II, ESA, Inc.).
Statistical Analysis
Linear Mixed Effect (LME) models (Laird and Ware, 1982) were implemented to analyze the data, using SPSS software (version 16). These multi-level models are suitable for designs that include repeated measures across multiple factors and levels as utilized in this study. Within-subjects factors of Imagery Condition (stress, alcohol cue, neutral), Time-point (0, +15, +30, +45, +60, +75) and the Between-subjects factors of Medication Group (prazosin /placebo) were the fixed effects. Subjects represented the random effect. As the goal of the study was to assess the effects of medication on provocation of stress and alcohol cue exposure, change from baseline delta scores were analyzed for all dependent measures. The Bonferroni test for multiple comparisons was used to analyze simple effects. All T-tests and Chi-square analyses were used to compare the medication groups on demographic variables.
Results
Participants
Both placebo and prazosin medication groups were statistically matched for gender, race, IQ, age, recent prior alcohol and drug use and other clinical characteristics (Table 1). None of the participants met DSM-IV criteria for current mood or anxiety disorders.
Table 1.
Demographics and Clinical Characteristics of Alcohol Dependent Individuals in each Medication Group
| Placebo n = 9 | Prazosin n = 8 | |
|---|---|---|
| Gender -% male | 66.67 % | 75.0 % |
| Race | ||
| - % African American | 33.33 % | 50 % |
| - % Caucasian | 66.67 % | 37.50 % |
| - % Hispanic | 0 | 12.5 % |
| Agea | 36.11 ± 8.24 | 35.75 ± 7.67 |
| IQ (Shipley)a | 110 ± 8.52 | 102.75 ± 11.52 |
| Smoking status - % regular smokers | 100 % | 100 % |
| Years of Alcohol usea | 18.22 ± 10.31 | 13.00 ± 10.55 |
| No. of days used in past montha | 24.22 ± 7.10 | 22.50 ± 11.56 |
| No. of drinks per montha | 274.67 ± 143.05 | 434.88 ± 399.89 |
| Lifetime depression - % | 0 | 0 |
| Lifetime anxiety (incl PTSD) - % | 44.44 % | 25.0 % |
| Lifetime anxiety (without PTSD) - % | 11.11 % | 0 |
Note. All group comparisons are insignificant.
Data indicate means and standard deviations
Safety Measures
Preliminary findings from both the safety measures and laboratory sessions indicate that prazosin was well-tolerated, with mild levels of reported side effects. Findings indicated that 1/8 (12.5%) of the prazosin group reported a mild sensation of numbness following four weeks of prazosin aministration. In addition, 1/7 (14%) of the placebo group reported moderate and mild symptoms of nausea, light-headedness, lethargy and chest pain, and 2/7 (28%) reported moderate levels of fatigue. All symptoms dissipated over time without further intervention and chi-square analysis indicated no significant difference between medication groups in the frequency of patients reporting each of these symptoms.
Medication Group Effects at Baseline
The prazosin group demonstrated significantly higher levels of basal (pre-guided imagery) plasma norepinephrine across all three testing days [F (1, 10) = 20.1; p = 0.001]. No other measures showed baseline differences between the placebo and the prazosin groups (see Table 2).
Table 2.
Showing means (standard errors) for all measures at baseline.
| Dependent Variables* | Placebo (n = 9) | Prazosin (n=8) | p |
|---|---|---|---|
| Alcohol Craving (0-10) | 1.12 ± .21 | 0.96 ± .29 | ns |
| DES - Anxiety (3-15) | 4.62 ± .34 | 5.29 ± .47 | ns |
| DES - Negative Emotion (15-75) | 18.19 ± .82 | 20.29 ± 1.33 | ns |
| Heart Rate (bpm) | 66.56 ± 1.47 | 72.00 ± 1.61 | ns |
| SBP (mm/hg) | 114.25 ± 2.09 | 119.91 ± 1.97 | ns |
| DBP (mm/hg) | 69.89 ± 1.63 | 73.13 ± 1.57 | ns |
| ACTH (μg/dL) | 61.00 ± 4.89 | 62.86 ± 3.49 | ns |
| Cortisol (μg/dL) | 12.23 ± .87 | 11.14 ± .80 | ns |
| NE (pg/mL) | 155.80 ± 29.35 | 342.68 ± 28.63 | .001 |
Note. Range of scores and units are in parentheses
Condition Main Effects in Response to Imagery
As expected and reported in our previous studies (Fox et al., 2007; Sinha et al., 2009), there were significant main effects of imagery condition for the following response measures: alcohol craving [F(2, 30) = 13.78; p<. 0001] (stress > neutral, p<.0001; cue > neutral, p<.0001), anxiety [F(2, 30) = 11.54; p <0001] (stress > neutral, p<.0001; cue > neutral, p<.0001), negative emotion [F(2, 30) = 16.35; p < .0001] (stress > neutral, p<.0001; cue > neutral, p<.0001), cortisol [F(2, 26) = 6.14; p < .007] (stress > neutral, p<.003; cue > neutral, p=.009). These data indicate that the stress and alcohol cue versus neutral imagery manipulation was successful in inducing stress and cue-related provocation states.
Medication Group Effects in Response to Imagery
Alcohol craving
A significant Medication Group X Condition effect was observed [F (2, 30) = 5.40, p=.005; effect size: f =.80]. This resulted from significantly lower alcohol craving in the prazosin versus the placebo group for the stress condition (p<.04), and from no differences in stress-induced and alcohol cue-induced craving relative to the neutral condition in the prazosin group, but significantly higher alcohol craving in response to stress (p <.0001) and alcohol cue (p <.0001) relative to neutral in the placebo group (see Figure 1a). A significant Time-point X Condition effect [F (10, 150) = 2.73, p=.003] indicated that alcohol craving in both the placebo and prazosin groups decreased significantly from the initial peak response compared with all other recovery time-points, in the stress condition only (p<.0001, in all cases). No significant time-point X medication group effects were observed.
Figure 1. Line graphs showing subjective response to stress, alcohol cue and neutral imagery.
- PZ < PL following exposure to stress imagery (p<.04)
- PZ < PL following exposure to stress imagery (p<.0001)
- PZ < PL following exposure to stress imagery (p=.003)
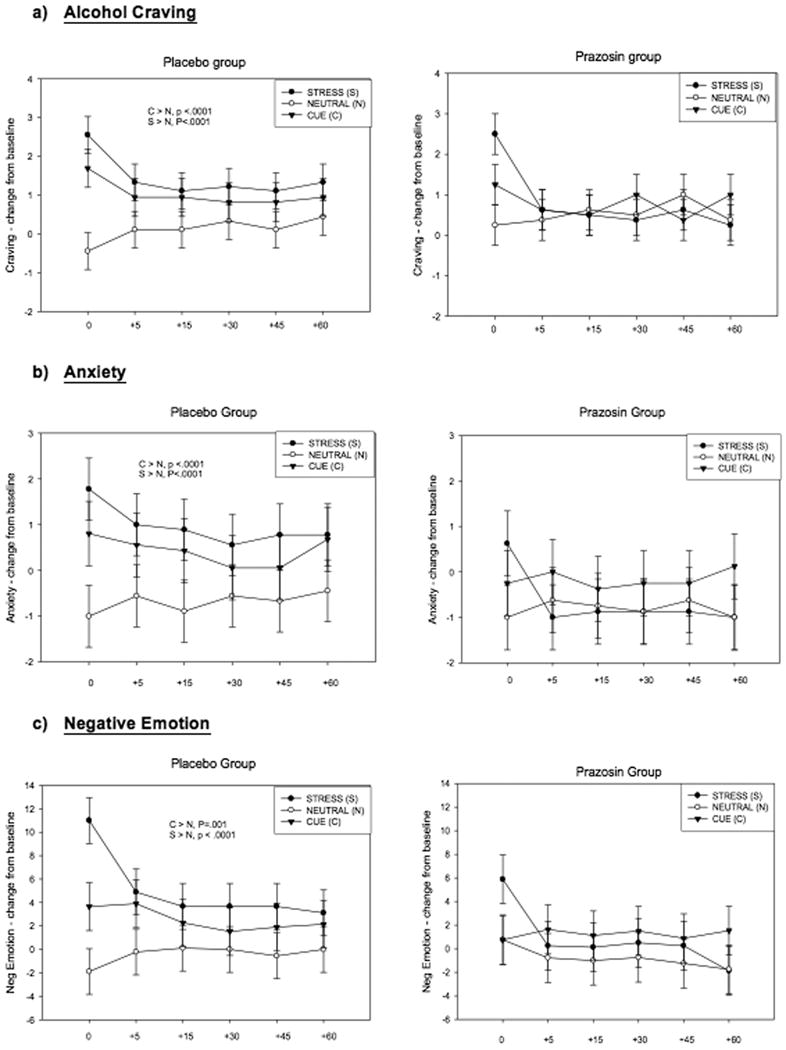
Anxiety
A significant Medication Group X Condition effect was observed for anxiety [F (2, 30) = 6.53; p =.002; effect size: f =.88]. Again, this interaction was a result of significantly lower anxiety in the prazosin versus the placebo group for the stress condition (p<.0001), and no differences in anxiety ratings during the stress and alcohol cue relative to neutral condition in the prazosin group, but significantly higher anxiety ratings in the placebo group following exposure to both stress (p<.0001) and alcohol cue (p<.0001) compared with the neutral imagery condition (see Figure 1b). No significant main effect of time-point was observed and no time-point interactions with Medication Group, or Imagery Condition.
Negative Emotion
A significant Medication Group X Condition effect was observed for negative emotion [F (2, 30) = 4.83; p =.009; effect size: f =.75]. This resulted from placebo group reporting significantly higher negative emotion ratings following exposure to stress compared with the prazosin group (p=.003), and also from significant increases in negative emotion ratings following both stress (p <.0001) and alcohol cue (p= .001) relative to neutral (control) imagery in the placebo group, but no such increases in negative emotion after stress and alcohol cue exposure relative to response in the neutral condition in the prazosin group (see Figure 1c). No significant main effect of time-point was observed and no time-point interactions with Medication Group, or Imagery Condition.
Blood Pressure Responses
A trend for a Medication Group main effect was observed for SBP [F (115) = 3.93; p = 0.06] where the prazosin group showed lower SBP than the placebo group. A significant Medication Group X Condition effect was observed for DBP [F (2, 30) = 4.93; p = 0.01; effect size: f =.76] which resulted from decreased DBP in the alcohol cue relative to neutral condition in the prazosin group (p=.0003). No differences between DBP responses were observed across conditions in the placebo group. The prazosin group also demonstrated significantly lower levels of DBP following alcohol cue exposure compared with the placebo group (p=.01). (see Figures 2a-2b). No significant main effects of time-point were observed for either SBP or DBP, and no significant time-point interactions with Medication Group, or Imagery Condition.
Figure 2. Line graphs showing blood pressure and HPA response to stress, alcohol cue and neutral imagery.
- Main effect of Medication Group (PLA >PZ: p=.06)
- PZ < PLA following exposure to cue imagery (p=.01)
-
PZ > PLA following exposure to stress (p=0.1) and cue (p=0.1)Note: * Cortisol levels in the neutral condition are decreased compared to baseline due to the expected diurnal drop in plasma cortisol over the course of the laboratory sessions conducted between 8:00 and 10:30 AM in the morning. In the Placebo group, cortisol levels following exposure to stress and cue are decreased reflecting a blunted response. In the Prazosin group, the drop in cortisol is attenuated following exposure to stress (p<.0001) and cue (p<.0001) relative to neutral, indicative of a significant stress and cue-induced increase in cortisol response.
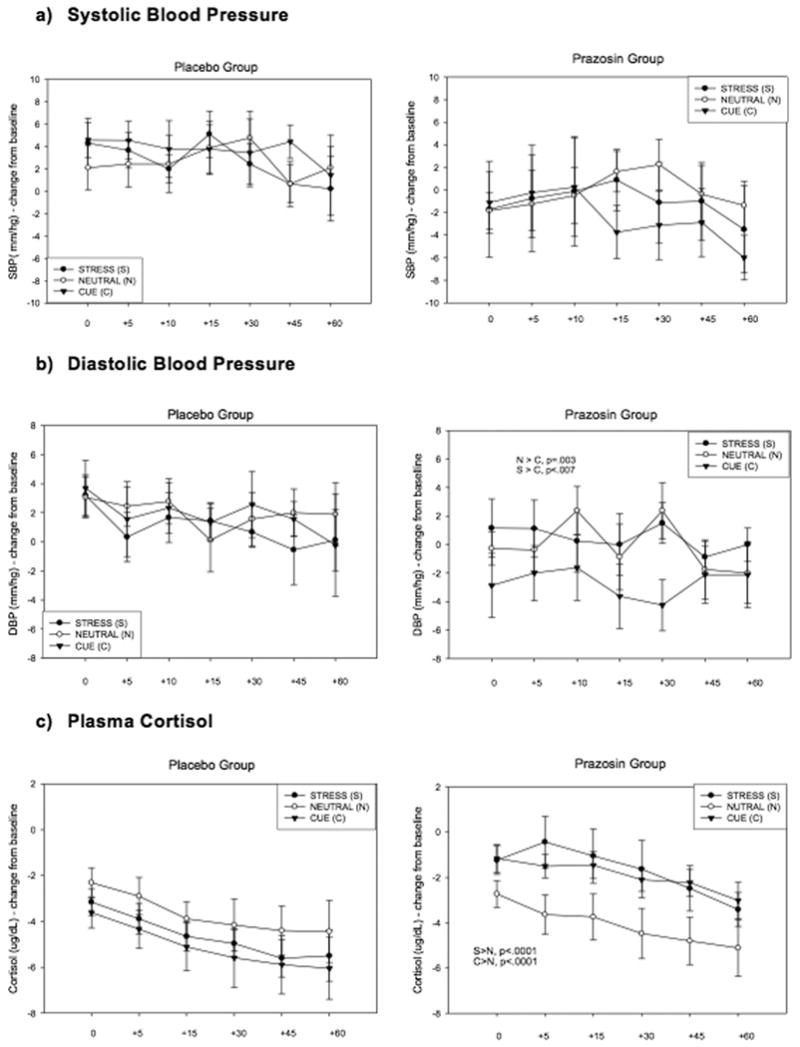
HPA axis measures
A main effect of Medication Group approached statistical significance for cortisol [F (1, 14) = 3.62; p = 0.07] indicating that the prazosin group demonstrated higher levels overall compared with the placebo group. A significant Medication Group X Condition interaction [F (2, 26) = 18.99; p < 0.0001; effect size: f =1.49] indicated that the prazosin group demonstrated significantly higher levels of cortisol following exposure to stress (p=.01) and cue (p=.01) compared with the placebo group. The prazosin group also showed a relative increase in cortisol following exposure to stress (p<.0001) and cue (p<.0001) compared to the neutral condition. This condition effect was not observed in the placebo group (see Figure 2c). A main effect of Time-point [F (5, 70) = 11.79; p< 0.0001] reflected an incremental increase in cortisol across all time-points. No main effects of Medication Group, Time-points or interaction effects were observed for ACTH.
Extended analysis
We further examined whether prazosin-related sympathetic basal adaptations were associated with the physiological and emotional responses to stress and alcohol cue observed in the current study.
Elevated basal levels of norepinephrine
Were shown to be associated with significantly lower stress-induced anxiety (r = -.24, p=.04). Elevated basal norepinephrine levels were also associated with significantly lower SBP following exposure to stressful (r = -0.25, p = .02) and neutral (control) imagery (r = -0.38, p < .0001) as well as significantly lower DBP following exposure to all three imagery conditions: (stress, r = -.32, p = .003; alcohol cue, r = -.32, p = .003; neutral, r = -.36, p = .001). Elevated basal levels of norepinephrine were also associated with increased plasma cortisol levels following exposure to both stressful imagery (r = .52, p <.0001) and alcohol cue related imagery (r = .55, p < .0001).
Discussion
In the current study, prazosin was found to be safe and well-tolerated in alcohol dependent patients during five weeks of inpatient stay. The prazosin group showed significantly lower stress-induced alcohol craving, anxiety and negative emotions relative to the placebo group. They also showed no significant increases in stress-induced and alcohol cue-induced craving as well as anxiety and negative emotion as compared to these responses in the neutral imagery condition, which was in contrast to the significant increases in these stress and cue-related alcohol craving, anxiety and negative emtoion responses in the placebo group. Furthermore, as expected and serving as a manipulation check for prazosin treatment, we found that prazosin increased basal sympathetic norepinephrine release, not seen in the placebo group. Higher norepinephrine levels were associated with an increased stress-induced cortisol response relative to neutral condition in the prazosin group. Conversely, the placebo group showed low basal norepinephrine levels alongside blunted cortisol responses to stress, typically observed in early abstinent alcoholics and individuals at risk for dependency (Adinoff et al., 2005; Brady et al., 2006; Junghanns et al., 2003; Fox et al., 2009; Sinha et al., 2009, 2011). These preliminary findings support the need for further clinical testing and assessment of prazosin in the treatment of alcoholism.
Direct medication group effects were seen in the stress condition with significantly lower alcohol craving, anxiety and negative emotion in the prazosin versus the placebo group. In addition, compared with the response to stress and cue exposure in the placebo group, alcohol dependent individuals given prazosin reported no significant increases in alcohol craving, anxiety and negative emotion following exposure to both stress and alcohol cue related imagery relative to neutral condition. These findings are clinically relevant as a greater sensitivity to stress, anxiety and negative emotion and stress-induced and cue-induced craving has been observed in alcohol dependent patients compared with social drinkers (Glautier et al., 1992; Kaplan et al., 1985; Pomerleau et al., 1983; Sinha et al., 2009; Wand and Dobs, 1991). Furthermore, stress, negative mood and cue-induced alcohol craving in the laboratory have been shown to predict alcohol relapse risk in prospective outcome studies (Breese et al., 2005; Cooney et al., 1997; Fox et al, 2007, 2009; Fox and Sinha, 2009; Litt et al., 1999; Sinha et al., 2011). Consistent with previous research, current findings show both stress and alcohol cue induced increases in craving were accompanied by enhanced ratings of negative affect and anxiety in the placebo group, and the ability of prazosin to reduce this sensitized negative emotional response to stress and cue, may be one of the processes by which prazosin exerts its effects on alcohol craving.
Alcohol dependent individuals who were administered prazosin also demonstrated lower blood pressure levels following exposure to all three imagery conditions compared with the placebo group. Although this medication effect only approached statistical significance, the data are consistent with the known vasodilatory therapeutic effects of prazosin (Bawaskar and Bawaskar, 1987; Bayliss et al., 1985; Colucci, 1982). Transient withdrawal hypertension is a common feature of alcohol abstinence (Ceccanti et al., 2006; King et al., 1994). Moreover, sensitized blood pressure increases following stress and cue in both alcohol dependent and cocaine dependent individuals, are associated with higher craving ratings (Fox et al., 2007, Fox et al., 2008; Sinha et al., 2009). Additionally, observational studies have highlighted a relationship between three or more alcoholic drinks daily and hypertension (Cushman, 2001) and alcohol consumption has been associated with hypertension even in light/social drinkers (Nakanishi et al., 2002). As such, the hypotensive effects of prazosin may also contribute to the decrease in subjective negative emotion following stress and cue and the concomitant reduction in alcohol craving.
In this study, prazosin was also shown to alter cortisol response to stress and cue as well as baseline norepinephrine levels. Following exposure to stress imagery, individuals in the prazosin group demonstrated significantly increased levels of plasma cortisol compared with the neutral (control) condition. Again, no condition differences were observed in the placebo group. These findings are clinically important as alcohol related dampening and blunting of the stress response has been well documented in active alcoholics and in abstinent alcoholics in early recovery (Adinoff et al., 2005; Fox et al., 2009; Sinha et al., 2009; Wand & Dobs, 1991) and has been associated with both alcohol craving and relapse factors in dependent individuals (Adinoff et al., 2005; Breese et al., 2005; Fox et al., 2009; Junghanns et al., 2005; Sinha, 2007; Sinha et al., 2011). For example, in prior research we demonstrated that a decrease in both HPA drive, characterized by suppressed cortisol and ACTH response to stress may be representative of the alcohol craving state in abstinent early recovering substance-abusing males (Fox et al., 2009). Blunted HPA axis response to stress might therefore increase relapse vulnerability and craving by representing a chronic inability to respond to threat and stress system challenge over time (Adinoff et al., 1998; Fox et al., 2009; Sinha et al., 2009; Wand and Dobs, 1991). In turn, increased stress-related levels of cortisol, observed in the prazosin group, could reflect a more appropriate mobilization of the HPA response to challenge as documented in healthy controls (Sinha et al., 2009; 2011) Such increases in stress-induced cortisol responses in the prazosin group may represent a return to adaptive coping in the face of challenge (Adinoff et al., 1998) which may in turn contribute to the decrease in stress-related and cue-related negative emotion and craving in a population of individuals with a dampened and highly dysregulated stress system (Fox and Sinha, 2009 for review).
The prazosin group also demonstrated significantly elevated basal levels of peripheral norepinephrine compared with the placebo group. An increase in the neural release of norepinephrine during alpha blockade may initially appear paradoxical, however, the therapeutic effects of prazosin are related to the selective blockade of post-synaptic uptake, not pre-synaptic release (Cambridge et al., 1977; Colucci, 1982; Stein et al., 1981). Sympathetic vascular effects are therefore reduced, although feedback control of norepinephrine release from alpha2 receptors is preserved. The apparent increase in tonic sympathetic activity is therefore presumably due to compensatory processes acting to partially counter prasozin's alpha-1 blocking effects. In corroboration with this, many earlier studies assessing the efficacy of prazosin in individuals with chronic congestive heart failure also document elevated plasma norepinephrine levels (Bayliss et al., 1985; Colucci et al., 1980; Stein et al., 1981). Moreover, in the current study high basal plasma norepinephrine levels in the prazosin-treated group were associated with significantly lower blood pressure following stress and cue exposure. In view of this, prior studies have shown similar blood pressure adaptations to accompany reduced stress and cue-related craving in both alcoholic and co-morbid cocaine and alcohol dependent individuals (Fox et al., 2005; Sinha et al., 2009).
Basal on-drug plasma norepinephrine levels were also associated with an increased HPA axis response to stress and alcohol cue exposure. These findings are in line with studies which have shown alpha1 adrenergic receptors to exert regulatory control over cortisol secretion (Dunn and Swiergiel, 2008). For example, in laboratory rats, increases in stress-induced norepinephrine release have been shown to induce an increase in hippocampal corticosteroid receptors facilitating a more efficient feedback of cortisol following stress (Kabbaj et al., 1995, 2007; Kiss and Aguilera, 1992; Meaney et al., 1988; Takao et al., 1988). Such changes in the HPA stress system response and decreasing blood pressure by increasing sympathetic tone may play a role in the reduction of anxiety, negative emotion and subsequently alcohol craving by prazosin.
The current study is limited by the small subject sample and hence is presented as preliminary data. However, large effect sizes for the observed effects were obtained supporting further research with prazosin. In addition, this study comprised predominantly male alcoholics (12/17) and gender differences in effects of PZ need to be further assessed. Despite such limitations, to our knowledge, these preliminary findings are the first to demonstrate that prazosin decreased stress-induced and cue-induced alcohol craving and related stress and emotional responses. As previous studies have shown that stress and cue-induced alcohol craving and stress dysregulation are associated with alcohol relapse risk (Adinoff et al., 2005; Breese et al., 2005; Cooney et al., 1997; Sinha, 2008; Sinha et al., 2011), the current data support further testing of prazosin in decreasing alcohol relapse risk and improving treatment outcomes.
Acknowledgments
We would like to thank the staff at the Substance Abuse Treatment Unit, Clinical Neuroscience Research Unit and the Yale Center for Clinical Investigation for their assistance in completing this study.
This study was supported in part by Grants R01-AA013892 (Sinha), UL1-DE019586 (Sinha), PL1-DA024859 (Sinha), PL1-DA024860 (Anderson), the NIH/NCRR/CTSA Program Grant no.1 UL1 RR024139 (Yale Center for Clinical Investigation) and the Connecticut Department of Mental Health and Addiction Services.
Footnotes
Disclosure/Conflict of Interest: The authors declare that they have no competing financial interests pertaining to the aims and results of this study.
References
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22(1):67–72. Review. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: Implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawaskar HS, Bawaskar PH. Prazosin for vasodilator treatment of acute pulmonary oedema due to scorpion sting. Ann Trop Med Parasitol. 1987;81(6):719–23. doi: 10.1080/00034983.1987.11812175. [DOI] [PubMed] [Google Scholar]
- Bayliss J, Norell MS, Canepa-Anson R, Reid C, Poole-Wilson P, Sutton G. Clinical importance of the renin-angiotensin system in chronic heart failure: double blind comparison of captopril and prazosin. Br Med J (Clin Res Ed) 1985;290(6485):1861–5. doi: 10.1136/bmj.290.6485.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton L, Bentley J, Strachan E, Barbato A, Raskind M. Preliminary findings concerning the use of prazosin for the treatment of posttraumatic nightmares in a refugee population. J Psychiatr Pract. 2009;15(6):454–9. doi: 10.1097/01.pra.0000364287.63210.92. [DOI] [PubMed] [Google Scholar]
- Brady KT, Waldrop AE, McRae AL, Back SE, Saladin ME, Upadhyaya HP, Anton RF, Randall PK. The impact of alcohol dependence and posttraumatic stress disorder on cold pressor task response. J Stud Alcohol. 2006;67(5):700–6. doi: 10.15288/jsa.2006.67.700. [DOI] [PubMed] [Google Scholar]
- Brandon, et al. Relapse and relapse prevention. Annual Review of Clinical Psychology. 2007;3:257–285. doi: 10.1146/annurev.clinpsy.3.022806.091455. [DOI] [PubMed] [Google Scholar]
- Breese GR, Chu K, Dayas CV, et al. Stress enhancement of craving during sobriety: a risk for relapse. Alcohol Clin Exp Res. 2005;29(2):185–95. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambridge D, Davey MJ, Massingham R. The pharmacology of antihypertensive drugs with special reference to vasodilators, alpha-adrenergic blocking agents and prazosin. Med J Aust. 1977;2(1 Suppl):2–6. doi: 10.5694/j.1326-5377.1977.tb107750.x. 20. [DOI] [PubMed] [Google Scholar]
- Ceccanti M, Sasso GF, Nocente R, Balducci G, Prastaro A, Ticchi C, Bertazzoni G, Santini P, Attilia ML. Hypertension in early alcohol withdrawal in chronic alcoholics. Alcohol Alcohol. 2006;41(1):5–10. doi: 10.1093/alcalc/agh221. [DOI] [PubMed] [Google Scholar]
- Colucci WS. Alpha-adrenergic receptor blockade with prazosin. Consideration of hypertension, heart failure, and potential new applications. Ann Intern Med. 1982;97(1):67–77. doi: 10.7326/0003-4819-97-1-67. Review. [DOI] [PubMed] [Google Scholar]
- Colucci WS, Williams GH, Braunwald E. Increased plasma norepinephrine levels during prazosin therapy for severe congestive heart failure. Ann Intern Med. 1980;93(3):452–3. doi: 10.7326/0003-4819-93-3-452. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Morse PA, Bauer LO, Gaupp L. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J Abnorm Psychol. 1997;106:243–50. doi: 10.1037//0021-843x.106.2.243. [DOI] [PubMed] [Google Scholar]
- Cukor J, Spitalnick J, Difede J, Rizzo A, Rothbaum BO. Emerging treatments for PTSD. Clin Psychol Rev. 2009;29(8):715–26. doi: 10.1016/j.cpr.2009.09.001. Review. [DOI] [PubMed] [Google Scholar]
- Cushman WC. Alcohol consumption and hypertension. J Clin Hypertens (Greenwich) 2001;3(3):166–70. doi: 10.1111/j.1524-6175.2001.00443.x. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–28. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. The role of corticotropin-releasing factor and noradrenaline in stress related responses, and the inter-relationships between the two systems. Eur J Pharmacol. 2008;583(2-3):186–93. doi: 10.1016/j.ejphar.2007.11.069. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Schuck J, Stender N, Pilz J, Gefeller O, Schilling L, Poser W, Kaw S. Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin Exp Res. 1997;21(7):1285–93. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0, 4/97 revision) New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- Fox HC, Berquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol dependent individuals. Alcoholism: Clinical and Experimental Research. 2007;31(3):395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz, Bergquist KL, Anderson GM, Kreek MJ, Sinha R. Gender dissociations in autonomic and HPA responses to stress and cues in alcohol dependent patients with cocaine abuse. Alcohol and Alcoholism Alcohol Alcohol. 2009;44(6):575–85. doi: 10.1093/alcalc/agp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz KM, Sinha R. Enhanced Sensitivity to Stress and Drug/Alcohol Craving in Abstinent Cocaine Dependent Individuals Compared to Social Drinkers. Neuropsychopharmacology. 2008;33(4):796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Sinha R. Sex differences in drug-related stress system changes: implications for treatment in substance-abusing women. Harvard Review of Psychiatry. 2009;17(2):103–19. doi: 10.1080/10673220902899680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and to drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Glautier S, Drummond DC, Remington B. Different drink cues elicit different physiological responses in non-dependent drinkers. Psychopharmacology. 1992;106(4):550–554. doi: 10.1007/BF02244829. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12 month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74(3):223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hughes JC, Cook CC. The efficacy of Disulfiram. Addiction. 1997;92:381–95. Review. [PubMed] [Google Scholar]
- Izard C. Patterns of emotions: A new analysis of anxiety and depression. New York: Academic Press; 1972. [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38:189–93. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Tietz U, Dibbelt L, Kuether M, Jurth R, Ehrenthal D, et al. Attenuated salivary cortisol secretion under cue exposure is associated with early relapse. Alcohol Alcohol. 2005;40:80–85. doi: 10.1093/alcalc/agh107. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Morley-Fletcher S, Le Moal M, Maccari S. Individual differences in the effects of chronic prazosin hydrochloride treatment on hippocampal mineralocorticoid and glucocorticoid receptors. Eur J Neurosci. 2007;25(11):3312–8. doi: 10.1111/j.1460-9568.2007.05585.x. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Piazza PV, Simon H, Le Moal M, Maccari S. Opposite effects on hippocampal corticosteroid receptors induced by stimulation of b and a1 noradrenergic receptors. Neuroscience. 1995;66:539–549. doi: 10.1016/0306-4522(94)00620-k. [DOI] [PubMed] [Google Scholar]
- Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerleau OF. Reactivity to alcohol-related cues: Physiological and subjective responses in alcoholics and nonproblem drinkers. Journal of Studies on Alcohol. 1985;46(4):267–272. doi: 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- King AC, Parsons OA, Bernardy NC, Lovallo WR. Drinking history is related to persistent blood pressure dysregulation in postwithdrawal alcoholics. Alcohol Clin Exp Res. 1994;18(5):1172–6. doi: 10.1111/j.1530-0277.1994.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Kiss A, Aguilera G. Participation of alpha 1-adrenergic receptors in the secretion of hypothalamic corticotropin-releasing hormone during stress. Neuroendocrinology. 1992;56(2):153–60. doi: 10.1159/000126223. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. rain Res. 2009 doi: 10.1016/j.brainres.2009.11.008. Nov 11. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–59. doi: 10.1176/appi.ajp.2007.05030503. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Sher KJ, Erickson DJ. Prospective analysis of the relation between DSM-III anxiety disorders and alcohol use disorders. Am J Psychiatry. 1999;156(5):723–32. doi: 10.1176/ajp.156.5.723. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: Conceptual structure and pattern of somatovisceral response. Psychophysiology. 1980;17(2):179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: The problem of affective response integration. Journal of Abnormal Psychology. 1983;92(3):276–306. doi: 10.1037//0021-843x.92.3.276. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre B, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2178-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Cooney NL. Inducing craving for alcohol in the laboratory. Alcohol Res Health. 1999;23:174–8. [PMC free article] [PubMed] [Google Scholar]
- Meaney M, Viau V, Aitken DH, Bhatnagar S. Stress induced ccupancy and translocation of hippocampal glucocorticoid receptors. Brain Res. 1988;445:198–203. doi: 10.1016/0006-8993(88)91093-1. [DOI] [PubMed] [Google Scholar]
- Mercer D, Woody G. Addiction Counseling. University of Pennsylvania/VAMC Center for Studies of Addiction; 1992. Unpublished manuscript. [Google Scholar]
- Miller WR, Brown JM, Simpson TL, et al. What works? A summary of alcohol treatment outcome research. In: Hester RK, Miller WR, editors. Handbook of Alcoholism Treatment Approaches: Effective Alternatives. 3rd. Boston, Mass: Allyn and Bacon; 2003. pp. 13–63. [Google Scholar]
- Miller G, Levin DN, Kozak MJ, Cook EW, McLean A, Lang PJ. Individual differences in imagery and the psychophysiology of emotion. Cognitive Emotion. 1987;1:367–390. [Google Scholar]
- Nakanishi N, Makino K, Nishina K, Suzuki K, Tatara K. Relationship of light to moderate alcohol consumption and risk of hypertension in Japanese male office workers. Alcohol Clin Exp Res. 2002;26(7):988–94. doi: 10.1097/01.ALC.0000021161.94001.33. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Gopalakrishnan R, Naik PC, Murray HW, Vergare MJ, Marsden CA. Changes in plasma noradrenaline and serotonin levels and craving during alcohol withdrawal. Alcohol Alcohol. 2003;38(3):224–31. doi: 10.1093/alcalc/agg055. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Marsden CA, Naik PC, Kendall DA, Gopalakrishnan R, Vergare MJ, Weinstein SP. Differences in peripheral noradrenergic function among actively drinking and abstinent alcohol-dependent individuals. Am J Addict. 2004;13(3):225–35. doi: 10.1080/10550490490459898. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Fertig J, Baker L, Cooney N. Reactivity to alcohol cues in alcoholics and non-alcoholics: Implications for a stimulus control analysis of drinking. Addictive Behaviors. 1983;8(1):1–10. doi: 10.1016/0306-4603(83)90048-5. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O'Connell J, Taylor F, Gross C, Rohde K, McFall ME. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928–34. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Rasmussen DD, Alexander LL, Raskind MA, Froehlich JC. The alpha1-adrenergic receptor antagonist, prazosin, reduces alcohol drinking in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2009;33(2):264–72. doi: 10.1111/j.1530-0277.2008.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Wilkinson CW, Raskind MA. Chronic daily ethanol and withdrawal: 6. Effects on rat sympathoadrenal activity during “abstinence”. Alcohol. 2006;38(3):173–7. doi: 10.1016/j.alcohol.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Peselow E. Pharmacotherapy of addictive disorders. Clin Neuropharmacol. 2009;32:277–89. doi: 10.1097/wnf.0b013e3181a91655. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33(2):255–63. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–59. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiatry Rep. 2007;9(5):388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic alcohol related neuroadaptations in stress responses: Effects on compulsive alcohol seeking and relapse outcomes. Alcoholism: Clinical and Experimental Research. 2008;32(1):274. [Google Scholar]
- Sinha R. Stress and addiction: a dynamic interplay of genes, environment, and drug intake. Biol Psychiatry. 2009;66(2):100–1. doi: 10.1016/j.biopsych.2009.05.003. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist KL, Bhagwagar Z, Siedlatz K. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34(5):1198–208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Siedlarz KA, Bergquist KT, Kreek MJ. Effects of adrenal sensitivity, stress and cue-induced craving and anxiety on subsequent alcohol relapse and treatment outcomes. Archives of General Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.49. epub online May 2nd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O'Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152(2):140–8. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sinha R, Hong KA, Seo D, Fox HC, Berguist K. Neural and endocrine predictions of alcohol relapse risk. Alcoholism: Clin Exp Res. 2010;34(6) Supplement:290A. [Google Scholar]
- Sinha R, Lovallo WR, Parsons OA. Cardiovascular differentiation of emotions. Psychosomatic Medicine. 1992;54(4):422–435. doi: 10.1097/00006842-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-ACDrenal axis and sympatho-ACDreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology (Berl) 2003;170:62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- Stein L, Henry DP, Weinberger MH. Increase in plasma norepinephrine during prazosin therapy for chronic congestive heart failure. Am J Med. 1981;70(4):825–32. doi: 10.1016/0002-9343(81)90539-8. [DOI] [PubMed] [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, Del Boca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. J Stud Alcohol Suppl. 1994;12:70–5. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Takao T, Hashimoto K, Ota Z. Central catecholaminergic control of ACTH secretion. Regul Peptides. 1988;21:301–308. doi: 10.1016/0167-0115(88)90013-4. [DOI] [PubMed] [Google Scholar]
- Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm. 2008;65(8):716–22. doi: 10.2146/ajhp070124. Review. [DOI] [PubMed] [Google Scholar]
- Thompson CE, Taylor FB, McFall ME, Barnes RF, Raskind MA. Nonnightmare distressed awakenings in veterans with posttraumatic stress disorder: response to prazosin. J Trauma Stress. 2008;21(4):417–20. doi: 10.1002/jts.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Surgeon General's Call to Action To Prevent and Reduce Underage Drinking. U.S. Department of Health and Human Services, Office of the Surgeon General; 2007. [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42(2):91–7. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. Journal of Clincal Endocrinology & Metabolism. 1991;72(6):1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Williams SH. Medications for treating alcohol dependence. Am Fam Physician. 2006;72:1775–80. [PubMed] [Google Scholar]
- Zimmerman U, Spring K, Koller G, Holsboer F, Soyka M. Hypothalamic-pituitary-ACDrenal system regulation in recently detoxified alcoholics is not altered by one week of treatment with acamprosate. Pharmacopsychiatry. 2004;37:98–102. doi: 10.1055/s-2004-818986. [DOI] [PubMed] [Google Scholar]


