Abstract
Background
Advanced glycations end products increase oxidant stress, inflammation, and neurotoxicity. Serum levels are increased in diabetes and aging. We examined the relationship between serum methylglyoxal derivatives (sMG), and cognitive decline, in 267 non-demented elderly.
Methods
Tobit mixed regression models assessed the association of baseline sMG with cognitive decline in the Mini Mental State Exam (MMSE) over time, controlling for sociodemographic factors (age, sex, and years of education), cardiovascular risk factors (diabetes and presence of an APOE4 allele), and kidney function. sMG was assessed by ELISA.
Results
The fully adjusted model showed an annual decline of 0.26 MMSE points per unit increase in baseline sMG (p=0.03). Significance was unchanged as additional risk factors were added to the model. The interactions of sMG with diabetes, sex, age, kidney function, and APOE4 genotype were not significant.
Conclusions
Higher levels of baseline sMG were associated with a faster rate of cognitive decline, after adjusting for several sociodemographic and clinical characteristics. This relationship did not differ by sex, APOE4 genotype, or diabetes status suggesting its generality. Since subjects were cognitively normal at the beginning of the study, elevated sMG may be indicative of brain cell injury initiated before clinically evident cognitive compromise.
Keywords: methylglyoxal, advanced glycation end products, type 2 diabetes, cognitive decline, Alzheimer's disease
Introduction
Advanced glycation end products (AGEs) are a heterogeneous group of endogenous and exogenous glucose-derived compounds, with significant pro-inflammatory and pro-oxidative stress effects(1). AGEs play a significant pathogenic role in numerous age-related diseases, e.g. diabetes, cardiovascular disease, and kidney disease, and may also have a role in Alzheimer's disease (AD)(2). Elderly and diabetic individuals have increased AGEs and a higher risk of AD(3). AGEs are higher in serum(4) and cerebrospinal fluid (CSF)(5, 6) of AD patients than in normal controls. Due to their chemical structure, AGEs may take part in the transformation of soluble into insoluble β amyloid and the aggregation of microtubule associated protein tau(7). AGE-modified Aβ ve as a “seed” in the acceleration and stabilization of amyloid plaques in the AD brain(8). Based on immuno-evidence, histochemical levels of AGEs in the brain of AD patients are higher than in normal controls, constituting components of senile plaques and neurofibrillary tangles(8).
Among other reactive AGE precursors, glyoxal and methylglyoxal are common dicarbonyl compounds that contribute to AGE formation, via derivatives, such as imidazolones. Methylglyoxal is endogenously produced at a low rate during autoxidation of reducing sugars and lipids(9) and at higher rates under conditions of increased oxidant stress, while its methylglyoxal-lysine derivatives can also be acquired from the diet(10). The neurotoxic effects of methylglyoxal and its reactive derivatives have been widely discussed in the literature(11-13). Methylglyoxal is associated with enhanced aggregation of Aβ(14), and CSF methylglyoxal concentrations in AD patients are substantially higher than in normal elderly controls(11, 15). In this study we examined the relationship between circulating levels of methylglyoxal derivatives (sMG) and cognitive decline over time in a sample of very elderly non-demented subjects, who are thus at high risk of AD. We hypothesized that higher sMG concentrations would be associated with a faster rate of cognitive decline.
Methods
Subjects
Data are from an ongoing, NIA-funded, longitudinal research project at the Mount Sinai School of Medicine (MSSM) that began at the end of 2005, investigating risk factors for AD in the very elderly. Subjects were recruited from senior centers and by newspaper ads, or were outpatients of the James J. Peters Veteran Affairs Medical Center (VAMC) affiliated with the MSSM and assessed approximately annually. Subjects were at least 75 years old and cognitively intact at entry into the project. Subjects with a diagnosis of stroke or other neuropsychiatric disease that could compromise cognition (such as Parkinson's disease or schizophrenia) based on medical chart reviews were excluded from the study. Normal cognition was defined by a Clinical Dementia Rating (CDR) scale score of 0 (non-demented) and by a Mini Mental State Exam (MMSE) score above the 10th percentile of the age and education norms(16). Those with CDR score equal to zero but with a discrepancy between informant and subject's reports, raising doubt about cognitive intactness, were referred to the Alzheimer's Disease Research Center for a full dementia workup. All subjects so referred (n=6) had their non-dementia status (CDR=0) confirmed. The study was approved by the MSSM and VAMC Institutional Review Boards, and all subjects signed informed consent.
At the time of this analysis the study has recruited 443 subjects. Of those, 176 had no follow up (69 dropped out or died and 106 did not reach the follow up assessment time), 111 had one follow up, 129 had two follow ups, and 28 had three follow ups or more. Since the objective of this study was to examine the relationship between baseline sMG and cognitive decline, the 267 subjects who had at least one follow up were included in the analyses.
Methylglyoxal analysis
Serum derivatives of methylglyoxal (sMG) were measured by a competitive enzyme-linked immunosorbent assay (ELISA), using a monoclonal anti-MG-BSA antibody (MG3D11) raised against MG-modified BSA (22 MG-modified arg/mol BSA, by high performance liquid chromatography and GC-MS). In our laboratory, the minimum detection limit is 0.03 nmol/ml, and the intra- and inter-assay coefficients of variation are 5.5% and 8.3%, respectively. MG3D11 has been found to be strongly immunoractive against MG-ovoalbumin and AGE-BSA but not with CML-BSA or unmodified BSA(17).
Diabetes assessment
Diagnosis of diabetes was determined by consensus conference based on the presence of diabetes diagnosis in the medical chart, blood glucose measures in the chart, and use of medications for the treatment of diabetes. Assessments were done at the home of each participant, where the interviewer recorded medication information directly from the labels of participants’ medications, providing good reliability of this data.
Other covariates
ApoE4 genotype was defined by the presence of at least one ApoE4 allele. ApoE genotype was determined by PCR run from blood(18). Fasting plasma glucose and total cholesterol were determined using standard methods. HDL cholesterol was determined after precipitation of apolipoprotein B-containing lipoproteins with phosphotungstic acid. LDL cholesterol levels were calculated using the Friedewald formula(19). HbA1c was determined using ion exchange, high performance liquid chromatography (20). Kidney function or glomerular filtration rate was estimated using the Cockroft-Gault formula(21). Smoking was dichotomized into subjects who ever or never smoked. Body Mass Index (BMI) was calculated as weight in kilograms divided by height in meters square.
Statistical analyses
Tobit mixed regression models assessed the association of baseline sMG with cognitive decline in the MMSE score over time, controlling for sociodemographic factors (age, sex, and years of education), cardiovascular risk factors (diabetes, BMI, and presence of an APOE4 allele) associated with dementia(22), and kidney function. Although generalized estimating equations (GEE) models are appropriate for most longitudinal data sets, by modeling the correlation among repeated observations within a given subject, they do not accommodate situations where there are either floor or ceiling effects in the response variable. The ceiling effect is in fact a type of censoring that is present in data using the MMSE, particularly in the context of intact cognition at baseline, and the model should account for this aspect of the data. The original Tobit model introduced by Tobin (1958) was for ceiling effects (censoring) in cross-sectional data (rather than repeated measurements). However, Twisk and Rijmen (2009) extended the Tobit model to the setting of longitudinal studies with repeated measurements, compared the longitudinal Tobit model to the regular linear mixed model on a data set that had repeated measures as well as ceiling effects, and found that the longitudinal Tobit performed better than the linear mixed model. The general idea of Tobit regression is that it models both the probability of reaching either the floor or ceiling and the development over time between the floor and ceiling. The GEE approach only models the development over time between the floor and ceiling and ignores the probability of reaching either a floor or ceiling. Additionally, similarly to GEE, Tobit regression can handle differences in length of follow up as well as missing values. Thus, we selected Tobit regression as the most appropriate for the data presented herein. Additional models evaluated the interactions of diabetes, sex, age, APOE4 genotype, BMI, and kidney function with MG.
Results
The 267 subjects who had at least two MMSEs and complete data on all variables of interest were included in this study. Subjects averaged 83.5 (±5.3) years of age, 14.3 (±3.1) years of education, 35.7 (±15.7) months of follow up. Their mean sMG was 0.93 nmol/ml (±0.57), estimated GFR 56ml/min (±19.5), BMI 26.13 (±3.9), HbA1c 5.8% (±0.87), fasting glucose 108.5 (±40.1), total cholesterol 178.5 (±38.0), LDL 104.8 (±42.1), and HDL 50.9 (14.7); 76% of the sample were men (since a major source of recruitment was the VAMC), 18% had diabetes, 20% carried an APOE4 allele, and 66.4% ever smoked. The average MMSE at baseline was 28.2 (±1.7) reflecting a cognitively normal sample, and at last visit it was 27.3 (±2.3). For descriptive purposes, Table 1 presents the characteristics of the sample stratified by sMG quartiles. There were significantly more women in the upper quartiles of sMG (p=0.008). All other variables were not significantly associated with sMG levels.
Table 1.
Description of the sample characteristics stratified by sMG quartiles
| Clinical variable | sMG quartiles | F (3,263) or χ2 (3) | P-value | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Age (mean; SE) | 84.5 (0.7) | 82.6 (0.6) | 83.8 (0.7) | 83.4 (0.6) | 1.55 | 0.20 |
| Sex (% F) | 28 | 13 | 22 | 37 | 11.91 | 0.008 |
| Years of Education (mean; SE) | 14.2 (0.4) | 13.9 (0.4) | 14.7 (0.4) | 14.6 (0.4) | 1.04 | 0.38 |
| Baseline MMSE (mean; SE) | 27.9 (0.2) | 28.4 (0.2) | 28.3 (0.2) | 28.3 (0.2) | 1.03 | 0.38 |
| Diabetes (%) | 20 | 27 | 31 | 22 | 1.52 | 0.68 |
| Fasting glucose (mean; SD) | 106.8 (38.5) | 105.8 (41.0) | 109.2 (37.3) | 112.7 (44.0) | 0.715 | 0.54 |
| HbA1c (mean; SD) | 5.8 (.69) | 5.7 (.75) | 5.9 (.88) | 5.8 (1.1) | 0.645 | 0.59 |
| Total cholesterol (mean; SD) | 176.8 (38.3) | 178.4 (33.5) | 179.5 (40.8) | 178.9 (39.2) | 0.114 | 0.95 |
| LDL cholesterol (mean; SD) | 102.6 (2.1) | 103.4 (32.7) | 108.9 (64.6) | 104.6 (29.9) | 0.512 | 0.67 |
| HDL cholesterol (mean; SD) | 48.4 (14.2) | 50.9 (14.3) | 53.3 (13.9) | 51.1 (15.3) | 2.294 | 0.08 |
| BMI (mean; SE) | 26.2 (0.5) | 26.4 (0.5) | 26.3 (0.5) | 25.7 (0.5) | 0.52 | 0.67 |
| APOE4 (%) | 28 | 22 | 25 | 25 | 0.76 | 0.86 |
| Kidney function (mean; SE) | 56.4 (2.6) | 60.2 (2.5) | 54.7 (2.6) | 52.4 (2.4) | 1.78 | 0.15 |
| Smoking (ever/never) % | 63.2 | 68.7 | 66.4 | 67.9 | 0.880 | 0.83 |
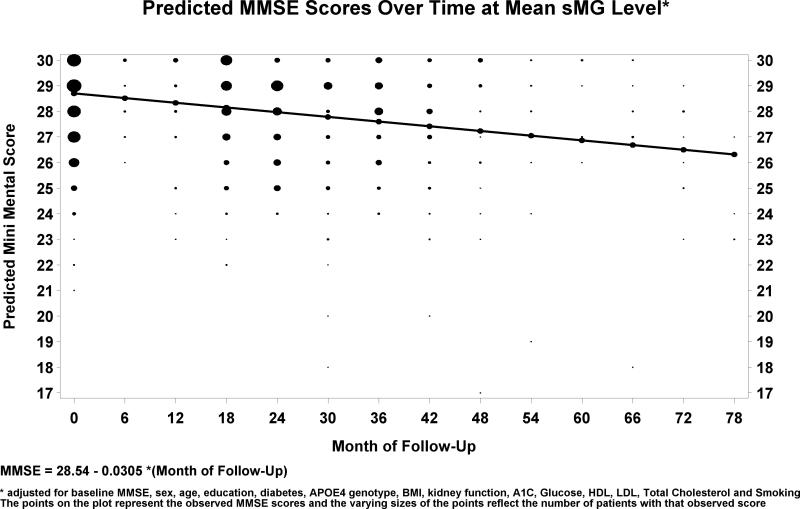
Table 2 presents the results of successive analyses of the association of sMG with cognitive decline over time controlling for additional risk factors. For each model, we estimated the regression coefficient of annual MMSE change per increase in sMG of 1 nmol/ml. Significance was essentially unchanged as several additional risk factors were added to the model suggesting they do not contribute substantially to the relationship between MG and the MMSE. The fully adjusted model showed an average annual decline of 0.39 MMSE points per year, with an additional annual decline of 0.36 MMSE points per unit increase in sMG (p=0.012), i.e. higher MG levels were associated with increasing rates of cognitive decline. For descriptive purposes, Figure 1 presents the estimated regression line of MMSE decline over time for the mean level of sMG, controlling for baseline MMSE and for all covariates. Based on the fully adjusted model, lower baseline MMSE, male gender, lower education, older age, higher HDL, and lower total cholesterol were associated with poorer overall cognitive performance. Diabetes, kidney function, the APOE4 genotype, BMI, LDL, and smoking were not associated with cognitive performance. Finally, the interactions of sMG with diabetes, sex, age, kidney function, BMI, and APOE4 genotype were not significant and did not substantially improve the model fit.
Table 2.
Tobit regression estimates for longitudinal MMSE as dependent variable and baseline sMG as the primary predictor
| Parameter | Estimate | StandardError | tValue | P-value | Lower 95% CL | Upper 95% CL |
|---|---|---|---|---|---|---|
| INTERCEPT | 6.3413 | 2.0902 | 3.0300 | 0.0027 | 2.2258 | 10.4567 |
| MONTH OF FOLLOW UP | -0.0177 | 0.0068 | -2.6100 | 0.0095 | -0.0310 | -0.0044 |
| BASELINE NEWMG | -0.0751 | 0.1750 | -0.4300 | 0.6683 | -0.4196 | 0.2695 |
| MONTH OF FOLLOW UP X BASELINE NEWMG | -0.0137 | 0.0063 | -2.1700 | 0.0311 | -0.0261 | -0.0013 |
| BASELINE MINI MENTAL | 0.8745 | 0.0487 | 17.9600 | <.0001 | 0.7787 | 0.9703 |
| FEMALE | 0.4839 | 0.2051 | 2.3600 | 0.0190 | 0.0802 | 0.8877 |
| YEARS OF EDUCATION | 0.0730 | 0.0255 | 2.8600 | 0.0045 | 0.0228 | 0.1232 |
| AGE | -0.0420 | 0.0168 | -2.5100 | 0.0127 | -0.0751 | -0.0090 |
| MONTH OF FOLLOW UP | -0.0181 | 0.0070 | -2.6000 | 0.0098 | -0.0319 | -0.0044 |
| BASELINE NEWMG | -0.0920 | 0.1762 | -0.5200 | 0.6022 | -0.4389 | 0.2550 |
| MONTH OF FOLLOW UP X BASELINE NEWMG | -0.0142 | 0.0064 | -2.2000 | 0.0285 | -0.0268 | -0.0015 |
| BASELINE MINI MENTAL | 0.8615 | 0.0497 | 17.3200 | <.0001 | 0.7636 | 0.9594 |
| FEMALE | 0.5699 | 0.2124 | 2.6800 | 0.0077 | 0.1517 | 0.9880 |
| YEARS OF EDUCATION | 0.0652 | 0.0260 | 2.5100 | 0.0128 | 0.0140 | 0.1163 |
| AGE | -0.0584 | 0.0172 | -3.3900 | 0.0008 | -0.0922 | -0.0245 |
| DIABETES | -0.1801 | 0.2131 | -0.8400 | 0.3989 | -0.5997 | 0.2395 |
| APOE4CH | -0.3898 | 0.1965 | -1.9800 | 0.0484 | -0.7767 | -0.0028 |
| BMI | -0.0135 | 0.0201 | -0.6700 | 0.5017 | -0.0532 | 0.0261 |
| INTERCEPT | 7.8554 | 2.4871 | 3.1600 | 0.0018 | 2.9560 | 12.7548 |
| MONTH OF FOLLOW UP | -0.0191 | 0.0070 | -2.7100 | 0.0072 | -0.0330 | -0.0052 |
| BASELINE NEWMG | -0.0522 | 0.1784 | -0.2900 | 0.7702 | -0.4036 | 0.2993 |
| MONTH OF FOLLOW UP X BASELINE NEWMG | -0.0135 | 0.0064 | -2.1100 | 0.0363 | -0.0262 | -0.0009 |
| BASELINE MINI MENTAL | 0.8453 | 0.0525 | 16.1100 | <.0001 | 0.7419 | 0.9487 |
| FEMALE | 0.6674 | 0.2203 | 3.0300 | 0.0027 | 0.2335 | 1.1014 |
| YEARS OF EDUCATION | 0.0685 | 0.0267 | 2.5600 | 0.0110 | 0.0158 | 0.1212 |
| AGE | -0.0450 | 0.0202 | -2.2300 | 0.0270 | -0.0848 | -0.0052 |
| DIABETES | -0.1608 | 0.2252 | -0.7100 | 0.4760 | -0.6044 | 0.2829 |
| APOE4CH | -0.2750 | 0.2053 | -1.3400 | 0.1817 | -0.6794 | 0.1294 |
| BMI | -0.0368 | 0.0228 | -1.6200 | 0.1075 | -0.0817 | 0.0081 |
| KIDNEY FUNCTION | 0.0106 | 0.0054 | 1.9600 | 0.0515 | -0.0001 | 0.0213 |
| INTERCEPT | 9.0411 | 2.8383 | 3.1900 | 0.0017 | 3.4445 | 14.6377 |
| MONTH OF FOLLOW UP | -0.0148 | 0.0074 | -2.0000 | 0.0464 | -0.0294 | -0.0002 |
| BASELINE NEWMG | 0.0649 | 0.1870 | 0.3500 | 0.7289 | -0.3038 | 0.4335 |
| MONTH OF FOLLOW UP X BASELINE NEWMG | -0.0170 | 0.0067 | -2.5300 | 0.0123 | -0.0303 | -0.0037 |
| BASELINE MINI MENTAL | 0.7946 | 0.0565 | 14.0700 | <0.0001 | 0.6832 | 0.9059 |
| FEMALE | 0.7769 | 0.2450 | 3.1700 | 0.0018 | 0.2939 | 1.2599 |
| YEARS OF EDUCATION | 0.0778 | 0.0301 | 2.5900 | 0.0103 | 0.0185 | 0.1371 |
| AGE | -0.0607 | 0.0225 | -2.7000 | 0.0075 | -0.1049 | -0.0164 |
| DIABETES | -0.3545 | 0.3300 | -1.0700 | 0.2840 | -1.0051 | 0.2962 |
| APOE4CH | -0.2447 | 0.2158 | -1.1300 | 0.2581 | -0.6702 | 0.1807 |
| KIDNEY FUNCTION | 0.0058 | 0.0054 | 1.0800 | 0.2829 | -0.0049 | 0.0165 |
| A1C | 0.0767 | 0.1543 | 0.5000 | 0.6196 | -0.2275 | 0.3809 |
| GLUCOSE | -0.0022 | 0.0034 | -0.6500 | 0.5166 | -0.0090 | 0.0045 |
| HDL | -0.0174 | 0.0077 | -2.2500 | 0.0257 | -0.0326 | -0.0021 |
| LDL | -0.0119 | 0.0072 | -1.6500 | 0.0996 | -0.0261 | 0.0023 |
| TOTAL CHOLESTEROL | 0.0136 | 0.0065 | 2.0900 | 0.0376 | 0.0008 | 0.0265 |
| EVER SMOKED | 0.1039 | 0.1822 | 0.5700 | 0.5690 | -0.2553 | 0.4631 |
Figure 1. Predicted MMSE scores over time at mean sMG level.
Adjusted for baseline MMSE, sex, age, education, diabetes, APOE4, genotype, BMI, kidney function, HbA1c, glucose, LDL, HDL, total cholesterol, and smoking. The points on the plot represent the observed MMSE scores and the varying sizes of the points reflect the number of patients with that observed score.
Discussion
In this longitudinal study of initially non-demented community dwelling very elderly, higher sMG levels were associated with a faster rate of cognitive decline as measured by the MMSE, after adjusting for several sociodemographic and clinical characteristics. In addition, this relationship did not differ by sex, APOE4 genotype, BMI, or diabetes status suggesting its generality. MG derivatives are common reactive AGE intermediates found elevated in older subjects, and together with terminal AGEs, such as carboxy-methyllysine (CML) correlate with increased levels of oxidative stress(17) and markers of inflammation(10), processes which are inherently associated with AD neuropathology(2, 23, 24). Elevated AGEs are also associated with diabetes(1) and vascular disease(25), conditions also associated with AD(3, 26). Thus, elevated sMG may provide an indicator of brain injury preceding clinical dementia in older persons.
Several potential mechanisms can explain the neurotoxicity of MG and its reactive derivatives. MG impairs glucose metabolism and leads to energy depletion in neuronal cells(27). Mutations in the glycolytic enzyme Tpi lead to accumulation of MG, increased production of AGEs, and subsequent dysfunction and death of neurons(27). MG alters intracellular Aβ/carboxy-terminal fragment aggregation and is cytotoxic(14). Several counteracting processes normally ameliorate the deleterious effects of MG: most cells possess glyoxalases and other aldehyde-scavenging enzymes(28) that convert MG into less harmful molecules. Up-regulation of glyoxalase-1 activity can lower cell-associated MG, possibly suppressing AD neuropathology(28).
AGE concentrations in CSF of AD patients were approximately double that of controls in two studies(5, 6). MG is a highly reactive substance(13), promoting glycation and cross-linking of proteins, and eventually damage to lipids and DNA(29). Therefore, it has been proposed that glycation of CSF proteins early in the course of cognitive decline may stimulate the formation and deposition of AGEs, raising oxidative stress and inflammatory response in the brain(5), and leading to increased production of neuritic plaques and neurofibrillary tangles. Since subjects were cognitively normal at the beginning of the study, our results suggest that the deleterious effects of elevated circulating MG may take effect even before overt cognitive compromise.
Since AGEs are increased in diabetes, the accumulation of AGEs in the brain has been proposed as a possible mechanism linking diabetes to cognitive impairment. In this study, sMG levels were nominally, but not statistically significantly higher in diabetic subjects (0.96 nmol/ml±0.58) compared to non-diabetic subjects (0.91nmol/ml±0.57; p=0.65). This may reflect a survivor effect with reduced mortality for diabetic subjects with relatively low sMG levels(30). Additionally, the lack of a significant interaction between sMG and diabetes in this study suggests that the effect of sMG on cognitive compromise is similar in diabetic and non-diabetic individuals. Alternatively, it is possible that anti-diabetic medications alter the association between sMG and diabetes or counteract the effect of sMG on cognitive compromise(31); indeed all diabetic subjects in this study were medicated.
It has been demonstrated that in addition to endogenous mechanisms that reduce MG concentrations, dietary restriction of caloric intake or of AGE intake without altering calories, reduces serum concentrations of MG derivatives and its terminal product CML, together with markers of oxidative stress and inflammation (10). The independent association of dietary AGE content with circulating AGE levels and the significant decrease of serum MG derivatives following dietary AGE restriction even in healthy older subjects(25) suggests that western diets, which are AGE-rich may potentially play a role in the development of AD. This may be clinically relevant since the dietary AGE load can be easily and safely reduced by using food processing methods that limit high or prolonged heat application and preserve food moisture(10, 17).
This study was limited by the use of a convenience sample that was not epidemiologically representative because it consisted of highly educated very elderly subjects. Since the very elderly are the fastest growing segment of the population(32) and have the highest risk for cognitive compromise(33), they are worthy of particular attention. Additionally, the relationships between age, sex, and education, with cognition in this sample are consistent with those of younger (34) and similarly aged samples(33). Furthermore, the lack of association of diabetes and the APOE4 genotype with cognition is consistent with that of other samples of very elderly participants(35, 36). Although the MMSE was sensitive enough to detect cognitive changes associated with sMG levels, it is a global cognitive measure and cannot assess changes in specific cognitive domains for which a more in depth neuropsychological battery would be required. Finally, since sMG has pro-inflammatory and pro-oxidative stress effects(37, 38), such markers, although not assessed in the current study, may modulate the relationship between sMG and cognition. Among the strengths of the study were the longitudinal assessment of cognitive changes by an average of four annual assessments, and the availability of several important potential confounders.
Highlights.
Higher sMG levels at baseline were associated with faster rate of cognitive decline. Results were unchanged when controlling for sociodemographic/clinical confounders. Lack of significant interactions of sMG with confounders suggests results’ generality.
Acknowledgments
Study funding: supported by NIA grants K01 AG023515-01 and R01 AG034087 to Dr. Beeri, Project 4 in P01 AG02219 to Dr. Silverman, P50 AG05138 to Dr. Mary Sano, and R37AG023188 (MERIT) to Dr. Vlassara, as well as by the Irma T. Hirschl Award to Dr. Beeri and the Berkman Trust to Dr. Vahram Haroutunian.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors and Contributions: Drs. Beeri, Silverman, Vlassara, Sano, Uribarri, and Schmeidler designed the study; Mrs. Moshier and Drs. Schmeidler and Godbold analyzed the data; Drs. Cai and Grossman vouch for the data and its analyses; Dr. Beeri wrote the report.
Conflicts of Interest: The authors report no conflict of interest.
Reference List
- 1.Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994 Feb;70(2):138–151. [PubMed] [Google Scholar]
- 2.Munch G, et al. Alzheimer's disease--synergistic effects of glucose deficit, oxidative stress and advanced glycation endproducts. J Neural Transm. 1998;105(4-5):439–461. doi: 10.1007/s007020050069. [DOI] [PubMed] [Google Scholar]
- 3.Schnaider BM, et al. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004 Nov 23;63(10):1902–1907. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- 4.Meli M, et al. Serum pentosidine as an indicator of Alzheimer's disease. J Alzheimers Dis. 2002 Apr;4(2):93–96. doi: 10.3233/jad-2002-4203. [DOI] [PubMed] [Google Scholar]
- 5.Shuvaev VV, et al. Increased protein glycation in cerebrospinal fluid of Alzheimer's disease. Neurobiol Aging. 2001 May;22(3):397–402. doi: 10.1016/s0197-4580(00)00253-0. [DOI] [PubMed] [Google Scholar]
- 6.Bar KJ, et al. Pentosidine and N(epsilon)-(carboxymethyl)-lysine in Alzheimer's disease and vascular dementia. Neurobiol Aging. 2003 Mar;24(2):333–338. doi: 10.1016/s0197-4580(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 7.Yan SD, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996 Aug 22;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 8.Vitek MP, et al. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao D, et al. Methylglyoxal modification of mSin3A links glycolysis to angiopoietin-2 transcription. Cell. 2006 Jan 27;124(2):275–286. doi: 10.1016/j.cell.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 10.Vlassara H, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhla B, et al. Methylglyoxal, glyoxal, and their detoxification in Alzheimer's disease. Ann N Y Acad Sci. 2005 Jun;1043:211–216. doi: 10.1196/annals.1333.026. [DOI] [PubMed] [Google Scholar]
- 12.Webster J, et al. The carbonyl scavengers aminoguanidine and tenilsetam protect against the neurotoxic effects of methylglyoxal. Neurotox Res. 2005;7(1-2):95–101. doi: 10.1007/BF03033780. [DOI] [PubMed] [Google Scholar]
- 13.Ramasamy R, Yan SF, Schmidt AM. Methylglyoxal comes of AGE. Cell. 2006 Jan 27;124(2):258–260. doi: 10.1016/j.cell.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Woltjer RL, et al. Advanced glycation endproduct precursor alters intracellular amyloid-beta/A beta PP carboxy-terminal fragment aggregation and cytotoxicity. J Alzheimers Dis. 2003 Dec;5(6):467–476. doi: 10.3233/jad-2003-5607. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed N, et al. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer's disease and link to cognitive impairment. J Neurochem. 2005 Jan;92(2):255–263. doi: 10.1111/j.1471-4159.2004.02864.x. [DOI] [PubMed] [Google Scholar]
- 16.Beeri MS, et al. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology. 2006 Sep 26;67(6):1006–1010. doi: 10.1212/01.wnl.0000237548.15734.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai W, et al. Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med. 2002 Jul;8(7):337–346. [PMC free article] [PubMed] [Google Scholar]
- 18.Ossendorf M, Prellwitz W. Rapid and easy apolipoprotein E genotyping using an improved PCR-RFLP technique. Qiagen. 2000 January;11:11–13. [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972 Jun;18(6):499–502. [PubMed] [Google Scholar]
- 20.Goldstein DE, et al. Tests of glycemia in diabetes. Diabetes Care. 2003 Jan;26(Suppl 1):S106–S108. doi: 10.2337/diacare.26.2007.s106. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep. 2004 Jul;6(4):261–266. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- 23.Dickson DW, et al. Glycation and microglial reaction in lesions of Alzheimer's disease. Neurobiol Aging. 1996 Sep;17(5):733–743. doi: 10.1016/0197-4580(96)00116-9. [DOI] [PubMed] [Google Scholar]
- 24.Weisman D, Hakimian E, Ho GJ. Interleukins, inflammation, and mechanisms of Alzheimer's disease. Vitam Horm. 2006;74:505–530. doi: 10.1016/S0083-6729(06)74020-1. [DOI] [PubMed] [Google Scholar]
- 25.Cerami A, Vlassara H, Brownlee M. Protein glycosylation and the pathogenesis of atherosclerosis. Metabolism. 1985 Dec;34(12 Suppl 1):37–42. doi: 10.1016/s0026-0495(85)80008-1. [DOI] [PubMed] [Google Scholar]
- 26.Beeri MS, et al. Coronary artery disease is associated with Alzheimer disease neuropathology in APOE4 carriers. Neurology. 2006 May 9;66(9):1399–1404. doi: 10.1212/01.wnl.0000210447.19748.0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Arriba SG, et al. Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells--protection by carbonyl scavengers. Neurobiol Aging. 2007 Jul;28(7):1044–1050. doi: 10.1016/j.neurobiolaging.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Thornalley PJ. Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003 Dec;31(Pt 6):1343–1348. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- 29.Maeta K, et al. Diagnosis of cell death induced by methylglyoxal, a metabolite derived from glycolysis, in Saccharomyces cerevisiae. FEMS Microbiol Lett. 2005 Feb 1;243(1):87–92. doi: 10.1016/j.femsle.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 30.Kilhovd BK, et al. Increased serum levels of advanced glycation endproducts predict total, cardiovascular and coronary mortality in women with type 2 diabetes: a population-based 18 year follow-up study. Diabetologia. 2007 Jul;50(7):1409–1417. doi: 10.1007/s00125-007-0687-z. [DOI] [PubMed] [Google Scholar]
- 31.Beeri MS, et al. Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. Neurology. 2008 Sep 2;71(10):750–757. doi: 10.1212/01.wnl.0000324925.95210.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinsella K, Velkoff VA. Life expectancy and changing mortality. Aging Clin Exp Res. 2002 Oct;14(5):322–332. doi: 10.1007/BF03324458. [DOI] [PubMed] [Google Scholar]
- 33.Corrada MM, et al. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010 Jan;67(1):114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall CB, et al. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007 Oct 23;69(17):1657–1664. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- 35.van den BE, et al. The impact of diabetes mellitus on cognitive decline in the oldest of the old: a prospective population-based study. Diabetologia. 2006 Sep;49(9):2015–2023. doi: 10.1007/s00125-006-0333-1. [DOI] [PubMed] [Google Scholar]
- 36.Carrion-Baralt JR, et al. Impact of APOE epsilon4 on the cognitive performance of a sample of non-demented Puerto Rican nonagenarians. J Alzheimers Dis. 2009;18(3):533–540. doi: 10.3233/JAD-2009-1160. [DOI] [PubMed] [Google Scholar]
- 37.Brouwers O, et al. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010 May;53(5):989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nobecourt E, et al. Nonenzymatic glycation impairs the antiinflammatory properties of apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 2010 Apr;30(4):766–772. doi: 10.1161/ATVBAHA.109.201715. [DOI] [PMC free article] [PubMed] [Google Scholar]