Abstract
Tumors are thought to be sustained by a reservoir of self-renewing cells, termed tumor initiating cells or cancer stem cells. Osteosarcomas are high-grade sarcomas derived from osteoblast progenitor cells and are the most common pediatric bone malignancy. In this report we show that the stem cell transcription factor Sox2 is highly expressed in human and murine osteosarcoma cell lines as well as in tumor samples. Osteosarcoma cells have increased ability to grow in suspension as osteospheres, that are greatly enriched in expression of Sox2 and the stem cell marker, Sca-1. Depletion of Sox2 by shRNAs in independent murine osteosarcoma-derived cells drastically reduces their transformed properties in vitro and their ability to form tumors. Sox2-depleted osteosarcoma cells can no longer form osteospheres, and differentiate into mature osteoblasts. Concomitantly, they exhibit decreased Sca-1 expression and upregulation of the Wnt signaling pathway. Thus, despite other mutations, these tumor cells maintain a proliferative requirement for Sox2. Our data indicate that Sox2 is required for osteosarcoma cell self-renewal, and that Sox2 antagonizes the pro-differentiation Wnt pathway, that can in turn reduce Sox2 expression. These studies define Sox2 as a survival factor and a novel biomarker of self-renewal in osteosarcomas, and support a tumor suppressive role for the Wnt pathway in tumors of mesenchymal origin. Our findings could provide the basis for novel therapeutic strategies based on inhibiting Sox2 or enhancing Wnt signaling for the treatment of osteosarcomas.
Keywords: Sox2, osteosarcoma, Wnt signaling, cancer stem cell, tumor initiating cell, mesenchymal tumors, differentiation, sarcosphere
Introduction
The hypothesis that tumor maintenance depends on a subpopulation of cells that self-renew, and that are often referred to as tumor initiating or cancer stem cells (CSCs), has recently received considerable attention (Clarke et al 2006, Kim and Dirks 2008, Shackleton et al 2009). Self-renewing, stem cell-like, cells were first identified in acute myeloid leukemia, and have more lately been observed in diverse solid tumors (Buzzeo et al 2007, Clarke et al 2006, Kim and Dirks 2008). CSCs have the capacity of symmetric as well as of asymmetric division, and appear more drug-resistant than bulk tumor (Fujii et al 2009, Shackleton et al 2009).
Osteosarcomas, the most common primary, non-hematologic malignant tumors in childhood and adolescence, comprise almost 60% of the common histologic subtypes of bone sarcomas (Cormier and Pollock 2004, Heare et al 2009). Since the advent of chemotherapy, the long-term cure rate for non-metastatic osteosarcomas following amputation has risen from 25% to 60-70%. However, the survival rate for metastatic osteosarcoma has not improved over the last 30 years, and ∼ 40% of osteosarcoma patients succumb to the disease (Heare et al 2009, Levings et al 2009, Tabone et al 1994). A better understanding of how these tumors originate and progress is needed for the development of targeted therapies for both primary and metastatic osteosarcomas.
Molecular genetic analysis of sporadic and hereditary osteosarcomas in humans demonstrated that inactivation of the tumor suppressors Rb and p53 plays a role in their development. Osteosarcomas are the second most frequent tumor in patients with hereditary retinoblastoma, and individuals with germ line mutation in the RB-1 gene have a 500- fold increased risk of osteosarcoma as compared to the general population (Helman and Meltzer 2003, Wadayama et al 1994). Osteosarcomas contain highly proliferative malignant cells that are largely arrested in their differentiation (Tang et al 2008, Thomas et al 2004). Although osteosarcomas derive from the osteoblastic lineage, the nature of the cell of origin is unclear. However, murine osteosarcomas originate at high frequency in mice following conditional knock-out (KO) of the Rb-1 and p53 genes in the osteoblastic lineage (Berman et al 2008, Walkley et al 2008). These osteosarcoma cells express several genes characteristic of stem cells, such as the hematopoetic and mesenchymal stem cell antigen, Sca-1 (Morikawa et al 2009, Walkley et al 2008) The presence of osteosarcoma stem-like cells has been reported in patient tumors as well as in established human osteosarcoma cell lines (Gibbs et al 2005, Levings et al 2009). While these studies have alluded to the existence of osteosarcoma stem cells, they have not identified a direct mechanism by which these cells originate and how these stem cells can be targeted for tumor abrogation. Activation of several signaling pathways such as fibroblast growth factor (FGF) and Wnt signaling have been implicated in the etiology of osteosarcomas and could contribute to the maintenance of an osteosarcoma stem cells niche.
We and others have previously shown that FGF stimulates proliferation and blocks differentiation of immature osteoblasts (Mansukhani et al 2000, Mansukhani et al 2005, Marie 2003). Furthermore FGF signaling antagonizes the Wnt pathway, that promotes differentiation (Ambrosetti et al 2008). In osteoblasts, FGF induces the transcription factor, Sox2, and similar to FGF treatment, constitutive Sox2 expression can by itself prevent differentiation (Mansukhani et al 2005). Sox2 is a transcription factor of the HMG domain family that plays a critical role in embryonic development and in maintaining pluripotency and self-renewal in embryonic stem (ES) cells (Avilion et al 2003, Lefebvre et al 2007, Niwa 2007, Yuan et al 1995) and several cell lineages such as neural (Pevny and Nicolis 2010) and tracheal cells (Que et al 2009). We have recently demonstrated that Sox2 is necessary for the self-renewal of the osteoblast lineage. Conditional, bone-specific Sox2 knock-out leads to an osteopenic phenotype in mice, and inactivation of Sox2 in cell culture abolishes the proliferative capacity of normal osteoblasts (Basu-Roy et al 2010). In addition, “osteospheres”, osteoblasts growing as spherical clusters in suspension and believed to represent a self-renewing, multipotent, stem cell-like population (Gutierrez et al 2008), exhibit high Sox2 expression, consistent with Sox2 serving as a marker of a subpopulation of self-renewing osteoprogenitors (Basu-Roy et al 2010).
In the present study, we have investigated the role of Sox2 in osteosarcoma. Our data show that Sox2 is highly expressed in human and murine osteosarcoma cells lines as compared to normal osteoblasts. Knock-down of Sox2 by shRNA in murine osteosarcoma cell lines decreases colony formation. Cells stably expressing Sox2 shRNA show reduced Sox2 expression, invasion and migration, as well as tumor formation in immunocompromised mice. Furthermore, downregulation of Sox2 expression results in increased activity of the Wnt signaling pathway. Following Sox2 depletion, osteosarcoma cells display accelerated differentiation into mature bone-forming cells and decreased Sca-1 expression. Based on these findings, we propose that Sox2 marks a population of tumor initiating, cancer stem cells and that its function is required for their self-renewal and tumorigenicity.
Results
Sox2 is highly expressed in human and mouse osteosarcoma cell lines
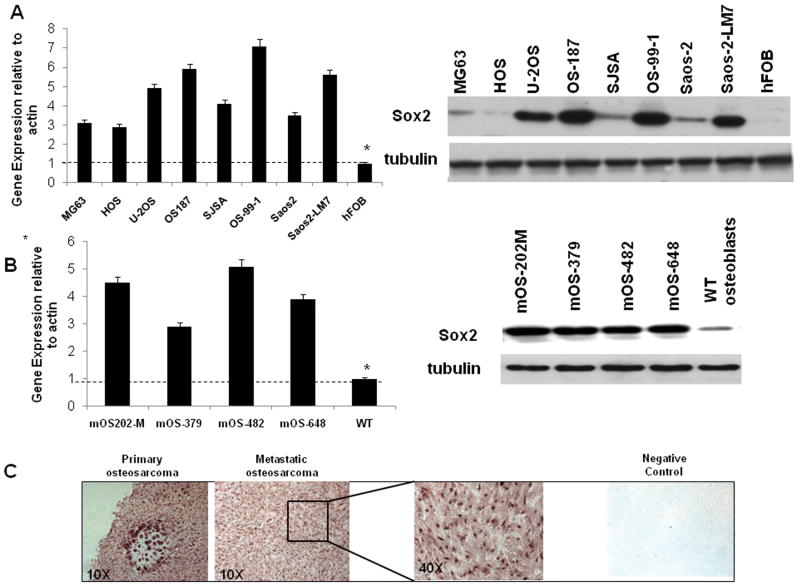
Previously we reported that Sox2 is expressed in U-2OS osteosarcoma cells (Mansukhani et al 2005). We have now examined Sox2 expression in seven independent human osteosarcoma cell lines and have found that Sox2 mRNA and protein are over-expressed in all cell lines as compared to primary human osteoblasts (Figure 1A). We also tested Sox2 expression by immunohistochemistry in a panel of human osteosarcomas and found that all of them (18/18) expressed Sox2 at variably high levels (Fig. 1C). We then examined osteosarcoma cell lines established from p53-/-Rb-/- conditional (bone-specific) knockout mice (Berman et al 2008, Walkley et al 2008). Such tumors arising in the conditional mice mimic the human malignancy with respect to gene expression and metastasis (Walkley et al 2008). As shown in Figure 1B, Sox2 is highly expressed in four independent murine osteosarcoma-derived cell lines (mOS), as compared to wild type (WT) primary osteoblasts both at the mRNA and protein levels. Since the murine osteosarcoma cell lines display the high levels of Sox2 expression observed in human cell lines and their origin and history are well controlled, we have used these cells to carry out the subsequent experiments reported here.
Figure 1. Sox2 is highly expressed in human and murine osteosarcomas.
Sox2 mRNA (left) and protein expression (right) in human (A) and murine (B) osteosarcoma cell lines. Quantitative RT-PCR for mRNA levels and Western blot of Sox2 protein in the indicated cell lines. hFOB, Normal Fetal Human Osteoblast; WT osteoblasts, wild type murine primary calvarial osteoblasts.
(C) Sox2 expression in human osteosarcoma tissue. Immunohistochemistry on paraffin-embedded human osteosarcoma tissue samples using a polyclonal antibody against Sox2 (Magnification 10×). Representative images of osteosarcoma samples are shown. Higher magnification (40×) at which nuclear Sox2 staining is evident is shown. Secondary antibody alone was used as a negative control. We examined a total of 18 samples. Tumors were identified as Sox2-positive when nuclear staining was present in more than 50% cells per field. All samples were scored positive by these criteria.
Sox2 expression is induced by FGF signaling in preosteoblasts (Mansukhani et al 2005). To determine whether FGF signaling might account for the high Sox2 expression in osteosarcomas, we used the pan-FGFR inhibitor PD173074 to block FGF signaling in these cells (Beenken and Mohammadi 2009). Increasing doses of PD173074 block FGF signaling, as measured by a decrease in phosphorylated FRS2 (a downstream adaptor protein highly specific to FGF signaling) and Erk1 and 2. Sox2 expression decreased in a dose-dependent manner 48 hours after treatment with 10μM PD173074 (Fig. S1) and cell proliferation was retarded. These findings indicate that the high Sox2 expression in osteosarcoma cells may be dependent on FGF signaling, and suggested that reducing Sox2 expression affects osteosarcoma cell proliferation.
Sox2 is required for osteosarcoma cell survival, in vitro transformed properties and tumor formation
To determine the role of Sox2 in osteosarcoma cells, we knocked down Sox2 expression using shRNA against Sox2 in the four murine osteosarcoma lines whose genotypes are indicated in Fig.2B. Cells were infected with pBABE-hygro retrovirus expressing the two Sox2-specific shRNAs or a scrambled shRNA control, and selected for hygromycin resistance.
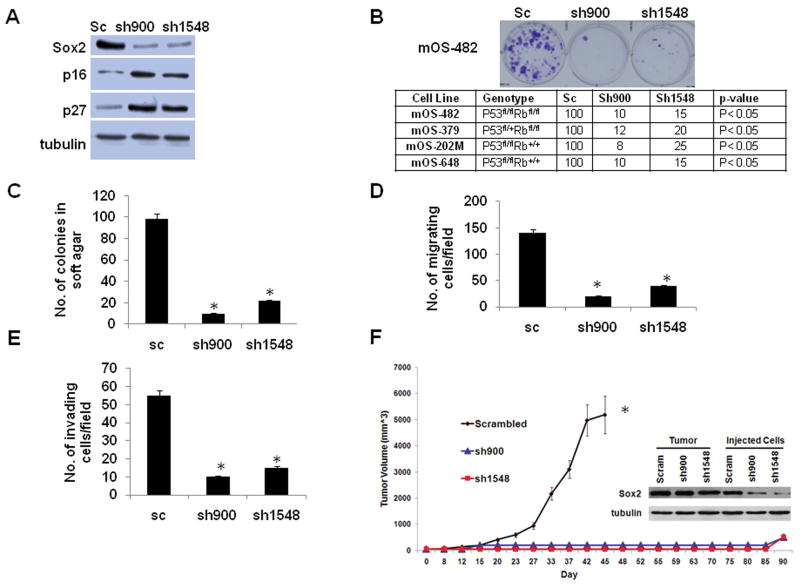
Figure 2. Sox2 depletion decreases transformation properties and tumorigenesis.
(A) Sox2 protein expression in osteosarcoma cells stably expressing Sox2 shRNA. Western blot of mOS-482 osteosarcoma cells expressing scrambled (sc) shRNA or shRNAs against Sox2 were analyzed by immunoblotting. Tubulin was used as a loading control.
(B) Colony formation. Osteosarcoma cells infected with either scrambled (sc) or Sox2 shRNA viruses were selected in Hygromycin B for 7-10 days. Colonies were counted following staining with Crystal Violet. Representative plates of mOS-482 cells are shown. Table shows genotypes and colony counts from four independent cell lines.
(C) Soft Agar Colony Formation; (D), Migration and (E) Invasion. mOS-482 cells expressing sc or Sox2 shRNA were analyzed in the indicated assays as described in Materials and Methods. All experiments were performed in at least two independent cell lines. Results shown are an average of triplicate plates. * = p<0.05
(F) Tumorigenicity assay: Tumor-forming ability of osteosarcoma cell lines (mOS-482) expressing either scrambled or Sox2 shRNAs, was measured in NOD/SCID mice. Injection of 1 × 106 cells gave similar results (not shown). Tumor volume was monitored and measured bi-weekly. Difference between groups was measured by ANOVA. * = p<0.05
The inset shows a western blot of Sox2 protein expression in the injected cells and isolated tumors from either scrambled or long latency tumors arising from cells expressing Sox2 shRNAs.
As shown in Figure 2A, two different shRNAs against Sox2 substantially reduce Sox2 expression. Osteosarcoma cells infected with high titer retroviruses expressing the Sox2-specific shRNAs showed a drastically reduced (80-90%) ability to form colonies in the presence of hygromycin (Fig. 2B). However hygromycin resistant, Sox2-shRNA stably expressing cells could be easily isolated. These cells had low levels of Sox2 protein and were compared with cells expressing scrambled shRNA for ability to proliferate as well as for in vitro properties of transformation, including growth in soft agar and ability to migrate and invade (Fig. 2C-E). These results were reproduced an at least two other murine osteosarcoma cell lines. Sox2 depletion slightly decreased proliferation rate (Fig.S2A-B) with concomitant upregulation of the CDK inhibitors p16 and p27, but these cells proliferate well without evidence of increased apoptosis or senescence (Fig. S2C, D). On the other hand, the growth of osteosarcoma cells in soft-agar was drastically impaired by Sox2 depletion. Sox2 downregulation also dramatically decreased migration and invasion. (Fig.2C-E). As the modest decrease in proliferation in the Sox2-depleted cells was not sufficient to explain these effects, we examined whether Sox2 depletion alters attachment of cells to substratum. We employed an in vitro adhesion assay (Douglas et al 2008) and determined that cells expressing Sox2 shRNAs do not show altered adherence to substratum (Fig.S3). These results indicate that depletion of Sox2 markedly reduces the in vitro transformed phenotype of osteosarcoma cells. Although the data shown are only for the mOS-482 cells, these results were replicated in the mOS-379 and mOS-202M cell lines.
We tested the ability of the parental osteosarcoma cells, cells expressing scrambled shRNA or Sox2 shRNAs to form tumors in immunocompromised NOD/SCID mice. While parental cells and cells expressing scrambled shRNA readily implanted and formed tumors within two weeks, Sox2 knockdown cells failed to form palpable tumors within 10 weeks. (Fig.2F). Notably, after about 12 weeks 5/16 of the animals injected with the Sox2 knockdown cells developed tumors that grew progressively. When these tumors were excised and examined for Sox2 expression, they all showed high levels of Sox2 protein expression (Fig. 2F). The absence of detectable Rb and p53 protein expression in the tumor lysates confirmed that the tumors were derived from the originally injected p53-/- Rb-/- cells (Figure S4). Thus down-regulation of Sox2 attenuates in vivo tumor formation by osteosarcoma cells, and cells that could form tumors had reacquired high Sox2 expression.
Sox2 marks a population of osteosarcoma stem cells
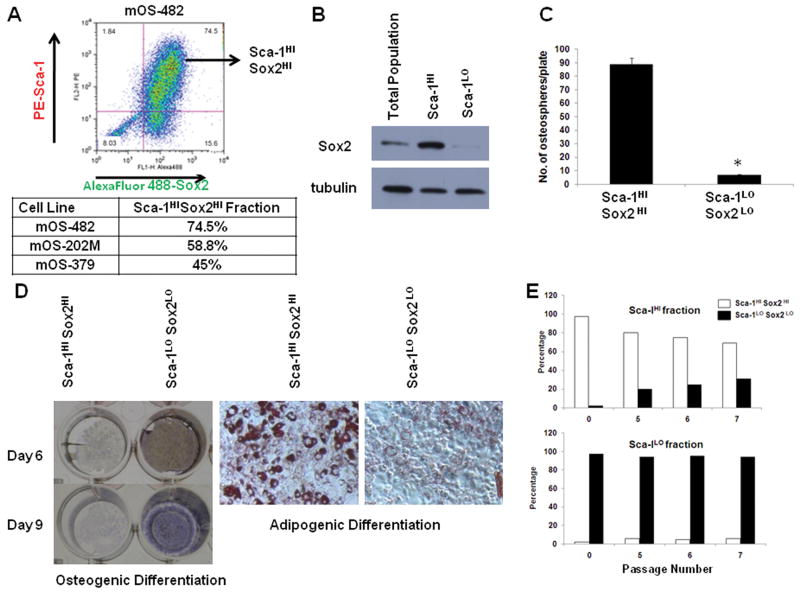
As previously mentioned, osteosarcomas may contain a sub-population of tumor initiating stem cells (Gibbs et al 2005). The murine osteosarcoma cell lines that we have used support this notion as they contain multipotent cells that are capable of differentiating into different lineages such as the adipocytic and osteoblastic lineage (Berman et al 2008) as well as a population of Sca-1 positive cells, that appears to represent the tumor-initiating fraction (Walkley et al 2008). Futhrmore, sphere-forming osteosarcoma cells (also referred to as sarcospheres or osteospsheres) have increased tumorigenic capacity (TICs) (Rainusso et al 2011). We grew osteosarcoma cells in suspension in defined serum-free medium and determined that they are capable of forming osteospheres, spherical colonies forming in non-adherent conditions that are generally considered to represent self-renewing, stem-like cells (Gutierrez et al 2008, Rainusso et al 2011). These osteospheres are enriched for Sox2 and Sca-1, a stem cell antigen of the hematopoietic system. Osteospheres also exhibit low expression of osterix (OSX), a marker of more mature osteoblasts (discussed later). We labeled the cell lines with antibodies to Sca-1 and Sox2 and analyzed the proportion of cells expressing either antigen by flow cytometry. As shown in figure 3A, the majority of the cells co-expressed both Sca-1 and Sox2, consistent with Sca-1 expression marking a population of Sox2 positive cells. Since all cell expressing high levels of Sca-1 antigen were also strongly Sox2 positive, we sorted two of the cell lines into Sca-1HI and Sca-1LO fractions by magnetic separation. The purity of each fraction was determined by flow cytometry for Sca-1 (Fig. S5) and Western analysis for Sox2 (Fig.3B). After verifying that each fraction indeed consisted of Sca-1HI Sox2HI and Sca-1LO Sox2LO cells, these fractions are hereafter referred to as Sca-1HI Sox2HI and Sca-1LO Sox2LO cells. Each live cell fraction was plated to test a) the ability to form osteospheres and b) ability to differentiate into the osteoblastic and adipogenic lineage. The Sca-1HI Sox2HI fraction contained a higher proportion of cells capable of forming osteospheres (Fig.3C). To confirm that fewer Sca-1HI Sox2HI cells can form spheres, we performed a limiting dilution assay for sphere formation. While the Sca-1HI Sox2HI cells could form spheres at a frequency of 1/18, this was decreased to 1/50 in the Sca-1LO Sox2LO cells (Table 1). Additionally, while their osteoblastic differentiation ability was strongly reduced as compared to the Sca-1LO Sox2LO fraction, the Sca-1HI Sox2HI fraction produced significantly more adipocytes than the Sca-1LO Sox2LO fractions (Fig. 3D). This result indicates that Sox2 expression marks a population of Sca-1 positive cells that have a low capacity for osteoblastic differentiation, but have maintained the ability to enter the adipocytic fate.
Figure 3. Sox2 marks a population of Sca-1 positive, multipotent osteosarcoma stem cells.
(A) Flow cytometric analysis of Sox2 and Sca-1 doubly-labeled osteosarcoma cells. Osteosarcoma cells (mOS-482) were labeled to detect membrane Sca-1 (PE- Sca-1, Phycoerythrin-Sca-1 conjugated antibody) and intracellular Sox2 (Sox2 polyclonal antibody followed by Alexa Fluor 488 conjugated secondary antibody). Forward scatter-Side scatter (FSC-SSC) profile shows that 90% cells were viable (Fig. S4) and was used for anaysis. Sca-1HISox2HI population is indicated in top right quadrant. The percentage of Sca-1HISox2HI population from three independent cell lines is shown in the adjoining Table.
(B) Sox2 protein expression in Sca-1HI and Sca-1LO fraction. mOS-482 cells were separated into Sca-1HI and Sca-1LO cells using Sca-1 magnetic bead separation. Western blot of Sox2 expression in the total adherent population and the two fractions is shown.
(C) Osteosphere Assay. Equal numbers of cells from Sca-1HI and Sca-1LO fractions from mOS-482 cells were plated in suspension culture in triplicate. Spheres were counted after 10 days. Each experiment was repeated at least twice. Results from a representative experiment are shown. * = p<0.05
(D) Osteogenic and Adipogenic Differentiation Assay. Sca-1HI and Sca-1LO fractions from mOS-482 cells were plated in either osteogenic or adipogenic medium. Osteogenic differentiation was detected using alkaline phosphatase staining. Results from representative plates at the indicated time period are shown. Adipogenesis was detected by Oil Red O staining of cells induced for 15 days (magnification 100×).
(E) Serial propagation of Sca-1HI and Sca-1LO fractions. Sca-1HI and Sca-1LO fractions from mOS-482 cells were doubly purified by magnetic bead and FACS. Each fraction was plated separately and serially passaged. After labeling for both Sox2 and Sca-1, the proportion of cells that were doubly positive (Sca-1HI Sox2HI or Sca-1LOSox2LO) at each passage was determined by flow cytometry.
Table 1. Limiting dilution analysis of osteosphere-forming frequency of Sca-1HI Sox2HI and Sca-1LO Sox2LO cells from mOS-482.
| Number of cells/well | Number of wells plated | Number of wells with osteospheres | |
|---|---|---|---|
| Sca-1HI Sox2HI | Sca-1LO Sox2LO | ||
| 1000 | 24 | 24 | 24 |
| 100 | 84 | 84 | 78 |
| 10 | 84 | 34 | 5 |
| 1 | 84 | 4 | 0 |
| Sphere-forming frequency (95% CI) | 1/18 (1/14-1/24) | 1/50 (1/40-1/64) | |
| P value | <0.0001 | ||
A property of stem cells is the ability to undergo symmetric as well as asymmetric division, generating either undifferentiated or both undifferentiated and differentiated daughter cells. To confirm that the Sca-1HI Sox2HI fraction represented a stem cell population, we propagated doubly purified populations (Fig.S5) of Sca-1HI and Sca-1LO cells for several passages, and determined the proportion cells with high or low Sca-1 expression as well as high or low Sox2 at each passage. While the Sca-1LO fraction remained stable over 7 passages, the Sca-1HI fraction generated an increasing proportion of Sca-1LO cells, that were also low in Sox2 expression, indicating that the Sca-1HI Sox2HI cells are capable of self-renewal as well of generating Sca-1LO Sox2LO progeny (Fig. 3E). As the Sca-1HI Sox2HI cells grow somewhat faster than the Sca-1LO Sox2LO cells, it is unlikely that the Sca-1LO Sox2LO cells are repopulating the Sca-1HI Sox2HI fraction (Fig. S6E).
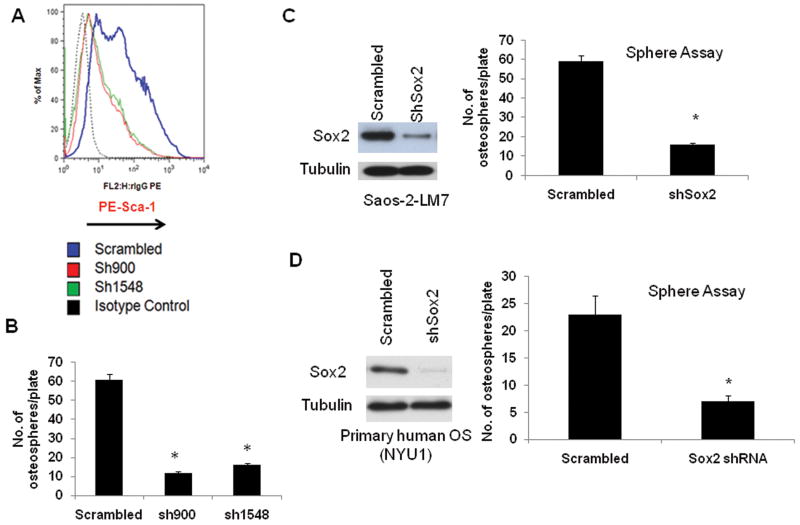
To test whether Sox2 is required to maintain a subpopulation of self-renewing osteosarcoma cells, we determined the ability of the control and Sox2-shRNA expressing cells to form osteospheres. As shown in Figure 4, osteosarcoma cells expressing Sox2 shRNAs have reduced Sca-1 expression and show greatly reduced ability to form osteospheres (Fig. 4B). A similar reduction in sphere-forming ability is also seen in human osteosarcoma cells in which Sox2 has been depleted. Both Saos-2-LM7 cells (an established metastatic osteosarcoma cell line) and OS-NYU1 (primary osteosarcoma-derived cells from a fresh tumor biopsy), infected with a human Sox2shRNA, show decreased Sox2 expression and reduced sphere formation (Fig. 4C and D), confirming that the requirement for Sox2 is not limited to murine osteosarcoma cells. To strengthen the notion that high levels of Sox2 expression correlate with the ability of cells of the osteoblast lineage to form osteospheres, we over-expressed Sox2 in murine primary osteoblasts using lentivirus vectors, and found that Sox2 over-expression is sufficient to enhance osteosphere formation (Fig. S6). Additionally, over-expression of Sox2 was sufficient to reduce osteogenic and increase adipogenic differentiation (Fig. S6). These gain-of-function studies in primary osteoblasts produced the opposite effects seen by the depletion of Sox2.
Figure 4. Sox2 down-regulation decreases the stem cell population in murine and human osteosarcomas.
(A) Surface Sca-1 expression is reduced in Sox 2-depleted cells. mOS-379 cells expressing either scrambled or Sox2 shRNA were stained with a PE-conjugated anti-Sca-1 antibody and analyzed by flow cytometry. Mean fluorescence intensity (MFI) of the indicated cells is plotted. Y axis = % of Max (Percentage of Maximum MFI). X axis = rIgG-PE (rat-PE-conjugated-anti-Sca-1)
(B) Osteosphere Assay. Equal numbers of cells expressing scrambled or Sox2 shRNA were plated in suspension culture in triplicate. Spheres were counted after 10 days. Each experiment was repeated at least twice. Results from a representative experiment are shown. * = p<0.05. Similar results were obtained with the mOS-682 and mOS-202M cell lines.
(C) and (D) Western Analysis ad Osteosphere Assay in human osteosarcoma samples. Saos-2-LM7 (Figure 4C) or primary osteosarcoma cells from fresh biopsy (OS-NYU1) (Figure 4D) cells expressing either scrambled or human Sox2 shRNA were analyzed for Sox2 expression by Western analysis. For osteosphere assay, equal numbers of cells expressing scrambled or human Sox2 shRNA were plated in suspension culture in triplicate. Spheres were counted after 10 days. Each experiment was repeated at least twice. Results from a representative experiment are shown. * = p<0.05.
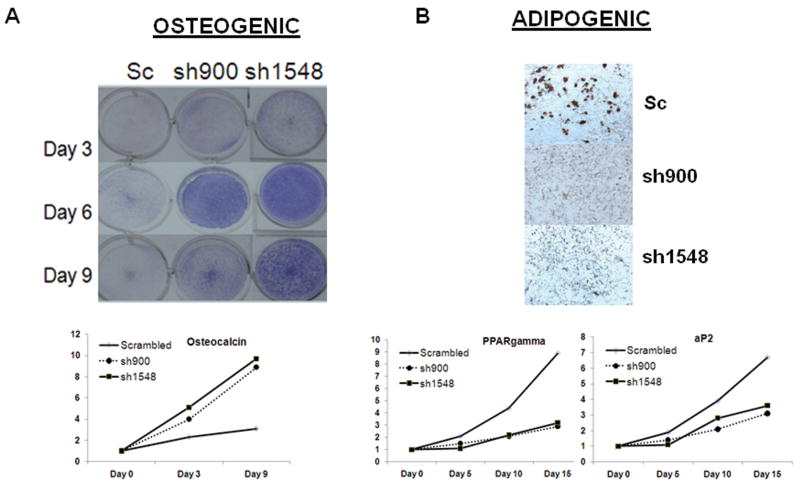
We then determined the ability of the parental and Sox2 knockdown osteosarcoma cells to differentiate into osteoblastic and adipocytic lineages (Fig.5). Parental osteosarcoma cells, or those expressing scrambled shRNAs, are impaired in their ability to differentiate into osteoblasts (Fig. 5A). However, the Sox2 shRNA-expressing cell lines rapidly differentiate into mature osteoblasts, as shown by an increase expression of alkaline phosphatase and osteocalcin (Fig.5A), as well as Runx2 and OSX (not shown). Like the Sca-1LO Sox2LO cells, these Sox2-depleted cells are impaired in their ability to enter the adipocytes lineage, as revealed by the absence of Oil Red O positive granules (Fig. 5B). Adipogenesis can occur in the parental cells, as shown by the presence of Oil Red O stained lipid granules, and expression of the adipocyte-specific genes PPARγ and AP2 (Fig.5B). Together the results presented in this section indicate that high Sox2 expression marks a population of Sca-1 positive stem-like cells and is required for their maintenance. Furthermore Sox2 downregulation results in a lineage shift in the differentiation ability of osteosarcoma cells, such that cells with high Sox2 expression preferentially differentiate into the adipogenic pathway, while Sox2 depleted cells only undergo osteogenic differentiation.
Figure 5. Sox2 down-regulation increases osteoblastic differentiation of murine osteosarcoma cells.
(A) Osteoblastic differentiation. mOS-379 cells expressing scrambled or Sox2 shRNA were plated in osteogenic for the indicated time periods. Differentiation was assessed using alkaline phosphatase staining. Results from representative plates at the indicated time period are shown. For gene expression analysis, real-time quantitative PCR analysis using primers for osteocalcin at the indicated times is shown. All values are normalized to actin and are expressed relative to expression in uninduced cells at Day 0.
(B) Adipogenic differentiation. mOS-379 cells expressing scrambled or Sox2 shRNA were plated in adipogenic medium for 15 days. Differentiation was measured using Oil Red O staining (magnification 100×). For gene expression analysis, real-time quantitative PCR analysis using primers for PPARγ and aP2 at the indicated days. All values are normalized to actin and are expressed relative to uninduced cells at Day 0.
Sox2 depletion in osteosarcoma cells results in activation of Wnt signaling
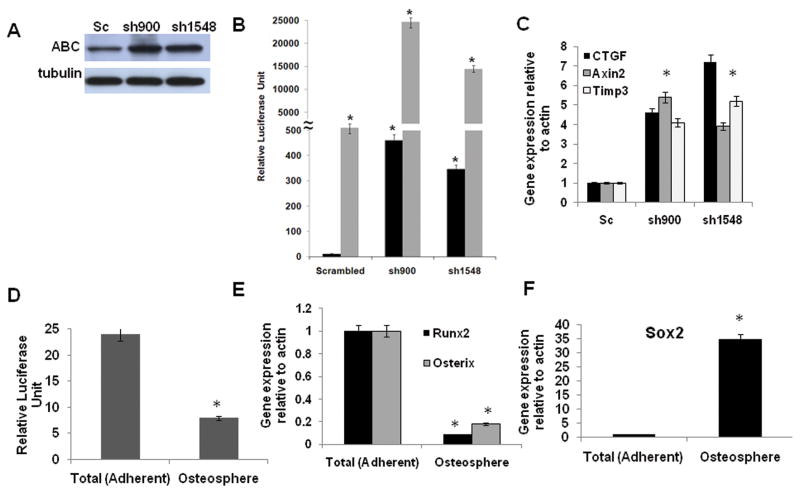
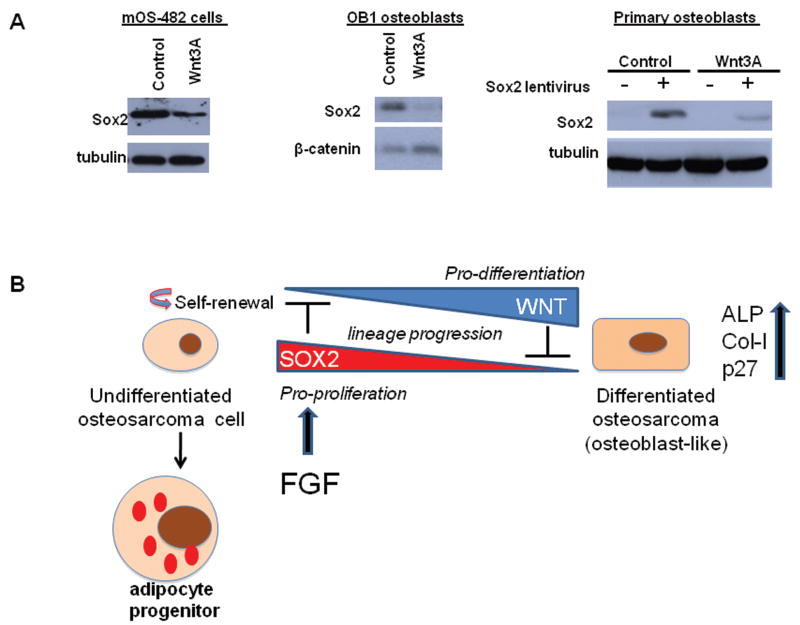
Wnt signaling is a critical pathway for proper bone formation, and stimulates osteoblast lineage commitment and maturation (Krishnan et al 2006, Milat and Ng 2009). Sox2 plays a role in the antagonistic effect of FGF on the Wnt pathway (Ambrosetti et al 2008, Mansukhani et al 2005). Therefore, we analyzed Wnt signaling in control and Sox2 knock down osteosarcoma cells. Activation of Wnt signaling results in stabilization and nuclear translocation of β-catenin, that together with TCF/LEF factors regulates Wnt-target genes (Clevers 2006). We first assessed the levels of active β-catenin (ABC) (ABC - β-catenin that is unphosphorylated at critical serine and threonine residues and hence, resistant to proteasomal degradation) (van Noort et al 2002). As shown in Figure 6A, ABC levels are strongly increased in cells expressing Sox 2-shRNAs, suggesting that Wnt signaling is active in these cells. We then established stable cell lines expressing a Wnt-responsive TOPFLASH reporter (Ambrosetti et al 2008), and determined whether basal Wnt signaling was higher in the cells expressing Sox2-shRNA. We found that the basal levels of TOPFLASH reporter activity were much higher in cells expressing Sox2 shRNAs, as compared to the control cells. In addition, these cells responded more strongly to exogenous Wnt3A stimulation (Fig 6B). Osteoblast-specific Wnt targets, such as Axin2, Timp3 and CTGF (Ambrosetti et al 2008), were also up-regulated in Sox2- shRNA-expressing osteosarcoma cells (Fig. 6C). We established osteospheres from osteosarcoma cells expressing the Wnt reporter. As expected, Sox2 expression was dramatically increased in osteospheres as compared to the total adherent cell population, while the levels of Runx2 and Osterix, markers of more mature osteoblasts, were decreased (Fig. 6E and F). The activity of the Wnt reporter was strongly decreased in the osteospheres (Fig. 6D). Together these results indicate that Wnt signaling is repressed in osteosarcoma cells expressing Sox2 and that Sox2 downregulation results in increased Wnt signaling. While we cannot conclusively prove that the increased Wnt signaling requires inhibition of Sox2 expression or function, we observed that activation of Wnt signaling in osteoblasts or osteosarcoma cells induces slow but very significant downregulation of Sox2 protein expression (Fig.7A). Thus, high Sox2 expression is accompanied by reduced Wnt signaling while activation of the Wnt pathway results in low Sox2 expression, suggesting that activation of Wnt signaling antagonizes the effect of Sox2 in maintaining osteosarcoma cells in an undifferentiated stem-like state. A model depicting the regulation of osteosarcoma cells self-renewal and differentiation by Sox2 and Wnt is shown in Fig. 7B.
Figure 6. Sox2 depletion leads to activation of Wnt signaling in osteosarcoma cells.
(A) Activated β-catenin (ABC) protein expression. Immunoblot of mOS-379 cells expressing either scrambled or Sox2 shRNA with anti-ABC antibody. γ- tubulin was used as a loading control.
(B) Wnt reporter activity. Relative TOPFLASH luciferase reporter activity in mOS-379 cells expressing scrambled or Sox2 shRNA. Basal (black bars) or Wnt 3A stimulated (gray bars) activity at 48 hours was measured and normalized to total protein.* = p<0.05
(C) Wnt target gene expression. Real-time quantitative PCR analysis of CTGF, Axin and Timp3 expression in mOS-379 cells expressing scrambled or Sox2 shRNAs as indicated. All values are normalized to actin and expressed relative to expression in cells expressing scrambled shRNA.
(D) Wnt reporter activity in osteospheres. Relative luciferase activity in total (adherent) cells and osteospheres from mOS-379 cells expressing TOPFLASH luciferase reporter plasmid.
(E) Expression of Osterix and Runx2 in osteospheres. Real-time quantitative PCR analysis of Runx2 and Osterix expression in osteospheres and adherent cells, as in (D).
(F) Expression of Sox2 in osteospheres. Real-time quantitative PCR analysis of Sox2 expression in osteospheres and adherent cells, as in (D).
Figure 7. Activation of Wnt signaling decreases Sox2 expression in osteoblasts and osteosarcoma.
(A) Sox2 Expression in Wnt-treated cells. Western blot of Sox2 in osteosarcoma cells (mOS-482), immortalized osteoblasts (OB1). and primary osteoblasts transduced with empty vector (-) or Sox2 (+) lentivirus, after treatment with control or Wnt 3A conditioned medium for 48 hours. γ- tubulin was used as a loading control.
(B) Model for Sox2-Wnt antagonism in osteosarcoma. Undifferentiated osteosarcoma cells with high Sox2 expression and low Wnt pathway activity are in a stem-like, multipotent, self-renewing state.suggesting that Sox2 and Wnt pathway are mutually antagonistic in osteosarcoma development. Sox2 depletion or activation of Wnt signaling leads to a more differentiated, osteoblast-like state.
Discussion
Osteosarcomas are tumors of mesenchymal origin that are derived from immature osteoblasts. We show here that Sox2 expression is up-regulated in human and murine osteosarcoma cell lines, and in primary human osteosarcomas. Sox2 knock-down markedly reduces in vitro transformed properties, including, migration, invasion and colony formation in soft agar. Interestingly, Sox2-depleted osteosarcoma cells show a dramatic decrease in osteosphere formation and expression of the Sca-1 stem cell antigen, accompanied by enhanced differentiation into mature bone forming osteoblasts with activation of Wnt signaling. Consistent with these findings, cells depleted of Sox2 fail to form tumors in murine xenografts. These data identify a critical role of Sox2 in the maintenance of a population of self-renewing, stem-like osteosarcoma cells.
Osteosarcomas overexpress Sox2
As Sox2 is highly expressed in several osteosarcoma cell lines we reasoned that increased FGF signaling may be responsible, at least in part, for high Sox2 expression in these cells. Indeed, we found that inhibition of FGF signaling decreased Sox2 expression and proliferation. Sox2 has been identified as an oncogene and is amplified in squamous cell carcinoma of the lung and esophagus (Bass et al 2009, Xiang et al 2011). Genomic analysis of osteosarcomas does not indicate amplification of the Sox2 gene and we have not detected increased expression of FGFR1 and 2 genes in those cells. It is possible that the relatively high Sox2 expression in osteosarcomas reflects their higher content of immature, stem-like cells and that FGF signaling is kept active by an autocrine mechanism sustained by an endogenously produced FGF ligand. The high expression of Sox2 in those cells is not due to loss of p53 function, since ectopic expression of wild type p53 does not affect Sox2 expression in our murine osteosarcoma cell lines.
Sox2 regulates osteosarcoma cell tumorigenesis and differentiation
We have shown that Sox2 is an essential gene for osteoblast survival and self-renewal in culture, where it exerts multiple functions, including direct transcriptional regulation and inhibition of osteoblast differentiation (Basu-Roy et al 2010, Mansukhani et al 2005). The results shown here demonstrate that Sox2 is also required for self-renewal and tumorigenicity of osteosarcoma cells. Acute Sox2 downregulation results in considerable loss of colony-forming ability in tumor-derived murine cell lines, but differently from what observed in normal osteoblasts, that enter a non–proliferative senescent-like state following Sox2 inactivation (Basu-Roy et al 2010, Mansukhani et al 2005), osteosarcoma cells with strongly reduced Sox2 expression can be easily isolated and propagated in culture. Murine osteosarcomas contain a population of cells with high Sox2 and Sca-1 expression, as well as cells with low levels of Sox2 and Sca-1. Sox 2 highly expressing osteosarcoma cells are tumorigenic, form osteospheres, express the Sca-1 antigen, are inhibited in osteogenic differentiation, and are capable of symmetric and asymmetric division. In contrast, cells with reduced Sox2 expression, whether spontaneously occurring (Sca-1LO Sox2LO) or created by Sox2 knock-down, have reduced ability to form osteospheres, do not express Sca-1, and readily undergo osteogenic differentiation. Importantly they do not appear to be capable of generating Sca-1HI Sox2HI dedifferentiated cells.
Osteosarcoma cells with reduced Sox2 expression fail to form tumors in xenograft assays. Interestingly, the few tumors that arose with long latency in animals injected with Sox2-depleted cells had reacquired high levels of Sox2. This is likely because of loss of the shRNA plasmid in the absence of positive selection, thus strengthening the notion that Sox2 is required for the tumorigenic phenotype. In addition, Sox2-depleted cells exhibit reduced Sca-1 expression, increased expression of the cell cycle inhibitors p16 and p27, and readily differentiate into mature osteoblasts. Disruption of p27 function has been reported in osteosarcomas (Thomas et al 2004). Although the distinction may be semantic, we do not think that Sox2 is a canonical osteosarcoma oncogene, but rather that its function is essential for self-renewal of osteoblast precursors. Thus its inactivation would lead to the reduction of a subpopulation of stem-like cells or promote a terminally differentiated state that is incompatible with sustained proliferation.
Together, the data presented in this report strongly support the hypothesis that Sox2 marks a population of tumor-initiating osteosarcoma cells with stem cell properties and is necessary for their renewal and tumorigenic ability. In apparent contrast with this notion however, Sox2 highly expressing cells are a sizable portion of our osteosarcoma cell populations, while cancer stem cells are generally thought to represent a minority of the tumor cells. This prevailing notion however mostly derives from studies of hematologic malignancies, but even in this case there are exceptions (Adams et al 2008, Kelly et al 2007, Rosen and Jordan 2009). For solid tumors several reports have identified a large portion (up to 90%) of tumor initiating cells with stem-like properties (Boiko et al 2010, Gangemi et al 2009, Gong et al 2010, Rosen and Jordan 2009, Takenobu et al 2011). Furthermore, in our case, the high proportion of stem-like cells is to a good extent the result of propagation in culture, as shown in the original characterization of the murine osteosarcomas used in the present studies (Walkley et al 2008). This report showed quite clearly that the proportion of Sca-1HI cells in the tumors (that we have shown to correspond to the Sox2HI population) increases substantially with establishment and further propagation in tissue culture. The definition or concept of “cancer stem” cells has been the subject of considerable debate and is still open to different interpretations (Rosen and Jordan 2009). Our work identifies Sox2 as a transcription factor which marks and maintains a distinct cell population in osteosarcomas that has stem cell properties and is responsible for their tumorigenic potential. A recent study reported expression of Sox2 in sphere-forming cells from human sarcomas (Tirino et al 2011).
Although the mechanisms that disrupt osteogenic differentiation are not well understood, our data suggest that Sox2 is a key player in this process. Sox2 downregulation promotes osteoblast differentiation, while restricting entry to the adipogenic pathway. Few determinants of mesenchymal stem cell fate are known. Taz and Rb have been shown to play a role in the balance of bone versus adipocyte lineages (Berman et al 2008, Calo et al 2010, Hong and Yaffe 2006)., Our findings suggest that Sox2 levels may be important for fate choices in the mesenchymal lineage. High Sox2 appears to favor the adipogenic fate, whereas low Sox2 may favor the osteoblastic fate. As discussed later, this hypothesis fits with the antagonism of Sox2 with the Wnt pathway which has pro- osteoblastic and anti-adipogenic properties (Takada et al 2009).
Sox2 antagonizes Wnt signaling in osteosarcomas
An extensive body of evidence indicates that Wnt signaling promotes osteoblast differentiation and function (Krishnan et al 2006), and we had previously shown that Sox2 played a role in the antagonistic effect of FGF signaling in inhibiting Wnt-induced osteoblast differentiation (Ambrosetti et al 2008). The mechanism by which Sox2 inhibits Wnt signaling is complex. While it clearly involves binding of Sox2 to β-catenin, it may also include transcriptional regulation of several Wnt pathway genes. Following Sox2 downregulation in osteosarcoma cells, we found a striking increase in Wnt pathway activity, including increasing amounts of active β-catenin, activation of endogenous Wnt target genes, and increased activity of a Wnt-reporter. Furthermore, inactivating phosphorylation of GSK3β, that would result in increased abundance of active β-catenin, was significantly increased in Sox2 knock-down cells. While these findings confirm our previous identification of Sox2 as an antagonist of Wnt signaling in osteoblasts (Mansukhani et al 2005), they are in contrast with the notion that increased Wnt signaling promotes osteosarcomagenesis. The pro-tumorigenic role of Wnt signaling is well-established in epithelial cancers (Clevers 2006), but its role in tumors of mesenchymal origin is controversial. Although the inactivation of a Wnt inhibitor has been linked to radiation-induced osteosarcoma (Kansara et al 2009), other groups have found that Wnt signaling is down-regulated in osteosarcomas (Cai et al 2010, Cleton-Jansen et al 2009). Wnt signaling could therefore be active in less aggressive tumors, but does not appear to play a pro-tumorigenic role in the osteosarcoma model we studied, where it clearly correlates with a reversal of transformed growth properties.
Sox2 appears to maintain osteosarcoma cells in a self-renewing state, in part through inhibition of the pro-differentiation Wnt pathway. The notion that Wnt signaling plays a tumor suppressive role in these tumors is not limited to osteosarcomas, but has been proposed for other mesenchymal tumors, such as malignant fibrous histocytoma, where establishment of Wnt signaling prevents differentiation (Matushansky et al 2007). Our finding that Sox2 might play a critical role in tumors of mesenchymal origin is also consistent with a similar finding in Ewings' sarcoma, where Sox2 maintains a stem cell-like signature (Riggi et al 2010).
Thus, more generally, Sox2 may be responsible for maintaining self-renewal of a subpopulation of mesenchymal tumor cells. Interestingly, the effect of Sox2 depletion on in vitro tumorigenic self-renewing properties is apparent in cells that lack both Rb and p53, indicating that Sox2 may be an effective target in cancers that lack these tumor suppressors.
The mechanism(s) by which Sox2 maintains self-renewal in osteosarcoma cells are probably complex but likely result mainly from its function as a direct transcriptional regulator. Our preliminary analysis of the gene expression profiles of parental and Sox2 depleted osteosarcoma cells shows that a number of genes are downregulated in the Sox2 depleted cells. The ability of Sox2 to inhibit canonical Wnt signaling, that does not require its DNA binding domain (Ambrosetti et al 2008, Mansukhani et al 2005), may also play an important role. Sox2 could also directly activate the expression of specific inhibitors of the Wnt pathway, such as APC. In addition, Sox2 protein levels are down-regulated by Wnt signaling. This finding may fit with a recent report that Sox2 could be a target of GSK3β in Wnt-dependent protein metabolism (Taelman et al 2010).
In short, our study highlights the role of Sox2 in the maintenance of self-renewing cells in osteosarcoma and identifies the Wnt pathway as a potential anti-tumorigenic pathway in these tumors. The osteosphere forming population in osteosarcomas has been reported recently to be refractory to chemotherapeutic drugs (Fujii et al 2009). This observation may provide an explanation for the poor response of osteosarcomas to chemotherapy. Blocking Sox2 function in osteosarcoma may be considered a basis for novel and effective therapeutic strategies for the treatment of these tumors.
Materials and Methods
Cell culture
The human osteosarcoma cell lines, U-2OS, MG63 and HOS were obtained from the American Type Culture Collection. The OS-187 cells were a gift from Dr. Nancy Gordon from M.D. Anderson Cancer Center, TX The OS-99-1 cells were obtained from Dr. Shiela Nielsen-Preiss, University of Montana, Missoula, MO (Gillette et al 2008). The parental Saos-2 cells and the Saos-2-LM7 cells that are a metastatic variant of Saos-2 were provided by Dr. Eugenie Kleinerman, M. D. Anderson Cancer Center, TX (Jia et al 1999). The murine osteosarcoma cells used in this study (mOS-482, mOS-379, mOS-648 and mOS-202M) were derived from spontaneous osteosarcomas and isolated as previously described (Walkley et al 2008). All cells were maintained in DMEM supplemented with 10% fetal bovine serum. Human osteosarcoma tissue samples were obtained from INGENEX (Super Biochip) and New York University Medical Center Tissue Acquisition Banking Services (TABS). These samples were obtained following the NIH guidelines for research on human specimens. Fresh human osteosarcoma tissue was obtained from TABS. Tumor tissues were minced asceptically after diagnostic biopsy and digested in 2U/ml dispase for 1 hour at 37 °C. Cells were grown in RPMI medium supplemented with 10% fetal bovine serum for all experiments.
Sox2 knockdown and over-expression
pBabe-Hygro-retrovirus- vector containing scrambled or Sox2-specific shRNA (described in (Basu-Roy et al 2010) was used to infect four independent osteosarcoma cell lines. For colony formation assay, murine osteosarcoma cells were plated at a density of 5000cells/well in 6-well plates and infected for 24 hours in the presence of 8 μg/ml polybrene at an MOI of ∼ 50. Following infection, colonies were selected in 200 μg/ml Hygromycin B for 7-10 days and stained with Crystal Violet. For selection of cells expressing Sox2 shRNA, cells were infected as in the colony forming assay and selected for 14 days in 200 μg/ml Hygromycin B. Pools of cells expressing scrambled or Sox2 shRNA were used in all experiments. For Sox2 over-expression studies, primary osteoblasts were infected with a lentivirus expressing full length human Sox2 cDNA as described before (Basu-Roy et al 2010).
Establishment of Wnt reporter osteosarcoma cell lines
Osteosarcoma cell lines expressing scrambled or Sox2 shRNA were transfected with a 8× TOP-Luciferase construct that is responsive to Wnt-dependent induction in osteoblasts (described previously in (Ambrosetti et al 2008). Stable cell lines expressing both shRNA and Wnt reporter were selected in the presence of 200 μg/ml Hygromycin B and 500 μg/ml G418. Pools were analyzed based on their responsiveness to Wnt 3A conditioned medium.
In vitro transformation and in vivo tumorigenicity assay
Cells expressing scrambled or Sox2 shRNA were used in these assays. In vitro migration, invasion and soft agar growth were assessed as described (Cantiani et al 2007). To assay for cell migration, 105 osteosarcoma cells were plated in Transwell chambers (Costar). Cells (105) in serum-free DMEM were seeded in the upper compartment, whereas DMEM plus 10% FBS was placed in the lower compartment of the chamber. Cells migrated to the lower side of the membrane was counted after crystal violet staining. Experiments were done in triplicate. To assay for cell invasion, cells (5 × 105) were seeded in the upper compartment of Biocoat Matrigel invasion chambers (BD Biosciences). In these chambers, the upper compartment is coated with Matrigel and migrating cells must pass through the Matrigel layer. This assay measures the ability of cells to degrade the Matrigel layer before migrating to the lower compartment. Assay was done according to the manufacturer's instructions similar to cell migration. All experiments were made in triplicate. For soft agar assays to assess anchorage-independent growth, 10,000 cells were suspended in semi-solid medium (DMEM with 10% FBS and 0.33% agarose) with a 0.5% agarose underlay, in 6-well plates and incubated at 37°C. Colonies were counted after 10-12 days.
For assay of in vivo tumorigenicity, 5 × 106 cells were injected subcutaneously into NOD/SCID mice. Tumor growth was monitored bi-weekly and tumor volume was measured with an electronic caliper using the formula Tumor Volume (TV) = L * W2 * Π / 6. All tumorigenesis studies were performed at the Antitumor Assessment Facility of Memorial Sloan Kettering Cancer Center under IACUC Approved Protocol Number A3311-01.
Isolation of Sox2-Sca-1 double-positive cells
Flow cytometry of intracellular Sox2 and membrane Sca-1 double-labeled cells was carried out as follows. Phycoerythrin (PE)-conjugated Sca-1 and anti-rabbit Alexa Fluor 488 secondary antibody were from Invitrogen, Carlsbad, CA. Sox2 polyclonal antibody used for flow cytometry was obtained from Millipore, Billerica, MA. For labeling 1 × 106 cells, 0.1 μg/ml of PE-Sca-1 antibody or isotype control was used. Briefly, cells were washed in cold FACS buffer (5% FBS in PBS with 0.02% sodium azide). Cells were incubated on ice with diluted Sca-1 antibody for 20 minutes and washed three times with FACS buffer. For Sca-1 staining, cells were then resuspended in 4% paraformaldehyde (PFA) and analyzed by flow cytometry. For intracellular staining for Sox2, cells were first stained for surface Sca-1 as described above. After washing, cells were permeabilized in 1% saponin in FACS buffer on ice for 20 minutes, followed by incubation with 0.5 μg/ml of either Sox2 or rabbit IgG as isotype control on ice for 30 minutes. Cells were then washed three times with permeabilization buffer, followed by incubation with a 1:400 dilution of the anti-rabbit secondary antibody in permeabilization buffer. After washing three times with permeablization buffer, cells were resuspended in PFA for flow cytometric analysis. Data were collected on a FACScan flow cytometer using CellQuest software and analyzed using FlowJo software (TreeStar, Ashland, OR). 30,000–50,000 events were collected per sample. Osteosarcoma cells were fractionated into Sca-1HI and Sca-1LO cells using the Sca-1 Cell Separation Kit (Stem Cell Technologies, Vancouver, Canada) as described by the manufacturer's instructions. Fractionated cells were used immediately for all experiments.
Osteosphere Assay and Limiting Dilution Analysis
Osteosphere assay was carried out as described (Gutierrez et al 2008). Briefly, 1000 cells were plated in triplicate in 24-well Corning ultra-low attachment plates for 7-10 days in N2B27 defined serum free medium. Osteospheres were collected by sieving through a 40 μM strainer. RNA was extracted from the spheres for gene expression analysis. Spheres were counted in each plate using a Leica MZ12 inverted microscope. For limiting dilution analysis of sphere-forming cells, cells were plated at limiting dilutions in 96-well plates in N2B27 defined serum free medium for 10 days. Wells were scored as positive or negative based on the presence or absence of sphere, and data was analyzed using ELDA software (Hu and Smyth 2009).
Gene expression analysis by quantitative real-time RT-PCR and Western Blotting
mRNA was prepared using Trizol Reagent (Invitrogen, USA). Reverse transcription and real-time PCR analysis was carried out as previously described (Mansukhani et al 2005) using specific primers. Actin was used as a normalization control. Antibodies used in the study were Sox2 (Cell Signaling, Danvers, MA), active β-catenin or ABC (Millipore, Billerica, MA) and γ-tubulin (Sigma, St. Louis, MO).
Osteogenic and Adipogenic Differentiation
Cells were plated at a density of 50,000 cells/well of 24-well plates or 0.5 × 106 cells/well of 6-well plate. For osteogenic differentiation, cells were incubated in the presence of 10 mM β-glycerol phosphate and 100 μg/ml ascorbic acid for the indicated times. Differentiation was assessed by staining for alkaline phosphatas. For adipogenic differentiation, cells were incubated in the presence of 100 nM dexamethasone, 250 μM Iso-butyl-methyl-xanthine (IBMX), 100 μM indomethacin and 10 μg/ml insulin. Adipocytes were detected by staining with Oil Red O.
Supplementary Material
Acknowledgments
We thank Dr. E. De Stanchina (Antitumor Assessment Facility, Memorial Sloan Kettering Cancer Center, New York, NY) for excellent services and advice with the xenograft assays. Thanks to Dr. Joan Durbin, NYU TABS, for help with tissue acquisition.
This investigation was supported by PHS grants AR051358 from the NIAMS and DE013745 from the NIDCR, and by an NCI UO1 award (to SHO). UBR is a recipient of a fellowship from The Children's Cancer Research Fund in memory of Dr. Aaron Rausen. AM is a recipient of a research grant from St. Baldrick's Foundation. JAP is a postdoctoral fellow of the American Cancer Society. SHO is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 001.Adams JM, Kelly PN, Dakic A, Carotta S, Nutt SL, Strasser A. Role of “cancer stem cells” and cell survival in tumor development and maintenance. Cold Spring Harb Symp Quant Biol. 2008;73:451–459. doi: 10.1101/sqb.2008.73.004. [DOI] [PubMed] [Google Scholar]
- 002.Ambrosetti D, Holmes G, Mansukhani A, Basilico C. Fibroblast growth factor signaling uses multiple mechanisms to inhibit Wnt-induced transcription in osteoblasts. Mol Cell Biol. 2008;28:4759–4771. doi: 10.1128/MCB.01849-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 003.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 004.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 005.Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A, Basilico C. The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 2010;17:1345–1353. doi: 10.1038/cdd.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 006.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 007.Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC, et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci U S A. 2008;105:11851–11856. doi: 10.1073/pnas.0805462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 008.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, Ly DP, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 009.Buzzeo MP, Scott EW, Cogle CR. The hunt for cancer-initiating cells: a history stemming from leukemia. Leukemia. 2007;21:1619–1627. doi: 10.1038/sj.leu.2404768. [DOI] [PubMed] [Google Scholar]
- 0010.Cai Y, Mohseny AB, Karperien M, Hogendoorn PC, Zhou G, Cleton-Jansen AM. Inactive Wnt/beta-catenin pathway in conventional high-grade osteosarcoma. J Pathol. 2010;220:24–33. doi: 10.1002/path.2628. [DOI] [PubMed] [Google Scholar]
- 0011.Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–1114. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0012.Cantiani L, Manara MC, Zucchini C, De Sanctis P, Zuntini M, Valvassori L, et al. Caveolin-1 reduces osteosarcoma metastases by inhibiting c-Src activity and met signaling. Cancer Res. 2007;67:7675–7685. doi: 10.1158/0008-5472.CAN-06-4697. [DOI] [PubMed] [Google Scholar]
- 0013.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 0014.Cleton-Jansen AM, Anninga JK, Briaire-de Bruijn IH, Romeo S, Oosting J, Egeler RM, et al. Profiling of high-grade central osteosarcoma and its putative progenitor cells identifies tumourigenic pathways. Br J Cancer. 2009;101:1909–1918. doi: 10.1038/sj.bjc.6605405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0015.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 0016.Cormier JN, Pollock RE. Soft tissue sarcomas. CA Cancer J Clin. 2004;54:94–109. doi: 10.3322/canjclin.54.2.94. [DOI] [PubMed] [Google Scholar]
- 0017.Douglas D, Hsu JH, Hung L, Cooper A, Abdueva D, van Doorninck J, et al. BMI-1 promotes ewing sarcoma tumorigenicity independent of CDKN2A repression. Cancer Res. 2008;68:6507–6515. doi: 10.1158/0008-5472.CAN-07-6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0018.Fujii H, Honoki K, Tsujiuchi T, Kido A, Yoshitani K, Takakura Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol. 2009;34:1381–1386. [PubMed] [Google Scholar]
- 0019.Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, et al. SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells. 2009;27:40–48. doi: 10.1634/stemcells.2008-0493. [DOI] [PubMed] [Google Scholar]
- 0020.Gibbs CP, Kukekov VG, Reith JD, Tchigrinova O, Suslov ON, Scott EW, et al. Stem-like cells in bone sarcomas: implications for tumorigenesis. Neoplasia. 2005;7:967–976. doi: 10.1593/neo.05394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0021.Gillette JM, Gibbs CP, Nielsen-Preiss SM. Establishment and characterization of OS 99-1, a cell line derived from a highly aggressive primary human osteosarcoma. In Vitro Cell Dev Biol Anim. 2008;44:87–95. doi: 10.1007/s11626-007-9075-8. [DOI] [PubMed] [Google Scholar]
- 0022.Gong C, Yao H, Liu Q, Chen J, Shi J, Su F, et al. Markers of tumor-initiating cells predict chemoresistance in breast cancer. PLoS One. 2010;5:e15630. doi: 10.1371/journal.pone.0015630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0023.Gutierrez GM, Kong E, Sabbagh Y, Brown NE, Lee JS, Demay MB, et al. Impaired bone development and increased mesenchymal progenitor cells in calvaria of RB1-/- mice. Proc Natl Acad Sci U S A. 2008;105:18402–18407. doi: 10.1073/pnas.0805925105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0024.Heare T, Hensley MA, Dell'Orfano S. Bone tumors: osteosarcoma and Ewing's sarcoma. Curr Opin Pediatr. 2009;21:365–372. doi: 10.1097/MOP.0b013e32832b1111. [DOI] [PubMed] [Google Scholar]
- 0025.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3:685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 0026.Hong JH, Yaffe MB. TAZ: a beta-catenin-like molecule that regulates mesenchymal stem cell differentiation. Cell Cycle. 2006;5:176–179. doi: 10.4161/cc.5.2.2362. [DOI] [PubMed] [Google Scholar]
- 0027.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 0028.Jia SF, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;17:501–506. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- 0029.Kansara M, Tsang M, Kodjabachian L, Sims NA, Trivett MK, Ehrich M, et al. Wnt inhibitory factor 1 is epigenetically silenced in human osteosarcoma, and targeted disruption accelerates osteosarcomagenesis in mice. J Clin Invest. 2009;119:837–851. doi: 10.1172/JCI37175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0030.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 0031.Kim CF, Dirks PB. Cancer and stem cell biology: how tightly intertwined? Cell Stem Cell. 2008;3:147–150. doi: 10.1016/j.stem.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 0032.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0033.Lefebvre V, Dumitriu B, Penzo-Mendez A, Han Y, Pallavi B. Control of cell fate and differentiation by Sry-related high-mobility-group box (Sox) transcription factors. Int J Biochem Cell Biol. 2007;39:2195–2214. doi: 10.1016/j.biocel.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0034.Levings PP, McGarry SV, Currie TP, Nickerson DM, McClellan S, Ghivizzani SC, et al. Expression of an exogenous human Oct-4 promoter identifies tumor-initiating cells in osteosarcoma. Cancer Res. 2009;69:5648–5655. doi: 10.1158/0008-5472.CAN-08-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0035.Mansukhani A, Bellosta P, Sahni M, Basilico C. Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol. 2000;149:1297–1308. doi: 10.1083/jcb.149.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0036.Mansukhani A, Ambrosetti D, Holmes G, Cornivelli L, Basilico C. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol. 2005;168:1065–1076. doi: 10.1083/jcb.200409182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0037.Marie PJ. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene. 2003;316:23–32. doi: 10.1016/s0378-1119(03)00748-0. [DOI] [PubMed] [Google Scholar]
- 0038.Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA, et al. Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J Clin Invest. 2007;117:3248–3257. doi: 10.1172/JCI31377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0039.Milat F, Ng KW. Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol. 2009;310:52–62. doi: 10.1016/j.mce.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 0040.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0041.Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- 0042.Pevny LH, Nicolis SK. Sox2 roles in neural stem cells. Int J Biochem Cell Biol. 2010;42:421–424. doi: 10.1016/j.biocel.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 0043.Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0044.Rainusso N, Man TK, Lau CC, Hicks J, Shen JJ, Yu A, et al. Identification and gene expression profiling of tumor-initiating cells isolated from human osteosarcoma cell lines in an orthotopic mouse model. Cancer Biol Ther. 2011;12 doi: 10.4161/cbt.12.4.15951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0045.Riggi N, Suva ML, De Vito C, Provero P, Stehle JC, Baumer K, et al. EWS-FLI-1 modulates miRNA145 and SOX2 expression to initiate mesenchymal stem cell reprogramming toward Ewing sarcoma cancer stem cells. Genes Dev. 2010;24:916–932. doi: 10.1101/gad.1899710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0046.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0047.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 0048.Tabone MD, Kalifa C, Rodary C, Raquin M, Valteau-Couanet D, Lemerle J. Osteosarcoma recurrences in pediatric patients previously treated with intensive chemotherapy. J Clin Oncol. 1994;12:2614–2620. doi: 10.1200/JCO.1994.12.12.2614. [DOI] [PubMed] [Google Scholar]
- 0049.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, et al. Wnt Signaling Requires Sequestration of Glycogen Synthase Kinase 3 inside Multivesicular Endosomes. Cell. 2010;143:1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0050.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol. 2009;5:442–447. doi: 10.1038/nrrheum.2009.137. [DOI] [PubMed] [Google Scholar]
- 0051.Takenobu H, Shimozato O, Nakamura T, Ochiai H, Yamaguchi Y, Ohira M, et al. CD133 suppresses neuroblastoma cell differentiation via signal pathway modification. Oncogene. 2011;30:97–105. doi: 10.1038/onc.2010.383. [DOI] [PubMed] [Google Scholar]
- 0052.Tang N, Song WX, Luo J, Haydon RC, He TC. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res. 2008;466:2114–2130. doi: 10.1007/s11999-008-0335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0053.Thomas DM, Johnson SA, Sims NA, Trivett MK, Slavin JL, Rubin BP, et al. Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol. 2004;167:925–934. doi: 10.1083/jcb.200409187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0054.Tirino V, Desiderio V, Paino F, De Rosa A, Papaccio F, Fazioli F, et al. Human primary bone sarcomas contain CD133+ cancer stem cells displaying high tumorigenicity in vivo. Faseb J. 2011;25:2022–2030. doi: 10.1096/fj.10-179036. [DOI] [PubMed] [Google Scholar]
- 0055.van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- 0056.Wadayama B, Toguchida J, Shimizu T, Ishizaki K, Sasaki MS, Kotoura Y, et al. Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res. 1994;54:3042–3048. [PubMed] [Google Scholar]
- 0057.Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev. 2008;22:1662–1676. doi: 10.1101/gad.1656808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0058.Xiang R, Liao D, Cheng T, Zhou H, Shi Q, Chuang TS, et al. Downregulation of transcription factor SOX2 in cancer stem cells suppresses growth and metastasis of lung cancer. Br J Cancer. 2011;104:1410–1417. doi: 10.1038/bjc.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 0059.Yuan H, Corbi N, Basilico C, Dailey L. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 1995;9:2635–2645. doi: 10.1101/gad.9.21.2635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.