Abstract
Recent evidence suggests that the persistence of cocaine seeking during periods of protracted drug abstinence following chronic cocaine exposure is mediated, in part, by neuroadaptations in the mesolimbic dopamine system. Specifically, incubation of cocaine-seeking behavior coincides with increased brain-derived neurotrophic factor (BDNF) protein expression in the ventral tegmental area (VTA). However, the molecular mechanisms that regulate time-dependent changes in VTA BDNF protein expression during cocaine abstinence are unclear. The goal of these experiments was to determine whether VTA BDNF transcript levels are altered following cocaine abstinence and identify the molecular mechanisms regulating cocaine-induced changes in VTA BDNF transcription. Rats were allowed to self-administer cocaine (0.25 mg/infusion, i.v.) for 14 d on a fixed-ratio schedule of reinforcement followed by 7 days of forced drug abstinence. BDNF protein and exon I-containing transcripts were significantly increased in the VTA of cocaine-experienced rats following 7 days of forced drug abstinence compared to yoked saline controls. Cocaine-induced changes in BDNF mRNA were associated with increased acetylation of histone 3 (AcH3) and binding of CREB binding protein (CBP) to exon I-containing promoters in the VTA. Taken together, these results suggest that drug abstinence following cocaine self-administration remodels chromatin in the VTA resulting in increased expression of BDNF, which may contribute to neuroadaptations underlying cocaine craving and relapse.
Keywords: self-administration, transcription, chromatin remodeling, addiction, neurotrophic factor, psychostimulant
Introduction
Mesocorticolimbic brain-derived neurotrophic factor (BDNF) plays a critical role in the reinstatement of cocaine-seeking behavior. Cue-induced cocaine seeking progressively increases during periods of prolonged drug abstinence and is associated with time-dependent increases in BDNF protein expression in the ventral tegmental area (VTA), nucleus accumbens, and amygdala (Grimm et al. 2003). Consistent with these findings, acute administration of recombinant BDNF directly into the VTA following the last day of cocaine self-administration potentiates cue-induced cocaine seeking during extended periods of drug abstinence (Lu et al. 2004). Moreover, increased BDNF-TrkB signaling in the nucleus accumbens augments cocaine seeking and blocking BDNF signaling in the accumbens decreases cocaine seeking (Graham et al. 2007). In contrast to effects in the VTA and accumbens, acute BDNF administration into the medial prefrontal cortex (mPFC) attenuates cocaine seeking (Berglind et al. 2007, Berglind et al. 2009, Whitfield et al. 2011). Taken together, these results suggest that BDNF in various limbic nuclei differentially regulates drug-induced long-term neuroadaptations that contribute to cocaine craving and relapse (Thomas et al. 2008).
Repeated exposure to drugs of abuse including cocaine induces alterations in gene expression profiles within the mesocorticolimbic dopamine system that underlie addiction (Kalivas 2005, Renthal & Nestler 2008, Shaham & Hope 2005). Cocaine-induced changes in gene expression profiles are mediated, in part, by persistent changes in genome-wide regulatory networks and chromatin remodeling (Maze & Nestler 2011). A growing body of evidence indicates that cocaine alters the expression of BDNF protein and mRNA in mesocorticolimbic nuclei (Ghitza et al. 2010, McGinty et al. 2010). For example, acute injections of cocaine increase BDNF mRNA in the striatum and mPFC (Le Foll et al. 2005, Liu et al. 2005, Fumagalli et al. 2007). Similarly, a sensitizing regimen of cocaine injections or cocaine self-administration results in increased BDNF mRNA in the VTA, mPFC, nucleus accumbens, and striatum (Le Foll et al. 2002, Zhang et al. 2002, Kumar et al. 2005, Graham et al. 2007, Sadri-Vakili et al. 2010). Increased BDNF mRNA expression in the striatum and mPFC, in particular, is associated with cocaine-induced alterations in chromatin remodeling, including histone acetylation (Kumar et al. 2005, Sadri- Vakili et al. 2010, Schroeder et al. 2008). Acetylation of histone proteins is associated with an open chromatin conformation and increased gene transcription (Jenuwein & Allis 2001, Keppler & Archer 2008). These findings indicate that cocaine-induced behavioral plasticity is mediated, in part, by changes in chromatin structure surrounding mesocorticolimbic BDNF promoters that lead to increased BDNF transcription.
The mechanisms underlying increased BDNF protein expression in the VTA during forced cocaine abstinence (Grimm et al. 2003) are unclear. The goal of these experiments was to determine whether VTA BDNF transcripts are altered following 7 days of forced drug abstinence in rats with a history of self-administering cocaine and whether these changes in gene expression are associated with chromatin modifications. We hypothesized that increases in VTA BDNF protein are associated with increased BDNF mRNA expression and increased histone acetylation. Here, we report that BDNF protein and mRNA levels are increased in the VTA following 7 days of forced cocaine abstinence and that these changes are associated with increased acetylation of histone H3 at BDNF exon I-containing promoters.
Materials and Methods
Animals and housing
Male Sprague-Dawley rats (Rattus norvegicus) weighing 250–300 g were obtained from Taconic Laboratories (Germantown, NY). Animals were individually housed with food and water available ad libitum in their home cage. A 12/12 hr light/dark cycle was used with the lights on at 7:00 a.m. All experimental procedures were performed during the light cycle. The experimental protocols were all consistent with the guidelines issued by the U.S. National Institutes of Health and were approved by the University of Pennsylvania School of Medicine and the University of Pennsylvania School of Medicine’s Institutional Animal Care and Use Committee.
Surgery
Prior to surgery, the rats were anesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine (i.p.; Sigma-Aldrich, St. Louis, MO). An indwelling silastic catheter (inner diameter 0.33 mm, outer diameter 0.64 mm) was inserted into the right jugular vein and sutured in place. The catheter was then passed subcutaneously over the shoulder blade and routed to a mesh backmount platform (CamCath, Cambridge, UK) that was sutured below the skin between the shoulder blades. Catheters were flushed daily with 0.3 ml of the antibiotic Timentin (ticarcillin disodium/potassium clavulanate, 0.93 mg/ml; Henry Schein, Melville, NY) dissolved in heparinized saline (10 U/ml). The catheters were sealed with plastic obturators when not in use.
Cocaine self-administration
Rats were allowed 7 days to recover from surgery before cocaine self-administration commenced. Rats were assigned to one of two groups: cocaine self-administering animals and yoked saline controls. Within each individual experiment, rats were randomly assigned to experimental and control groups. Each rat trained to respond for contingent cocaine infusions was paired with a yoked subject that received infusions of saline. Lever pressing for the saline-yoked rats had no scheduled consequences, but these animals received the same number and temporal pattern of infusions as self-administered by the paired cocaine-experimental rat. Rats responded for infusions of cocaine in the absence of cues (i.e., lights and tones).
Initially, cocaine-experimental rats were placed in the modular operant chambers (Med Associates, St. Albans, VT) and allowed to lever press for intravenous cocaine infusions (0.25 mg cocaine/59 µl saline, infusion over 5 s) on a fixed-ratio 1 (FR1) schedule of reinforcement. Once a cocaine-experimental rat achieved at least 20 infusions of cocaine in a single operant session under the FR1 schedule, the response requirement was switched to a FR5 schedule of reinforcement. For responding on both FR1 and FR5 schedules, the maximum number of cocaine infusions was limited to 30 per daily self-administration session and a 20 s time-out period followed each cocaine infusion, during which time active lever responses were tabulated but had no scheduled consequences. Daily 2 h operant sessions (7 days/week) were conducted for a total of 14 days. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during both the FR1 and FR5 training sessions.
After the 14th daily operant session, cocaine-experimental and yoked saline control rats were returned to their home cages where they underwent 7 days of forced drug abstinence. On the 7th day of cocaine abstinence, brains were removed and the VTA was dissected on ice. Seven days of cocaine abstinence were chosen in order to draw comparisons to our previously published study examining cocaine-induced changes in transcriptional regulation of BDNF promoters in the prefrontal cortex (Sadri-Vakili et al. 2010). Furthermore, cocaine-induced changes in chromatin structure at striatal BDNF promoters persist for at least 7 days of forced drug abstinence (Kumar et al. 2005). VTA dissections were based upon standard anatomical landmarks and stereotaxic coordinates commonly used in studies examining biochemical changes in this brain region (Choi et al. 2011, Fitzgerald et al. 1996, Russo et al. 2007). Each VTA was dissected from a coronal slice that spanned approximately −5.2 to −6.3 mm relative to bregma (Paxinos & Watson 1997). VTA tissue samples were stored at -80°C until assayed using Western blot, ELISA, and ChIP methods.
Enzyme-linked Immunosorbant Assay
VTA tissue homogenates were diluted to 10 µg/µl and 20 µg/µl. The concentration of BDNF in the diluted lysates was quantified using the Chemikine™ Brain Derived Neurotrophic Factor (BDNF) Sandwich ELISA kit (Chemicon International Inc., CA). Diluted VTA tissue homogenates and serial dilutions of BDNF standards were loaded in triplicate onto a 96 well plate coated with rabbit anti-human BDNF polyclonal antibodies and incubated overnight at 4°C. Each microplate was washed 4 times and biotinylated mouse anti-human BDNF monoclonal antibody (1:1000) was added to each well for 2.5 h at room temperature. Subsequently, the plates were washed 4 times and a strepavidin-enzyme conjugate was added to each well and allowed to incubate for 1 h. After further washing, tetramethylbenzidine chromagenic substrate was added to each well. Fifteen minutes later the reaction was stopped. Absorbance at 450 nm was measured with a plate reader. BDNF concentration in the VTA tissue homogenates was measured by comparing values to the prepared standard curve.
Chromatin immunoprecipitation (ChIP) assay
A modified ChIP technique was adapted from previously published studies from our laboratory in order to analyze DNA/protein complexes in dissected brain tissue (Braveman et al. 2004, Chen-Plotkin et al. 2006). Briefly, the VTA underwent a formaldehyde cross-linking step to link the transcription factors or histone proteins to DNA. Samples then were homogenized and subjected to immunoprecipitation with antibodies specific for the CBP or di-acetyl lysine 9, lysine14 histone H3 (H3K9K14Ac2; AcH3). The antibody-transcription factor-DNA complexes were washed to reverse the crosslinks, and the DNA was detected by qPCR using specific primers for the multiple BDNF promoters. Threshold amplification cycle numbers (Tc) using iCycler software were used to calculate IP DNA quantities as percentage of corresponding inputs. The following exon specific BDNF primers were designed based on previously published sequences (Chen et al. 2003, Martinowich et al. 2003, Jiang et al. 2008) and used for real-time PCR analysis: BDNF exon I: forward 5’-GCAGTTGGACAG TCATTGGTAACC-3’ and reverse 5’-ACGCAAACGCCCTCATTCTG-3’; BDNF exon IV: forward 5’-AACAAGAGGCTGTGACAC TATGCTC-3’ and reverse 5’-CAGTAAGTAAAGGCTAGGGCAGGC-3’.
RNA extraction and reverse transcription
RNA was extracted from dissected VTA following 14 days of cocaine self-administration and 1 or 7 days of forced abstinence using a RNeasy kit (Qiagen, Valencia, CA) according to manufacturer’s instructions and as described previously (Sadri-Vakili et al. 2010). Reverse transcription reactions were performed using specific primers in an iCycler (Bio-Rad) (25°C for 10 min, 42°C for 50 min, 70°C for 15 min) in an iCycler (Bio-Rad) to quantify the amount of gene expression as compared to a standard curve (Superscript First Strand Synthesis System; Invitrogen, Carlsbad, CA). The following primers were used: BDNF exon I: forward 5’-GCGTTGAGAAAGCTGCTTCAG-3’ and reverse 5’-GAATGAGCGAGGTTACCAATGA-3’; BDNF exonII (a, b, c): forward 5’-GCAGAGTCCATTCAGCACCTTG-3’ and reverse 5’-TGGCTTGACAGCGAGGAAAAG-3’; BDNF exon IV: forward 5’-TTCCACTATCAATAATTTAACTTCTTTGC-3’ and reverse 5’-CTCTTACTATATATTTCCCCTTCTCTTCAGT-3’; BDNF exon VI: forward 5’- TTTGGGGCAGACGAGAAAGC-3’ and reverse 5’-GGCAGT GGAGTCACATTGTTGTC-3’; GAPDH: forward 5’-AACAGCAACTCCCATTC TTC-3’ and reverse 5’-TGGTCCAGGGTTTCTTACTC-3’;. BDNF primers were based on previously published sequences (Aid et al. 2007). Quantitative real time-PCR was performed using 50 PCR cycles (95°C for 30 s, 57°C for 60 s, 72°C for 90 s) in an iCycler (Bio-Rad) with the use of SYBR-green PCR Master Mix (Applied Biosystems, Foster City, CA). The threshold cycle for each sample was chosen from the linear range and converted to a starting quantity by interpolation from a standard curve run on the same plate for each set of primers. For each replicate, mRNA levels were normalized to their respective GAPDH mRNA levels.
Statistical Analysis
In each experiment, we used data from 3 to 6 individual rats per treatment condition. Differences between groups were analyzed for statistical significance by using two-tailed Student’s t-test. When more than two groups of data were compared two-way ANOVA was used followed by Bonferroni post-hoc test.
Results
VTA BDNF protein is increased in cocaine-experienced animals following 7 days of forced drug abstinence
Cocaine-experienced animals self-administered an average of 25.14±1.56 (mean±SEM) infusions of cocaine on the last day of self-administration. Following 7 d of forced drug abstinence, cocaine-experienced and yoked saline control animals were sacrificed and their VTA dissected. Figure 1A depicts a coronal section of the rat brain at the level of the VTA (−5.30 mm posterior to bregma according to the atlas of Paxinos and Watson (1997)). The portion of the midbrain including the VTA that was dissected for these studies is indicated by the black triangle. There was a significant increase in BDNF protein in the VTA of cocaine-experienced rats when compared to yoked saline controls as measured by ELISA [t(6) = 4.314, p<0.01](Figure 1B).
Figure 1. BDNF protein levels are increased in the VTA following 7 days of forced cocaine abstinence.
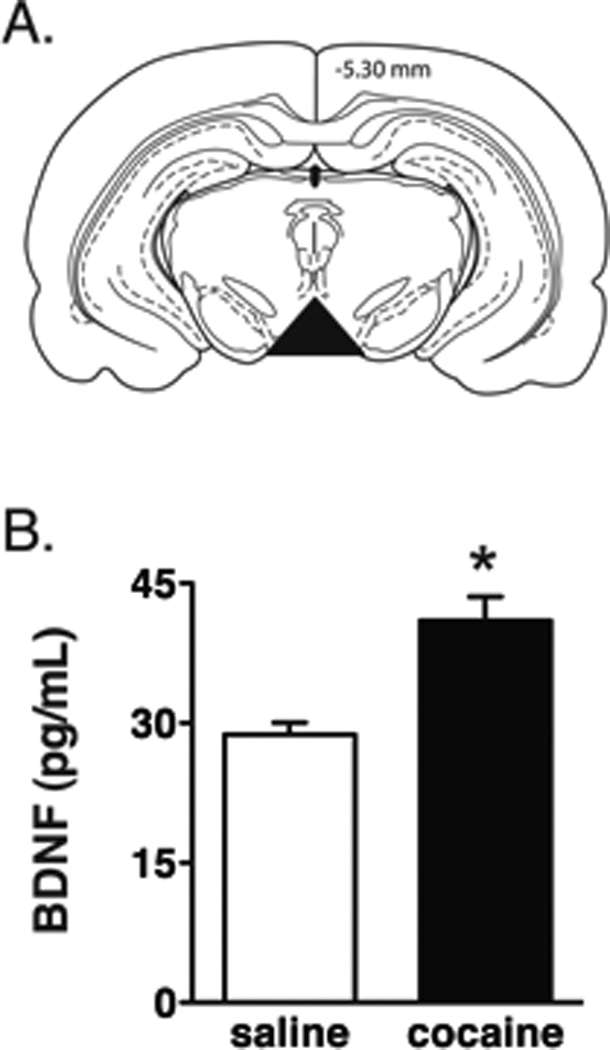
(A) Coronal section at the level of the VTA. The black triangle represents the region of the midbrain dissected for these studies. (B) VTA BDNF protein was significantly increased in cocaine-experienced animals when compared to yoked saline controls (unpaired t-test, p<0.05). n=4 rats/treatment
VTA BDNF exon I-containing transcripts are selectively increased in cocaine-experienced animals following 7 days of forced drug abstinence
The BDNF gene is comprised of eight untranslated 5’ exons that are spliced onto a 3’ coding exon. Therefore, multiple mRNA species are transcribed from the BDNF gene (Timmusk et al. 1993, Aid et al. 2006, Liu et al. 2006). While the specific function of these individual transcripts is not known, the expression of exon-specific BDNF transcripts differs between brain regions and they are differentially targeted and translated within cells. Expression of BDNF exon I, II, IV, and VI transcripts was assessed in the VTA of cocaine-experienced rats and compared to yoked saline controls following 1 and 7 days of forced abstinence. There was no significant difference in BDNF mRNA expression in the VTA of cocaine-experienced rats when compared to yoked saline controls following 1 day of forced abstinence as measured RT-qPCR [saline: 5.15 ± 0.636 BDNF transcript (% GAPDH); cocaine: 5.35 ± 1.626 BDNF transcript (% GAPDH)]. However, there was a significant alteration in BNDF mRNA levels in the VTA of cocaine-experienced rats following 7 days of forced abstinence. These data were analyzed with a two-way ANOVA, which revealed a significant main effect of exon [F(3,21) = 31.01, p<0.0001] as well as a significant drug treatment×exon interaction [F(3,21) = 3.09, p<0.0491] (Figure 2). Subsequent pairwise comparisons (Bonferroni post-hoc test, p<0.01) showed a significant difference between treatments for BDNF exon I. No significant differences between treatments were noted for BDNF exon II, IV, or VI promoters in the VTA of cocaine-experienced rats when compared to yoked saline controls.
Figure 2. BDNF exon I-containing transcripts are increased specifically in the VTA following 7 days of forced cocaine abstinence.
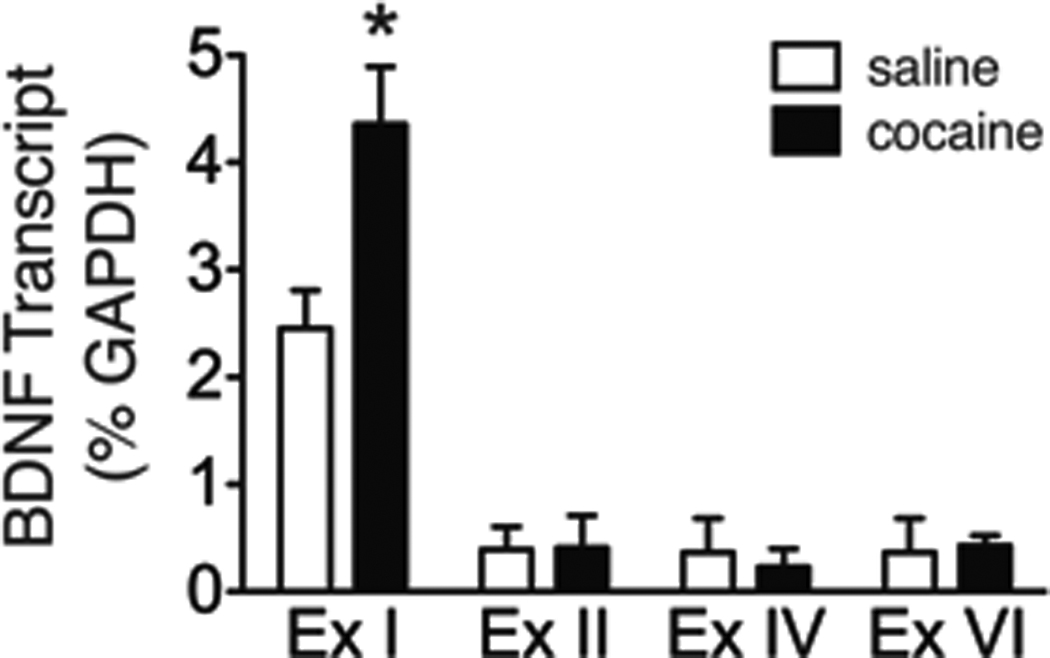
BDNF exon I, II, IV and VI transcripts were assessed in the VTA of cocaine self-administration rats and yoked saline controls. The asterisk represents a significant increase in BDNF exon I-containing transcript in cocaine-experienced rats as compared to yoked saline control animals (p<0.01, Bonferroni post hoc test). n=3–6 rats/treatment
VTA BDNF exon I-containing promoters are associated with increased CBP binding following 7 days of forced cocaine abstinence
One mechanism whereby gene transcription is regulated is by remodeling chromatin structure through modifications of histone proteins. CREB binding protein (CBP) displays histone acetyltransferase (HAT) activity and leads to alterations in chromatin structure by acetylating specific lysine residues on histone proteins (Lonze & Ginty 2002, Chan & La Thangue 2001). Thus, CBP acetylates histone proteins and promotes an open chromatin configuration thereby facilitating increased gene transcription. Changes in CBP binding to BDNF promoters were next measured following 7 days of forced cocaine abstinence. As shown in Figure 3A, cocaine self-administration followed by 7 days of forced abstinence significantly increased CBP associations with the BDNF exon I-containing promoter, but not with promoter IV. These data were analyzed with a two-way ANOVA, which indicated a significant main effect of exon [F(2,16)=3.733, p<0.05]. Subsequent pairwise comparisons (Bonferroni post-hoc test, p<0.05) showed a significant difference between treatments for exon I. These results indicate that one mechanism whereby cocaine increases VTA BDNF mRNA expression is by increasing specifically the association of CBP with BDNF exon I promoters.
Figure 3. Cocaine self-administration results in increased CBP binding and H3 acetylation at BDNF exon I promoters in the VTA.
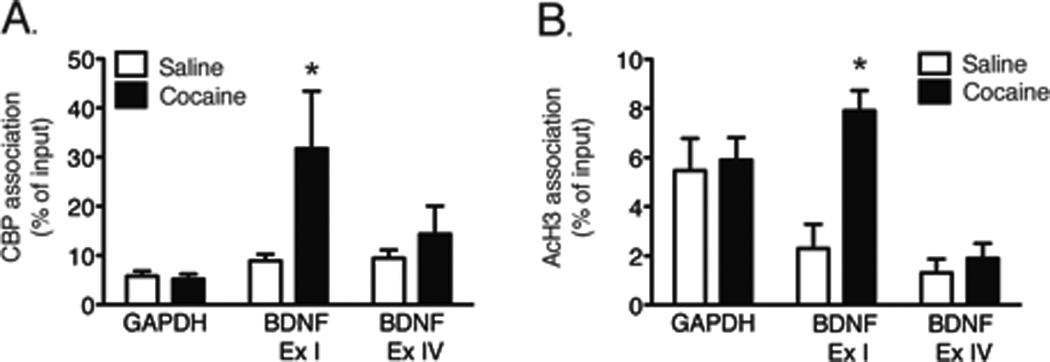
(A) 7 days of forced cocaine abstinence was associated with a significant increase in CBP association with BDNF exon I, but not exon IV, -containing or GAPDH promoters in the VTA (p<0.05, Bonferroni post hoc test). (B) Cocaine self-administration also results in a significant increase in AcH3 association with BDNF exon I-containing promoters in the VTA (p<0.05, Bonferroni post hoc test). n=3–4 rats/treatment.
VTA BDNF promoters are associated with increased acetylation of histone 3 following 7 days of forced cocaine abstinence
Results from Figure 3A demonstrate that there is an increase in CBP association with BDNF exon I-containing promoters in the VTA. CBP acetylates histone proteins and promotes an open chromatin configuration thereby facilitating increased gene transcription. Therefore, alterations in histone acetylation at BDNF promoters are one potential mechanism underlying cocaine-induced increases in BDNF mRNA expression in the VTA. In order to test this hypothesis, ChIP was used to assess the association of acetylated histone H3 (AcH3) with BDNF promoters in the VTA. As shown in Figure 3B, there was a significant increase in the association of AcH3 with BDNF exon I-, but not exon IV-, containing promoters in the VTA following cocaine self-administration. These data were analyzed with a two-way ANOVA which revealed significant main effects of drug treatment [F(1, 12) = 8.941, p<0.05] and exon [F(2, 12) = 11.89, p<0.01] as well as a significant treatment×exon interaction [F(2, 12) = 5.255, p<0.05]. Subsequent pairwise comparisons (Bonferroni post-hoc test) showed that there was a significant difference between treatments for BDNF exon I (p<0.01). Taken together, these findings suggest that cocaine self-administration followed by 7 days of forced abstinence results in enhanced association of acetylated histone H3 with BDNF exon I-containing promoters in the VTA.
Discussion
In the current study, cocaine self-administration followed by 7 days of forced drug abstinence increased BDNF protein levels in the rat VTA similar to previous findings (Grimm et al. 2003). However, this is the first study to demonstrate that increased BDNF protein corresponds with increased transcription of BDNF in the VTA of animals with a history of cocaine self-administration. Specifically, forced cocaine abstinence selectively increased BDNF exon I-containing transcripts in the VTA. Cocaine-induced increases in VTA BDNF exon I-containing transcripts were associated with increased acetylation of histone H3, which confers an open chromatin conformation, within these promoters. Increased CBP association with VTA BDNF exon I-containing promoters also was observed following 7 days of forced cocaine abstinence. Thus, the molecular mechanisms underlying increases in VTA BDNF expression during forced cocaine abstinence appear to focus, at least in part, on chromatin modifications that induce subsequent transcription of BDNF exon I-containing transcripts.
Increased BDNF transcription is differentially regulated in brain nuclei following cocaine self-administration
An expanding literature indicates that alterations in gene expression underlie cocaine-induced neuronal and behavioral plasticity (Nestler 2001). Both acute and repeated cocaine administration result in transient changes in gene expression in a number of brain regions including the VTA (Yuferov et al. 2005). Recent studies demonstrate that BDNF mRNA is increased in the dorsal striatum, mPFC, and nucleus accumbens following a sensitizing regimen of cocaine injections or cocaine self-administration (Le Foll et al. 2002, Zhang et al. 2002, Kumar et al. 2005, Fumagalli et al. 2007, Sadri-Vakili et al. 2010). BDNF mRNA is also increased in the VTA of mice following repeated experimenter-delivered infusions of cocaine (Le Foll et al. 2002). However, this study did not assess exon-specific changes in BDNF transcripts or the effects of cocaine self-administration on VTA BDNF mRNA levels (Le Foll et al. 2002). Recent evidence indicates that there are clear differences in transcriptional regulation of BDNF promoters depending upon whether an animal self-administers cocaine or receives experimenter-delivered injections of cocaine (Kumar et al. 2005). In the present study, we expand these findings and demonstrate for the first time that BDNF exon I-containing transcripts are increased in the VTA following 7 days of forced drug abstinence in rats with a history of cocaine self-administration. Increased BDNF transcription is time-dependent in that exon I-containing transcripts were significantly increased following 7, but not 1, days of forced cocaine abstinence. Furthermore, these changes were specific to exon I- and not exon IV-containing transcripts, which suggests that different mechanisms are responsible for altering chromatin structure for each of these promoters in response to cocaine. Taken together, these results suggest that one mechanism underlying increased VTA BDNF during periods of drug abstinence is increased transcription.
The rodent BDNF gene consists of eight 5’ untranslated exons with separate promoter regions and a 3’ protein coding exon (Aid et al. 2007). Expression of exon-specific BDNF transcripts is differentially regulated such that distinct expression profiles are observed in different brain nuclei (Aid et al. 2007). Here, we demonstrate that 7 days of forced cocaine abstinence is associated with increased expression of BDNF exon I- and not exon IV-containing transcripts in the VTA. In contrast, a previous study from our laboratory demonstrated that BNDF exon IV- and not exon I-containing transcripts were increased in the mPFC following 7 days of forced cocaine abstinence (Sadri-Vakili et al. 2010). Taken together, these findings indicate that cocaine self-administration differentially regulates BDNF mRNA expression in the brain by promoting region- and exon-specific transcription. Specifically, cocaine self-administration increases exon IV-containing transcripts in the mPFC (Sadri-Vakili et al. 2010) while exon I-containing transcripts are increased in the VTA (present findings). These are the first data to demonstrate that forced cocaine abstinence regulates BDNF gene expression through different transcriptional mechanisms in distinct brain nuclei. These results add to an expanding literature demonstrating differential regulation of BDNF transcripts in response to a variety of environmental (Lubin et al. 2008, Russo-Neustadt et al. 2004, Roth et al. 2011) and pharmacological (Caldwell et al. 2008).
One limitation of using ELISAs to measure BDNF protein levels is that this method does not distinguish between proBDNF and mature BDNF. Both proBDNF and mature BDNF are biologically active; however, they elicit opposing synaptic effects through distinct receptors (Greenberg et al. 2009). Future studies are required to distinguish the roles of proBDNF and mature BDNF in cocaine-seeking behavior.
Molecular mechanisms underlying cocaine-induced increases in BDNF transcription
Tissue- and region-specific expression of BDNF transcripts is regulated by chromatin remodeling at 5’ BDNF exons (Aid et al. 2007). Recent evidence suggests that cocaine exposure hyper-acetylates histones associated with gene promoters resulting in an active chromatin configuration (Brami-Cherrier et al. 2005, Kumar et al. 2005, Levine et al. 2005, Freeman et al. 2008, Sadri-Vakili et al. 2010). With regard to BDNF, there is increased acetylation of histone H3 at BDNF exon IV promoters within the striatum (Kumar et al. 2005) and mPFC (Sadri-Vakili et al. 2010) following cocaine self-administration. Histone acetylation at striatal BDNF promoters is relatively long-lasting and increases during forced cocaine abstinence (Kumar et al. 2005). Consistent with the time course of these findings, we show that BDNF exon I promoters in the VTA are associated with increased acetylation of histone H3 following 7 days of forced cocaine abstinence. Increased histone acetylation of BDNF promoters in the VTA (present findings), striatum (Kumar et al. 2005), and mPFC (Sadri-Vakili et al. 2010) coincides with increased BDNF protein expression in these brain regions during protracted periods of drug abstinence. Thus, drug-induced remodeling of chromatin structure in the VTA, striatum, and mPFC increases BDNF exon-specific mRNA expression, which may mediate enhanced cocaine craving and relapse during periods of prolonged drug abstinence (Grimm et al. 2003).
Chronic cocaine exposure decreases histone deacetylase (HDAC) function in the nucleus accumbens and decreased HDAC5 expression enhances cocaine-induced behavioral responses (Renthal et al. 2007). In addition, administration of HDAC inhibitors, which result in increased histone acetylation, potentiates cocaine-induced behavioral effects (Kumar et al. 2005, Renthal et al. 2007). For example, systemic administration of HDAC inhibitors enhances cocaine-induced histone 3 phosphoacetylation in the striatum and increases the locomotor-activating and conditioning effects of cocaine (Kumar et al. 2005). Consistent with these findings, systemic administration of an HDAC inhibitor blocks the attenuating effects of HDAC5 overexpression in the accumbens on cocaine conditioned place preference (Renthal et al. 2007). Moreover, altered histone acetylation levels in the mPFC including BDNF exon IV-containing promoters are associated with changes in the reinforcing efficacy of cocaine as measured using a progressive ratio schedule of reinforcement (Romieu et al. 2008, Sadri-Vakili et al. 2010). Consistent with these results, mutant mice that have decreased levels of the histone acetyltransferase CREB-binding protein (CBP) have reduced behavioral sensitivity to cocaine that is associated with decreased histone acetylation in the striatum (Levine et al. 2005). Moreover, the current findings indicate that increased acetylation of VTA BDNF exon I promoters is associated with enhanced binding of CBP to these promoter sequences. Taken together, these results suggest that repeated exposure to cocaine modifies chromatin, in part, via the recruitment of CBP and an increase in histone acetylation at gene promoters. These alterations, in turn, facilitate an active, permissive chromatin state at VTA BDNF exon I promoters such that other regulators of transcription can bind.
Functional consequences of increased VTA BDNF
BDNF in the mesocorticolimbic dopamine system plays a critical role in cocaine craving and relapse (Ghitza et al. 2010, McGinty et al. 2010). Recent evidence indicates that exogenous BDNF infusions into the mPFC suppress the reinstatement of cocaine seeking (Berglind et al. 2007) by normalizing cocaine-induced alterations in glutamate transmission the nucleus accumbens (Berglind et al. 2009, McGinty et al. 2010). These results are consistent with the notion that increases in mPFC BDNF protein reflect a homeostatic mechanism that diminishes the reinforcing efficacy of cocaine (Sadri-Vakili et al. 2010). In contrast to its role in the mPFC, BDNF in the nucleus accumbens and VTA promotes or facilitates cocaine-seeking behavior. Cue-induced cocaine seeking progressively increases during protracted drug abstinence and is paralleled by increases in BDNF protein levels in the VTA, nucleus accumbens and amygdala (Grimm et al. 2003). Moreover, acute administration of BDNF directly into the VTA enhanced cocaine seeking (Lu et al. 2004). Repeated administration of BDNF into the nucleus accumbens also results in enduring enhancement of the reinstatement of cocaine seeking (Graham et al. 2007). These findings indicate that alterations in BDNF protein levels in the accumbens and VTA contributes significantly to cocaine-seeking behavior and that these behavioral responses are opposite to those mediated by BDNF in the mPFC. While increased BDNF transcription in the mPFC may represent a compensatory reaction to decrease the reinforcing efficacy of cocaine (Sadri-Vakili et al. 2010), the overall effect of cocaine-induced increases in mesocaccumbens BDNF protein appears to enhance drug-seeking behavior (Lu et al. 2004, Graham et al. 2007). However, it remains to be determined whether increasing endogenous BDNF levels in the VTA or nucleus accumbens promotes cocaine-seeking behavior.
Conclusions
The present findings indicate that cocaine self-administration increases BDNF mRNA expression in the VTA and that these changes in gene transcription result from alterations in chromatin structure. Specifically, following cocaine self-administration the chromatin associated with BDNF exon I becomes acetylated, which shifts it into an open configuration conducive to transcriptional regulation. One mechanism through which BDNF exon I becomes acetylated is via increased association with CBP, a known histone acetyltransferase, at this promoter region. These results define a specific epigenetic mechanism whereby cocaine experience increases BDNF transcription in the VTA, which has previously been associated with animal models of cocaine craving and relapse. Defining the molecular mechanisms that regulate differential cocaine-induced effects at specific BDNF promoters may lead to novel treatments for cocaine addiction.
Acknowledgments
This work was supported by NIDA grants DA22339 (R.C.P. & G.S.V.) and DA18678 (R.C.P.). H.D.S. was supported by an individual K01 award (DA030445).
Footnotes
The authors declare no conflicts of interest.
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2006 doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Herve D, Darragh J, Corvol JC, Pages C, Simon AJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman MW, Chen-Plotkin AS, Yohrling GJ, Cha JH. Chromatin immunoprecipitation technique for study of transcriptional dysregulation in intact mouse brain. Methods Mol Biol. 2004;277:261–276. doi: 10.1385/1-59259-804-8:261. [DOI] [PubMed] [Google Scholar]
- Caldwell KK, Sheema S, Paz RD, Samudio-Ruiz SL, Laughlin MH, Spence NE, Roehlk MJ, Alcon SN, Allan AM. Fetal alcohol spectrum disorder-associated depression: evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse model. Pharmacol biochem behavior. 2008;90:614–624. doi: 10.1016/j.pbb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114:2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Chen-Plotkin AS, Sadri-Vakili G, Yohrling GJ, et al. Decreased association of the transcription factor Sp1 with genes downregulated in Huntington's disease. Neurobiol Dis. 2006;22:233–241. doi: 10.1016/j.nbd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Choi KH, Edwards S, Graham DL, et al. Reinforcement-related regulation of AMPA glutamate receptor subunits in the ventral tegmental area enhances motivation for cocaine. J Neurosci. 2011;31:7927–7937. doi: 10.1523/JNEUROSCI.6014-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, Roberts DC, Vrana KE. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva M. Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci. 2007;26:2756–2763. doi: 10.1111/j.1460-9568.2007.05918.x. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jiang X, Tian F, Du Y, et al. BHLHB2 controls Bdnf promoter 4 activity and neuronal excitability. J Neurosci. 2008;28:1118–1130. doi: 10.1523/JNEUROSCI.2262-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. How do we determine which drug-induced neuroplastic changes are important? Nat Neurosci. 2005;8:1440–1441. doi: 10.1038/nn1105-1440. [DOI] [PubMed] [Google Scholar]
- Keppler BR, Archer TK. Chromatin-modifying enzymes as therapeutic targets--Part 1. Expert Opin Ther Targets. 2008;12:1301–1312. doi: 10.1517/14728222.12.10.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Diaz J, Sokoloff P. A single cocaine exposure increases BDNF and D3 receptor expression: implications for drug-conditioning. Neuroreport. 2005;16:175–178. doi: 10.1097/00001756-200502080-00022. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Frances H, Diaz J, Schwartz JC, Sokoloff P. Role of the dopamine D3 receptor in reactivity to cocaine-associated cues in mice. Eur J Neurosci. 2002;15:2016–2026. doi: 10.1046/j.1460-9568.2002.02049.x. [DOI] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, Schwartz JH. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A. 2005;102:19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Maze I, Nestler EJ. The epigenetic landscape of addiction. Ann N Y Acad Sci. 2011;1216:99–113. doi: 10.1111/j.1749-6632.2010.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, Zwiller J. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J Neurosci. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011;45:919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Russo-Neustadt AA, Alejandre H, Garcia C, Ivy AS, Chen MJ. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29:2189–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, Akbarian S. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology. 2008;33:2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Whitfield TW, Jr, Shi X, Sun WL, McGinty JF. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci. 2011;31:834–842. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuferov V, Nielsen D, Butelman E, Kreek M. Microarray studies of psychostimulant-induced changes in gene expression. Addict Biol. 2005;10:101–118. doi: 10.1080/13556210412331308976. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Lou DW, Nakabeppu Y, Zhang J, Xu M. The dopamine D1 receptor is a critical mediator for cocaine-induced gene expression. J Neurochem. 2002;82:1453–1464. doi: 10.1046/j.1471-4159.2002.01089.x. [DOI] [PubMed] [Google Scholar]


