Abstract
Developmental exposure to multiple-ortho substituted polychlorinated biphenyls (PCBs) causes adverse neurodevelopmental outcomes in laboratory animals and humans by mechanisms involving the sensitization of Ryanodine receptors (RyRs). In the case of PCB 136, the sensitization of RyR is enantiospecific, with only (-)-PCB 136 being active. However, the role of enantioselective metabolism in the developmental neurotoxicity of PCB 136 is poorly understood. The present study employed hepatic microsomes from phenobarbital (PB-), dexamethasone (DEX-) and corn oil (VEH-)treated male Sprague-Dawley rats to investigate the hypothesis that PCB 136 atropisomers are enantioselectively metabolized by P450 enzymes to potentially neurotoxic, hydroxylated PCB 136 metabolites. The results demonstrated the time- and isoform-dependent formation of three metabolites, with 5-OH-PCB 136 (2,2',3,3',6,6'-hexachlorobiphenyl-5-ol) being the major metabolite. The formation of 5-OH-PCB 136 increased with the activity of P450 2B enzymes in the microsomal preparation, which is consistent with PCB 136 metabolism by rat P450 2B1. The minor metabolite 4-OH-PCB 136 (2,2',3,3',6,6'-hexachlorobiphenyl-4-ol) was produced by a currently unidentified P450 enzymes. An enantiomeric enrichment of (-)-PCB 136 was observed in microsomal incubations due to the preferential metabolism of (+)-PCB 136 to the corresponding 5-OH-PCB 136 (2,2',3,3',6,6'-hexachlorobiphenyl-5-ol) atropisomer. 4-OH-PCB 136 displayed an enrichment of the atropisomer formed from (-)-PCB 136; however, the enrichment of this metabolite atropisomer didn't affect the enantiomeric enrichment of the parent PCB because 4-OH-PCB 136 is only a minor metabolite. Although the formation of 5- and 4-OH-PCB 136 atropisomers increased with time, the enantioselective formation of the OH-PCB metabolites resulted in constant enantiomeric enrichment, especially at later incubation times. These observations not only demonstrate that the chiral signatures of PCBs and their metabolites in wildlife and humans are due to metabolism by P450 enzymes, but also suggest that the enantioselective formation of neurotoxic PCB 136 metabolites, such as 4-OH-PCB 136, may play a role in the developmental neurotoxicity of PCBs.
INTRODUCTION
Polychlorinated biphenyls (PCBs) are an important class of ubiquitous and persistent organic pollutants in which one or more hydrogen atoms on biphenyl are replaced by chlorine atoms.1 There are a total of 209 individual PCB components, known as congeners, which differ in the degree of chlorination and the chlorine substitution pattern. Technical PCB mixtures were employed for a range of commercial and industrial applications, including plasticizers, lubricants, and hydraulic fluids, and are still in use in certain enclosed applications, such as transformers, in the United States.2 Due to the global contamination of the environment with PCBs and their adverse effects on the environment and human health, the production of PCBs was banned in the United States in the late 70s. Exposure to PCBs has been implicated in a number of human diseases, including cancer, cardiovascular and immune disease.3 Of special concern are epidemiological studies that have shown a negative correlation between PCB exposure during early development and cognitive function in infancy or childhood.4 In particular, exposure to PCB congeners with multiple ortho chlorine substituents have been implicated in the adverse neurodevelopmental effects of PCBs.5-7
PCB congeners are metabolized by mammalian P450 enzymes in the liver to hydroxylated metabolites (OH-PCBs).8 In principle, the 209 possible PCB congeners can be converted to 847 OHPCBs,9 but only a small number of the more persistent OH-PCBs are routinely quantified in wildlife 10-12 or human blood samples.13-16 There is evidence that OH-PCBs can cross the placenta,13, 14 resulting in fetal exposure. The extent of fetal exposure may change during pregnancy, with OH-PCB levels tending to decline between the first and second trimester.15 The effects of OH-PCBs can be analogous to those of the parent PCBs and can contribute to developmental neurotoxicity by altering Ca2+ signaling, interfering with thyroid hormone signaling and decreasing dopamine content.5, 6 Sensitization of Ryanodine receptors (RyRs) appears to be a key mechanism by which PCBs with multiple ortho chlorine substituents and their hydroxylated metabolites (e.g., PCB 136 and 4-OH-PCB 136) alter Ca2+ signaling.17-19
Seventy-eight PCB congeners, such as PCB 136, and their hydroxylated metabolites are chiral because they exist as non-superimposable mirror images of each other.20 Nineteen PCB congeners with three or four ortho chlorine substituents are chiral because rotation around the phenyl-phenyl bond is hindered by the bulky chlorine substituents. These chiral PCB congeners exist as stable rotational isomers, known as atropisomers, which display different biological activities. For example, the PCB 136 atropisomers display enantiospecific activity towards RyRs, and only the (-)-PCB 136 atropisomer is active.21 Metabolites of chiral PCBs, such as 4-OH-PCB 136, are also potent sensitizers of RyRs7 and it is likely that OH-PCB atropisomers enantiospecifically sensitize RyRs. Therefore, it is critical to investigate the enantioselective metabolism of chiral, neurotoxic PCB congeners to OH-PCBs.
It is well known that chiral PCBs,20, 22 OH-PCBs23 and methylsulfonyl PCBs24, 25 undergo enantiomeric enrichment in mammals, but it is unclear if oxidative metabolism of chiral PCBs by P450 enzymes results in the enantioselective formation of OH-PCBs and how the resulting enantiomeric enrichment, expressed as enantiomeric fraction (EF), changes with time. In the present study, the time-and isoform-dependent formation of the atropisomers of mono-hydroxylated PCB 136 metabolites by P450 enzymes was investigated by utilizing rat liver microsomes from phenobarbital (PB)-, dexamethasone (DEX)- and vehicle (VEH)-treated male Sprague-Dawley rats. The results demonstrated a pronounced enantiomeric enrichment of (-)-PCB 136, the 5-OH-PCB 136 atropisomer formed from (+)-PCB 136 and the 4-OH-PCB 136 atropisomer formed from (-)-PCB 136. Furthermore, the enantioselective formation of OH-PCB metabolites resulted in constant EF values with time. The observation that PCBs are metabolized enantioselectively to OH-PCBs demonstrates that the chiral signatures of PCBs and their metabolites in wildlife, laboratory animals and humans20 are due to metabolism by P450 enzymes and not a result of other biotransformation or transport processes. Furthermore, the enantiomeric enrichment of OH-PCBs suggests that the enantioselective formation of neurotoxic PCB 136 metabolites, such as 4-OH-PCB 136,7 may play an important role in the developmental neurotoxicity of PCBs.
EXPERIMENTAL PROCEDURES
PCB 136 and PCB 136 metabolite standards
Racemic PCB 136 was synthesized by the Ullmann coupling of 2,3,6-trichloro-1-iodobenzene26. The atropisomers of PCB 136 were separated using two serially connected Nucleodex β-PM columns. The enantiomeric fractions (EF = Area(+)-PCB 136 /(Area(+)-PCB 136 + Area(-)-PCB 136)) of 2-PCB 136 and (+)-PCB 136 were 0.01 and 1.00, respectively.27 4-OH-PCB 136, 5-OH-PCB 136, 4,5-dimethoxy-2,2',3,3',6,6'-hexachlorobiphenyl and 2,2',3',4,6,6'-hexachloro-3-methoxybiphenyl were prepared as described elsewhere.28
Aqueous Solubility of PCB 136
The solubility of PCB 136 in the incubation buffer was assessed by light scattering as previously described.29 The determination was performed at 37 °C using a LS55 Perkin-Elmer luminescence spectrophotometer equipped with a PTP-1 Peltier system (Perkin-Elmer, Waltham, MA, USA), with excitation and emission wavelength set to 400 nm, 5 nm excitation and emission slits, 5 s integration time, 5 min pre-recording and 5 min recording time. PCB 136 solutions in dimethyl sulfoxide (DMSO) (15 μL) were added to phosphate buffer (0.1 M, containing 3 mM of MgCl2) to give a total volume of 3 mL.
Animal treatment
Experiments involving animals were approved by the Institutional Animal Care and Use Committee at the University of Iowa. Eight week old male Sprague-Dawley rats (231 ± 12 g) were purchased from Harlan, Inc. (Indianapolis, IN, USA) and allowed to acclimatize for one week before random assignment to the respective treatment groups. Rats received intraperitoneal injections of phenobarbital (PB), 102 mg/kg b.w./day in saline for three consecutive days, dexamethasone (DEX), 50 mg/kg b.w./day in corn oil for four consecutive days, or corn oil alone (VEH), 5 mL/kg b.w./day for four consecutive days.27, 30 Rats were sacrificed by CO2 asphyxiation followed by cervical dislocation 24 h after the last treatment. Livers were excised en bloc and immediately placed in ice-cold 0.05 M Tris-HCl buffer (pH 7.5) containing 0.15 M KCl for the preparation of microsomes. The effects of the different inducers on final body and liver weights are summarized in Table S1.
Microsome preparation and characterization
Liver microsomes were prepared by differential centrifugation as described previously.27 In short, liver homogenates were prepared from individual livers, spun in a centrifuge at 9,000 g for 20 min and the supernatant was subjected to two additional centrifugation steps at 100,000 g for 60 min. The microsomal pellets were re-suspended in 0.25 M sucrose, pooled for animals within same treatment group and ~1 mL aliquots were stored at -80 °C. Microsomal protein concentrations were measured as described by Lowry et al., with bovine serum albumin as standard.31 The P450 enzyme activity was measured using 7-ethoxyresorufin-O-deethylase (EROD), 7-benzyloxyresorufin-O-debenzylase 32 and 7-benzyloxyquinoline debenzylation (BQ) assays employing established methods (Table S2).33, 34
Hepatic microsomal metabolism experiments
A high PCB 136 concentration (50 μM) was selected for the hepatic microsomal metabolism experiments based on work by Schnellmann et al. to ensure that the P450-mediated metabolism of PCB 136 is not substrate limited.35 In addition, metabolism experiments were performed at a low PCB 136 concentration (5 μM). This low concentration was selected because preliminary experiments demonstrated the disappearance of PCB 136 from incubations with PB-microsomes at this concentration, thus allowing an assessment of the enantiomeric enrichment of PCB 136. Furthermore, this concentration is comparable to the apparent Km of 8.8 μM for the metabolism of PCB 136 by human liver microsomes.35 Both PCB 136 concentrations are above the aqueous solubility of PCB 136, which was estimated to be 0.25 μM according to light scattering experiments; however, the microsomal lipid facilitated the dissolution of PCB 136 and no visible precipitate was observed.
Incubations consisted of 0.1 M phosphate buffer, pH 7.4, 3 mM magnesium chloride and 0.5 mg of hepatic microsomal protein in a final volume of 2 mL. In the case of the carbon monoxide inhibition study, carbon monoxide was slowly passed through the incubation mixtures for 2 min. NADPH (10 mM NADPH in phosphate buffer, 100 μL) was added to the incubation mixture at the final concentration of 0.5 mM. After 5 min pre-incubation at 37 ± 1 °C in shaking water bath, PCB 136 in DMSO (10 μL, < 1% of the total volume) was added to give final PCB 136 concentrations of 5 or 50 μM and the samples were incubated at 37 ± 1 °C for 5 min. Control samples containing phosphate buffer and DMSO (10 μL), but no microsomes or NADPH, or containing heat-inactivated microsomes (inactivated for 10 min in boiling water) were included with each incubation. Reactions were stopped by adding ice-cold sodium hydroxide (0.5 M, 2 mL). The incubation mixture was kept at 100 °C for 10 min and extracted as described below. Assay conditions were optimized by measuring metabolite formation as a function of NADPH concentration (0.1 to 2.0 mM) and microsomal protein concentration (0.1 to 2.0 mg/mL). NADPH was found to be in excess at 0.5 mM and metabolite formation was linear with respect to protein concentration between 0.1 to 1 mg/mL, after a 10 min incubation with PCB 136 at 50 μM.
Time-course experiments
Incubations with a total volume of 16 mL were performed to obtain metabolites for GC/MS analysis or for time course experiments. After a 5 min pre-incubation with a buffer containing NADPH at 37 ± 1 °C, PCB 136 in DMSO (80 μL) was added to give final concentrations of PCB 136 of 5 μM and 50 μM. For time-course experiments, an aliquot (2 mL) was removed from the primary incubation mixture after 1, 2, 3, 5, 10, 20 and 30 min. For the 0 min time point, a separate sample was pre-incubated for 5 min as described above, followed by sequential addition of the sodium hydroxide stop solution (2 mL) and of PCB 136. Control incubations were performed in parallel.
Extraction and separation of PCB 136 and its hydroxylated metabolites
Extraction of PCB 136 and its hydroxylated metabolites was performed using a published method.36, 37 In short, surrogate standards (2,3,4,4',5,6-hexachlorobiphenyl, 500 ng; 2',3,3',4,5,5'-hexachlorobiphenyl-4-ol, 274 ng) were added to each sample, followed by hydrochloric acid (6 M, 1 mL) and 2-propanol (3 mL). The samples were extracted with hexane-MTBE (1:1 v/v, 5 mL) and hexane (3 mL), and the combined organic extracts were washed with an aqueous KCl solution (1%, 3 mL). After removal of the organic phase, the KCl phase was re-extracted with hexane (3 mL), and the combined extracts were reduced under a gentle stream of nitrogen to ~ 1 mL. After addition of methanol (5 drops), the hydroxylated PCBs were derivatized with diazomethane in diethyl ether (0.5 mL) and subjected to a sulfur cleanup as described previously.36 The mean recovery rates were 93 ± 17 % and 93 ± 16 % for 2,3,4,4',5,6-hexachlorobiphenyl and 2',3,3',4,5,5'-hexachlorobiphenyl-4-ol, respectively. The concentrations were corrected for recovery rates below 100 %. Concentrations of PCB 136 and its metabolites were determined using PCB 204 as internal standard. All data are presented as mean ± SD (n = 3).
Gas chromatographic determinations
Analysis of PCB 136 and the methylated derivatives of hydroxylated PCB 136 metabolites was carried out using an Agilent 6890N gas chromatograph with 63Ni μECD detector and DB1-MS capillary column (60 m × 0.25 mm ID × 0.25 μm film thickness; Agilent, Santa Clara, CA, USA). The injector and detector temperatures were 280 °C and 300 °C, respectively. The temperature program was as follows: 100 °C for 1 min, 5 °C/min to 250 °C, hold for 20 min, 5 °C/min to 280 °C, hold for 3 min.
Enantioselective analysis of PCB 136 and the derivatized hydroxylated PCB 136 atropisomers was performed using an Agilent 7890A gas chromatograph equipped with two 63Ni- μECD detectors. The injector and detector temperatures were kept at 250 °C. PCB 136 and 5-OH PCB 136 atropisomers were separated using several Chirasil-Dex capillary columns (25 m × 0.25 mm ID × 0.25 μm film thickness; Varian, Palo Alto, CA, US). The temperature program was as follows: 15 °C/min from 50 to 143 °C (or 139 °C), hold for 410 min (or 600 min), 15 °C/min to 200 °C, hold for 10 min. To account for variations in the performance of different Chirasil-Dex columns, the analysis time and temperature were adjusted to optimize resolution. 4-OH PCB 136 atropisomers were separated with a Cyclosil-B capillary column (30 m × 0.25 mm ID × 0.25 μm film thickness; Agilent, Santa Clara, CA, US). The temperature program was as follows: 15 °C/min from 50 to 160 °C, hold for 360 min, 15 °C/min to 200 °C, hold for 10 min. 4,5-diOH-PCB 136 (2,2',3,3',6,6'-hexachlorobiphenyl-4,5-diol) atropisomers did not resolve on either column. The EF values for 4-OH and 5-OH-PCB 136 were determined as EF = Area E(2)/(Area E(1) + Area E(2)). The limits of detection, resolution and background levels are presented in Table S3. A comprehensive summary of PCB and OH-PCB levels in the various incubations is provided in Figures S4-S8.
RESULTS AND DISCUSSION
Enantiomeric enrichment of PCB 136 in microsomal incubations
Phase I metabolism of PCBs in vivo is complex and involves oxidation by different P450 isoforms. The present study investigates the metabolism of PCB 136 using liver microsomes prepared from PB-, DEX- and VEH-treated rats as the source of P450 enzymes. This approach is expected to better approximate the phase I metabolite profile of PCB 136 observed in vivo. Furthermore, microsomes from induced animals were used to gain insights into the role of different P450 enzymes, such as P450 2B and 3A, on the OH-PCB metabolite profile and the EF of PCB 136 and its metabolites (i.e., the OH-PCB 136 atropisomer profile). While it is known that PCB 136 is enantioselectively metabolized by recombinant rat P450 2B1 to OH-PCB metabolites (Figure 1),28, 35 with (-)-PCB 136 undergoing enantiomeric enrichment,22 the enantioselective metabolism of PCB 136 to OH-PCBs with rat liver microsomes has not been investigated previously. In addition to measuring the enantiomeric enrichment of OH-PCB 136 metabolites, time-dependent changes in the enantiomeric enrichment of PCB 136 were studied to determine if the enantiomeric enrichment of the parent PCB correlated with the enrichment of its hydroxylated metabolites.
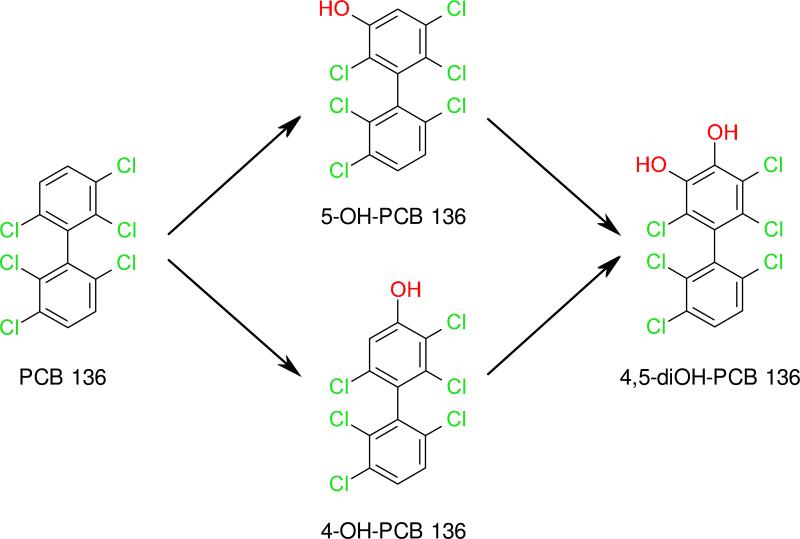
Figure 1.
Metabolism of PCB 136 to mono- and dihydroxylated metabolites by microsomal P450 enzymes. This metabolism scheme is in agreement with previous metabolism studies.22, 28, 35
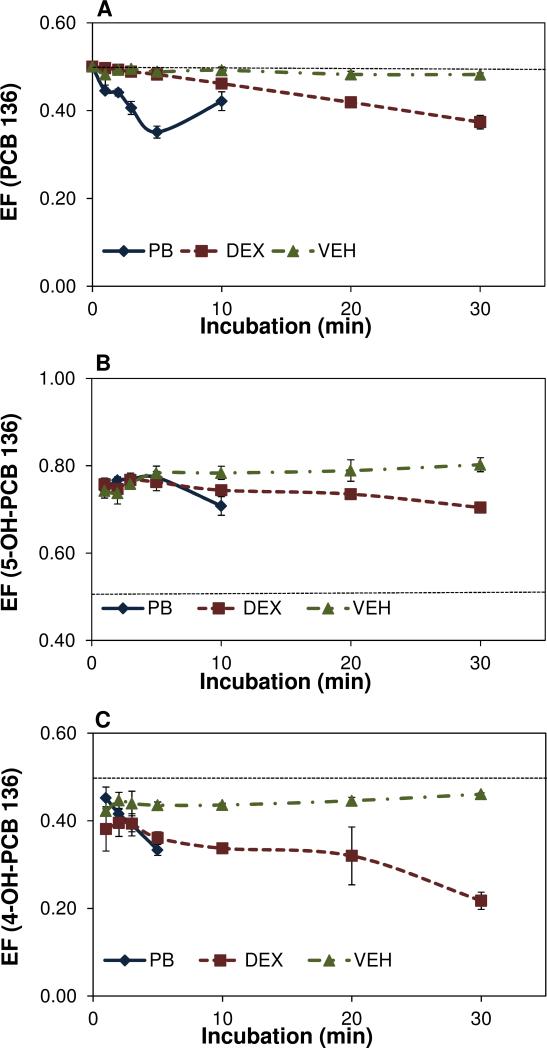
The PCB 136 EF values were only investigated in 5 μM PCB 136 incubations (Figure 2A) because high concentrations of racemic PCB136 were expected to mask changes in the EF. Microsomal metabolism resulted in time-dependent enrichment of (-)-PCB 136 in all three microsomal preparations, which is in agreement with previous in vitro 22 and in vivo studies.38 The EF values of PCB 136 decreased in a time-dependent manner for PB- and DEX-microsomes. Only a slight decrease in the EF value was observed for hepatic microsomes from VEH-treated rats. The enrichment of (-)-PCB 136 occurred faster with hepatic microsomes from PB-treated rats, with a rank order of PB- >> DEX- > VEH treatment. For example, the EF values of PCB 136 were 0.35, 0.48 and 0.49 for PB, DEX and VEH-microsomes, respectively, following a 5 min incubation. This observation suggests that induction of P450 2B enzymes may result in a more pronounced enantiomeric enrichment of the neurotoxic (-)-PCB 136 in vivo, which may affect the developmental toxicity of PCB 136 in rats. Since BROD activity, a measure of the activity of P450 2B enzymes, also decreased in the order PB- > DEX- > VEH-treatment, the time-dependent changes in the EF values are consistent with enantioselective metabolism of PCB 136 by P450 2B enzymes. It is interesting to note that the EF value of PCB 136 in incubations with hepatic microsomes from VEH-treated rats was comparable to the EF values of 0.49 observed in Sprague-Dawley rats after intraperitoneal administration of racemic PCB 136.38 This indicates that the enantiomeric enrichment of PCB 136 in incubations with microsomes obtained from untreated animals is representative of the enantiomeric enrichment observed in vivo.
Figure 2.
Hepatic microsomal metabolism of PCB 136 results in a time-dependent enrichment of (A) (-)-PCB 136, (B) the second eluting atropisomer of 5-OH PCB 136 and (C) the first eluting atropisomer of 4-OH PCB 136. Hepatic microsomes were incubated with 5 μM PCB 136 at 37 °C for 30 min. The dotted line represents the EF value of 0.5 in a racemic mixture. No PCB 136 or its mono-hydroxylated metabolites were detected after 10 min in incubations with hepatic microsomes from PB-treated rats.
Identification of PCB 136 metabolites
A large scale metabolism experiment was performed with hepatic microsomes from PB-treated rats to identify the hydroxylated metabolites formed from PCB 136 and to compare their retention times and mass spectra to authentic standards. Analysis of the methylated OH-PCB derivatives by GC/MS showed that 5-OH-PCB 136 was the major metabolite and that 4-OH-PCB 136 and 4,5-diOH-PCB 136 were formed as minor metabolites of PCB 136 (Figures S1 and S2). No other hydroxylated metabolites, such as NIH shift products, PCB dihydrodiols or dechlorination products, were observed. Formation of all three metabolites increased with incubation time at PCB concentrations of 5 and 50 μM with all microsomal preparations (Figures S4-S5). Formation of 4,5-diOH-PCB 136 occurred with a delay (Figures S4-S5). This is consistent with the formation of 4,5-diOH-PCB 136 from either 4-OH-PCB 136 or 5-OH-PCB 136, as reported previously by Waller and coworkers (Figure 1).28 The three metabolites observed in the present study, along with a small concentration of a fourth metabolite, 3'-OH-PB136, were previously detected in the liver of rats that were treated in vivo with PCB136,38 but only 5-OH-PCB136 and 4,5-diOH-PCB136 were formed when PCB136 was incubated in vitro with rat recombinant P450 2B1.28 In comparison, 4-OH-PCB136 and 5-OH-PCB136 were identified as major metabolites produced by human liver microsomes.35
PCB 136 metabolite profile and ratios
The extent of the PCB 136 metabolism depended on the microsomal preparation used (Table 1). For example, PCB 136 was completely metabolized after 5 min following incubation of 5 μM PCB 136 with hepatic microsomes from PB-treated rats, with approximately 89% of PCB 136 being converted to 5-OH-PCB 136. When 50 μM PCB 136 was incubated with hepatic microsomes from PB-, DEX- or VEH-treated rats, approximately 22 %, 2 % and 0.4 % of PCB 136 was converted to 5-OH-PCB 136, respectively, and 0.1 % and < 0.1% of PCB 136 was metabolized to 4-OH-PCB 136 and 4,5-diOH-PCB 136, respectively. Significant metabolism was observed in experiments with PB-microsomes and 5 μM PCB 136, where over 20 % of PCB 136 was converted via the monohydroxylated metabolites to 4,5-diOH-PCB 136 after 5 min. Overall, the sum of OH-PCB metabolites was greater with hepatic microsomes from PB- than DEX-treated rats and lowest with hepatic microsomes from VEH-treated rats for both PCB 136 concentrations investigated (Table 1). Carbon monoxide was used as a non-selective inhibitor to verify that P450 enzymes were responsible for the formation of hydroxylated metabolites. Pretreatment of rat liver microsomes with carbon monoxide drastically reduced formation of 4-OH and 5-OH-PCB 136 by hepatic microsomes from PB-, DEX- or VEH-treated rats at both PCB 136 concentrations (Figure S3).
Table 1.
Formation of hydroxylated PCB 136 metabolites by hepatic microsomes from PB-, DEX- or VEH-treated rats.*
| Inducer treatment | PCB 136 concentration in μM (nmol per incubation) | Metabolite formation | 5-OH-PCB 136:4-OH-PCB 136 ratio | |||||
|---|---|---|---|---|---|---|---|---|
| 5-OH-PCB 136 | 4-OH-PCB 136 | 4,5di-OH-PCB 136 | ||||||
| nmol | Percent of ΣOH-PCB | nmol | Percent of ΣOH-PCB | nmol | Percent of ΣOH-PCB | |||
| PB | 5 (10) | 8.9 | 78 | T | T | 2.4 | 22 | 540 |
| 50 (100) | 22.4 | 99 | 0.2 | 1 | T | T | 137 | |
| DEX | 5 (10) | 2.9 | 94 | 0.1 | 3 | 0.1 | 3 | 30 |
| 50 (100) | 2.3 | 98 | 0.1 | 2 | T | T | 43 | |
| VEH | 5 (10) | 0.4 | 83 | 0.1 | 15 | 0 | 2 | 8 |
| 50 (100) | 0.4 | 81 | 0.1 | 18 | <0.1 | 1 | 5 | |
PCB 136 was incubated with rat liver microsomes from PB, DEX and VEH-treated rats for 5 min at 37°C, as described under Materials and Methods; T – a trace of the respective metabolite was detected.
Hepatic microsomes from PB- and, to a lesser extent, DEX-treated rats produced more 5-OH-PCB 136 compared with hepatic microsomes from VEH-treated rats. For example, hepatic microsomes from PB- or DEX-treated rats formed 84- and 8-times more 5-OH-PCB 136 after a 2 min incubation time compared with hepatic from VEH-treated rats (Table S4). The increased formation of 5-OH-PCB 136 is in agreement with the 94- and 7-fold increase in BROD activity measured in hepatic microsomes from PB- and DEX-treated rats, respectively (Table S2), and consistent with the formation of 5-OH-PCB 136 by P450 2B enzymes.28 Interestingly, there was only a small change in the concentration of 4-OH-PCB 136 in PB- and DEX-microsomes relative to VEH-microsomes (Table S5). Since BQ activity, a catalytic measure of P450 3A, was increased by 84-fold in hepatic microsomes from DEX-treated rats (Table S2), the minimal change in 4-OH-PCB 136 levels in experiments using DEX- relative to VEH-microsomes suggests that P450 3A enzymes are not be involved in the formation of this minor metabolite. Analogously, formation of 4-OH-PCB 136 was unlikely due to P450 2B enzymes in the rat. This interpretation is consistent with the work by Waller et al., who did not detect the formation of 4-OH-PCB 136 in incubations with recombinant P450 2B1.28 Overall, these finding suggest that 4-OHPCB 136 may be formed by other P450 enzymes.
The 5-OH-PCB 136 : 4-OH PCB 136 ratio decreased in the order PB >> DEX > VEH, which suggests that induction of P450 2B enzymes increases meta hydroxylation of PCB 136 (Table 1). A preference for meta hydroxylation has also been observed for other PCB congeners with a 2,5-dichloro substitution pattern with liver microsomes from PB-treated rats.39 Preston and co-workers demonstrated that meta hydroxylation occurs by direct insertion of oxygen into an aromatic C-H bond and does not involve an arene oxide intermediate; however, formation of 4-OH-PCB 136 is thought to be due to rearrangement of an arene oxide.39 Depending on the PCB 136 concentration, the metabolite ratio observed in experiments with hepatic microsomes from VEH-treated rats ranged from 8:1 to 5:1 (Table 1). A comparable ratio of approximately 5:1 has also been reported in male and female rats in vivo.38 Previous studies with human liver microsomes yielded both monohydroxylated metabolites in a 1:1 ratio,35 whereas rat recombinant P450 2B1 yielded no detectable quantities of 4-OH-PCB 136.28
Characterization of atropisomer elution order
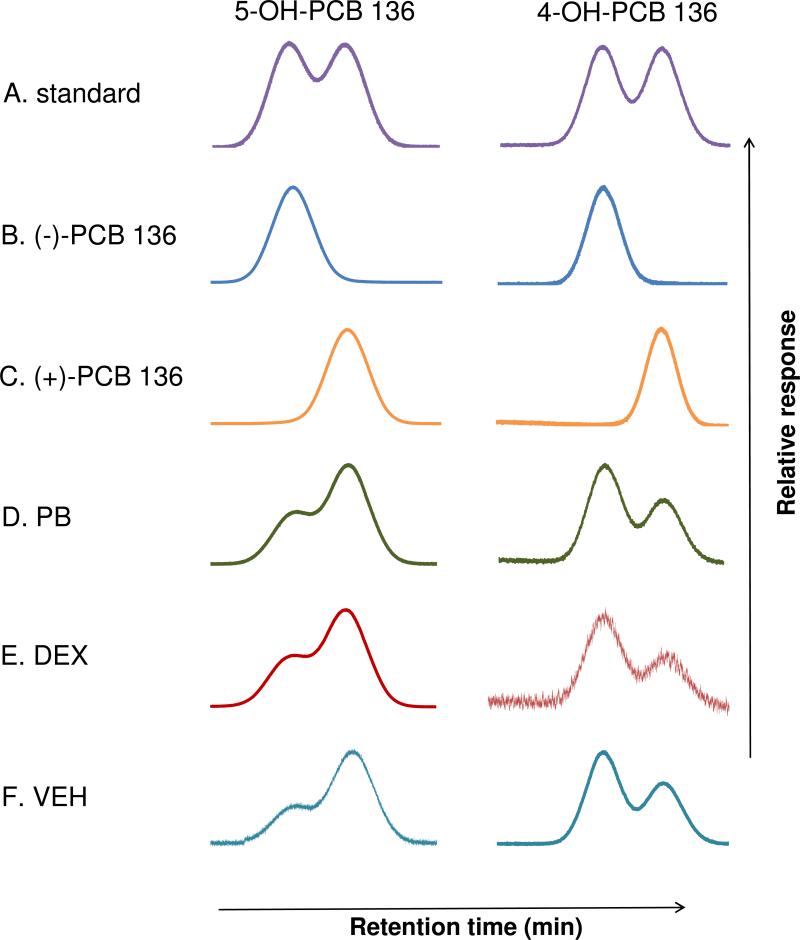
Metabolism experiments were also performed with pure PCB 136 atropisomers to determine which OH-PCB 136 atropisomers are formed from (+)- or (-)-PCB 136. As shown in Figures 3A and 3B, the 5-OH-PCB 136 and 4-OH-PCB 136 metabolites formed from (-)-PCB 136 corresponded to the atropisomer eluting first on both the Chirasil-Dex and the Cyclosil-B columns (i.e., E(1)-5-OH-PCB 136 and E(1)-4-OH-PCB 136, respectively). Metabolites formed from (+)-PCB 136 corresponded to the atropisomers eluting second on both columns (E(2)-5-OH-PCB 136 and E(2)-4-OH-PCB 136, respectively) (Figures 3A and 3C). Since (-)-PCB 136 is the first and (+)-PCB 136 is the second eluting atropisomer on both columns,40 hydroxylation in 4- and 5-position did not change the elution order of the metabolite atropisomers relative to the parent compound.
Figure 3.
The second eluting atropisomer of 5-OH PCB 136 (E(2)-5-OH-PCB 136) and the first eluting atropisomer of 4-OH PCB 136 (E(1)-4-OH-PCB) are formed enantioselectively by rat liver microsomes, with the first and second eluting atropisomer of both metabolites being formed from (-)-PCB 136 and (+)-PCB 136, respectively. Representative, normalized chromatograms of the methylated derivatives of (A) racemic 5-OH-PCB 136 and 4-OH-PCB 136 standards. (B) E(1)-5-OH-PCB 136 and E(1)-4-OH-PCB 136 formed from (-)-PCB 136. (C) E(2)-5-OH-PCB 136 and E(2)-4-OH-PCB 136 formed from (+)-PCB 136. Chiral signature of 5-OH-PCB 136 and 4-OH-PCB 136 obtained from incubations with hepatic microsomes from rats treated with (D) PB, (E) DEX or (F) VEH. All microsomal incubations were performed with 50 μM PCB 136 at 37 °C for 10 min. Atropisomers of 5-OH PCB 136 and 4-OH PCB 136 were separated on Chirasil-Dex and Cyclosil-B columns, respectively.
Enantioselective formation of OH-PCB 136 metabolites
Enantioselective gas chromatography of the extracts from the microsomal incubations revealed an enantiomeric enrichment of the second eluting atropisomer of 5-OH-PCB 136 (E(2)-5-OH-PCB 136) (Figure 3), the major metabolite of PCB 136 (EF > 0.5). This metabolite was formed from (+)-PCB 136. The enantiomeric enrichment of all three microsomal preparations showed the same direction (Figures 2B and 4A-C). The EF values of 5-OHPCB 136 ranged from 0.70-0.72, 0.73-0.68 and 0.62-0.75 for PB, DEX and VEH-microsomes with 50 μM PCB 136, respectively, which is comparable to EF values of 0.64 observed previously in male Sprague-Dawley rats after intraperitoneal administration of racemic PCB 136.38 Overall, these observations are consistent with the preferential metabolism of (+)-PCB 136 to the corresponding 5-OH-PCB 136 atropisomer by P450 enzymes and, thus, explain the enrichment of (-)-PCB 136 in incubations with hepatic microsomes (Figure 2A), recombinant P450 2B1 enzymes22 and in vivo (EF = 0.49 in male and female Sprague-Dawley rats).38
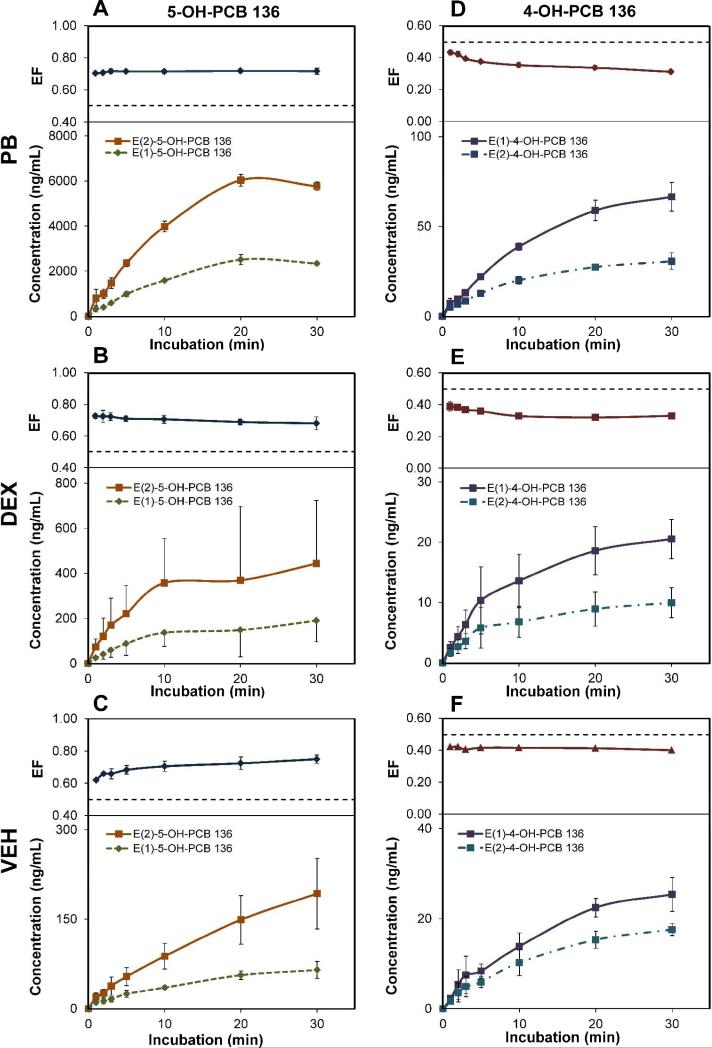
Figure 4.
Time-dependent formation of hydroxylated PCB 136 metabolites results in enantiomeric enrichment of the second eluting atropisomer of 5-OH PCB 136 (E(2)-5-OH-PCB 136) and the first eluting atropisomer of 4-OH PCB 136 (E(1)-4-OH-PCB) in incubations with rat liver microsomes. Time-dependent changes of atropisomer levels and enantiomeric fractions (EF) of 5-OH-PCB 136 from incubations with hepatic microsomes from PB-, DEX- and VEH-treated rats are shown in panels A, B and C, respectively. Changes of atropisomer levels and enantiomeric fractions of 4-OH-PCB 136 from incubations with hepatic microsomes from PB-, DEX- and VEH-treated rats are presented in panels D, E and F, respectively. Hepatic microsomes were incubated with 50 μM PCB 136 at 37 °C for 30 min. The dotted line in the upper panels represents the EF value of 0.5 in a racemic mixture.
The minor mono-hydroxylated metabolite of PCB 136, 4-OH-PCB 136, was also formed enantioselectively by all three microsomal preparations; however, the first eluting atropisomers of 4-OH-PCB 136 (E(1)-4-OH-PCB 136) was enriched (EF < 0.5) (Figures 2C and 4D-F). This atropisomer was a metabolite of (-)-PCB 136. The enantiomeric enrichment of 4-OH-PCB 136 in 50 μM PCB 136 incubation ranged from 0.43-0.31, 0.39-0.33 and 0.42-0.40 for PB, DEX and VEH-microsomes, respectively, which is comparable to the extent of the enrichment observed with 5-OH-PCB 136. The metabolism of (-)-PCB 136 to E(1)-4-OH-PCB 136 does not alter the direction of the enrichment of PCB 136 in the incubation mixtures and in vivo because 4-OH-PCB 136 is only a minor metabolite.
The extent of enantiomeric enrichment of both metabolites typically increased slightly within the first five to ten minutes in 50 μM PCB 136 incubations and remained relatively constant in incubations with all three microsomal preparations (Figure 4, upper panels). A clear change in the EF values of 5-OH-PCB was observed for incubations with VEH-microsomes, where the EF increased from 0.62 at 1 min. to 0.71 at 10 min (Figure 4C). In contrast, the EF values of 4-OH-PCB 136 showed the most pronounced change for incubations with PB and DEX-microsomes (0.43 at 1 min to 0.35 at 10 min for PB-microsomes; 0.39 at 1 min to 0.33 at 10 min for DEX-microsomes) (Figures 4D and 4E). Despite the fairly minimal changes in the EF values for incubation times between 10 to 30 min, the concentration of the respective OH-PCB 136 atropisomers typically increased with time, with more E(2)-5-OH-PCB 136 and E(1)-4-OH-PCB 136 formed compared to the respective other atropisomer (Figure 4, lower panels). The exception was experiments with PB-microsomes, where the concentration of the 5-OH-PCB 136 atropisomers showed a maximum at 20 minutes and subsequently decreased slightly (Figure 4A). One likely reason for this decrease in the concentration is the metabolism of the monohydroxylated metabolites to 4,5-diOH-PCB 136 (Figure S4).
The EF values of the two monohydroxylated metabolites also showed pronounced, time-dependent changes in 5 μM incubations with PB- and DEX- and, to a lesser extent, VEH-microsomes (Figures 2B and 2C). E(2)-5-OH-PCB 136 was enriched in all incubations at this PCB 136 concentration (EF > 0.5). For example, the EF values of 5-OH-PCB 136 were 0.77, 0.76, and 0.78 at 5 min for incubations with PB-, DEX- and VEH-microsomes, respectively. Since E(2)-5-OH-PCB 136 is formed from (+)-PCB 136 and (+)-PCB 136 is apparently more rapidly metabolized in the microsomal incubations (Figures 2A and S6), the present study demonstrates that the enantiomeric enrichment of (-)-PCB 136 in rats is due to the enantioselective metabolism of (+)-PCB 136 to E(2)-5-OH-PCB 136. It is interesting to note that, as observed in experiments with hepatic microsomes from PB-treated rats, the enantiomeric enrichment of both PCB 136 and 5-OH-PCB 136 reached a maximum at incubation times of approximately 5 minutes and subsequently decreased again (Figures 2A and 2B). Although further studies are needed, this change in the extent of the enantiomeric enrichment of 5-OH-PCB 136 at longer incubation times may be due to the enantioselective metabolism of 5-OH-PCB 136. For example, 5-OH-PCB 136 may be enantioselectively metabolized to 4,5-diOH-PCB 136 by P450 2B1 enzymes.22
In the 5 μM incubations, 4-OH-PCB 136 also displayed an enrichment of E(1)-4-OH-PCB 136 (EF < 0.5) (Figure 2C), which is formed from (-)-PCB136 in the microsomal incubations (Figure 3). In 5 min incubations, the EF values of 4-OH-PCB 136 decreased in the order PB > DEX > VEH. The most pronounced enrichment of E(1)-4-OH-PCB 136 was observed in incubations with DEX-microsomes at an incubation time of 30 min (EF = 0.22). The EF values in incubations with PB microsomes displayed no maximum with time. These observations are consistent with the formation of 4-OH-PCB 136 by P450 enzymes other than P450 2B1. Overall, the enantioselective metabolism of (-)-PCB 136 to E(1)-4-OH-PCB 136 did not make a contribution to the enantiomeric enrichment of PCB 136 because this pathway is only a minor metabolism pathway.
Although the direction of the enantiomeric enrichment of PCB 136 and its metabolites was identical in all hepatic microsomal incubations (i.e., enrichment of (-)-PCB 136, E(2)-5-OH-PCB 136 formed from (+)-PCB 136 and E(1)-4-OH-PCB 136 formed from (-)-PCB 136), there were differences in the time-dependent changes in enantiomeric enrichment between 5 and 50 μM PCB 136, especially with hepatic microsomes from PB- and DEX-treated rats. For example, the EF values increased with time when hepatic microsomes from DEX-treated rats were incubated with 5 μM PCB 136 (Figure 2B), whereas the EF values were near constant in incubations with 50 M PCB 136 between 10 and 30 min (Figures 4B and 4E). Several factors likely contribute to these differences, including induction of P450 2B (PB > DEX > VEH), differences in the velocities due to the different PCB concentrations (i.e., Vmax is probably only reached in 50 μM PCB 136 incubations) and the metabolism of both OH-PCB metabolites to 4,5-diOH-PCB 136.
Implications for toxicokinetic and toxicological studies
The time-dependent enantiomeric enrichment of several PCB congeners, including PCB 136, and methylsulfonyl PCB metabolites has been studied in vivo.41-44 These studies show time-dependent changes in the EF values of the parent PCB or the metabolites that are comparable to the trends observed in the present study for the EF values of PCB 136 and its hydroxylated metabolites. Specifically, a toxicokinetic study investigating the enantioselective disposition of chiral PCB congeners in female mice revealed that the enantiomeric fraction of all chiral PCB congeners investigated changed significantly within the first 24 hours after administration, but remained almost constant at later time points.41 Similarly, the enantiomeric fraction of PCB 84 (2,2',3,3',6-pentachlorobiphenyl) and PCB 136 did not change significantly between 3 and 6 days after intraperitoneal or oral administration.42, 43 A study investigating the enantiomeric enrichment of methylsulfonyl PCBs after administration of a single oral dose of Clophen A50, a technical PCB mixture manufactured in Germany, also showed no significant changes in the tissue EF values of the metabolites over the eight week study period.44
One possible explanation for the similar trends of the EF values in the in vitro and in vivo experiments is that enantioselective metabolism of PCBs by P450 enzymes is the biological process ultimately responsible for their enantiomeric enrichment in vivo. Furthermore, the apparent metabolism rates appear to be approximately constant with time, thus resulting in a constant ratio of the atropisomers of the parent PCB and its metabolites and, ultimately, similar EF values. Therefore, the EF values of PCB, which are frequently used in environmental source apportioning and food web transport studies,20 may provide a qualitative assessment insight into the differences in the apparent metabolism rates of PCB atropisomers in wildlife and in humans. However, further studies, including in-depth studies of the phase I and phase II metabolism of chiral PCBs and their metabolites, are needed to confirm this hypothesis.
The observation that OH-PCBs are formed enantioselectively by P450 enzymes and incubations with hepatic microsomes obtained from PB-, DEX- and VEH-treated rats display different OH-PCB atropisomer profiles raises questions that need to be considered in toxicological studies. OH-PCBs are potent sensitizers of RyRs, an important cellular target for PCB-mediated developmental neurotoxicity. For example, the concentration of 4-OH-PCB 136 needed to double basal RyR activity (EC2x) is 1.60 μM.7 Since (-)-PCB 136, but not (+)-PCB 136, displays activity towards RyRs, the enantioselective formation of OH-PCB 136 metabolites raises the question if OH-PCB atropisomers also display enantiospecificity towards RyRs. Furthermore, the induction of P450 enzymes by other xenobiotics, such as PB, DEX or PCBs themself, may alter the OH-PCB atropisomer profile in vivo and, thus, play a currently overlooked role in the developmental neurotoxicity of PCBs.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Michael A. Cripps, Jarline Encarnacion Medina, Thomas T. Rhoads and Hakkon Rosendahl for help with the microsomal metabolism studies.
FUNDING SOURCES The project described was supported by Award numbers ES05605, ES013661 and ES017425 from the National Institute of Environmental Health Sciences. S. Bandiera wishes to acknowledge the Natural Sciences and Engineering Research Council of Canada for their financial support. The hydroxylated PCB 136 metabolites were a generous gift from E.A. Mash and S.C. Waller of the Synthetic Chemistry Facility Core of the Southwest Environmental Health Sciences Center, funded by NIH grant ES06694. The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health or the Natural Sciences and Engineering Research Council of Canada.
ABBREVIATIONS
- 4,5-diMeO-PCB 136
4,5-dimethoxy-2,2',3,3',6,6'-hexachlorobiphenyl (methylated derivative of 4,5-diOH-PCB 136)
- 4-MeO-PCB 136
2,2',3,3',6,6'-hexachloro-4-methoxybiphenyl (methylated derivative of 4-OH-PCB 136)
- 5-MeO-PCB 136
2,2',3,3',6,6'-hexachloro-5-methoxybiphenyl (methylated derivative of 5-OH-PCB 136)
- 4,5-diOH-PCB 136
2,2',3,3',6,6'-hexachlorobiphenyl-4,5-diol
- 4-OHPCB 136
2,2',3,3',6,6'-hexachlorobiphenyl-4-ol
- 5-OH-PCB 136
2,2',3,3',6,6'-hexachlorobiphenyl-5-ol
- BROD
7-benzyloxyresorufin-O-debenzylase
- BQ
7-benzyloxyquinoline debenzylation
- DEX
dexamethasone
- DMSO
dimethyl sulfoxide
- EC2x
concentration needed to double basal receptor activity
- EF
enantiomeric fraction
- EROD
7-ethoxyresorufin-O-deethylase
- ID
inner diameter
- MTBE
methyl tert-butyl ether
- OH-PCB
hydroxylated polychlorinated biphenyl metabolite
- PB
phenobarbital
- PCBs
polychlorinated biphenyls
- PCB 136
2,2',3,3',6,6'-hexachlorobiphenyl
- PCB 84
2,2',3,3',6-pentachlorobiphenyl
- RyR
Ryanodine receptor
- SAR
structure-activity relationship
- μECD
microelectron capture detector
- VEH
vehicle (corn oil)
Footnotes
SUPPORTING INFORMATION AVAILABLE
Effect of PB, DEX and VEH treatment on body and liver weights in male rats used for preparation of microsomes; activities of P450 enzymes in male rat liver microsomes; limits of detection and mean levels of PCB 136 and hydroxylated metabolites in control samples in the microsomal incubations; metabolite ratios in different microsomal incubations; identification of hydroxylated PCB 136 metabolites by GC/MS; inhibition of the metabolism of PCB 136 by carbon monoxide; time-dependent formation of hydroxylated PCB 136 atropisomers by liver microsomes at PCB 136 concentrations of 5 and 50 μM; time-dependent metabolism of PCB 136 atropisomers by liver microsomes. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Robertson LW, Hansen LG. PCBs: Recent Advances in Environmental Toxicology and Health Effects. University Press of Kentucky; Lexington, KY: 2001. p. 461. [Google Scholar]
- 2.Erickson MD, Kaley RG. Applications of polychlorinated biphenyls. Environ. Sci. Pollut. Res. 2011;18:135–151. doi: 10.1007/s11356-010-0392-1. [DOI] [PubMed] [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry . Toxicological profile for polychlorinated biphenyls (PCBs) Department of Health and Human Services, Public Health Service; Atlanta, GA: 2000. [PubMed] [Google Scholar]
- 4.Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ. Health Perspect. 2003;111:357–376. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariussen E, Fonnum F. Neurochemical targets and behavioral effects of organohalogen compounds: an update. Crit Rev Toxicol. 2006;36:253–89. doi: 10.1080/10408440500534164. [DOI] [PubMed] [Google Scholar]
- 6.Kodavanti PRS. Intracellular signaling and developmental neurotoxicity. In: Zawia NH, editor. Molecular Neurotoxicology: Environmental Agents and Transcription-Transduction Coupling. CRC press; Boca Raton, FL: 2004. pp. 151–182. [Google Scholar]
- 7.Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1). Chem. Res. Toxicol. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- 8.Letcher RJ, Klasson-Wehler E, Bergman A. Methyl sulfone and hydroxylated metabolite of polychlorinated biphenyls. In: Passivirta J, editor. The handbook of environmental chemistry - New types of persistent halogenated compounds. 3K. Springer-Verlag; Berlin Heidelberg: 2000. pp. 315–359. [Google Scholar]
- 9.Nezel T, Müller-Plathe F,D,M, Müller DM, Buser HR. Theoretical considerations about chiral PCBs and their methylthio and methylsulfonyl metabolites being possibly present as stable enantiomers. Chemosphere. 1997;35:1895–1906. [Google Scholar]
- 10.Buckman AH, Wong CS, Chow EA, Brown SB, Solomon KR, Fisk AT. Biotransformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish. Aquat. Toxicol. 2006;78:176–185. doi: 10.1016/j.aquatox.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Kunisue T, Tanabe S. Hydroxylated polychlorinated biphenyls (OH-PCBs) in the blood of mammals and birds from Japan: lower chlorinated OH-PCBs and profiles. Chemosphere. 2009;74:950–61. doi: 10.1016/j.chemosphere.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 12.Nomiyama K, Murata S, Kunisue T, Yamada KT, Mizukawa H, Takahashi S, Tanabe S. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in the blood of toothed and baleen whales stranded along Japanese coastal waters. Environ. Sci. Technol. 2010;44:3732–3738. doi: 10.1021/es1003928. [DOI] [PubMed] [Google Scholar]
- 13.Guvenius DM, Aronsson A, Ekman-Ordeberg G, Bergman A, Norén K. Human prenatal and postnatal exposure to polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorobiphenylols, and pentachlorophenol. Environ Health Perspect. 2003;111:1235–1241. doi: 10.1289/ehp.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soechitram SD, Athanasiadou M, Hovander L, Bergman Å, Sauer PJJ. Fetal exposure to PCBs and their hydroxylated metabolites in a Dutch cohort. Environ Health Perspect. 2004;112:1208–12. doi: 10.1289/ehp.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glynn A, Larsdotter M, Aune M, Darnerud PO, Bjerselius R, Bergman Å . Changes in serum concentrations of polychlorinated biphenyls (PCBs), hydroxylated PCB metabolites and pentachlorophenol during pregnancy. Chemosphere. 2011;83:144–151. doi: 10.1016/j.chemosphere.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 16.Dirtu AC, Jaspers VLB, Cernat R, Neels H, Covaci A. Distribution of PCBs, their hydroxylated metabolites, and other phenolic contaminants in human serum from two European countries. Environ. Sci. Technol. 2010;44:2876–2883. doi: 10.1021/es902149b. [DOI] [PubMed] [Google Scholar]
- 17.Wong P, Brackney W, Pessah I. Ortho-substituted polychlorinated biphenyls alter microsomal calcium transport by direct interaction with ryanodine receptors of mammalian brain. J. Biol. Chem. 1997;272:15145–15153. doi: 10.1074/jbc.272.24.15145. [DOI] [PubMed] [Google Scholar]
- 18.Wong P, Joy R, Albertson T, Schantz S, Pessah I. Ortho-substituted 2,2',3,5',6-pentachlorobiphenyl (PCB 95) alters rat hippocampal ryanodine receptors and neuroplasticity in vitro: evidence for altered hippocampal function. Neurotoxicology. 1997;18:443–456. [PubMed] [Google Scholar]
- 19.Wong PW, Pessah IN. Ortho-substituted polychlorinated biphenyls alter calcium regulation by a ryanodine receptor-mediated mechanism: structural specificity toward skeletal- and cardiac-type microsomal calcium release channels. Mol Pharmacol. 1996;49:740–51. [PubMed] [Google Scholar]
- 20.Lehmler HJ, Harrad SJ, Hühnerfuss H, Kania-Korwel I, Lee CM, Lu Z, Wong CS. Chiral polychlorinated biphenyl transport, metabolism, and distribution: A review. Environ Sci Technol. 2010;44:2757–66. doi: 10.1021/es902208u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pessah IN, Lehmler H-J, Robertson LW, Perez CF, Cabrales E, Bose DD, Feng W. Enantiomeric specificity of (-)-2,2',3,3',6,6'-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem. Res. Toxicol. 2009;22:201–207. doi: 10.1021/tx800328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warner NA, Martin JW, Wong CS. Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ. Sci. Technol. 2009;43:114–121. doi: 10.1021/es802237u. [DOI] [PubMed] [Google Scholar]
- 23.Kania-Korwel I, Vyas SM, Song Y, Lehmler H-J. Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomers. J. Chromatogr. A. 2008;1207:146–154. doi: 10.1016/j.chroma.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norstrom K, Eriksson J, Haglund J, Silvari V, Bergman A. Enantioselective formation of methyl sulfone metabolites of 2,2',3,3',4,6'-hexachlorobiphenyl in rat. Environ. Sci. Technol. 2006;40:7649–7655. doi: 10.1021/es061584t. [DOI] [PubMed] [Google Scholar]
- 25.Larsson C, Ellerichmann T, Huehnerfuss H, Bergman A. Chiral PCB methyl sulfones in rat tissues after exposure to technical PCBs. Environ. Sci. Technol. 2002;36:2833–2838. doi: 10.1021/es025512n. [DOI] [PubMed] [Google Scholar]
- 26.Shaikh N, Parkin S, Lehmler HJ. The Ullmann coupling reaction: A new approach to tetraarylstannanes. Organometallics. 2006;25:4207–4214. [Google Scholar]
- 27.Kania-Korwel I, Hrycay EG, Bandiera SM, Lehmler HJ. 2,2',3,3',6,6'-hexachlorobiphenyl (PCB 136) atropisomers interact enantioselectively with hepatic microsomal cytochrome P450 enzymes. Chem. Res. Toxicol. 2008;21:1295–1303. doi: 10.1021/tx800059j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waller SC, He YA, Harlow GR, He YQ, Mash EA, Halpert JR. 2,2',3,3',6,6'-hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem. Res. Toxicol. 1999;12:690–699. doi: 10.1021/tx990030j. [DOI] [PubMed] [Google Scholar]
- 29.Blomquist CH, Kotts CE, Hakanson EY. A simple method for detecting steroid aggregation and estimating solubility in aqueous solutions. Anal. Biochem. 1978;87:631–635. doi: 10.1016/0003-2697(78)90714-5. [DOI] [PubMed] [Google Scholar]
- 30.Thomas PE, Reik LM, Ryan DE, Levin W. Induction of two immunochemically related rat liver cytochrome P-450 isozymes, cytochromes P-450c and P-450d, by structurally diverse xenobiotics. J. Biol. Chem. 1983;258:4590–4598. [PubMed] [Google Scholar]
- 31.Lowry OH, Rosenbrough NJ, Rarr AL, Randall RJ. Protein measurement with Folin Phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Weisbrod AV, Shea D, Moore MJ, Stegeman JJ. Bioaccumulation patterns of polychlorinated biphenyls and chlorinated pesticides in Northwest Atlantic pilot whales. Environ. Toxicol. Chem. 2000;19:667–677. [Google Scholar]
- 33.Lagueux J, Affar EB, Adeau D, Ayotte P, Dewailly D, Poirer GG. A microassay for the detection of low levels of cytochrome P450 O-deethylation activities with alkoxyresorufin substrates. Mol. Cell. Biochem. 1997;175:125–129. doi: 10.1023/a:1006835712436. [DOI] [PubMed] [Google Scholar]
- 34.Renwick AB, Lavignette G, Worboys PD, Williams B, Surry D, Lewis DFV, Price RJ, Lake BG, Evans DC. Evaluation of 7-benzyloxy-4-trifluoromethyl-coumarin, some other 7-hydroxy-4-trifluoro-methylcoumarin derivatives and 7-benzyloxyquinoline as fluorescent substrates for rat hepatic cytochrome P450 enzymes. Xenobiotica. 2001;31:861–878. doi: 10.1080/00498250110074063. [DOI] [PubMed] [Google Scholar]
- 35.Schnellmann R, Putnam C, Sipes I. Metabolism of 2,2',3,3',6,6'-hexachlorobiphenyl and 2,2',4,4',5,5'-hexachlorobiphenyl by human hepatic microsomes. Biochem. Pharmacol. 1983;32:3233–3239. doi: 10.1016/0006-2952(83)90209-5. [DOI] [PubMed] [Google Scholar]
- 36.Kania-Korwel I, Hornbuckle KC, Peck A, Ludewig G, Robertson LW, Sulkowski WW, Espandiari P, Gairola CG, Lehmler HJ. Congener specific tissue distribution of Aroclor 1254 and a highly chlorinated environmental PCB mixture in rats. Environ. Sci. Technol. 2005;39:3513–3520. doi: 10.1021/es047987f. [DOI] [PubMed] [Google Scholar]
- 37.Kania-Korwel I, Zhao H, Norstrom K, Li X, Hornbuckle KC, Lehmler HJ. Simultaneous extraction and clean-up of polychlorinated biphenyls and their metabolites from small tissue samples using pressurized liquid extraction. J. Chromatogr. A. 2008;1214:37–46. doi: 10.1016/j.chroma.2008.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kania-Korwel I, Vyas S, Song Y, Lehmler HJ. Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomer. J. Chromatogr. A. 2008;1207:146–154. doi: 10.1016/j.chroma.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Preston BD, Miller JA, Miller EC. Non-arene oxide aromatic ring hydroxylation of 2,2',5,5'-tetrachlorobiphenyl as the major metabolic pathway catalyzed by phenobarbital-induced rat liver microsomes. J Biol Chem. 1983;258:8304–11. [PubMed] [Google Scholar]
- 40.Haglund P, Wiberg K. Determination of the gas chromatographic elution sequences of the (+) and (-) enantiomers of stable enantiomeric PCBs on Chirasil-Dex. J. High Resol. Chromatogr. 1996;19:373–376. [Google Scholar]
- 41.Kania-Korwel I, El-Komy MH, Veng-Pedersen P, Lehmler HJ. Clearance of polychlorinated biphenyl atropisomers is enantioselective in female C57Bl/6 mice. Environ Sci Technol. 2010;44:2828–35. doi: 10.1021/es901781p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kania-Korwel I, Shaikh N, Hornbuckle KC, Robertson LW, Lehmler H-J. Enantioselective disposition of PCB 136 (2,2',3,3',6,6'-hexachlorobiphenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality. 2007;19:56–66. doi: 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- 43.Lehmler H-J, Price DJ, Garrison AW, Birge WJ, Robertson LW. Distribution of PCB 84 enantiomers in C57Bl/6 mice. Fresenius’ Environ. Bull. 2003;12:254–260. [Google Scholar]
- 44.Larsson C, Ellerichmann T, Hühnerfuss H, Bergman A. Chiral PCB methyl sulfones in rat tissues after exposure to technical PCBs. Environ Sci Technol. 2002;36:2833–8. doi: 10.1021/es025512n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.