Introduction
Multiple lines of evidence are converging on the concurrence that temporal processing is increasingly compromised in the aging auditory system. One line of evidence is psychophysical wherein behavioral responses to tasks that reflect temporal coding indicate increased deficits in older listeners (for review, see Fitzgibbons and Gordan-Salant 2010; Walton 2010). These tasks include measurements of ‘envelope level’ temporal processing such as temporal modulation transfer functions (He et al. 2008; Purcell et al. 2004) and gap detection/discrimination (Grose et al. 2006; He et al. 1999; Heinrich and Schneider 2006; Lister et al .2002; Pichora-Fuller et al. 2006; Schneider et al. 1998; Schneider and Hamstra 1999; Schneider et al. 1994; Snell 1997; Snell and Frisina 2000; Strouse et al. 1998). Age-related reductions insensitivity to arrhythmias in ongoing sounds (Fitzgibbons and Gordon-Salant 2010; Fitzgibbons et al. 2007) can also be considered a deficit in temporal envelope processing. In addition to deficits in envelope processing, age-related deficits at the ‘fine structure level’ of temporal processing are also observed. Evidence for this comes from tasks such as frequency modulation detection at low frequencies (He et al. 2007)and sensitivity to interaural phase differences (Grose and Mamo 2010; Ross et al. 2007).
A second line of evidence for temporal processing deficits in the aging auditory system is electrophysiological wherein potentials evoked by temporal aspects of stimuli show age-related changes. These include evoked potentials that occur in response to stimulus onsets, including the auditory brainstem response (Poth et al. 2001; Vander Werff and Burns 2011; Walton et al. 1999) and cortical evoked potentials (Harris et al. 2007; Ostroff et al. 2003; Tremblay et al. 2004). Age-related changes in cortical evoked potentials have also been demonstrated for responses elicited by temporal gaps in stimuli (Lister et al. 2011), as well as steady-state potentials that follow the stimulus envelope (Grose et al. 2009; Leigh-Paffenroth and Fowler 2006; Purcell et al. 2004). Finally, age-related deficits are evident in potentials evoked by changes in ongoing fine structure (Ross et al. 2007; Wambacq et al. 2009) or in steady-state potentials that follow the fine structure of the stimulus itself (Clinard et al. 2010). It is this latter aspect that is the topic of this study; i.e., the electrophysiological measurement of fine structure coding particularly as it relates to the aging auditory system.
One evoked potential that would appear well suited to the study of fine structure processing is the frequency following response (FFR), reviewed by Krishnan (2007). Classically, this potential refers to the recording of electrophysiological activity that follows the cycle-by-cycle oscillation of the stimulus above and below ambient pressure. The term FFR, however, has become somewhat more generically used to refer also to electrophysiological activity that follows the periodicity of the stimulus (e.g., Johnson et al. 2005). In that the periodicity of a complex stimulus may be represented by its envelope as well as by its fine structure, this usage of FFR blurs the distinction between frequency following responses and envelope following responses. In this study, we use FFR to refer strictly to the response to tonal stimuli such that it is the encoding of fine structure and not envelope that is the focus. Clinard et al. (2010) measured the FFR elicited by tones as a function of age. They observed that, under some conditions, both the amplitude of the FFR and its phase coherence declined with age.
One challenge to the interpretation of the FFR in humans is that the electrical activity recorded by scalp electrodes in response to tonal stimulation can consist of at least three components: (1) stimulus artifact; (2) cochlear microphonic (CM); and (3) phase-locked neural response. Removing the contribution of the stimulus artifact involves such techniques as maximizing the physical separation between transducers and electrodes, minimizing radiation by shielding the transducers with mu-metal casings, and/or alternating the stimulus phase in the averaging process to cancel out the artifact. This latter technique removes the stimulus artifact but also has ramifications for the recording of the veridical electrophysiological response. First, alternating phase will also tend to cancel out the CM. Second, given that neural transduction in regions of strong phase locking amounts to half-wave rectification because of the directional sensitivity of the hair cells, alternating stimulus phase will double the fundamental frequency of the neural response; i.e., the averaged neural response to alternating phase amounts to full-wave rectification(Sohmer and Pratt 1977). Various studies have attempted to distinguish between CM and neural contributions to the tone-evoked FFR without using alternating phase by: (a) capitalizing on the susceptibility of neural responses to adaptation and masking, in contrast to hair cell responses (Chimento and Schreiner 1990; De Boer et al. 1977); (b) gauging the latency of the response (Yamada et al. 1977); (c)capitalizing on the frequency dependence of phase locking (i.e., phase locking declines above about 1000 Hz (Kiang et al. 1965; Rose et al. 1967)); (d)making the assumption that the CM response will be generally uni-frequency whereas the neural response will be spectrally complex (Krogh et al. 1977); or (e) assessing responses in patients with specific known lesions in the auditory system (Sohmer et al. 1977). [Support for these approaches also comes from animal studies using invasive techniques but a full review of those studies is not warranted here; the interested reader is referred to original works by Worden and colleagues as a starting point (Marsh et al. 1972; Worden and Marsh 1968).]Although these techniques are successful to varying degrees, the fact remains that isolating the neural FFR elicited by a tonal stimulus from other frequency-following elements can be challenging. A more attractive solution would be to employ an electrophysiological measure of fine structure coding that depends on neural phase locking but is not subject to the response complexities of the FFR; i.e., one where the response frequency is not present in the stimulus. One such possibility is the electrophysiological response to binaural beats.
The binaural beat refers to the perception of a fluctuation or flutter resulting from the dichotic presentation of two spectrally distinct tones (primaries) over headphones. That is, each ear receives a tone of a different frequency and the perceptible fluctuation occurs at the difference frequency between the two tones. Psychophysically, the binaural beat is dominant for low-frequency primaries and for relatively small frequency separations between primaries (i.e., low modulation rates) (Licklider et al. 1950; Perrott and Musicant 1977). These findings are in line with the assumption that the binaural beat reflects an interaction between phase-locked responses ascending from each ear. As such, the binaural beat presents two important potential advantages over monaural measures. First, any disruption in phase locking in the monaural pathway is likely to be compounded at the level of binaural interaction. This has been elegantly shown by Batra et al.(1997) who demonstrated that, in cells in the superior olive sensitive to interaural time differences, the synchronization coefficient (strength of phase locking) to binaural input is the product of the coefficients to monaural input. Since the monaural synchronization coefficients (left and right inputs) are typically less than 1.0, the binaural synchronization coefficient must necessarily be still lower. Thus, any compromise in monaural phase locking will be exacerbated in the binaural response. This suggests that the binaural beat may actually be a more sensitive indicator of fine structure coding deficits than a monaural indicator. The second potential advantage of the binaural beat over monaural measures is that the frequency of the binaural beat does not exist in the stimulus presented to either ear. Thus, issues related to the contribution of stimulus artifact or CM to the FFR are moot.
Recent studies have demonstrated the feasibility of recording the binaural beat electrophysiologically (Draganova et al. 2008; Karino et al. 2006; Pratt et al. 2009; 2010; Schwarz and Taylor 2005). While these studies differ somewhat in their methodology and source analyses, all found the beat frequency to be present as a spectrally distinct steady-state potential; i.e., the magnitude of the response component at the beat frequency was significantly larger than that of surrounding components comprising the noise floor. Schwarz and Taylor (2005) measured a 40-Hz beat frequency in the electroencephalogram (EEG) with a pair of low-frequency primaries (380 & 420 Hz) and a pair of high-frequency primaries (3180 & 3220 Hz). Consistent with the finding that the upper frequency limit for the perception of binaural beats is about 1000 Hz (Licklider et al. 1950), no significant beat frequency responses were recorded for the high frequency tone pair. Pratt et al. (2009) also studied frequency effects using primaries in the 250-and 1000-Hz regions and frequency separations of the primary pairs yielding 3-and 6-Hz binaural beats. Waveform analysis of the recorded EEG indicated larger response amplitudes for the lower beat frequency and the lower frequency region. Two magnetoencephalographical (MEG) binaural beat studies suggested that the binaural beat component was present in the response recording; however, the amplitudes were low and the phase of the response was inconsistent (Draganova et al. 2008; Karino et al. 2006). These results support the use of the binaural beat as an indicator of fine structure coding but raise questions about the stability of the response. In addition, further investigation into the frequency effects of the response is warranted in the context of determining age-related differences.
In summary, the purpose of this study was to determine the reliability of the electrophysiological binaural beat steady-state response as a gauge of temporal fine structure coding, particularly as it relates to the aging auditory system. Two experiments were undertaken: (1) a study of the effects of primary tone frequency, and (2) a study of the effects of participant age.
Experiment 1. Effects of primary tone frequency
The purpose of experiment 1 was to determine whether the response to the individual primary tones, as well as to the binaural beat elicited by those primaries, was greater in a lower frequency region than in a higher frequency region. This hypothesis has already received some support, as noted above, and is based on the premise that the synchrony index (degree of phase locking) of the neural substrate responding to the stimulus is higher in the lower frequency region. A second purpose of experiment 1 was to assess the stability of the binaural beat as a steady-state response relative to the FFRs elicited by the monaural tones themselves.
Methods
Participants
Ten young adults ranging in age from 21 – 29 years (mean=25.2 yrs; 8 female) participated. All participants had normal audiometric hearing (≤20 dBHL) from 250 – 8000 Hz. (Mean audiograms are included in Table 1 from experiment 2.)
Table 1.
Mean audiometric thresholds in dB HL (with associated standard deviations) for the older participants of experiment 2 and the younger participants of experiment 1.
| Age group | Ear | 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | 8000 Hz |
|---|---|---|---|---|---|---|---|
| Older | L | 10.4 (5.0) | 8.9 (4.5) | 8.2 (6.4) | 9.3 (6.5) | 19.3 (3.9) | 42.5 (15.8) |
| R | 12.5 (7.3) | 8.6 (5.7) | 9.6 (4.6) | 10.7 (8.7) | 20.0 (9.8) | 41.8 (14.2) | |
| Younger | L | 5.0 (5.3) | 2.0 (4.8) | 2.5 (3.5) | 1.5 (6.7) | 2.0 (5.4) | 6.5 (9.7) |
| R | 4.0 (5.2) | 0.5 (3.7) | 1.5 (2.4) | 1.0 (3.9) | 1.5 (5.3) | 3.0 (5.4) |
Stimuli
Two pairs of primary tones were employed: (1) 390 and 430 Hz, and (2) 810 and 850 Hz. Based on the function relating synchrony index to frequency (Kiang et al. 1965; Rose et al. 1967), the strength of phase locking was expected to be higher for the lower-frequency pair than the higher-frequency pair. For both pairs, the frequency difference – and therefore the binaural beat rate – was 40 Hz. This binaural beat frequency was selected because it tends to be associated with a robust steady-state response (Picton et al. 2003) and was successfully used by Schwarz and Taylor (2005). The duration of each primary tone was 2070 ms, including a raised cosine rise/fall ramp of 20 ms, but an onset asynchrony was incorporated into the dichotic presentation such that the left ear (receiving the higher-frequency primary of the pair) led the right ear by 410 ms. As a result, both ears were simultaneously stimulated for 1660 ms(1620 ms at full amplitude), after which the trailing right ear continued for a further 410 ms. This configuration was implemented to permit – if of interest retrospectively – the measurement of the FFR to each tone in isolation, as well as the response to the simultaneous tones; however, the focus of this study was on the steady-state response during the period of binaural stimulation. The tones were presented at levels designed to be comfortably loud for the listener; the level was 78 dB SPL for the lower-frequency pair and 75 dB SPL for the higher-frequency pair. Each presentation of the pair of primary tones was separated by ban inter-stimulus interval of 840 ms, resulting in an overall presentation rate of about 0.3 Hz. Note that the onset phase of the stimulus was constant across presentations (i.e., alternating phase was not employed). Coincident with the onset of the leading tone for each presentation, a 1-ms rectangular pulse was output through a digital port to time stamp the continuously recorded EEG trace. To prevent stimulus artifact contamination, the transducers (KOSS/90) were located outside the double-walled sound-attenuating booth in custom casings and were coupled to the foam ear tips by 3 m of tubing.1 Stimulus generation and timing were controlled by a digital signal processing platform (Tucker-Davis Technologies) operating at a base sampling rate of 24,414 Hz.
Recording and Analysis
For the recording session, which typically lasted between 1–2 hours for a single pair of primary tones, the participants at in the sound-attenuating booth and relaxed in a reclined chair while watching a muted movie of choice with subtitles. The EEG was recorded differentially between the high forehead (Fz) and nape of neck using one bipolar channel of a multi-channel recording system (Compumedics Neuroscan). The ground electrode was situated on the low forehead (Fpz) and all electrode impedances were maintained at less than 2 kΩ throughout the recording session. The continuous EEG was sampled at a rate of 5000 Hz, band-passed filtered between 0.05 and 1000 Hz and saved to disk. A sufficiently long recording was acquired to ensure that, following off-line conditioning, about 1000 acceptable time-marked response epochs were available for analysis. The off-line conditioning began with visual inspection of the entire record for periods of artifactually large amplitude – such as from participant movement or eye blink – and these were demarcated for exclusion. Next, the channel was digitally re-filtered from 20 – 1000 Hz at 24 dB/octave. After this conditioning, the recording was segmented into epochs (referenced to the time markers), such that each epoch encompassed a designated segment of the response. As noted above, the analysis of the binaural beat and FFRs elicited by the primary tone pairs was conducted only on the response segment during which the two primary tones were simultaneously present at full amplitude at the two ears; i.e., the analysis segment was 1620 ms in duration. This segmentation took account both of the acoustic delay from the transducers to the ear inserts and the rise/fall time of the 20-ms gates. The epochs were then submitted to two different analyses. In the first analysis, all epochs were time-domain averaged together to yield a single mean waveform that was then submitted to spectral analysis via fast fourier transform (FFT), with 0.5-Hz resolution. A response was considered present if the magnitude of the component at the designated frequency was significantly greater (p < 0.05) than the surrounding noise floor, as determined by an F ratio statistic (Picton et al. 2003). The noise floor was derived from 47 spectral bins spanning approximately 5–15 Hz below and 5–15 Hz above the target response bin. The second analysis applied to the epochs was an assessment of magnitude-squared coherence (MSC) (Champlin 1992). Here, MSC was calculated across the set of epochs using the equation:
where n = number epochs and ∅ is the phase angle of the component at the response frequency of interest.
Results & Discussion
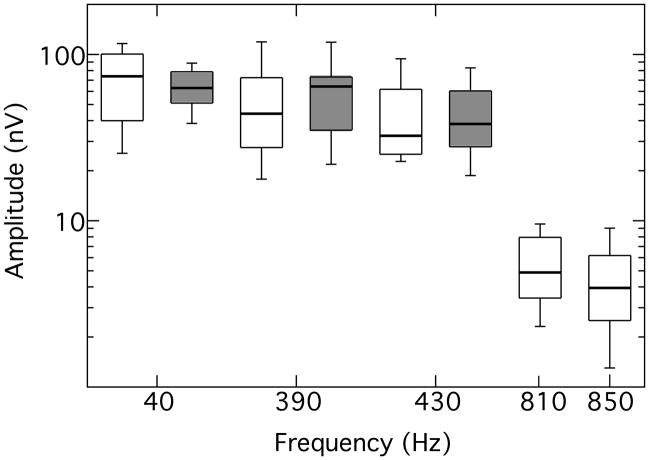
On average, about 1013 acceptable response epochs were obtained from each participant for each of the two primary tone pairs (range = 921 – 1078 epochs). All participants exhibited FFRs2 at all primary tone frequencies, as validated by both the F ratio statistic and the MSC measure, with the exception of one participant at 850 Hz. The distribution of FFR amplitudes is shown in Fig. 1, which also includes the results of experiment 2. The figure displays box-and-whisker plots of amplitude distributions (log scale) for all valid responses as a function of response frequency. For each distribution, the rectangle encompasses the 25th – 75th percentile, with the horizontal line indicating the median, and the capped bars marking the 10th – 90th percentile. The unfilled rectangles denote results from this experiment; the filled rectangles are from experiment 2 and will be discussed later. Comparing the distributions for the 390- and 430-Hz primary tone pair with those for the 810- and 850-Hz pair, it is evident that the FFR amplitudes are markedly greater for the lower-frequency primaries than for the higher-frequency primaries. This was confirmed with a repeated-measures analysis of variance (ANOVA) on the log transforms of the amplitudes that showed a significant effect of frequency region (F[1,8] = 149.37; p < 0.01), but no effect of primary tone within pairs (F[1,8] = 1.79; p = 0.22). Across the 10 participants, FFR amplitudes were highly correlated for the lower pair of primary tone frequencies (r = 0.90; p < 0.01) but not for the higher frequency pair(r = 0.01; p = 0.82). The MSC measures for the FFRs showed a similar pattern. All participants exhibited a significant MSC (p < 0.05) for all primary tone frequencies except for the one participant at 850 Hz. In parallel with the amplitude data, a repeated-measures ANOVA showed a significant effect of frequency region (F[1,8] = 7.60; p = 0.03), but no effect of primary tone within pairs (F[1,8] = 0.25; p = 0.63).
Fig. 1.
Amplitude distribution for valid responses at each response frequency. The 390-, 430-, 810-, and 850-Hz frequencies are FFRs; the 40-Hz frequency is the binaural beat elicited by the lower-frequency primaries. Each rectangle encompasses the 25th – 75th percentile, with the horizontal line indicating the median, and the capped bars encompass the 10th – 90th percentile. Open rectangles are results from younger listeners (exp. 1); filled rectangles are results from older listeners (exp. 2)
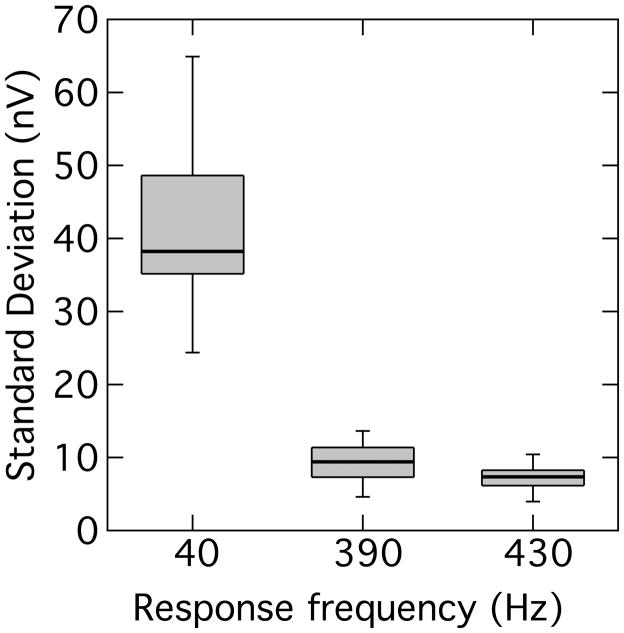
All 10 participants exhibited binaural beat responses to the lower-frequency pair of primaries, but only one participant exhibited a binaural beat response to the higher-frequency primaries. The distribution of binaural beat response amplitudes is also summarized in Fig. 1. In order to gauge the stability of the response, the amplitude of the 40-Hz binaural beat was sampled in 100-ms time windows shifted in 50-ms steps (50% overlap) along the response waveform. For comparison, the amplitudes of the 390- and 430-Hz components were also sampled in similar fashion. The standard deviation of these amplitude samples within each time-series trace was computed and the distribution of standard deviations compiled as a box-and-whisker plot shown in Fig. 2. It can be seen that the amplitude variation was markedly higher in the binaural beat response than in either FFR. Because the 100-ms window contained relatively few cycles of the 40-Hz component, a second exercise was also undertaken to compare two longer successive waveform segments of 800 ms each. This comparison showed that the absolute amplitude change (in dB) between the two segments was significantly greater for the 40-Hz component than for the average change of the FFR components (t[9] = 3.34; p < 0.01). This pattern of results suggests that the binaural beat is a more labile response than the FFR. Further support for this suggestion is provided by the finding that the MSC for the binaural beat was significantly lower than the average MSC for the FFRs (t[9] = 3.81; p < 0.01). As an aside, although it might be expected that the neural contribution to the FFR should also show adaption as a function of stimulus duration (Marsh et al. 1972; Worden and Marsh 1968), this was not observed: the amplitude of the FFR as a function of time appeared to remain relatively stable.
Fig. 2.
Distribution of amplitude standard deviations for individual response traces. Each rectangle encompasses the 25th – 75th percentile, with the horizontal line indicating the median, and the capped bars marking the 10th – 90th percentile.
In summary, the results of experiment 1 indicate that FFRs can be elicited by tones in both a lower-frequency region (390- and 430-Hz) and in a region approximately an octave higher (810- and 850 Hz). The responses are larger – and have a greater MSC – in the lower-frequency region, in line with the study of Clinard et al. (2010) who found larger FFR amplitudes in the 500-Hz region compared to the 1000-Hz region. The dependence of FFR on tone frequency likely reflects the roll-off of neural phase-locking with increasing frequency. Despite the presence of FFRs in both frequency regions, the binaural beat was essentially restricted to the lower-frequency primaries. The general absence of the binaural beat to the 810- and 850-Hz primaries, despite the presence of FFRs to these frequencies, is also compatible with an interpretation in terms of neural phase-locking: diminished phase-locking in the monaural pathway should be compounded at the level of binaural interaction (Batra et al. 1997). This observation supports the hypothesis that the binaural beat is a more sensitive gauge of fine structure coding than the FFR. However, the relative variability of the binaural beat within a participant over time would appear to undermine its potential utility as a gauge of fine structure coding.
Experiment 2. Effects of age
The main backdrop to this investigation concerns age-related deficits in temporal fine structure processing. Although the results of experiment 1 are cautionary in terms of the relative stability of the electrophysiological binaural beat response and therefore of its use as a gauge of fine structure coding, experiment 2 proceeded to examine the effects of age on this response. The purpose was to compare responses from a group of older participants having relatively normal audiometric hearing with those of the younger participants measured in experiment 1.
Method
Participants
Fourteen older adults ranging in age from 64 – 76 years (mean=69.1 yrs; 9 female) participated. All participants had audiometric thresholds ≤20 dBHL from 250 – 2000 Hz and ≤30 dBHL at 4000 Hz, with the exception of two participants who each had a single threshold of 25 dB HL at 250 Hz. There were no significant interaural threshold asymmetries across this frequency range, and the average difference between ears at each frequency was <5 dB. Mean audiograms for the older participants are shown in Table 1; also included in this table for comparison are the mean audiograms for the younger participants from experiment 1. Although the older participants had audio metrically normal thresholds through at least 2000 Hz (exceeding the frequency range of the stimuli in this experiment), a repeated measures ANOVA indicated that their thresholds were significantly higher than those of the younger participants at all audiometric frequencies (F(1,22) ranging from 9.8 – 71.6; p < 0.01).
Stimuli, Recording and Analysis
The same methodology was used as in experiment 1. However, because the binaural beat response was largely absent for the higher-frequency primary pair in the younger participants, only the lower-frequency primary pair (390 and 430 Hz) was tested here.
Results& Discussion
On average, about 1025 acceptable response epochs were obtained from each older participant (range = 870 – 1196 epochs). Dealing first with the FFRs elicited by the primary tones, assessment of the signal-to-noise ratios using the F statistic indicated that 12 of the 14 participants exhibited FFRs significantly above the noise floor at both the 390- and 430-Hz primary tone frequencies. Of the remaining two participants, one exhibited a response only at 430 Hz, and the other did not exhibit a response at either 390 or 430 Hz. The pattern of results from the MSC analysis was largely complementary: The two participants just noted who showed, respectively, no significant FFR at 390 Hz alone, and no significant FFR at both 390 Hz and 430 Hz, also showed no significant MSC at these frequencies. However, as a minor incongruity, one participant whose 430-Hz FFR was deemed significant by the F statistic exhibited a marginally insignificant MSC at this frequency (p = 0.065). Thus, only 11 participants exhibited a significant MSC for both the 390- and 430-Hz FFRs. For these 11 participants with valid FFRs at both primary tone frequencies, the response amplitudes for the two primaries were highly correlated (r = 0.91; p < 0.01). The distributions of valid FFR amplitudes are shown in Fig. 1 as shaded rectangles. In order to test for an age effect, the log-transformed FFR amplitudes for the 11 participants with valid FFRs by both statistical measures were compared to those of the young adults from exp. 1 using an ANOVA with age as a between-subjects factor (Younger, Older) and frequency as a within-subjects factor (390 Hz, 430 Hz). The results indicated no significant effect of age (F[1,19] = 0.22; p = 0.65), although a significant frequency effect reflected the somewhat larger amplitudes at 390 Hz re. 430 Hz (F[1,19] = 9.36; p = 0.01). There was no significant interaction between age and frequency (F[1,19] = 1.52; p = 0.23). A complementary analysis on the MSC data indicated no effect of age (F[1,19] = 1.47; p = 0.24) or frequency (F[1,19] = 1.81; p = 0.19) and no interaction between these factors (F[1,19] = 0.31; p= 0.58). Thus, there was no indication of an effect of age in this data set of valid FFRs. This result is compatible with Clinard et al. (2010) who found no effect of age in a lower-frequency region (463 – 500 Hz), although they did find a dependence of FFR amplitude and phase coherence on age at some frequencies in the 925 – 1000 Hz region. Recall that we did not test in a higher frequency region because of the failure to find robust binaural beat responses there in younger participants.
In terms of the binaural beat, only 9 of the 14 older participants exhibited a significant 40-Hz response, unlike the younger participants who uniformly showed a binaural beat response. The presence of a significant binaural beat in the 9 participants was confirmed by both the F ratio statistic and the MSC measure. The distribution of binaural beat amplitudes for the older age group is shown in Fig. 1. Responses were compared across age groups for the 9 older participants and the 10 younger participants of experiment 1. Preliminary assessment with Levene’s tests indicated that the variances of the log-transformed amplitudes and the MSC values were well matched across age groups, permitting the use of parametric t-tests. The t-tests indicated no significant differences in response amplitude (t[17] = 0.62, p = 0.55) or MSC (t[17] = 0.75, p = 0.46). Thus, for the stimulus configuration used here, the binaural beat exhibited by older adults did not differ from that exhibited by younger adults. However, proportionally fewer older adults exhibited a binaural beat. A Fisher’s Exact test comparing the proportion of older adults (9/14) to younger adults (10/10) with binaural beats indicated that this difference in proportionality was significant for p= 0.05. In summary, therefore, younger adults are more likely to exhibit a binaural beat response than are older adults, but when older adults do exhibit a response it is similar to that of younger adults.
The amplitudes of the binaural beat response, as well as the FFRs, varied widely across individuals, both younger and older. In order to determine whether there was an association between the amplitude of the binaural beat and the amplitude of the FFR, a bivariate correlation was undertaken. Because the FFR amplitudes were themselves highly correlated within an individual for the 390- and 430-Hz primary tones, as noted earlier, these amplitudes were averaged for an individual and then compared with the respective binaural beat amplitude. For the 10 younger and 9 older participants with valid binaural beat responses, the resulting Pearson product moment correlation was 0.47, which was significant at the p < 0.05 level.
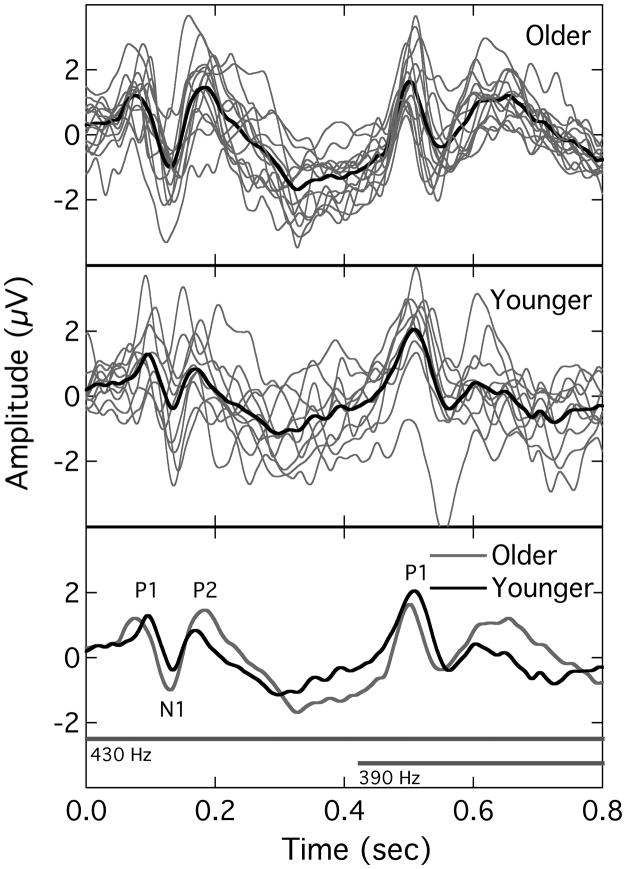
The results of this experiment provide no direct evidence for an age effect in terms of the characteristics of valid FFRs and binaural beat responses. This is not inconsistent with the findings of Clinard et al. (2010) who found no effect of age on FFR characteristics in a lower-frequency region (463 – 500 Hz), although they found some evidence for age effects at specific frequencies in a higher region (925 – 1000 Hz). The absence of a systematic age effect here raises the question of whether this absence was specific to the steady state responses measured in this study or whether it reflected a general lack of an age effect among this sample of participants. In order to test this, the EEG recordings collected in the present study were examined for other evidence of age effects. Specifically, the raw recordings were re-analyzed to extract another class of evoked potentials: the P1-N1-P2 complex. This is a conglomerate of potentials generated at the level of the cortex that can be evoked by the onset of a stimulus. Several studies have demonstrated age-related changes in this complex in response to parametric variation in, for example, stimulus duration (Ostroff et al. 2003), rate (Tremblay et al. 2004), and intensity (Harris et al. 2007). Because the raw data in the present study consisted of continuous EEG recordings time-stamped for stimulus presentation, it was possible to reanalyze the recordings to extract the P1-N1-P2 complex. However, it should be stressed that the stimuli used here were not optimal for eliciting the P1-N1-P2 complex because of their long duration (extending beyond that of the entire response latency) and relatively slow rise-time (20 ms). To obtain the P1-N1-P2 complex, the raw EEG data were re-filtered from 1 – 30 Hz (24 dB/octave), conditioned to remove eye-blink artifacts, segmented into 810-ms epochs relative to the onset of the leading primary tone, and then time-domain averaged. The resulting waveform represented the P1-N1-P2 complex evoked by the onset of the leading 430-Hz tone as well as a subsequent response evoked by the onset of the lagging 390-Hz tone 410 ms later. Fig. 3 shows the results for the older participants (upper panel) and younger participants (middle panel). In each panel, the gray traces are the individual responses and the thick black trace is the group average. For comparison, the lower panel re-plots the mean traces for the two age groups along with horizontal bars representing the time course of the two primary tones. Although there was relatively high variability across participants, the mean traces capture a regularity that was generally common across responses. Three independent judges evaluated the traces for occurrence/location of the P1-N1-P2 response peaks (one judge was an author). Whereas all three judges concurred on the presence of response peaks for the initial complex, there was uniform failure to identify a clear triad of peaks in many of the traces for the later complex. Indeed, the only peak that was marked with any consistency in the second complex was P1. Therefore, only the main vertex-positive peak was marked for the latter complex (in 9 of 10 younger participants and 13 of 14 older participants). Identified peaks are labeled in the lower panel of Fig. 3.
Fig. 3.
Cortical potentials evoked by the asynchronous stimulus onsets. In the upper (older) and middle (younger) panels, the individual traces are shown as gray lines, with the heavy dark trace being the mean response. The lower panel replots the mean responses for the two groups and includes gray bars that show the relative onsets of the left and right primary tones.
Dealing first with the initial P1-N1-P2 complex, an ANOVA was undertaken with peak latency as a within-subjects factor and age group as a between-subjects factor. The three sequential peaks differed, of course, in latency (F(2,44) = 356.43; p < 0.01) but there was no main effect of age (F(1,22) = 0.00; p = 0.99). However, there was a significant interaction between age group and peak latency. Analysis of simple main effects indicated that the two age groups did not differ in the latency of N1 and P2 (F(1,22) = 0.25 & 3.93; p = 0.62 & 0.06, respectively), but did differ in the latency of P1 (F(1,22) = 7.93; p = 0.01). Additionally, a t-test indicated that the log-transformed N1 amplitudes (N1-P2 peak voltage difference), as necessitated by a Levene’s test for equality of variances, was larger for the older participants than for the younger participants (t(22) = 2.72; p = 0.01). In terms of the later response complex, a t-test on the latency of the only ubiquitous peak (P1) indicated no effect of age (t(20) = 0.51; p = 0.61). In summary, therefore, an analysis of onset responses using the cortical P1-N1-P2 complex gave some indication of an age effect, with the older participants exhibiting an earlier P1 and an increased N1 amplitude. Harkrider et al. (2005) have also reported increased N1 amplitude with age. Thus, the recordings of this experiment contained some evidence of age-related differences in onset responses, although differences in steady state responses were less uniform across the two age groups.
Summary & Conclusion
The intent of this study was to determine the reliability of the electrophysiological binaural beat steady-state response as a gauge of temporal fine structure coding, and to examine the effects of age on this response. The theoretical advantages of the binaural beat response over the FFR as a neural metric of fine structure coding are that, (a) it is not susceptible to contamination by stimulus artifact or CM, and (b) deficits in fine structure coding at the monaural level might be compounded in the binaural response. In younger adults, FFRs were present at both a lower frequency region (390 Hz & 430 Hz) and at a higher frequency region (810 Hz & 850 Hz), although the amplitude and MSC of the FFR was reduced in the higher frequency region. A 40-Hz binaural beat response was consistently observed in the lower frequency region but not in the higher frequency region. When present, however, the binaural beat was a less stable response than the FFR, exhibiting greater amplitude fluctuation over time. Level fluctuation in the 40-Hz binaural beat is evident in the sample traces displayed by Schwarz and Taylor (2005) and they noted, anecdotally, that in their musically trained participants the amplitude of the binaural beat varied as a function of focused attention. In this vein, Karino et al. (2006) argued that the phase instability they observed in their MEG recordings of low-rate binaural beats might reflect contributions from higher-order cognitive processes. Pratt et al. (2009), in contrast, observed no such phase instability in their low-rate binaural beat responses. These related issues of binaural beat response stability and the role of higher-level cognitive processes remain unresolved but could have important ramifications for the use of the binaural beat as a measure of fine structure coding.
In terms of age effects, fewer older adults exhibited a binaural beat in response to 390- and 430-Hz primary tones compared to younger adults – a difference in proportionality that was significant. When present, both the FFRs and the binaural beat response were similar across age groups. Although Clinard et al. (2010) have observed an age-related decline in FFRs, this was observed only at primary frequencies in the 1000-Hz region; no age effects were noted in the 500-Hz region. This raises the issue that age effects might be more evident for combinations of primary tone frequency and beat rate not tested in the present study; further parametric investigation of this topic would be informative. In the context of aging effects, a comparison of onset P1-N1-P2 responses across the two age groups in the present study indicated that the older adults exhibited an earlier P1 latency and a larger N1 amplitude.
In conclusion, the results of this study advocate caution in the use of the binaural beat response as an objective measure of fine structure coding. Nevertheless, the lower prevalence of the response in older adults, despite the presence of FFRs, provides tentative support for the sensitivity of this measure to age-related deficits in temporal processing. Future research should include a systematic comparison of the behavior of the binaural beat measured electrophysiologically and psychophysically as this may shed additional light on the optimal approach to using this response as a gauge of temporal fine structure processing in the aging auditory system.
Acknowledgments
The assistance of Ellen Pearce in data collection is gratefully acknowledged. We thank Joseph W. Hall III, Emily Buss, and two anonymous reviewers for their helpful comments on a previous version of this paper. This work was supported by NIH NIDCD R01DC001507.
Work supported by NIDCD R01-DC01507
Footnotes
Cross-talk between the tubing was approximately 35 dB down (i.e., a primary tone presented through one tube was 35-dB down in the tube connected to the other transducer). A monaural control condition was undertaken where the primaries presented to one ear were either of equal level or one was 30-dB down from the other. A monaural 40-Hz steady-state response was recorded for the equal level primaries but no response was recorded for the unequal levels. This is in line with the chinchilla study by Arnold and Burkard (2000) who found no beat response in recordings from the inferior colliculus for a monaural presentation of primary tones that differed in level by more than 30 dB.
No attempt was made in this study to differentiate between the CM and the neural phase-locked response in the FFR. The wide physical separation between the transducers and the electrodes precluded the contamination of the response with stimulus artifact.
References
- Arnold S, Burkard R. Studies of interaural attenuation to investigate the validity of a dichotic difference tone response recorded from the inferior colliculus in the chinchilla. J Acoust Soc Am. 2000;107:1541–1547. doi: 10.1121/1.428439. [DOI] [PubMed] [Google Scholar]
- Batra R, Kuwada S, Fitzpatrick DC. Sensitivity to interaural temporal disparities of low- and high-frequency neurons in the superior olivary complex. II. Coincidence detection. J Neurophysiol. 1997;78:1237–1247. doi: 10.1152/jn.1997.78.3.1237. [DOI] [PubMed] [Google Scholar]
- Champlin CA. Method for detecting auditory steady-state potentials recorded from humans. Hear Res. 1992;58:63–69. doi: 10.1016/0378-5955(92)90009-c. [DOI] [PubMed] [Google Scholar]
- Chimento TC, Schreiner CE. Selectively eliminating cochlear microphonic contamination from the frequency-following response. Electroencephalogr Clin Neurophysiol. 1990;75:88–96. doi: 10.1016/0013-4694(90)90156-e. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings. Hear Res. 2010;264:48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer E, Machiels MB, Kruidenier C. Low-level frequency-following response. Audiol. 1977;16:229–240. [PubMed] [Google Scholar]
- Draganova R, Ross B, Wollbrink A, et al. Cortical steady-state responses to central and peripheral auditory beats. Cereb Cortex. 2008;18:1193–1200. doi: 10.1093/cercor/bhm153. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordan-Salant S. Behavioral studies with aging humans: Hearing sensitivity and psychoacoustics. In: Gordan-Salant S, Frisina RD, Fay RR, editors. The Aging Auditory System. Springer; 2010. [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Age-related differences in discrimination of temporal intervals in accented tone sequences. Hear Res. 2010;264:41–47. doi: 10.1016/j.heares.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S, Barrett J. Age-related differences in discrimination of an interval separating onsets of successive tone bursts as a function of interval duration. J Acoust Soc Am. 2007;122:458–466. doi: 10.1121/1.2739409. [DOI] [PubMed] [Google Scholar]
- Grose JH, Hall JW, 3rd, Buss E. Temporal processing deficits in the pre-senescent auditory system. J Acoust Soc Am. 2006;119:2305–2315. doi: 10.1121/1.2172169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Mamo SK. Processing of Temporal Fine Structure as a Function of Age. Ear Hear. 2010;31:755–760. doi: 10.1097/AUD.0b013e3181e627e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Mamo SK, Hall JW., 3rd Age effects in temporal envelope processing: speech unmasking and auditory steady state responses. Ear Hear. 2009;30:568–575. doi: 10.1097/AUD.0b013e3181ac128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkrider AW, Plyler PN, Hedrick MS. Effects of age and spectral shaping on perception and neural representation of stop consonant stimuli. Clin Neurophysiol. 2005;116:2153–2164. doi: 10.1016/j.clinph.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Harris KC, Mills JH, Dubno JR. Electrophysiologic correlates of intensity discrimination in cortical evoked potentials of younger and older adults. Hear Res. 2007;228:58–68. doi: 10.1016/j.heares.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Mills JH, Ahlstrom JB, et al. Age-related differences in the temporal modulation transfer function with pure-tone carriers. J Acoust Soc Am. 2008;124:3841–3849. doi: 10.1121/1.2998779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He NJ, Horwitz AR, Dubno JR, et al. Psychometric functions for gap detection in noise measures from young and aged subjects. J Acoust Soc Am. 1999;106:966. doi: 10.1121/1.427109. [DOI] [PubMed] [Google Scholar]
- He NJ, Mills JH, Dubno JR. Frequency modulation detection: effects of age, psychophysical method, and modulation waveform. J Acoust Soc Am. 2007;122:467–477. doi: 10.1121/1.2741208. [DOI] [PubMed] [Google Scholar]
- Heinrich A, Schneider B. Age-related changes in within- and between-channel gap detection using sinusoidal stimuli. J Acoust Soc Am. 2006;119:2316–2326. doi: 10.1121/1.2173524. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Nicol TG, Kraus N. Brain stem response to speech: a biological marker of auditory processing. Ear Hear. 2005;26:424–434. doi: 10.1097/01.aud.0000179687.71662.6e. [DOI] [PubMed] [Google Scholar]
- Karino S, Yumoto M, Itoh K, et al. Neuromagnetic responses to binaural beat in human cerebral cortex. J Neurophysiol. 2006;96:1927–1938. doi: 10.1152/jn.00859.2005. [DOI] [PubMed] [Google Scholar]
- Kiang NY-S, Watanabe T, Thomas EC, et al. Discharge Patterns of Single Fibres in the Cat’s Auditory Nerve. Cambridge, Mass: MIT Press; 1965. [Google Scholar]
- Krishnan A. Frequency-following response. In: Burkard RF, Don M, Eggermont JJ, editors. Auditory evoked potentials: Basic principles and clinical application. Baltimore: Lippincott Williams & Wilkins; 2007. pp. 313–333. [Google Scholar]
- Krogh HJ, Blegvad B, Stephens SDG. Harmonics in frequency-following responses: A preliminary report. Scand Audiol. 1977;6:157–162. doi: 10.3109/01050397709043117. [DOI] [PubMed] [Google Scholar]
- Leigh-Paffenroth ED, Fowler CG. Amplitude-modulated auditory steady-state responses in younger and older listeners. J Am Acad Audiol. 2006;17:582–597. doi: 10.3766/jaaa.17.8.5. [DOI] [PubMed] [Google Scholar]
- Licklider JCR, Webster JC, Hedlun JN. On the frequency limits of binaural beats. J Acoust Soc Am. 1950;22:468–473. [Google Scholar]
- Lister J, Besing J, Koehnke J. Effects of age and frequency disparity on gap discrimination. J Acoust Soc Am. 2002;111:2793–2800. doi: 10.1121/1.1476685. [DOI] [PubMed] [Google Scholar]
- Lister JJ, Maxfield ND, Pitt GJ, et al. Auditory evoked response to gaps in noise: older adults. Int J Audiol. 2011;50:211–225. doi: 10.3109/14992027.2010.526967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JT, Smith JC, Worden FG. Receptor and neural responses in auditory masking of low frequency tones. Electroencephalogr Clin Neurophysiol. 1972;32:63–74. doi: 10.1016/0013-4694(72)90228-3. [DOI] [PubMed] [Google Scholar]
- Ostroff JM, McDonald KL, Schneider BA, et al. Aging and the processing of sound duration in human auditory cortex. Hear Res. 2003;181:1–7. doi: 10.1016/s0378-5955(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Perrott DR, Musicant AD. Rotating tones and binaural beats. J Acoust Soc Am. 1977;61:1288–1292. doi: 10.1121/1.381430. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Benson NJ, et al. Effect of age on detection of gaps in speech and nonspeech markers varying in duration and spectral symmetry. J Acoust Soc Am. 2006;119:1143–1155. doi: 10.1121/1.2149837. [DOI] [PubMed] [Google Scholar]
- Picton TW, John MS, Dimitrijevic A, et al. Human auditory steady-state responses. Int J Audiol. 2003;42:177–219. doi: 10.3109/14992020309101316. [DOI] [PubMed] [Google Scholar]
- Poth EA, Boettcher FA, Mills JH, et al. Auditory brainstem responses in younger and older adults for broadband noises separated by a silent gap. Hear Res. 2001;161:81–86. doi: 10.1016/s0378-5955(01)00352-5. [DOI] [PubMed] [Google Scholar]
- Pratt H, Starr A, Michalewski HJ, et al. Cortical evoked potentials to an auditory illusion: binaural beats. Clin Neurophysiol. 2009;120:1514–1524. doi: 10.1016/j.clinph.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt H, Starr A, Michalewski HJ, et al. A comparison of auditory evoked potentials to acoustic beats and to binaural beats. Hear Res. 2010;262:34–44. doi: 10.1016/j.heares.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Purcell DW, John SM, Schneider BA, et al. Human temporal auditory acuity as assessed by envelope following responses. J Acoust Soc Am. 2004;116:3581–3593. doi: 10.1121/1.1798354. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brugge JF, Anderson DJ, et al. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967;30:769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Ross B, Fujioka T, Tremblay KL, et al. Aging in binaural hearing begins in mid-life: evidence from cortical auditory-evoked responses to changes in interaural phase. J Neurosci. 2007;27:11172–11178. doi: 10.1523/JNEUROSCI.1813-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider B, Speranza F, Pichora-Fuller MK. Age-related changes in temporal resolution: envelope and intensity effects. Can J Exp Psychol. 1998;52:184–191. doi: 10.1037/h0087291. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Hamstra SJ. Gap detection thresholds as a function of tonal duration for younger and older listeners. J Acoust Soc Am. 1999;106:371–380. doi: 10.1121/1.427062. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Pichora-Fuller MK, Kowalchuk D, et al. Gap detection and the precedence effect in young and old adults. J Acoust Soc Am. 1994;95:980–991. doi: 10.1121/1.408403. [DOI] [PubMed] [Google Scholar]
- Schwarz DW, Taylor P. Human auditory steady state responses to binaural and monaural beats. Clin Neurophysiol. 2005;116:658–668. doi: 10.1016/j.clinph.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Snell KB. Age-related changes in temporal gap detection. J Acoust Soc Am. 1997;101:2214–2220. doi: 10.1121/1.418205. [DOI] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am. 2000;107:1615–1626. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Sohmer H, Pratt H. Identification and separation of acoustic frequency following responses (FFRS) in man. Electroencephalogr Clin Neurophysiol. 1977;42:493–500. doi: 10.1016/0013-4694(77)90212-7. [DOI] [PubMed] [Google Scholar]
- Sohmer H, Pratt H, Kinarti R. Sources of frequency following responses (FFR) in man. Electroencephalogr Clin Neurophysiol. 1977;42:656–664. doi: 10.1016/0013-4694(77)90282-6. [DOI] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, et al. Temporal processing in the aging auditory system. J Acoust Soc Am. 1998;104:2385–2399. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Billings C, Rohila N. Speech evoked cortical potentials: effects of age and stimulus presentation rate. J Am Acad Audiol. 2004;15:226–237. doi: 10.3766/jaaa.15.3.5. quiz 264. [DOI] [PubMed] [Google Scholar]
- Vander Werff KR, Burns KS. Brain stem responses to speech in younger and older adults. Ear Hear. 2011;32:168–180. doi: 10.1097/AUD.0b013e3181f534b5. [DOI] [PubMed] [Google Scholar]
- Walton J, Orlando M, Burkard R. Auditory brainstem response forward-masking recovery functions in older humans with normal hearing. Hear Res. 1999;127:86–94. doi: 10.1016/s0378-5955(98)00175-0. [DOI] [PubMed] [Google Scholar]
- Walton JP. Timing is everything: temporal processing deficits in the aged auditory brainstem. Hear Res. 2010;264:63–69. doi: 10.1016/j.heares.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambacq IJ, Koehnke J, Besing J, et al. Processing interaural cues in sound segregation by young and middle-aged brains. J Am Acad Audiol. 2009;20:453–458. [PubMed] [Google Scholar]
- Worden FG, Marsh JT. Frequency-following (microphonic-like) neural responses evoked by sound. Electroencephalogr Clin Neurophysiol. 1968;25:42–52. doi: 10.1016/0013-4694(68)90085-0. [DOI] [PubMed] [Google Scholar]
- Yamada O, Yamane H, Kodera K. Simultaneous recordings of the brain stem response and the frequency-following response to low-frequency tone. Electroencephalogr Clin Neurophysiol. 1977;43:362–370. doi: 10.1016/0013-4694(77)90259-0. [DOI] [PubMed] [Google Scholar]