Abstract
ATP-binding cassette transporter A1 (ABCA1) is an integral cell membrane protein that protects cardiovascular disease by at least two mechanisms: by export of excess cholesterol from cells and by suppression of inflammation. ABCA1 exports cholesterol and phospholipids from cells by multiple steps that involve forming cell surface lipid domains, binding of apolipoproteins to ABCA1, activating signaling pathways, and solubilizing these lipids by apolipoproteins. ABCA1 executes its anti-inflammatory effect by modifying cell membrane lipid rafts and directly activating signaling pathways. The interaction of apolipoproteins with ABCA1 activates multiple signaling pathways, including Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3), protein kinase A, Rho family G protein CDC42 and protein kinase C. Activating protein kinase A and Rho family G protein CDC42 regulates ABCA1-mediated lipid efflux, activating PKC stabilizes ABCA1 protein, and activating JAK2/STAT3 regulates both ABCA1-mediated lipid efflux and anti-inflammation. Thus, ABCA1 behaves both as a lipid exporter and a signaling receptor. Targeting ABCA1 receptor-like property using agonists for ABCA1 protein could become a promising new therapeutic target for increasing ABCA1 function and treating cardiovascular disease.
Keywords: ATP-binding cassette transporter A1, signaling pathways, cholesterol efflux, lipid metabolism
1. Introduction
Atherosclerotic cardiovascular disease (CVD) remains one of the most common causes of morbidity and mortality in the Western world [1]. CVD is a complex disease that is initiated and propagated by disorders in cholesterol metabolism and by inflammatory processes [2]. Numerous population studies have shown that the prevalence and incidence of CVD is inversely correlated with plasma high density lipoprotein (HDL) cholesterol levels, implying that factors associated with HDL metabolism are cardioprotective [3, 4].
HDL is a heterogeneous population of particles that contain a variety of proteins of diverse functions, although the majority of the protein content represents apolipoprotein A-I (apoA-I) and apoA-II. ApoA-I is found in all serum HDL particles, while the other proteins are found only in some of the particles [5, 6]. There is evidence that HDL components can protect against CVD by several different mechanisms, including by promotion of cholesterol transport and inhibition of oxidative stress, inflammation, infection, thrombosis, and plaque rupture [4, 7]. The widely accepted view, however, is that HDL primarily prevents CVD by removing excess cholesterol from arterial macrophages and transporting it back to the liver [8–10]. The first step of this pathway, called reverse cholesterol transport, is mediated by an integral cell-membrane protein, ATP-binding cassette transporter A1 (ABCA1). Studies of human disease and mouse models have shown that ABCA1 is cardioprotective [11–14] and is essential for the generation of HDL [15]. ABCA1 loss-of-function mutations in Tangier disease patients increase the prevalence and severity of atherosclerosis [16, 17]. Over-expression of human ABCA1 in transgenic atherogenic mouse models protects against atherosclerosis [18, 19], and selective macrophage ablation of the ABCA1 gene increases atherosclerotic lesions in mice [20].
ABCA1 is a 2261-amino-acid integral membrane protein that is a member of a super-family of ABC transporters that utilize ATP as a source of energy for transporting lipids and other metabolites across membranes [21]. ABCA1 transports cholesterol and phospholipids from cells to lipid-poor apoA-I and other apolipoproteins, providing an efficient pathway for cells to unload excess cholesterol [22–25]. There is evidence that ABCA1 also has anti-inflammatory effects. The functions of ABCA1 are regulated both at the transcriptional and post-transcriptional level [26]. In addition, signaling cascades derived from the interaction of apoA-I with ABCA1 are involved in both ABCA1-mediated lipid efflux and anti-inflammation [27]. This review summaries the current knowledge of the molecular mechanisms by which signaling pathways regulate ABCA1 functions, and their potential therapeutic implications.
2. The mechanisms of ABCA1-mediated cholesterol efflux
One of the major functions of ABCA1 is to transport cellular cholesterol and phospholipids to lipid-poor apolipoproteins, such as apoA-I, to generate HDL particles and to provide cells with an efficient mean for unloading excess cholesterol [28]. Studies from several laboratories suggest the following model for the ABCA1-dependent lipid export pathway. When induced by cellular cholesterol loading, ABCA1 constitutively generates exovesiculated membrane domains, even in the absence of apolipoproteins [29]. These domains extrude from the plasma membrane to relieve the strain of the densely packed phospholipids, generating curved and disordered lipid surfaces that favor apolipoprotein interactions [30, 31]. The direct interaction of apolipoproteins with ABCA1 through the low capacity binding, activates several signaling pathway such as JAK2, which in turn increases binding of apolipoproteins to ABCA1 and to the high capacity binding sites (HCBS) [32–34]. Those interactions facilitate the contact of apoA-I with the protruding lipid domains, promoting solubilization of lipids and their release from the cells [35–37].
That ABCA1 can form exovesiculated membrane domains has been supported by immuno-gold electron micrographs, showing clusters of anti-apoA-I antibodies binding to cell surface protrusions of cells expressing high levels of ABCA1 [30]. However it is still unclear whether the exovesiculated lipid domains are the same sites as the HBCS, since they are identified by different methods and different groups. Additional studies have shown that independent of its interaction with apoA-I, ABCA1 expression increases cell surface cholesterol sensitive to oxidation by cholesterol oxidase, and decreases the lipid raft contents in the plasma membrane [29, 38]. However, it is still unclear how ABCA1 translocates cholesterol and phospholipids across membranes to form exovesiculated membrane domains. Electron microscopy and X-ray crystallography analyses of a limited number of ABC exporters suggest that the two symmetrical trans-membrane bundles come together to form a chamber that scans the inner leaflet of the membrane for substrates, incorporates them into the chamber, and flips them to the outer leaflet for extrusion from the cell [39–43]. Mutations of the nuclear binding domain (NBD) prevent the formation of these domains, suggesting that this involves a series of conformational changes in the ABC protein that is driven by ATP hydrolysis in the NBDs. In the case of ABCA1, amino acid motifs in the chamber are likely to bind phospholipids [44–46]. It is unknown whether the cholesterol in the inner leaflet is co-transported with these phospholipids, or phospholipid translocation alone increases the accessibility of cholesterol that flips to the outer leaflet by other processes [44–46].
ABCA1 is unique among ABC transporters in that it requires the direct binding of the acceptor for the transported substrates. Studies using chemical inhibitors or ABCA1 mutants indicate that apoA-I binding to ABCA1 and cells is required for ABCA1-mediated lipid efflux [47–49]. There are apparently two distinct sites that bind apoA-I: a relatively low binding capacity direct binding site with ABCA1; and a high capacity binding site (HCBS), which is not associated with ABCA1 [36, 37]. Evidence for direct binding of apoA-I with ABCA1 comes from chemical cross-linking studies, indicating that apoA-I and ABCA1 are in very close proximity (< 7Å) [50, 51]. Although studies indicate that the extracellular loops are important for the direct interaction of ABCA1 with apoA-I, the exact structural elements of apoA-I and ABCA1 responsible for this interaction are still to be identified. Interestingly, although the HCBS is distinct from the direct binding site for apoA-I, and is not associated with ABCA1, the direct apoA-I binding site and the HCBS are closely associated. Increasing the binding of apoA-I to ABCA1 promotes the binding of apoA-I to the HCBS, and inhibiting the binding of apoA-I to ABCA1 also inhibits its binding to the HCBS [36]. The HCBS is phospholipid-rich but it is still not clear how the HCBS is formed [37]. It is possible that the rapid, transient apoA-I binding to ABCA1 activates the signaling pathway such as JAK2, which allows secondary interactions of apoA-I with the HCBS. Alternatively, the initial direct binding of apoA-I with ABCA1 promotes the phospholipid translocation activity of ABCA1, or modifies adjacent phospholipid bilayer domains and the formation of HCBS. Defining the structural characteristics of apoA-I binding to the cell, and identifying the apoA-I binding sites within ABCA1 will be a key for understanding how ABCA1 regulates the association of apoA-I with different cellular compartments, and for clarifying the mechanisms by which ABCA1 promotes efflux of lipids to apolipoproteins.
It is proposed that after the binding of apoA-I to ABCA1 and the formation of highly curved exovesiculated lipid domains, the bound apoA-I spontaneously solubilizes the exovesiculated domains by inserting apoA-I into the membrane bilayer, leading to the simultaneous release of cellular phospholipids and cholesterol to apoA-I, allowing creation of nascent discoidal HDL particles [31]. The solubilization of apo A-I and its dissociations from the membrane does not occur within the context of the ABCA1/apoA-I protein complexes [31]. However, the analysis of naturally-occurring ABCA1 mutations indicates that the solubilization and release of apoA-I with lipids may not be a passive process, as the missense mutation W590S,which is still able to bind and release apoA-I back into the media, has a low capacity to transfer lipids to the released apoA-I [47, 48, 52]. In addition, a two-compartment efflux model, where ABCA1 effluxes to apoA-I occurs at both the cell surface and in a late endosomal compartment, has also be proposed. [53–57]. Both apoA-I and ABCA1 are actively endocytosed, and the deletion of the ABCA1 proline-glutamic acid-serine-threonine (PEST) motif prevents the trafficking of cell surface ABCA1 to the late endosome, which results in impaired ABCA1efflux capacity to apoA-I. Recent studies, however, have suggested that ABCA1 transports cholesterol to apolipoproteins exclusively at the cell surface [58, 59].
ABCA1 can efflux lipids to multiple HDL apolipoproteins, including apolipoproteins A-I, A-II, E, C-I, C-II, C-III, and A-IV [60]. These apolipoproteins contain 11–22 amino acid repeats of amphipathic α-helices [61]. Synthetic 18 amino acid peptides that are analogs of the type of amphipathic α-helices found in HDL apolipoproteins can mimic apoA-I in removing cholesterol and phospholipids by the ABCA1 pathway [62, 63]. A dimer of one of these peptides is more efficacious than its monomer [62, 64], suggesting cooperativity between tandem helices. Thus, the amphipathic α-helix is the major structural motif required for removing ABCA1-extruded lipids. Interestingly, the D-isomers of these α-helices are also active [65], implying that there are no stereoselective requirements for these peptides to accept lipids.
The broad specificity for amphipathic α-helices implies that proteins other than apolipoproteins containing this structural motif could remove cellular lipids by the ABCA1 pathway. Phospholipid transfer protein (PLTP), which contains amphipathic α-helices, can interact with ABCA1 and remove cellular cholesterol and phospholipids [66, 67]. Because of its low lipid-binding capacity, PLTP tends to transfer these lipids to HDL, rather than generate new lipoprotein particles. Serum amyloid A (SAA) also removes cellular lipids by the ABCA1 pathway [68, 69]. SAA is an acute phase protein that is induced over 1000-fold during inflammation [70–73]. SAA primarily associates with HDL in the plasma. It contains two tandem amphipathic α-helices that differ in amino acid charge distribution from those in apolipoproteins. The consequences of the SAA-ABCA1 interaction are unknown.
3. Anti-inflammatory activity
ABCA1 also has anti-inflammatory functions, which represent another putative mechanism for protection against CVD. Humans with dysfunctional ABCA1 and familial HDL deficiencies tend to have chronic low-grade inflammation [74, 75]. Mice lacking ABCA1 in all tissues or selectively in macrophages, have a heightened reaction in response to the lipopolysaccharides (LPS) inflammatory stimulus [76–78] This was evident by the increased appearance of inflammatory cytokines in the circulation and peritoneal fluid after mice were injected with LPS. These studies imply that macrophage ABCA1 has an anti-inflammatory function. Moreover, LDL−/− mice that are selectively deficient in leukocyte ABCA1 have increased peripheral blood leukocyte counts and increased macrophages infiltration in liver and spleen [79]. Bone marrow myeloid proliferation from ABCA1−/− mice is also increased, though not as dramatic as in bone myeloid from ABCA1−/−/ ABCG1−/− mice [80]. These studies suggest that ABCA1 plays a significant role in regulation of bone marrow-derived cell proliferation, and that ABCA1 and ABCG1-mediated lipid metabolism is of critical importance for normal proliferation of myeloid cells in bone marrow.
Studies with cultured cells have suggested that the anti-inflammatory activity of ABCA1 is secondary to its ability to modulate the cholesterol content of membrane lipid rafts [38, 77, 78, 81]. Murine macrophages lacking ABCA1 have increased cholesterol content in rafts, and enhanced signaling of LPS through its receptor, Toll-like receptor 4 (TLR4), leading to increased production of inflammatory cytokines [77, 78, 81]. Incubating human monocytes with apoA-I reduces the ability of the inflammatory stimuli to activate CD11b, and this effect is abolished in cells lacking ABCA1 [82]. ApoA-I also was shown to activate the raft-associated metalloprotease ADAM17 [83], which promotes proteolytic shedding of the pro-inflammatory cytokine tumor necrosis factor α (TNFα) and its receptors. All these responses appear to depend on the cholesterol content of plasma membrane rafts. These anti-inflammatory effects are not specific to ABCA1, as ABCA1-independent cholesterol acceptors, such as cyclodextrin, have the same effect [78, 84]. Moreover, the macrophage cholesterol exporter ABCG1, which promotes cholesterol efflux to mature HDL, is more potent with respect to the TLR-mediated pro-inflammatory pathways than ABCA1 [85].
Significantly, studies indicate that the interaction of apoA-I or its mimetic peptides with ABCA1 activates the transcription factor STAT3 [27, 28]. The STAT3 pathway is well-known to have an anti-inflammatory function in macrophages [86–89]. In fact, constitutive activation of STAT3 is sufficient to block production of most inflammatory cytokines in activated macrophages [90]. The interaction of apoA-I or its mimetic peptides with ABCA1-expressing macrophages activates STAT3, and markedly suppresses expression of these cytokines [27]. However, STAT3 activation is not required for ABCA1-mediated lipid efflux [27]. These findings suggest that, in addition to the ability of ABCA1 to influence the inflammatory signaling pathway indirectly by modifying cell surface domains, ABCA1 may also directly act as an anti-inflammatory receptor by inducing signaling through the JAK2/STAT3 pathway in response to apoA-I binding. Those results are very interesting in light of the profound remodeling that HDL undergoes in vivo, during which apoA-I is consistently displaced. These liberated lipid-poor apoA-I can interact with ABCA1 and induce the JAK2/STAT3 pathways to subdue the inflammatory response. Understanding the functions of ABCA1 as an anti-inflammatory receptor as well as a cholesterol exporter will open new opportunities in the development of therapeutic agents that target ABCA1 pathways for treating CVD. In addition, the anti-inflammatory properties of ABCA1 could potentially be exploited as a therapeutic target for other inflammatory diseases that involve macrophage infiltration and inflammation, such as inflammatory bowel disease and rheumatoid arthritis.
4.1 ABCA1-mediated lipid efflux is regulated by cAMP/PKA pathway
ABCA1 has been shown to be regulated by cAMP at the transcription level [91] and a cAMP responsive element has been identified in the mouse ABCA1 promoter [92–94]. cAMP/Protein kinase A (PKA) dependent pathway plays a pivotal role in ABCA1 phosphorylation, and modulates apoA-I-dependent cellular lipid efflux [95, 96]. Mutation of serine 2054 (S2054), located at the NBD of ABCA1, decreases ABCA1 phosphorylation and apoA-I-mediated lipid efflux, suggesting that ABCA1 is constitutively phosphorylated by the cAMP/PKA pathway, and that S2054 is essential for PKA phosphorylation and ABCA1-medaited lipid efflux [95]. How phosphorylation of ABCA1 by the cAMP/PKA pathway affects ABCA1-mediated lipid efflux is not yet known, although it appears that phosphorylation of ABCA1 at S2054 is not required for ABCA1 expression or trafficking, nor is it required for apoA-I binding to ABCA1. It is suggested that phosphorylation of ABCA1 by the cAMP/PKA pathway at S2045 and other residues may nudge the transporter toward adapting a more active conformation [95].
Treatment of ABCA1-expressing cells with apoA-I for short periods of time increases ABCA1 phosphorylation in a concentration and time-dependent manner by increasing cAMP production and activating the PKA pathway, without affecting ABCA1 expression level [96, 97]. Significantly, one naturally-occurring mutation of ABCA1 associated with Tangier disease, C1477R, severely reduces apoA-I-mediated cAMP production, ABCA1 phosphorylation and apoA-I-mediated lipid efflux, suggesting that direct interaction of apoA-I with a functional ABCA1 is required for the activation of cAMP/PKA pathway by apoA-I. It is proposed that the initial binding of apoA-I to ABCA1, which couples to a G protein, leads to activation of adenylyl cyclase, cAMP production, and subsequent PKA-mediated ABCA1 phosphorylation, allowing lipidation of apoA-I through ABCA1 [97]. Identifying the specific G proteins coupled to ABCA1, and elucidating the molecular interactions between the apoA-I/ABCA1/G protein complexes should help us understand the mechanisms by which ABCA1 transports lipid to apolipoproteins.
4.2 Regulation of ABCA1 functions by the JAK2/STAT3 pathway
The best characterized signaling molecule activated by apolipoprotein/ABCA1 interactions is JAK2, a tyrosine kinase that is activated by over half of the cytokine/hematopoietin superfamily of receptors [98]. Activation of JAK2 is the initiating step in downstream signaling for most of these receptors. A JAK2-specific inhibitor markedly reduces apoA-I-mediated lipid transport and apoA-I binding to ABCA1, and mutant cells lacking JAK2 have a severely impaired ABCA1-mediated lipid efflux [32, 33]. Inhibiting or ablating JAK2 has no effect on the intrinsic lipid translocase activity of ABCA1, but reduces the apolipoprotein binding to ABCA1, a step required for removal of the translocated lipids [32, 33]. Studies of ABCA1 mutants with variable severities of functional impairment show that apolipoprotein-stimulated phosphorylation of JAK2 is highly correlated with both the lipid export and apolipoprotein binding activities of ABCA1 [99], indicating that JAK2 activation is necessary for the optimum binding of apoA-I to ABCA1, which is required for lipid removal.
Even short interaction of apoA-I with ABCA1-expressing cells (minutes) stimulates autophosphorylation of JAK2 [32], thus generating the active JAK2 that phosphorylates its target proteins. For receptor systems, phosphorylated JAK2 activates downstream transcription factors called STATs, which regulate a wide variety of cellular pathways. The interaction of apoA-I with ABCA1 increases phosphorylation and thus activation of STAT3 [27]. However knockout of STAT3 has no effect on ABCA1-mediated cholesterol efflux. Moreover, mutating the STAT3 docking sites located within the ABCA1 NBD domains completely abolishes the ability of apoA-I to activate and bind STAT3 without affecting the cholesterol efflux export function of ABCA1, indicating that these sites are responsible for apoA-I-mediated STAT3 activation, but activation of STAT3 is not required for ABCA1-mediated lipid efflux [27]. It has been shown that activation of STAT3 has anti-inflammatory function in macrophages. Remarkably, pre-treating macrophages that express ABCA1 with apoA-I suppresses subsequent LPS-induced production of the inflammatory cytokines TNF-α, interleukin-1(IL-1) and (interleukin-6) IL-6 [27]. This effect of apoA-I is reduced by knockdown of STAT3 with siRNA, silencing STAT3 in myeloid-specific STAT3 knockout mice, or inhibiting STAT3 phosphorylation, indicating a role for STAT3 in the anti-inflammatory effects of apoA-I/ABCA1 interaction [27]. The anti-inflammatory effect of JAK2/STAT3 pathway induced by apoA-I/ABCA1 interaction in macrophages was further confirmed by another independent group [100], which showed that activation of the JAK2/STAT3 pathway is required for the anti-inflammatory effects of apoA-I in THP cells. ApoA-I loses its anti-inflammatory activity in macrophages from ABCA1−/− mice, indicating that ABCA1 is required for the anti-inflammatory effects of apoA-I. Silencing STAT3 in macrophages did not completely reverse the ability of apoA-I to suppress LPS-induced cytokine production, indicating that some of the anti-inflammatory effects of apoA-I/ABCA1 interaction are independent of STAT3 [27]. It has been shown that cholesterol efflux activity can also inhibit inflammatory cytokine production in macrophages by disrupting sterol-rich membrane rafts. It is therefore likely that apoA-I/ABCA1 interaction can suppress macrophages inflammation by at least two mechanisms.
The interaction of apoA-I with ABCA1-expressing cells rapidly activates JAK2, which in turn causes two independent effects: lipid export from cells, and STAT3-mediated anti-inflammatory activity. These studies raise the possibility that apolipoprotein/ABCA1 interactions may have multiple biological effects that are both dependent and independent of lipid export. Significantly, the JAK2/STAT3 pathway can also be activated by synthetic apoA-I mimetic peptides, and one of the peptides, peptide 5A, which has been shown to decrease atherosclerosis with very little side effects compared to other peptides [101, 102], is more specific than other peptides in activating the JAK2/STAT3 pathway (Tang, unpublished data), raising the possibility of finding peptides or small molecules for ligand/receptor interactions to regulate both the lipid transport and anti-inflammatory activity of ABCA1.
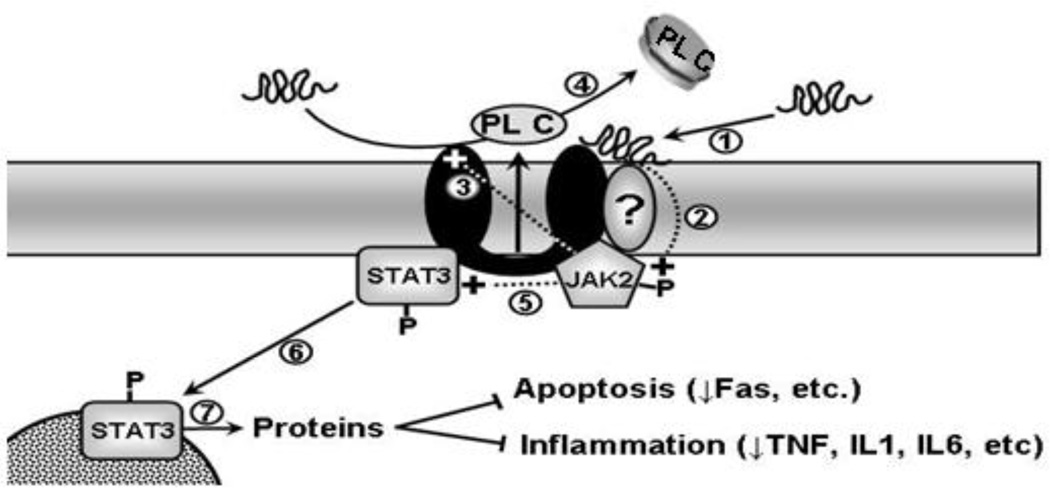
Taken together, those studies suggest the following model for the ABCA1/JAK2/STAT3 pathway (Fig. 1). Lipid-poor apoA-I or its mimetic peptides bind directly to ABCA1 or a closely associated protein (step 1), which rapidly stimulates JAK2 autophosphorylation (step 2). The activated JAK2 then has two distinct effects on the cells. First, it recruits and/or dissociates ABCA1 partner proteins and causes conformational change of ABCA1, thus enhancing apoA-I binding to ABCA1 (step 3) and lipid removal from the cell (step 4). Second, it makes the STAT3 docking sites accessible, leading to activation of STAT3 (step 5), which promotes translocation of STAT3 to the nucleus (step 6), where it regulates transcription of proteins that repress inflammatory processes (step 7). This model suggests that the ABCA1/JAK2/STAT3 pathway represents a direct link between the cardioprotective effects of reverse cholesterol transport and anti-inflammation.
Figure 1. Scheme for the apoA-I/ABCA1/JAK2/STAT3 pathway.
Lipid-poor apoA-I or its mimetic peptides bind directly to ABCA1 or a closely associated protein (step 1) that rapidly stimulates JAK2 autophosphorylation (step 2). The activated JAK2 then has two distinct effects on the cells. First, it recruit and/or dissociate ABCA1 partner proteins and causes confirmation change of ABCA1, thus enhancing apoA-I binding to ABCA1 (step 3) and lipid removal from the cells (step 4). Second, it generates STAT3 docking sites and activates STAT3 (step 5), which promotes translocation of STAT3 to the nucleus (step 6) where it regulates transcription of proteins that repress inflammatory processes (step 7). P: Phosphorylated.
4.3 Regulation of ABCA1-mediated cholesterol efflux by Rho family G protein CDC42
Incubating apoA-I with cells induces a marked stimulation of Rho family G protein Cdc42 [103, 104]. By using various tools, including pharmacological inhibitors and activators, bacterial toxins and dominant negative mutants, it is demonstrated that activation of Cdc42 regulates apoA-I-mediated lipid efflux [103]. Apparently, ABCA1 and CDC42 are associated [104]. ApoA-I also stimulates Rho family G protein Rac1, but selective inhibition of Rac1 has no effect on apoA-I-mediated cholesterol efflux [103]. Since ABCA1-containing vesicles were shown to shuttle between the trans-Golgi network and the plasma membrane [105], and Cdc42 could be involved in the vesicular trafficking of cholesterol between the trans-Golgi network and the plasma membrane [106, 107], it is proposed that apoA-I-induced Cdc42 activation enhances vesicular transport, and thereby promotes cholesterol export by ABCA1 from cells. One of the downstream signaling targets of apoA-I/Cdc42 is the stress kinase c-Jun N-terminal kinase (JNK). Inhibition of JNK by JNK inhibitors decreases cholesterol efflux, while activation of JNK by JNK activators increases apoA-I-induced cholesterol efflux [103]. Cdc42 can bind and interact with ABCA1 directly [104]. However, how apoA-1 activates Cdc42 and the identities of the kinases involved is not known. It is also unclear if direct interaction of apoA-I with ABCA1 is required for the activation of Rho family G protein signaling pathway. ABCA-mediated lipid efflux is, furthermore, regulated by casein kinase2 (CK2), though the mechanisms by which CK2 regulates ABCA1-mediated lipid efflux are poorly understood [108]. Additionally, the trafficking of ABCA1 appears to be regulated by palmitoylation [109].
4.4 Signaling pathways and ABCA1 degradation
The turnover of ABCA1 protein is rapid, with a half-life of less than 1 h in murine macrophages and differentiated THP-1 cells [57, 65]. Calpain protease inhibitors significantly decrease the rate of ABCA1 turnover, suggesting that ABCA1 protein degradation is mediated by calpain protease [57, 110, 111]. Significantly, studies from several different laboratories found that apoA-I and its synthetic mimetic peptides can decrease ABCA1 protein degradation [32, 110–112]. Wang and colleagues [57] have shown that deletion of the PEST sequence, which regulates protein degradation by proteolysis in many proteins [113], decreased the ABCA1 degradation rate and prevented apoA-I from stabilizing ABCA1 protein. It was further shown that threonine-1286 and threonine-1305 in the ABCA1 PEST sequence are constitutively phosphorylated, and that mutation of those two phosphorylated threonine sites abolishes the ability of apoA-1 to increase ABCA1 phosphorylation and to stabilize ABCA1 protein [110]. It is therefore proposed that apoA-I stabilizes ABCA1 by decreasing the phosphorylation of threonine-1286 and threonine-1305 in the PEST sequence, and thereby prevents ABCA1 from fast degradation by calpain protease. The mechanism linking the binding of apoA-I to ABCA1 PEST phosphorylation is unknown and the kinases/phosphatases responsible for the decreased ABCA1 PEST phosphorylation by apoA-I are still to be identified.
In another study [111], it was found that apoA-I stabilizes ABCA1 protein by increasing ABCA1 phosphorylation at a yet-unidentified site through protein kinase C α (PKCα). It was further proposed that apoA-I removes cellular free cholesterol and phosphatidylcholine, resulting in the generation of diacylglycerol (DAG), which in turn activates PKCα, leading to the phosphorylation of ABCA1 [111]. This model suggests that DAG, which is produced by apoA-I-mediated release of phosphatidylcholine-phospholipase C (PC-PLC), is the key activator of PKC, required for phosphorylation of ABCA1 and its protection against calpain-mediated proteolytic degradation. However, other studies suggest that apoA-I-mediated lipid efflux is not required for apoA-I to stabilize ABCA1, and DAG produced by unsaturated fatty acid destabilizes ABCA1 [32, 114, 115]. On the other hand, another study reported that ABCA1-mediated cholesterol efflux to taurocholate, in the absence of apoA-I, protects ABCA1 from degradation [116]. The cause of this discrepancy is unknown, and further work is required to sort out these observations. Moreover, it is not fully understood how the phosphorylation of ABCA1 by PKC is involved in stabilizing ABCA1 against calpain-mediated proteolysis. Identification of the apoA-I-induced phosphorylation sites of ABCA1 by PKC pathway and the kinases/phosphatase responsible for the dephosphorylation of the PEST sequence within ABCA1 will facilitate our understanding as to how apoA-I triggers signaling pathway to stabilize ABCA1.
5. ABCA1 as a therapeutic target
Current therapeutic approaches for treating heart disease have focused on lowering LDL cholesterol, but the most effective cholesterol-lowering drugs reduce CVD events by only one third [3, 117, 118]. Because of this large residual disease burden, the HDL pathway has become a new target for drug development [119, 120]. The inverse relationship between HDL levels and CVD has led to the widely-held view that raising HDL levels will be cardioprotective. Recent clinical trials using an inhibitor of cholesterol ester transfer protein to raise HDL had the unexpected effect of increasing cardiovascular complications [119, 121]. Although some of this may have been due to off-target drug effects [119], it is also possible that this approach generated dysfunctional HDL that had harmful, rather than beneficial effects. Thus, factors that raise HDL such as the expression of apoA-I or ABCA1, or factors that increase HDL functions, such as HDL efflux capacity, rather than HDL itself might be the important therapeutic targets [120, 122]. To date, the only tissue protein involved in HDL metabolism unequivocally shown to reduce CVD in both animal models and humans is ABCA1. Since ABCA1 is a major determinant of HDL levels, it is reasonable to assume that much of the association between high HDL levels and reduced CVD reflects an active ABCA1 pathway. This transporter has therefore become a new therapeutic target for drug development designed for clearing cholesterol from arterial macrophages and preventing CVD.
Multiple strategies have been initiated for enhancing the ABCA1 pathway [120, 123]. The activity of the ABCA1 pathway can be modulated at multiple levels, providing an array of potential therapeutic targets for enhancing this pathway. LXR/RXR ligands and agonists induce ABCA1 and other reverse cholesterol transport proteins and inhibit production of inflammatory cytokines [124–126], making these agonists potentially robust therapeutic agents for mobilizing cholesterol from tissues, reducing inflammation, and preventing CVD. The problem with current LXR agonists is that they also induce enzymes of fatty acid synthesis and production, thus causing fatty livers and hypertriglyceridemia when administered to animals [127–129]. The concept of targeting ABCA1 transcription has also come under question, since at least one animal study showed that tissue ABCA1 protein levels appear to be regulated mostly by post-transcriptional processes [130]. ABCA1 activity is also regulated by diverse intracellular signaling pathways.
Studies have shown that administering recombinant HDL, lipid free apoA-I or small apolipoprotein-mimetic peptides to atherogenic animals reduces atherosclerosis [131–133]. In addition, targeting ABCA1 receptor-like properties using agonists for ABCA1 protein would have several advantages over transcriptional agonists, since they could stabilize ABCA1 protein and stimulate its cholesterol export and anti-inflammatory activities. ABCA1 agonists would allow for targeting of cholesterol-laden cells, such as arterial macrophages, that express relatively high levels of ABCA1 and are a major source of arterial inflammatory reactions. By suppressing inflammation, small chemical agonists for ABCA1 could have cardioprotective effects even if they have little capacity to remove cholesterol.
5. Conclusions and future directions
Human genetic and mouse model studies have shown that ABCA1 is cardioprotective. Studies of human HDL deficiencies, transgenic mice, and cultured cells have shown that ABCA1 is the major exporter of cellular cholesterol and phospholipids to HDL apolipoproteins, and that this activity is essential for formation of HDL particles in vivo. There is emerging evidence that ABCA1 also plays a role in suppressing inflammatory cytokine production by macrophages through multiple mechanisms. ABCA1 is unique among transporters in that its interaction with apolipoproteins promotes its receptor-like properties and activates multiple signaling pathways to regulate its cholesterol efflux ability and anti-inflammatory functions.
Although we have gained a great deal of knowledge about the function of ABCA1 since its discovery in 1999, the ABCA1 pathway is highly complex and far from being completely understood. Additional studies are needed to identify the molecular components of the ABCA1 pathway, to characterize the receptor-like properties of ABCA1 and its anti-inflammatory activity, to elucidate the diverse processes that regulate ABCA1 expression and activity, and to determine its contribution to disease protection in humans. These studies would provide more insight into the role of ABCA1 in health and diseases, and potentially reveal novel therapeutic strategies for treating these diseases.
Highlights.
This review summarizes current knowledge of the regulation of ABCA1 functions by signaling pathways.> ABCA1 functions are regulated by multiple signaling pathways.> Activating PKA and Rho family G protein CDC42 regulates ABCA1-mediated lipid efflux.> Activating PKC stabilizes ABCA1 protein.>Activating JAK2/STAT3 regulates both ABCA1-mediated lipid efflux and anti-inflammation.
Acknowledgements
This review is dedicated to my mentor, Dr. Jack Oram, who was, and still is, a true inspiration for all of us. Jack was a great mentor and a brilliant scientist. ABCA1 field would not have been able to get where we are now without him.
The authors’ work described in this review was supported by National Institutes of Health grants HL18645, HL075340, HL55362, and DK02456 and Scientist Development Grant from the American Heart Association 10SDG3860011. We would also like to thank Drs. Jay Heinecke, John Albers and Simona Vuletic for their critical reading and editing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 4.Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero-protective effect of high density lipoproteins. J Intern Med. 2008;263:256–273. doi: 10.1111/j.1365-2796.2007.01898.x. [DOI] [PubMed] [Google Scholar]
- 5.Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, Byun J, Vuletic S, Kassim S, Singh P, Chea H, Knopp RH, Brunzell J, Geary R, Chait A, Zhao XQ, Elkon K, Marcovina S, Ridker P, Oram JF, Heinecke JW. Shotgun proteomics implicates protease inhibition and complement activation in the antiinflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McPherson PA, Young IS, McKibben B, McEneny J. High density lipoprotein subfractions: isolation, composition, and their duplicitous role in oxidation. J Lipid Res. 2007;48:86–95. doi: 10.1194/jlr.M600094-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58:342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 8.Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat Rev Cardiol. 2009;6:455–463. doi: 10.1038/nrcardio.2009.94. [DOI] [PubMed] [Google Scholar]
- 9.Rader DJ. Mechanisms of disease: HDL metabolism as a target for novel therapies. Nat Clin Pract Cardiovasc Med. 2007;4:102–109. doi: 10.1038/ncpcardio0768. [DOI] [PubMed] [Google Scholar]
- 10.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50 Suppl:S189–S194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singaraja RR, Brunham LR, Visscher H, Kastelein JJ, Hayden MR. Efflux and atherosclerosis: the clinical and biochemical impact of variations in the ABCA1 gene. Arterioscler Thromb Vasc Biol. 2003;23:1322–1332. doi: 10.1161/01.ATV.0000078520.89539.77. [DOI] [PubMed] [Google Scholar]
- 12.Frikke-Schmidt R, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Genetic variation in ABC transporter A1 contributes to HDL cholesterol in the general population. J Clin Invest. 2004;114:1343–1353. doi: 10.1172/JCI20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyce C, Freeman L, Brewer HB, Jr, Santamarina-Fojo S. Study of ABCA1 function in transgenic mice. Arterioscler Thromb Vasc Biol. 2003;23:965–971. doi: 10.1161/01.ATV.0000055194.85073.FF. [DOI] [PubMed] [Google Scholar]
- 14.Joyce CW, Wagner EM, Basso F, Amar MJ, Freeman LA, Shamburek RD, Knapper CL, Syed J, Wu J, Vaisman BL, Fruchart-Najib J, Billings EM, Paigen B, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr ABCA1 overexpression in the liver of LDLr-KO mice leads to accumulation of pro-atherogenic lipoproteins and enhanced atherosclerosis. J Biol Chem. 2006;281:33053–33065. doi: 10.1074/jbc.M604526200. [DOI] [PubMed] [Google Scholar]
- 15.Singaraja RR, Stahmer B, Brundert M, Merkel M, Heeren J, Bissada N, Kang M, Timmins JM, Ramakrishnan R, Parks JS, Hayden MR, Rinninger F. Hepatic ATP-binding cassette transporter A1 is a key molecule in high-density lipoprotein cholesteryl ester metabolism in mice. Arterioscler Thromb Vasc Biol. 2006;26:1821–1827. doi: 10.1161/01.ATV.0000229219.13757.a2. [DOI] [PubMed] [Google Scholar]
- 16.Clee SM, Kastelein JJ, van Dam M, Marcil M, Roomp K, Zwarts KY, Collins JA, Roelants R, Tamasawa N, Stulc T, Suda T, Ceska R, Boucher B, Rondeau C, DeSouich C, Brooks-Wilson A, Molhuizen HO, Frohlich J, Genest J, Jr, Hayden MR. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J Clin Invest. 2000;106:1263–1270. doi: 10.1172/JCI10727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer EJ, Zech LA, Schwartz DE, Brewer HB., Jr Coronary heart disease prevalence and other clinical features in familial high-density lipoprotein deficiency (Tangier disease) Ann Intern Med. 1980;93:261–266. doi: 10.7326/0003-4819-93-2-261. [DOI] [PubMed] [Google Scholar]
- 18.Singaraja RR, Bocher V, James ER, Clee SM, Zhang LH, Leavitt BR, Tan B, Brooks-Wilson A, Kwok A, Bissada N, Yang YZ, Liu G, Tafuri SR, Fievet C, Wellington CL, Staels B, Hayden MR. Human ABCA1 BAC transgenic mice show increased high density lipoprotein cholesterol and ApoAI-dependent efflux stimulated by an internal promoter containing liver X receptor response elements in intron 1. J Biol Chem. 2001;276:33969–33979. doi: 10.1074/jbc.M102503200. [DOI] [PubMed] [Google Scholar]
- 19.Singaraja RR, Fievet C, Castro G, James ER, Hennuyer N, Clee SM, Bissada N, Choy JC, Fruchart JC, McManus BM, Staels B, Hayden MR. Increased ABCA1 activity protects against atherosclerosis. J Clin Invest. 2002;110:35–42. doi: 10.1172/JCI15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aiello RJ, Brees D, Francone OL. ABCA1-deficient mice: insights into the role of monocyte lipid efflux in HDL formation and inflammation. Arterioscler Thromb Vasc Biol. 2003;23:972–980. doi: 10.1161/01.ATV.0000054661.21499.FB. [DOI] [PubMed] [Google Scholar]
- 21.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- 22.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 23.Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- 24.Bodzioch M, Orso E, Klucken J, Langmann T, Bottcher A, Diederich W, Drobnik W, Barlage S, Buchler C, Porsch-Ozcurumez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 25.Rust S, Rosier M, Funke H, Real J, Amoura Z, Piette JC, Deleuze JF, Brewer HB, Duverger N, Denefle P, Assmann G. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat Genet. 1999;22:352–355. doi: 10.1038/11921. [DOI] [PubMed] [Google Scholar]
- 26.Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–1372. doi: 10.1152/physrev.00005.2005. [DOI] [PubMed] [Google Scholar]
- 27.Tang C, Liu Y, Kessler PS, Vaughan AM, Oram JF. The macrophage cholesterol exporter ABCA1 functions as an anti-inflammatory receptor. J Biol Chem. 2009;284:32336–32343. doi: 10.1074/jbc.M109.047472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang C, Oram JF. The cell cholesterol exporter ABCA1 as a protector from cardiovascular disease and diabetes. Biochim Biophys Acta. 2009;1791:563–572. doi: 10.1016/j.bbalip.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Vaughan AM, Oram JF. ABCA1 redistributes membrane cholesterol independent of apolipoprotein interactions. J Lipid Res. 2003;44:1373–1380. doi: 10.1194/jlr.M300078-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Lin G, Oram JF. Apolipoprotein binding to protruding membrane domains during removal of excess cellular cholesterol. Atherosclerosis. 2000;149:359–370. doi: 10.1016/s0021-9150(99)00503-1. [DOI] [PubMed] [Google Scholar]
- 31.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 32.Tang C, Vaughan AM, Anantharamaiah GM, Oram JF. Janus kinase 2 modulates the lipid-removing but not protein-stabilizing interactions of amphipathic helices with ABCA1. J Lipid Res. 2006;47:107–114. doi: 10.1194/jlr.M500240-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Tang C, Vaughan AM, Oram JF. Janus kinase 2 modulates the apolipoprotein interactions with ABCA1 required for removing cellular cholesterol. J Biol Chem. 2004;279:7622–7628. doi: 10.1074/jbc.M312571200. [DOI] [PubMed] [Google Scholar]
- 34.Vaughan AM, Tang C, Oram JF. ABCA1 mutants reveal an interdependency between lipid export function, apoA-I binding activity, and Janus kinase 2 activation. J Lipid Res. 2009;50:285–292. doi: 10.1194/jlr.M800366-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassan HH, Bailey D, Lee DY, Iatan I, Hafiane A, Ruel I, Krimbou L, Genest J. Quantitative analysis of ABCA1-dependent compartmentalization and trafficking of apolipoprotein A-I: implications for determining cellular kinetics of nascent high density lipoprotein biogenesis. J Biol Chem. 2008;283:11164–11175. doi: 10.1074/jbc.M707720200. [DOI] [PubMed] [Google Scholar]
- 36.Hassan HH, Denis M, Lee DY, Iatan I, Nyholt D, Ruel I, Krimbou L, Genest J. Identification of an ABCA1-dependent phospholipid-rich plasma membrane apolipoprotein A-I binding site for nascent HDL formation: implications for current models of HDL biogenesis. J Lipid Res. 2007;48:2428–2442. doi: 10.1194/jlr.M700206-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Vedhachalam C, Ghering AB, Davidson WS, Lund-Katz S, Rothblat GH, Phillips MC. ABCA1-induced cell surface binding sites for ApoA-I. Arterioscler Thromb Vasc Biol. 2007;27:1603–1609. doi: 10.1161/ATVBAHA.107.145789. [DOI] [PubMed] [Google Scholar]
- 38.Landry YD, Denis M, Nandi S, Bell S, Vaughan AM, Zha X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J Biol Chem. 2006;281:36091–36101. doi: 10.1074/jbc.M602247200. [DOI] [PubMed] [Google Scholar]
- 39.Locher KP. Structure and mechanism of ATP-binding cassette transporters. Philos Trans R Soc Lond B Biol Sci. 2009;364:239–245. doi: 10.1098/rstb.2008.0125. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg MF, Callaghan R, Ford RC, Higgins CF. Structure of the multidrug resistance P-glycoprotein to 2.5 nm resolution determined by electron microscopy and image analysis. J Biol Chem. 1997;272:10685–10694. doi: 10.1074/jbc.272.16.10685. [DOI] [PubMed] [Google Scholar]
- 41.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci U S A. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 43.Procko E, O'Mara ML, Bennett WF, Tieleman DP, Gaudet R. The mechanism of ABC transporters: general lessons from structural and functional studies of an antigenic peptide transporter. Faseb J. 2009;23:1287–1302. doi: 10.1096/fj.08-121855. [DOI] [PubMed] [Google Scholar]
- 44.Yamauchi Y, Abe-Dohmae S, Yokoyama S. Differential regulation of apolipoprotein A-I/ATP binding cassette transporter A1-mediated cholesterol and phospholipid release. Biochim Biophys Acta. 2002;1585:1–10. doi: 10.1016/s1388-1981(02)00304-9. [DOI] [PubMed] [Google Scholar]
- 45.Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 46.Alder-Baerens N, Muller P, Pohl A, Korte T, Hamon Y, Chimini G, Pomorski T, Herrmann A. Headgroup-specific exposure of phospholipids in ABCA1-expressing cells. J Biol Chem. 2005;280:26321–26329. doi: 10.1074/jbc.M413993200. [DOI] [PubMed] [Google Scholar]
- 47.Fitzgerald ML, Morris AL, Chroni A, Mendez AJ, Zannis VI, Freeman MW. ABCA1 and amphipathic apolipoproteins form high-affinity molecular complexes required for cholesterol efflux. J Lipid Res. 2004;45:287–294. doi: 10.1194/jlr.M300355-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald ML, Morris AL, Rhee JS, Andersson LP, Mendez AJ, Freeman MW. Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I. J Biol Chem. 2002;277:33178–33187. doi: 10.1074/jbc.M204996200. [DOI] [PubMed] [Google Scholar]
- 49.Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem. 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- 50.Chroni A, Liu T, Fitzgerald ML, Freeman MW, Zannis VI. Cross-linking and lipid efflux properties of apoA-I mutants suggest direct association between apoA-I helices and ABCA1. Biochemistry. 2004;43:2126–2139. doi: 10.1021/bi035813p. [DOI] [PubMed] [Google Scholar]
- 51.Denis M, Haidar B, Marcil M, Bouvier M, Krimbou L, Genest J., Jr Molecular and cellular physiology of apolipoprotein A-I lipidation by the ATP-binding cassette transporter A1 (ABCA1) J Biol Chem. 2004;279:7384–7394. doi: 10.1074/jbc.M306963200. [DOI] [PubMed] [Google Scholar]
- 52.Rigot V, Hamon Y, Chambenoit O, Alibert M, Duverger N, Chimini G. Distinct sites on ABCA1 control distinct steps required for cellular release of phospholipids. J Lipid Res. 2002;43:2077–2086. doi: 10.1194/jlr.m200279-jlr200. [DOI] [PubMed] [Google Scholar]
- 53.Neufeld EB, Remaley AT, Demosky SJ, Stonik JA, Cooney AM, Comly M, Dwyer NK, Zhang M, Blanchette-Mackie J, Santamarina-Fojo S, Brewer HB., Jr Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem. 2001;276:27584–27590. doi: 10.1074/jbc.M103264200. [DOI] [PubMed] [Google Scholar]
- 54.Neufeld EB, Stonik JA, Demosky SJ, Jr, Knapper CL, Combs CA, Cooney A, Comly M, Dwyer N, Blanchette-Mackie J, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr The ABCA1 transporter modulates late endocytic trafficking: insights from the correction of the genetic defect in Tangier disease. J Biol Chem. 2004;279:15571–15578. doi: 10.1074/jbc.M314160200. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi Y, Smith JD. Cholesterol efflux to apolipoprotein AI involves endocytosis and resecretion in a calcium-dependent pathway. Proc Natl Acad Sci U S A. 1999;96:11358–11363. doi: 10.1073/pnas.96.20.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen W, Wang N, Tall AR. A PEST deletion mutant of ABCA1 shows impaired internalization and defective cholesterol efflux from late endosomes. J Biol Chem. 2005;280:29277–29281. doi: 10.1074/jbc.M505566200. [DOI] [PubMed] [Google Scholar]
- 57.Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR. A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J Clin Invest. 2003;111:99–107. doi: 10.1172/JCI16808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Denis M, Landry YD, Zha X. ATP-binding cassette A1-mediated lipidation of apolipoprotein A-I occurs at the plasma membrane and not in the endocytic compartments. J Biol Chem. 2008;283:16178–16186. doi: 10.1074/jbc.M709597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faulkner LE, Panagotopulos SE, Johnson JD, Woollett LA, Hui DY, Witting SR, Maiorano JN, Davidson WS. An analysis of the role of a retroendocytosis pathway in ABCA1-mediated cholesterol efflux from macrophages. J Lipid Res. 2008;49:1322–1332. doi: 10.1194/jlr.M800048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Remaley AT, Stonik JA, Demosky SJ, Neufeld EB, Bocharov AV, Vishnyakova TG, Eggerman TL, Patterson AP, Duverger NJ, Santamarina-Fojo S, Brewer HB., Jr Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem Biophys Res Commun. 2001;280:818–823. doi: 10.1006/bbrc.2000.4219. [DOI] [PubMed] [Google Scholar]
- 61.Segrest JP, Jones MK, De Loof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The amphipathic helix in the exchangeable apolipoproteins: a review of secondary structure and function. J Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- 62.Mendez AJ, Anantharamaiah GM, Segrest JP, Oram JF. Synthetic amphipathic helical peptides that mimic apolipoprotein A-I in clearing cellular cholesterol. J Clin Invest. 1994;94:1698–1705. doi: 10.1172/JCI117515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Remaley AT, Thomas F, Stonik JA, Demosky SJ, Bark SE, Neufeld EB, Bocharov AV, Vishnyakova TG, Patterson AP, Eggerman TL, Santamarina-Fojo S, Brewer HB. Synthetic amphipathic helical peptides promote lipid efflux from cells by an ABCA1-dependent and an ABCA1-independent pathway. J Lipid Res. 2003;44:828–836. doi: 10.1194/jlr.M200475-JLR200. [DOI] [PubMed] [Google Scholar]
- 64.Yancey PG, Bielicki JK, Johnson WJ, Lund-Katz S, Palgunachari MN, Anantharamaiah GM, Segrest JP, Phillips MC, Rothblat GH. Efflux of cellular cholesterol and phospholipid to lipid-free apolipoproteins and class A amphipathic peptides. Biochemistry. 1995;34:7955–7965. doi: 10.1021/bi00024a021. [DOI] [PubMed] [Google Scholar]
- 65.Arakawa R, Yokoyama S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J Biol Chem. 2002;277:22426–22429. doi: 10.1074/jbc.M202996200. [DOI] [PubMed] [Google Scholar]
- 66.Oram JF, Wolfbauer G, Tang C, Davidson WS, Albers JJ. An amphipathic helical region of the N-terminal barrel of phospholipid transfer protein is critical for ABCA1-dependent cholesterol efflux. J Biol Chem. 2008;283:11541–11549. doi: 10.1074/jbc.M800117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oram JF, Wolfbauer G, Vaughan AM, Tang C, Albers JJ. Phospholipid transfer protein interacts with and stabilizes ATP-binding cassette transporter A1 and enhances cholesterol efflux from cells. J Biol Chem. 2003;278:52379–52385. doi: 10.1074/jbc.M310695200. [DOI] [PubMed] [Google Scholar]
- 68.van der Westhuyzen DR, Cai L, de Beer MC, de Beer FC. Serum amyloid A promotes cholesterol efflux mediated by scavenger receptor B-I. J Biol Chem. 2005;280:35890–35895. doi: 10.1074/jbc.M505685200. [DOI] [PubMed] [Google Scholar]
- 69.Stonik JA, Remaley AT, Demosky SJ, Neufeld EB, Bocharov A, Brewer HB. Serum amyloid A promotes ABCA1-dependent and ABCA1-independent lipid efflux from cells. Biochem Biophys Res Commun. 2004;321:936–941. doi: 10.1016/j.bbrc.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 70.Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR, de Beer FC. HDL remodeling during the acute phase response. Arterioscler Thromb Vasc Biol. 2009;29:261–267. doi: 10.1161/ATVBAHA.108.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marsche G, Frank S, Raynes JG, Kozarsky KF, Sattler W, Malle E. The lipidation status of acute-phase protein serum amyloid A determines cholesterol mobilization via scavenger receptor class B, type I. Biochem J. 2007;402:117–124. doi: 10.1042/BJ20061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoffman JS, Benditt EP. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982;257:10510–10517. [PubMed] [Google Scholar]
- 73.de Beer MC, Ji A, Jahangiri A, Vaughan AM, de Beer FC, van der Westhuyzen DR, Webb NR. ATP binding cassette G1-dependent cholesterol efflux during inflammation. J Lipid Res. 2010;52:345–353. doi: 10.1194/jlr.M012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soro-Paavonen A, Westerbacka J, Ehnholm C, Taskinen MR. Metabolic syndrome aggravates the increased endothelial activation and low-grade inflammation in subjects with familial low HDL. Ann Med. 2006;38:229–238. doi: 10.1080/07853890500526352. [DOI] [PubMed] [Google Scholar]
- 75.Birjmohun RS, van Leuven SI, Levels JH, van 't Veer C, Kuivenhoven JA, Meijers JC, Levi M, Kastelein JJ, van der Poll T, Stroes ES. High-density lipoprotein attenuates inflammation and coagulation response on endotoxin challenge in humans. Arterioscler Thromb Vasc Biol. 2007;27:1153–1158. doi: 10.1161/ATVBAHA.106.136325. [DOI] [PubMed] [Google Scholar]
- 76.Francone OL, Royer L, Boucher G, Haghpassand M, Freeman A, Brees D, Aiello RJ. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1198–1205. doi: 10.1161/01.ATV.0000166522.69552.99. [DOI] [PubMed] [Google Scholar]
- 77.Koseki M, Hirano K, Masuda D, Ikegami C, Tanaka M, Ota A, Sandoval JC, Nakagawa-Toyama Y, Sato SB, Kobayashi T, Shimada Y, Ohno-Iwashita Y, Matsuura F, Shimomura I, Yamashita S. Increased lipid rafts and accelerated lipopolysaccharide-induced tumor necrosis factor-alpha secretion in Abca1-deficient macrophages. J Lipid Res. 2007;48:299–306. doi: 10.1194/jlr.M600428-JLR200. [DOI] [PubMed] [Google Scholar]
- 78.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Eck M, Bos IS, Kaminski WE, Orso E, Rothe G, Twisk J, Bottcher A, Van Amersfoort ES, Christiansen-Weber TA, Fung-Leung WP, Van Berkel TJ, Schmitz G. Leukocyte ABCA1 controls susceptibility to atherosclerosis and macrophage recruitment into tissues. Proc Natl Acad Sci U S A. 2002;99:6298–6303. doi: 10.1073/pnas.092327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu X, Owen JS, Wilson MD, Li H, Griffiths GL, Thomas MJ, Hiltbold EM, Fessler MB, Parks JS. Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor trafficking to lipid rafts by reduction of lipid raft cholesterol. J Lipid Res. 2010;51:3196–3206. doi: 10.1194/jlr.M006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Murphy AJ, Woollard KJ, Hoang A, Mukhamedova N, Stirzaker RA, McCormick SP, Remaley AT, Sviridov D, Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 83.Tellier E, Canault M, Poggi M, Bonardo B, Nicolay A, Alessi MC, Nalbone G, Peiretti F. HDLs activate ADAM17-dependent shedding. J Cell Physiol. 2008;214:687–693. doi: 10.1002/jcp.21265. [DOI] [PubMed] [Google Scholar]
- 84.Sun Y, Ishibashi M, Seimon T, Lee M, Sharma SM, Fitzgerald KA, Samokhin AO, Wang Y, Sayers S, Aikawa M, Jerome WG, Ostrowski MC, Bromme D, Libby P, Tabas IA, Welch CL, Tall AR. Free cholesterol accumulation in macrophage membranes activates Toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ Res. 2009;104:455–465. doi: 10.1161/CIRCRESAHA.108.182568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- 87.Levy DE, Lee CK. What does Stat3 do? J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murray PJ. STAT3-mediated anti-inflammatory signalling. Biochem Soc Trans. 2006;34:1028–1031. doi: 10.1042/BST0341028. [DOI] [PubMed] [Google Scholar]
- 89.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Williams LM, Sarma U, Willets K, Smallie T, Brennan F, Foxwell BM. Expression of constitutively active STAT3 can replicate the cytokine-suppressive activity of interleukin-10 in human primary macrophages. J Biol Chem. 2007;282:6965–6975. doi: 10.1074/jbc.M609101200. [DOI] [PubMed] [Google Scholar]
- 91.Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 92.Langmann T, Porsch-Ozcurumez M, Heimerl S, Probst M, Moehle C, Taher M, Borsukova H, Kielar D, Kaminski WE, Dittrich-Wengenroth E, Schmitz G. Identification of sterol-independent regulatory elements in the human ATP-binding cassette transporter A1 promoter: role of Sp1/3, E-box binding factors, and an oncostatin M-responsive element. J Biol Chem. 2002;277:14443–14450. doi: 10.1074/jbc.M110270200. [DOI] [PubMed] [Google Scholar]
- 93.Santamarina-Fojo S, Peterson K, Knapper C, Qiu Y, Freeman L, Cheng JF, Osorio J, Remaley A, Yang XP, Haudenschild C, Prades C, Chimini G, Blackmon E, Francois T, Duverger N, Rubin EM, Rosier M, Denefle P, Fredrickson DS, Brewer HB., Jr Complete genomic sequence of the human ABCA1 gene: analysis of the human and mouse ATP-binding cassette A promoter. Proc Natl Acad Sci U S A. 2000;97:7987–7992. doi: 10.1073/pnas.97.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Le Goff W, Zheng P, Brubaker G, Smith JD. Identification of the cAMP-responsive enhancer of the murine ABCA1 gene: requirement for CREB1 and STAT3/4 elements. Arterioscler Thromb Vasc Biol. 2006;26:527–533. doi: 10.1161/01.ATV.0000201042.00725.84. [DOI] [PubMed] [Google Scholar]
- 95.See RH, Caday-Malcolm RA, Singaraja RR, Zhou S, Silverston A, Huber MT, Moran J, James ER, Janoo R, Savill JM, Rigot V, Zhang LH, Wang M, Chimini G, Wellington CL, Tafuri SR, Hayden MR. Protein kinase A site-specific phosphorylation regulates ATP-binding cassette A1 (ABCA1)-mediated phospholipid efflux. J Biol Chem. 2002;277:41835–41842. doi: 10.1074/jbc.M204923200. [DOI] [PubMed] [Google Scholar]
- 96.Haidar B, Denis M, Krimbou L, Marcil M, Genest J., Jr cAMP induces ABCA1 phosphorylation activity and promotes cholesterol efflux from fibroblasts. J Lipid Res. 2002;43:2087–2094. doi: 10.1194/jlr.m200235-jlr200. [DOI] [PubMed] [Google Scholar]
- 97.Haidar B, Denis M, Marcil M, Krimbou L, Genest J., Jr Apolipoprotein A-I activates cellular cAMP signaling through the ABCA1 transporter. J Biol Chem. 2004;279:9963–9969. doi: 10.1074/jbc.M313487200. [DOI] [PubMed] [Google Scholar]
- 98.Kerr IM, Costa-Pereira AP, Lillemeier BF, Strobl B. Of JAKs, STATs, blind watchmakers, jeeps and trains. FEBS Lett. 2003;546:1–5. doi: 10.1016/s0014-5793(03)00411-3. [DOI] [PubMed] [Google Scholar]
- 99.Vaughan AM, Tang C, Oram JF. ABCA1 mutants reveal an interdependency between lipid export function, apoA-I binding activity, and Janus kinase 2 activation. J Lipid Res. 2009;50:285–292. doi: 10.1194/jlr.M800366-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yin K, Deng X, Mo ZC, Zhao GJ, Jiang J, Cui LB, Tan CZ, Wen GB, Fu Y, Tang CK. Tristetraprolin-dependent Post-transcriptional Regulation of Inflammatory Cytokine mRNA Expression by Apolipoprotein A-I: ROLE OF ATP-BINDING MEMBRANE CASSETTE TRANSPORTER A1 AND SIGNAL TRANSDUCER AND ACTIVATOR OF TRANSCRIPTION 3. J Biol Chem. 2011;286:13834–13845. doi: 10.1074/jbc.M110.202275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Amar MJ, D'Souza W, Turner S, Demosky S, Sviridov D, Stonik J, Luchoomun J, Voogt J, Hellerstein M, Sviridov D, Remaley AT. 5A apolipoprotein mimetic peptide promotes cholesterol efflux and reduces atherosclerosis in mice. J Pharmacol Exp Ther. 2010;334:634–641. doi: 10.1124/jpet.110.167890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D'Souza W, Sviridov D, Remaley AT. Asymmetry in the lipid affinity of bihelical amphipathic peptides. A structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J Biol Chem. 2008;283:32273–32282. doi: 10.1074/jbc.M804461200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nofer JR, Feuerborn R, Levkau B, Sokoll A, Seedorf U, Assmann G. Involvement of Cdc42 signaling in apoA-I-induced cholesterol efflux. J Biol Chem. 2003;278:53055–53062. doi: 10.1074/jbc.M305673200. [DOI] [PubMed] [Google Scholar]
- 104.Nofer JR, Remaley AT, Feuerborn R, Wolinnska I, Engel T, von Eckardstein A, Assmann G. Apolipoprotein A-I activates Cdc42 signaling through the ABCA1 transporter. J Lipid Res. 2006;47:794–803. doi: 10.1194/jlr.M500502-JLR200. [DOI] [PubMed] [Google Scholar]
- 105.Boadu E, Choi HY, Lee DW, Waddington EI, Chan T, Asztalos B, Vance JE, Chan A, Castro G, Francis GA. Correction of apolipoprotein A-I-mediated lipid efflux and high density lipoprotein particle formation in human Niemann-Pick type C disease fibroblasts. J Biol Chem. 2006;281:37081–37090. doi: 10.1074/jbc.M606890200. [DOI] [PubMed] [Google Scholar]
- 106.Schmitz G, Grandl M. Lipid homeostasis in macrophages - implications for atherosclerosis. Rev Physiol Biochem Pharmacol. 2008;160:93–125. doi: 10.1007/112_2008_802. [DOI] [PubMed] [Google Scholar]
- 107.Schmitz G, Grandl M. The molecular mechanisms of HDL and associated vesicular trafficking mechanisms to mediate cellular lipid homeostasis. Arterioscler Thromb Vasc Biol. 2009;29:1718–1722. doi: 10.1161/ATVBAHA.108.179507. [DOI] [PubMed] [Google Scholar]
- 108.Roosbeek S, Peelman F, Verhee A, Labeur C, Caster H, Lensink MF, Cirulli C, Grooten J, Cochet C, Vandekerckhove J, Amoresano A, Chimini G, Tavernier J, Rosseneu M. Phosphorylation by protein kinase CK2 modulates the activity of the ATP binding cassette A1 transporter. J Biol Chem. 2004;279:37779–37788. doi: 10.1074/jbc.M401821200. [DOI] [PubMed] [Google Scholar]
- 109.Singaraja RR, Kang MH, Vaid K, Sanders SS, Vilas GL, Arstikaitis P, Coutinho J, Drisdel RC, El-Husseini Ael D, Green WN, Berthiaume L, Hayden MR. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ Res. 2009;105:138–147. doi: 10.1161/CIRCRESAHA.108.193011. [DOI] [PubMed] [Google Scholar]
- 110.Martinez LO, Agerholm-Larsen B, Wang N, Chen W, Tall AR. Phosphorylation of a pest sequence in ABCA1 promotes calpain degradation and is reversed by ApoA-I. J Biol Chem. 2003;278:37368–37374. doi: 10.1074/jbc.M307161200. [DOI] [PubMed] [Google Scholar]
- 111.Yamauchi Y, Hayashi M, Abe-Dohmae S, Yokoyama S. Apolipoprotein A-I activates protein kinase C alpha signaling to phosphorylate and stabilize ATP binding cassette transporter A1 for the high density lipoprotein assembly. J Biol Chem. 2003;278:47890–47897. doi: 10.1074/jbc.M306258200. [DOI] [PubMed] [Google Scholar]
- 112.Lu R, Arakawa R, Ito-Osumi C, Iwamoto N, Yokoyama S. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler Thromb Vasc Biol. 2008;28:1820–1824. doi: 10.1161/ATVBAHA.108.169482. [DOI] [PubMed] [Google Scholar]
- 113.Rechsteiner M, Rogers SW. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 114.Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a phospholipase D2 pathway. J Biol Chem. 2005;280:35896–35903. doi: 10.1074/jbc.M506210200. [DOI] [PubMed] [Google Scholar]
- 115.Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize ABCA1 through a protein kinase C delta pathway. J Lipid Res. 2007;48:1062–1068. doi: 10.1194/jlr.M600437-JLR200. [DOI] [PubMed] [Google Scholar]
- 116.Nagao K, Zhao Y, Takahashi K, Kimura Y, Ueda K. Sodium taurocholate-dependent lipid efflux by ABCA1: effects of W590S mutation on lipid translocation and apolipoprotein A-I dissociation. J Lipid Res. 2009;50:1165–1172. doi: 10.1194/jlr.M800597-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 118.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 120.Khera AV, Rader DJ. Future therapeutic directions in reverse cholesterol transport. Curr Atheroscler Rep. 2010;12:73–81. doi: 10.1007/s11883-009-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tall AR. CETP inhibitors to increase HDL cholesterol levels. N Engl J Med. 2007;356:1364–1366. doi: 10.1056/NEJMe078029. [DOI] [PubMed] [Google Scholar]
- 122.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Degoma EM, Rader DJ. Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol. 2011;8:266–277. doi: 10.1038/nrcardio.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 125.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O'Connell R M, Cheng G, Saez E, Miller JF, Tontonoz P. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 126.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 127.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors. LXRalpha and LXRbeta, Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chisholm JW, Hong J, Mills SA, Lawn RM. The LXR ligand T0901317 induces severe lipogenesis in the DB/DB diabetic mouse. J Lipid Res. 2003 doi: 10.1194/jlr.M300135-JLR200. [DOI] [PubMed] [Google Scholar]
- 130.Wellington CL, Walker EK, Suarez A, Kwok A, Bissada N, Singaraja R, Yang YZ, Zhang LH, James E, Wilson JE, Francone O, McManus BM, Hayden MR. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Invest. 2002;82:273–283. doi: 10.1038/labinvest.3780421. [DOI] [PubMed] [Google Scholar]
- 131.Dimayuga P, Zhu J, Oguchi S, Chyu KY, Xu XO, Yano J, Shah PK, Nilsson J, Cercek B. Reconstituted HDL containing human apolipoprotein A-1 reduces VCAM-1 expression and neointima formation following periadventitial cuff-induced carotid injury in apoE null mice. Biochem Biophys Res Commun. 1999;264:465–468. doi: 10.1006/bbrc.1999.1278. [DOI] [PubMed] [Google Scholar]
- 132.Shah PK, Nilsson J, Kaul S, Fishbein MC, Ageland H, Hamsten A, Johansson J, Karpe F, Cercek B. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation. 1998;97:780–785. doi: 10.1161/01.cir.97.8.780. [DOI] [PubMed] [Google Scholar]
- 133.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Navab M, Reddy ST, Buga GM, Fogelman AM. Apolipoprotein A-I mimetic peptides. Curr Atheroscler Rep. 2009;11:52–57. doi: 10.1007/s11883-009-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]