Abstract
Pre-B-Cell Colony-Enhancing Factor (PBEF) is known as a rate-limiting enzyme that converts nicotinamide (NAM) to NMN in the salvage pathway of mammalian NAD+ biosynthesis. Previously we found PBEF is exclusively expressed in neurons in the mouse brain; heterozygous PBEF knockout (Pbef+/−) mice have larger ischemic lesion than wild type mice in photothrombosis-induced ischemia. For the mechanistic study of neuronal protective role of PBEF, we used in vitro oxygen-glucose deprivation (OGD) and glutamate excitotoxicity models of primary cultured neurons in current study. Our results showed that the treatments of neurons with NAM and NAD+, the substrate and downstream product of PBEF, respectively, significantly reduced neuronal death after OGD and glutamate excitotoxicity, while treatment of neurons treated with FK866, a PBEF inhibitor, increased neuronal death after OGD. Furthermore, overexpression of human PBEF (hPBEF) reduced glutamate excitotoxicity, while overexpression of hPBEF mutants (i.e., H247A, and H247E) without enzymatic activity had no effect on neuronal death. We further tested the effect of PBEF on mitocondrial function and biogenesis. Our results show that addition of NAD+ and NAM increased mitochondrial biogenesis in neurons after OGD. Overexpression of PBEF in neurons reduced mitochondrial membrane potential (MMP) depolarization following glutamate stimulation, while overexpression of H247A and H247E did not affect MMP depolarization. We conclude that PBEF has a neuroprotective effect in ischemia through its enzymatic activity for NAD+ production that can ameliorate mitochondrial dysfunction.
Keywords: Nicotinamide phosphoribosyltransferase (Nampt), NAD+, NAM, ischemia, mitochondrial biogenesis, mitochondrial membrane potential
Introduction
Stroke is the leading cause of long-term disability. Several different mechanisms regarding the neuronal death and brain damage following ischemia have been suggested, those including glutamate and Ca2+ toxicity, oxidative stress, acidosis, inflammation, and mitochondrial dysfunction (Barber and Demchuk 2003; Xiong et al. 2004; Mattiasson et al. 2003; Choi 1988; Lo et al. 2003; Swanson et al. 2004). Although these mechanisms demonstrate overlapping and redundant features because of their temporal and spatial dependence, energy depletion is the root cause of ischemia-induced brain damage.
Pre-B-cell colony-enhancing factor (PBEF), also known as Nicotinamide phosphoribosyltransferase (Nampt) is the rate-limiting enzyme to catalyze the conversion of nicotinamide (NAM) to NMN in the salvage pathway of mammalian NAD+ biosynthesis (Revollo et al. 2007; Luk et al. 2008), the predominant pathway for NAD+ biosynthesis in mammals (Magni et al. 1999; Imai 2009). The major cellular functions of NAD+ and its derivative compound NADH include modulating cellular energy metabolism and mitochondrial biogenesis (Ying 2006; Chen et al. 2010; Csiszar et al. 2009; Lagouge et al. 2006). The intracellular levels of NAD+ and NAM have recently been shown to be important for cell survival (Araki et al. 2004; Bitterman et al. 2002). Upregulation of Nampt increases the cellular NAD+ level and enhances the transcriptional regulatory activity of the catalytic domain of Sirt1 in mouse fibroblasts (Revollo et al. 2004). In HEK293 cells, Nampt is also an essential component of the mitochondrial NAD+ salvage pathway and promotes cell survival through stimulation of mitochondrial sirtuins, including Sirt3 and Sirt4 (Yang et al. 2007). Most recently, it is demonstrated that Nampt protects macrophages from ER stress-induced apoptosis through its non-enzymatic activity that triggers secretion of IL-6 and consequentially activates the pro-survival signal transducer STAT3 in an IL-6-mediated autocrine/paracrine manner (Li et al. 2008b). PBEF has also been shown to play a role in inflammatory, stress-related and metabolic response (Liu et al. 2009b; Li et al. 2008a) and mediate cardiac myocyte survival (Hsu et al. 2009).
Despite the various roles of PBEF in cellular function and cell survival in non-CNS, little has been explored regarding the function and the role of PBEF in health and diseases in CNS. Our recent study showed that PBEF is exclusively expressed in neurons in mouse brain and heterozygous PBEF knockout (Pbef+/−) mice have larger ischemic lesion than wild type mice, suggesting PBEF is important in neuronal survival after ischemia (Zhang et al. 2010). In this study we further investigated the effects and mechanisms of PBEF on ischemia using in vitro ischemia models including oxygen-glucose deprivation (OGD) as well as glutamate excitotoxicity of primary cultured neurons. We postulate that PBEF might be an important enzyme to regulate cellular energy metabolism and signaling pathways in neurons, and alterations in expression level or enzymatic activity may have significant impact on cellular function and survival under ischemic conditions. The effects of PBEF on neuronal protection, NAD+ synthesis, and mitochondria dysfunction in ischemic condition have been studied using both pharmacological and molecular approaches.
Materials and Methods
Primary neuronal cultures
Throughout the study, timely-pregnant C57BL/6J mice were either purchased from Jackson Laboratory or raised in the animal facility in the University of Missouri. All procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Missouri Animal Care Quality Assurance Committee. Cortical neurons were prepared from embryonic day 15/16 mice. Cortical tissues were dissociated by a mild mechanical triturating after digestion with trypsin. The dissociated cells were planted onto poly-D-lysine-coated (50 μg/ml) (Cat# P9155, Sigma) tissue culture plates or glass coverslips of 12 mm in diameter (Cat#1943-10012, Fisher Scientific) in a culture plate with Dulbecco’s modified Eagle medium/nutrient F12 (DMEM/F12) (Cat# 25030, Invitrogen, CA) supplemented with 10% heated-inactivated fetal bovine serum (Cat# S11150, Atlanta Biological) for 4 h, the medium was then changed to Neurobasal Media (Cat#21103, Invitrogen) containing 2% B-27 serum free supplements (Cat# 17504-044, Invitrogen). The cultures were maintained in an incubator at 37 °C with a humidified atmosphere of 5% CO2 and 95% air. Experiments were conducted within 7–12 days in vitro (DIV). We did immunostaining on MAP2 (Supplementary Fig 1A), a neuronal marker, to test the quality of cultured neurons. Our data show that 97.7±0.3 % (n=2 preparations, counted ~3000 cells in 6 image fields) cells expressed MAP2, suggesting high purity of cultured neurons.
In vitro models of ischemia
To mimic ischemia-like conditions in vitro, primary neuronal cultures in 24-well plates were exposed to transient OGD similar to previous report (Cao et al. 2003). In brief, the culture medium was rinsed out twice and replaced with serum- and glucose-free medium (Cat# 11966, Invitrogen), and culture plates were then placed in a modular chamber in a 37°C incubator. The chamber was sealed and flushed with 95% N2 and 5% CO2 for 90 min and then returned to 5% CO2 and 95% air and glucose-containing medium for the period of time indicated in each experiment. To induce glutamate excitotoxicity, neuronal cultures were exposed to 50 or 100 μM glutamate with 10μM glycine for 3 h.
Neuronal injury and death assay
Neuronal injury induced by OGD and glutamate excitotoxicity was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (He et al. 2011), a method used to assess mitochondrial function by measuring the ability of neurons to reduce MTT by reductase. Briefly, after OGD or glutamate stimulation, MTT (Cat# M2128, Sigma-Aldrich, MO) was added to neurons cultured in 48-well plates (~105 neurons in each well) for a final concentration of 0.5 mg/ml and incubated at 37°C for an additional 3 h. The supernatant was then removed and dimethyl sulfoxide (DMSO) was added to each well to dissolve the formed blue formazan. Absorbance was read at 570 nm on a Monochromatic Microplate Reader (BioTek, VT). Cell viability was expressed as a percentage of the control culture value in each experiment. Values from 3–5 wells of neurons from the same preparation were averaged as a single value for that experiment. Data from 4 to 6 experiments with the same condition were averaged.
We used propidium iodide (PI) staining as a complementary assay for neuronal death after OGD and glutamate stimulation (Taghibiglou et al. 2009). PI can intercalate into double-stranded nucleic acids. It is excluded by viable cells but can penetrate cell membranes of dying or dead cells. For this experiment, neurons were seeded on glass coverslips coated with poly-D-lysine. Neuronal cultures after OGD or glutamate stimulation were stained with 10 μg/mL PI (Cat# P4710, Sigma-Aldrich, MO) for 30 min, and subsequently with 4′, 6-diamidino-2-phenylindole (Dapi) (Cat# 36931, Invitrogen) to label nuclei. The total number of neuron was counted based on Dapi stained nuclei and PI+ cells were counted as dead neurons. Cell counting was done in a blinded manner in four to six randomly picked images (at least 500 neurons per image) from different places within each glass coverslip. Each experimental group was repeated in triplicate glass coverslips and averaged to produce a single value for that experiment group.
Mitochondrial staining with MitoTracker
MitoTracker Red FM (Cat# M22425, Invitrogen) was used to stain mitochondria in neurons to quantify mitochondrial mass by fluorescence intensity (Chen et al. 2010). MitoTracker Red FM was dissolved in DMSO to make a stock solution with a concentration of 1 mM. The cells were washed twice with 1× PBS diluted from 10× solution (Cat# 14200, Invitrogen) and then incubated with 500 nM MitoTracker Red FM for 30 min. After 3 washes with PBS, the cells were subjected to fluorescence detection using a Nikon FN1 epi-fluorescence microscopy equipped with a CoolSNAP-EZ CCD-camera. The average intensity or intensity distribution of MitoTracker Red fluorescence of an entire field was analyzed by MetaMorph Imaging software (Molecular Devices, CA).
Mitochondrial DNA (mtDNA) and nuclear DNA (nucDNA) analysis
PCR was used to quantify the relative abundance of intact mtDNA (Valerio et al. 2011; Yin et al. 2008). Total DNA of cultured neurons was extracted and purified using the genomic DNA extraction kit (Cat. No. 69504, Qiagen Sciences, Inc., MD). The total DNA (50 ng) derived from neurons in one well of 12-well plates was added to the polymerase chain reaction (PCR) mixture with GoTaq Flexi DNA Ploymerase (Cat# PRM8295, Promega, WI). The primers used for the amplification of mtDNA were: 5′-ATTTCGTGCCAGCCACCGCGG-3′ (forward); 5′-GGCTACACCTTGACCTAACGT-3′ (reverse). The primers for nucDNA were: 5′-GGAATAATGGAATAGGACCGCG-3′ (forward); 5′-GGACATCTAAGGGCATCACAG-3′ (reverse). PCR reaction was performed at 94°C for 3 min, then 18 cycles at 94°C for 30s, 55°C for 30s and 72°C for 1 min, followed by 72°C for 7 min for the final extension. PCR products are 655 bp for mtDNA and 464 bp for nucDNA. They were separated on a 1% agarose gel and stained by Ethidium Bromide (0.5 μg/ml). The band images were acquired using an Alpha Imager and analyzed by the AlphaEase Stand Alone Software (Alpha Innotech Corp., CA).
Determining mitochondrial membrane potential depolarization
Mitochondrial membrane potential (MMP) was evaluated with the fluorescent probe tetramethylrhodamine ethyl ester (TMRE, Molecular Probes) using time-lapse fluorescent imaging similar to methods described previously (Liu et al. 2009a). Neurons cultured on glass coverslips were loaded with 25 nM TMRE for 20 min at RT, in ACSF containing (in mM): 120 NaCl, 10 Hepes, 3.1 KCl, 2 CaCl2, 1.3 MgCl2, and 10 glucose (pH 7.4). Cells were perfused by ACSF containing 25 nM TMRE throughout the experiments. Time-lapse imaging of TMRE fluorescence was performed using an upright wide-field Nikon FN1 epi-fluorescence microscope with a 40x/0.8 water immersion objective. Excitation was generated with an X-Ford metal halide lamp filtered with a Nikon Y-2E/C fluorescence filter. Emission was detected by a CoolSNAP-EZ CCD-camera. Glutamate (100μM) and glycine (10 μM) were applied through a perfusion system equipped with a pinch valve that controls the duration of application. Images were acquired every 30 s using MetaMorph Imaging software. Fluorescent signals of TMRE were quantified by measuring the mean pixel intensities of the cell body of each neuron using the MetaMorph software. Fluorescence changes in individual neurons were calculated as ΔF/Fo values vs. time, where Fo was the baseline fluorescence and were normalized to its peak value of ΔF/Fo.
Statistical analysis
Data are expressed as means ± S.E.M obtained from 4–6 independent experiments. Statistical significance was assayed by Student’s t-test. A P<0.05 was considered to be statistically significant.
Results
NAM and NAD+ ameliorate neuronal death after OGD and glutamate excitotoxicity
To test the role of PBEF in neuronal protection in ischemia using primary cultured neurons, we initially did an immunostaining of PBEF in cultured cells (Supplementary Fig. 1B). Our results show that 90.4±1.8 % (n=3 preparations, counted ~1000 cells) of cells express PBEF based on the total number of cells evaluated by Dapi staining, consistent with our in vivo study showing that the majority of PBEF expressing cells were neurons in the mouse brain (Zhang et al. 2010).
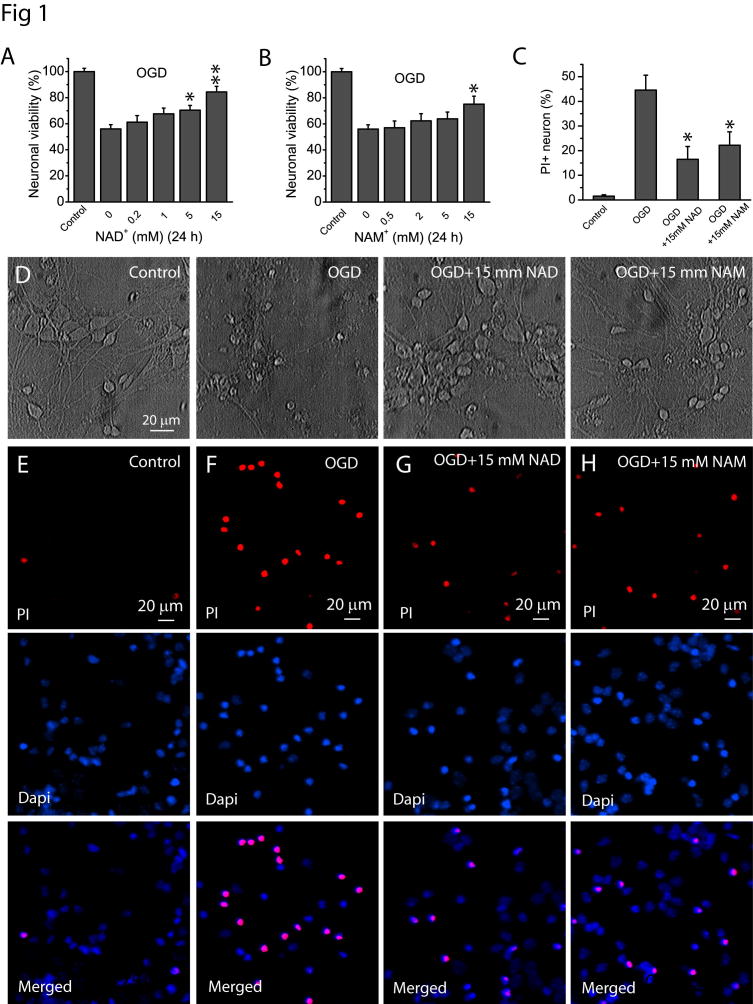
Our previous study showed that knockout of PBEF increased ischemia lesion in the mouse brain using a photothrombosis-induced ischemia model. To further test the role of PBEF in ischemia, we used two in vitro ischemic models, i.e., OGD and glutamate excitotoxicity in this study. These models can mimic in vivo ischemic conditions and have been widely used for mechanistic studies of ischemia. To test whether PBEF confers neuronal protection against ischemia, we first studied the effect of NAM and NAD+, which are the substrate and downstream product of PBEF, on neuronal viability after OGD and glutamate excitotoxicity. NAD+ and NAM at various concentrations were added directly to the neuronal cultures prior to OGD and kept in the medium for a total of 24 h. Cell viability was measured using MTT assay. The results showed that treatments of high concentration of NAD+ and NAM significantly reduced OGD-induced loss of neuronal viability (Fig. 1A–B). The protective effects of NAD+ and NAM were also confirmed using morphological assessments (Fig. 1D). Representative photomicrographs demonstrated that neurons in the control group exhibit bright cell body with intact processes. In contrast, a 90 min of OGD resulted in shrinkage of neuronal soma and beading and retraction of neurites. However, cultures treated with 15 mM NAD+ and NAM maintained relatively normal neuronal morphology after OGD. We used a complementary assay of PI staining and showed that treatments of neurons with 15 mM NAD+ and NAM remarkably attenuated cell death at 24 h after OGD (Fig. 1C, E–H), which is consistent with the findings via MTT assay.
Fig 1. NAD+ and NAM protect neurons after OGD.
(A–B) Effect of NAD+ (A) and NAM (B) on neuronal viability after OGD. Neuronal cultures in 48 well plates were subject to OGD for 1.5 h in the absence and presence of NAD+ and NAM of various concentrations. NAD+ and NAM were continuously present after OGD for a total time of 24 h from the beginning of OGD. MTT assay was then performed to determine the effect of NAD+ and NAM on neuronal viability. (C) Summary of PI assay to confirm the neuronal protective effect of NAD+ and NAM after 1.5 h of OGD. The percentage of PI+ cells was calculated based on the total number of cells determined by Dapi-stained nuclei. Data from (A–C) were the mean value from 6 independent experiments, for each experiment, 3–4 parallel assays were performed and averaged as a single value for that experiment. *t-test with p<0.05; **t-test with p<0.005 vs no NAD+ or NAM treatment for (A–C). (D) Phase contrast image of cultured neurons at 24 h after 1.5 h of OGD in the presence and absence of 15 mM NAD+ and NAM. Notice the normal morphology of neurons treated with NAD+ and NAM. (E–G) Representative images of PI staining in the absence or presence of NAD+ and NAM. Neuronal cultures on glass coverslips in 48 well plates were subject to OGD for 1.5 h in the absence and presence of 15 mM NAD+ and NAM. NAD+ and NAM were continuously present after OGD for a total time of 24 h from the beginning of OGD. Neurons were then stained with PI and Dapi for 30 min for imaging and cell counting.
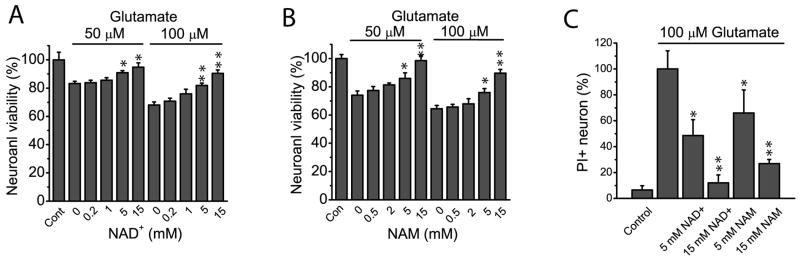
Ischemia induces glutamate elevation and subsequent Ca2+ overloading through the overstimulation of glutamate receptors-especially NMDA receptors, which are the primary mediators of acute neuronal death (Dirnagl et al. 1999). Thus glutamate has also been used as a model for excitotoxicity to mimic in vivo ischemia. We incubated neuronal culture with 50 and 100 μM glutamate for 3 h in the presence of various concentrations of NAD+ and NAM. Consistent with results using the OGD model, 5 mM and 15 mM of NAD+ and NAM significantly ameliorated cell viability reduction (Fig. 2A–B). Furthermore, 5 and 15 mM NAD+, and 15 mM NAM significantly reduced neuronal death based on PI staining (Fig. 2C and Fig. S3). Thus using two different in vitro ischemic models and two different assays our results demonstrated that NAM and NAD+ have a neuronal protective effect, suggesting PBEF plays a critical role in neuronal protection after ischemia through its enzymatic activity.
Fig 2. NAD+ and NAM protect neurons after excitotoxic glutamate stimulations.
(A–B)Effect of NAD+ and NAM on cell viability after 50 μM and 100 μM glutamate stimulations. Neurons were treated with glutamate for 3 h in the absence and in the presence of various concentrations of NAD+ (A) and NAM (B). NAD+ and NAM were continuously present after glutamate stimulation for a total time of 24 h. Neuronal viability was then determined by MTT assay. (C) Summary of PI staining confirming the neuronal protective effect of NAD+ and NAM after glutamate stimulations. The percentage of PI+ cells was calculated based on the total number cells determined by Dapi-stained nuclei and was normalized to the values in the absence of NAD+ and NAM. Data from (A–C) were the mean values from 5–6 independent experiments; for each experiment, 3–4 parallel assays were performed and averaged as a single value for that experiment. *t-test with p<0.05; **t-test with p<0.005 vs no NAD+ or NAM treatment for (A–C).
FK866 exacerbates OGD-induced neuronal injury and NAD+ depletion
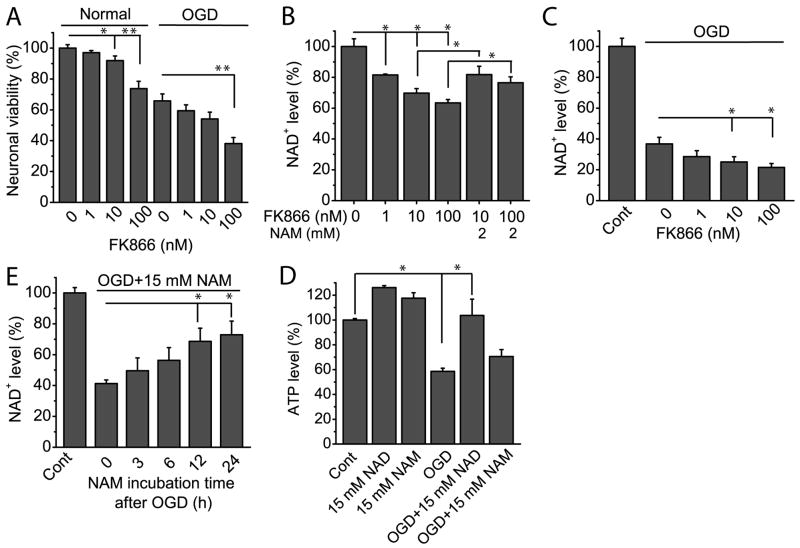
Although the above and our previous (Zhang et al. 2010) studies suggest NAD+ depletion would cause neuronal death in cerebral ischemia, whether modulation of NAD+ synthesis by PBEF affects neuronal survival is unclear. To inhibit the enzymatic activity of PBEF in neurons, we resorted to its specific inhibitor FK866 (Skokowa et al. 2009). Initially we studied whether FK866 affects neuronal viability under normal condition. Thus, neurons were exposed to different concentrations of FK866 for 4 h, and neuronal viability was evaluated using MTT assay. Our data showed that exposure to FK866 reduced neuronal viability in a dose dependent manner (Fig. 3A). A similar effect was observed on NAD+ levels in the presence of FK866 (Fig. 3B). Surprisingly, the addition of NAM also restored NAD+ levels (Fig. 3B). Being consistent with the fact that PBEF is a rate limiting enzyme in a salvage pathway of mammalian NAD+ synthesis in other systems, our data indicate that PBEF plays the same role in CNS.
Fig 3. Effect of the inhibition of PBEF by FK866 on cell viability and NAD+ and ATP levels of cultured neurons.
(A) FK866 reduced neuronal viability under normal and OGD conditions. For measurement of cell viability under normal condition, neurons were treated with indicated concentration of FK866 for 4 h, and cell viability was measured with MTT assay at 24 h starting from FK866 treatment. For measurement of cell viability after OGD, neurons were subject to OGD for 1.5 h in the presence of FK866. FK866 is present for a total of 4 h starting from OGD, and cell viability was measured with MTT assay at 24 h starting from OGD. Data were collected from 4 independent experiments. (B) Effect of FK866 and NAM on NAD+ levels in cortical neurons. Neurons were exposed to different concentrations of FK866 alone or together with 2 mM NAM for 4 h, and NAD+ levels were measured. Data were collected from 4 independent experiments. (C) Effect of FK866 on NAD+ levels after OGD. Neurons were subjected to 1.5 h OGD in the absence or presence of FK866. FK866 was present for a total of 4 h after OGD. NAD+ was measured 6 h after OGD or wash-away of FK866. Data were the mean values from 4 independent experiments. (D) The effect of incubation time of 15 mM NAM on NAD+ levels in OGD. Neurons were subjected to 1.5 h OGD in the presence of 15 mM NAM, and NAM was continuously present for different periods of time indicated in the figure. NAD+ levels were measured 6 h later following wash-away of NAM. Data were the mean value from 5 independent experiments. (E) Effect of NAD+ and NAM on ATP levels in normal and OGD conditions. Neurons were treated with 15 mM NAD+ or 15 mM NAM and were or were not subject to OGD for 1.5 h at the same time. NAD+ and NAM was continuously present for a total time of 24 h and ATP was measured 6 h later after wash-away of NAD+ and NAM. Data were the mean values from 4 independent experiments. *t-test with p<0.05; **t-test with p<0.005 vs the indicated conditions.
Next we tested whether the inhibition of PBEF exacerbates neuronal injury and reduces NAD+ content after ischemia. Neuronal cultures were treated with different concentrations of FK866 for 4 h starting at the same time as OGD, and cell viability was measured 24 h later. As shown in Fig. 3A, neurons treated with different concentrations of FK866 and subject to OGD showed a decrease in cell viability as compared with neurons subject to OGD but without FK866 treatment (Fig. 3A). Intracellular NAD+ levels are further decreased after OGD in the presence of FK866 (Fig. 3C). The results suggest that FK866 exacerbates neuronal death through inhibition of NAD+ production.
If that inhibition of PBEF reduces neuronal viability after ischemia is due to the reduction of NAD+, it is conceivable that the replenishment of NAM will increase NAD+ levels after OGD. Accordingly, neurons were subject to OGD in the absence and presence of 15 mM NAM for different time periods and were harvested for measurement of the NAD+ contents. The results show treatment of NAM significantly increase NAD+ levels after OGD as compared to control experiment (Fig. 3D).
Normal neuronal function heavily relies on ATP produced through mitochondrial oxidative phosphorylation as an energy source (Soane et al., 2007). Further, NAD+ is an essential coenzyme of ATP-synthesizing redox reactions implicated in glycolysis and oxidative phosphorylation. We next investigated the effect of PBEF on the cellular ATP content under OGD condition. In keep with NAD+ consumption, OGD lead to a sharp reduction of ATP level to ~50% of the control (Fig. 3E). Replenishment of NAD+ prevented ATP depletion that nearly restores it to a normal level. Similarly, NAM shows some suppressive effect on ATP decrease but with no statistical significance. Interestingly, under normal conditions, both NAD+ and NAM treatment each have a positive influence on ATP level (Fig. 3E).
Overexpression of PBEF decreases neuronal death after glutamate stimulation
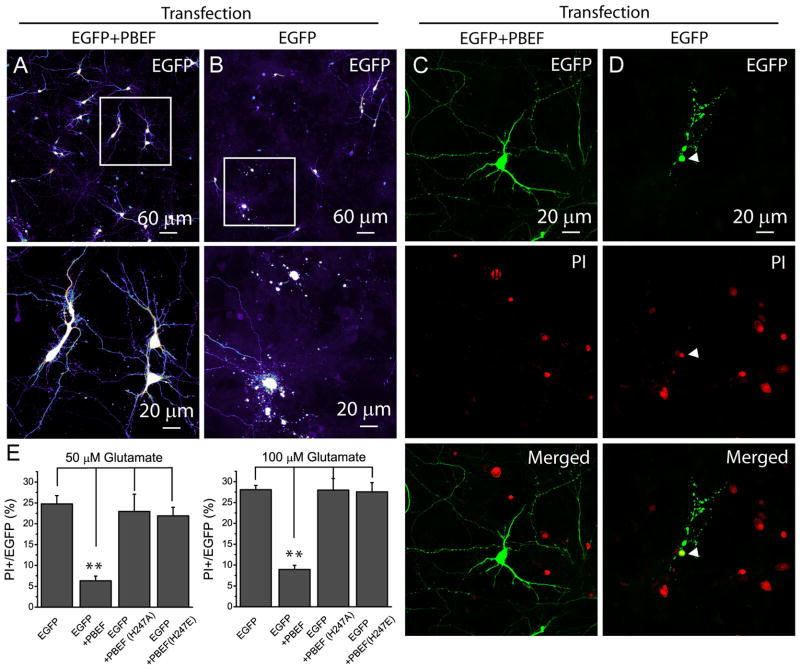
Our results using the inhibitor and the substrate and product of PBEF provide evidence that PBEF plays a neuronal protective role. To obtain direct evidence that PBEF exerts neuronal protective effect after ischemia, neurons were transiently overexpressed with PBEF by DNA transfection and were subsequently subject to glutamate excitotoxicity. PBEF overexpressing neurons can be identified by EGFP fluorescence through the cotransfection, which is a common approach to identify cells expressing the gene of interest (Semenova et al. 2007; Andrabi et al. 2011). We first confirmed that in co-transfected cultures, all of EGFP+ neurons were overexpressed with PBEF, as indicated by remarkable increase in PBEF signal in these neurons (Supplemental Fig. 2). We conducted PI staining after glutamate stimulation and calculated the percentage of PI+ cells cotransfected with PBEF and EGFP and cells transfected with EGFP alone. After a3h period of glutamate stimulation, the majority of neurons cotransfected with wild type (WT) human PBEF (hPBEF) and EGFP maintained structural integrity (Fig. 4A), while neurons transfected with EGFP alone exhibit severe neurite beading (Fig. 4B), an indication of neuronal injury. Results from PI staining showed that overexpression of WT hPBEF dramatically reduced neuronal death after glutamate stimulations (Fig. 4C–E). The data indicate that PBEF indeed can protect neurons from injury after ischemia. To test whether this effect requires its enzymatic activity, two different hPBEF point mutants, H247A and H247E, which have little enzymatic activities, were used for further study (Liu et al. 2009b). Strikingly, overexpression of those two mutants did not ameliorate glutamate excitotoxicity and has similar sensitivity to 50 and 100 μM glutamate stimulations as compared with neurons transfected with EGFP alone (Fig. 4E). Thus PBEF enzymatic activity is required to protect neurons after glutamate excitotoxicity.
Fig 4. Overexpression of hPBEF reduces glutamate excitotoxicity.
(A–B) The morphological changes of neurons determined by EGFP fluorescence after glutamate stimulation (50 μM, 3 h). The lower panels are the high resolution images of the boxed region in upper panels. Notice that the morphological differences between neurons transfected with (Left) and without (Right) PBEF manifested by EGFP fluorescence displayed as a pseudo color. Images were taken 24 h after 3 h treatment of glutamate. (C–D) PI staining detects degenerating neurons after glutamate stimulation (50 μM, 3 h). Notice the colocalization of EGFP and PI fluorescence in the neurons transfected by EGFP alone. (E) Summary of the effect of overexpressions of WT and mutant hPBEF on neuronal death after glutamate stimulation. Data were collected from 4 independent experiments and a total of 20–30 EGFP+ neurons were counted from each experiment. *t-test with p<0.05 vs neurons expressing EGFP alone and EGFP/mutant hPBEF.
Inhibition of PBEF enzymatic activity reduces mitochondrial biogenesis
A variety of cell death pathways during cerebral ischemia converge on mitochondrial dysfunction. As an important organelle, mitochondria functions to produce ATP through oxidative phosphorylation that consumes large amount of NAD+, maintains calcium homeostasis, and generates reactive oxygen species. Due to the coordinated action of numerous transcription factors and coactivators (Scarpulla 2008), healthy neurons regularly generate new functional mitochondria, while prolonged cerebral ischemia causes impairment of mitochondrial biogenesis (Chen et al. 2001b; Valerio et al. 2011). As our results have shown that NAD+ and NAM could significantly reduce neuronal death after OGD and glutamate stimulation, we hypothesized that replenishment of NAD+ and NAM could compensate for the deleterious effects of ischemia through enhanced mitochondrial biogenesis.
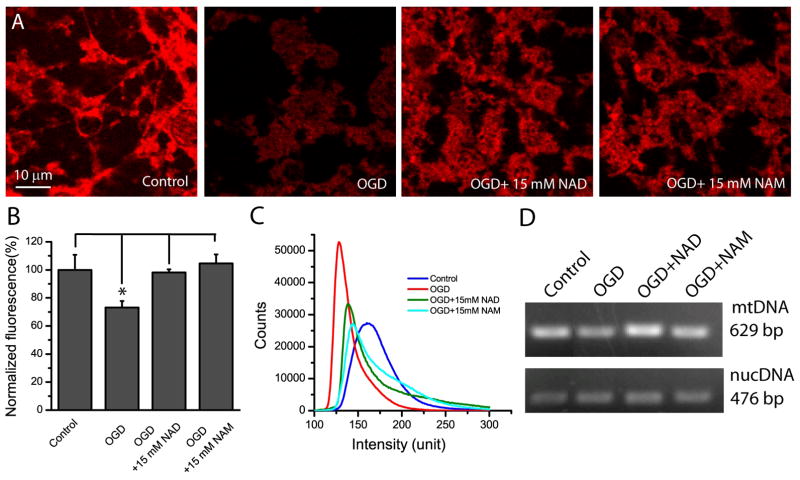
To assess the possible role of PBEF in mitochondrial biogenesis, neurons were stained with MitoTracker Red, a fluorescent dye that can label mitochondria and thus can assess mitochondria biogenesis (Schulz et al. 2008; Chen et al. 2010). When neurons were subject to OGD, significant reduction of MitoTracker Red fluorescence was observed as compared with control neurons (Fig. 5A–C), but both NAD+ and NAM rescued neurons from impaired mitochondrial biogenesis as indicated by increased MitoTracker Red fluorescence. Quantitative analysis of entire image fields showed NAD+ and NAM increased the average fluorescence intensity and shifted fluorescence distribution of neurons to high intensity as compared with fluorescence from neurons only subject to OGD (Fig. 5B–C). Using quantative PCR, we further measured mtDNA and nucDNA to study the effect of PBEF on mitochondrial biogenesis. OGD reduced mtDNA while NAD and NAM largely prevented the reduction of mtDNA (Fig. 5D). The data indicate that PBEF plays an important role in mitochondrial biogenesis and provide mechanistic evidence for our results that PBEF confers neuroprotection after OGD.
Fig 5. NAD+ and NAM decrease mitochondrial loss in neurons after OGD.
(A) Single optical section of confocal fluorescent images of MitoTracker Red-stained neurons under different conditions as indicated. Neurons were subject to OGD for 1.5 h in the absence or presence of NAD+ and NAM. NAD+ and NAM were continuously present after OGD for a total time of 24 h from the beginning of OGD. Neurons were then stained with MitoTracker Red for 20 min. (B) Summary of MitoTracker Red mean fluorescent intensities under different conditions. *t-test with p<0.05 vs the indicated conditions. Data were the mean values of fluorescence intensity from the entire image frame from 3 independent experiments. (C) MitoTracker Red fluorescence intensity distributions under different conditions. (D) NAD+ and NAM decreased the mtDNA loss after ischemia. Image shows mtDNA and nucDNA contents after PCR in agarose gel. The total amounts of DNA were adjusted so that nucDNA contents were similar in each condition to reveal the difference of mtDNA.
Overexpression of PBEF decreases mitochondrial membrane potential (MMP) depolarization induced by glutamate stimulation
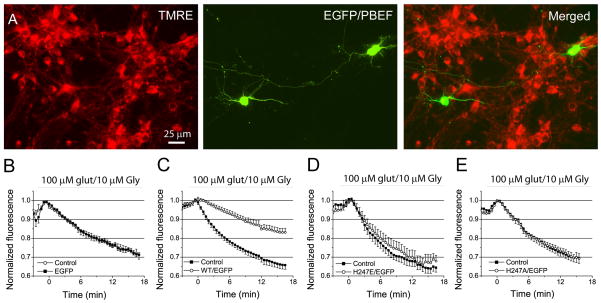
To further explore the role of PBEF in mitochondrial dysfunction in ischemia, we tested whether overexpression of PBEF affects MMP depolarization in neurons up to excitotoxic glutamate stimulation. We labeled cultured neurons with tetramethylrhodamine, ethyl ester (TMRE), a red fluorescent probe, to measure MMP using live cell fluorescence imaging (Fig. 6A). PBEF overexpressing neurons were identified by EGFP fluorescence (Fig. 6A and Supplemental Fig. 2). TMRE fluorescence was continuously monitored using time-lapse imaging before and during the exposure of 100 μM glutamate and 10 μM glycine. MMP depolarization is indicated by the loss of probe and thus the reduction of fluorescence intensity. Fluorescence change of individual neurons transfected with or without PBEF after glutamate stimulation were measured and compared. Our results showed that for non-transfected neurons or neurons transfected with EGFP alone, glutamate induced a rapid and progressive decrease of TMRE fluorescence with similar rates (Fig. 6B). Whereas WT hPBEF overexpressing neurons showed a slower fluorescence decrease as compared with non-transfected neurons or neurons transfected with EGFP alone, indicating overexpression of PBEF render neurons more resistant to excitotoxicity-induced MMP collapse (Fig. 6C). Point mutants H247A and H247E of hPBEF have similar sensitivity to glutamate stimulation to those of non-transfected neurons or neurons transfected with EGFP alone (Fig. 6D–E). Collectively, the above results indicate that the ability of PBEF to protect neurons from death is resulted from preserving MMP through its enzymatic activity.
Fig 6. Overexpression of PBEF with enzymatic activity reduces mitochondrial membrane (MMP) depolarization induced by excitotoxic glutamate stimulation.
(A) Fluorescence images of EGFP-expressing and TMRE-labeled neurons. (B–E) Comparison of time courses of TMRE fluorescence changes in neurons expressing EGFP alone (B), and neurons coexpressing EGFP and WT hPBEF (C), EGFP and H247E (D) and H247A (E) mutants with non-transfected neurons after glutamate stimulation. Notice PBEF-expressing neurons have much slower rate of MMP depolarization than neurons expressing PBEF mutants, EGFP alone, and non-transfected neurons. Data was normalized to control value before glutamate application. All data was collected from 3–4 preparations of primary cultured neurons.
Discussion
Stroke refers to the neurological condition that develops when a part of the whole brain is deprived of oxygen and glucose. In 70–80% of the cases, the precipitating cause is a blood clot that blocks the supply of oxygenated blood to a region of the brain, a situation termed ischemic stroke. The damage caused to the neurons during ischemia is due to a reduction in oxygen and glucose supply—that is, OGD. Subsequent energy depletion leads to neuronal membrane depolarization that results in excessive release of glutamate from the synaptic vesicles of injured neurons, and consequently Ca2+ overloading and excitotoxicity. Since energy loss is the root cause of glutamate and Ca2+ excitotoxicity, it is conceivable that mechanisms that can compensate for energy metabolism will ameliorate excitotoxicity and consequently reduce acute neuronal death as well as delayed neuronal death and brain damage.
PBEF or Nampt, is a rate limiting enzyme that converts NAM to NMN in the salvage pathway of mammalian NAD+ biosynthesis (Revollo et al. 2007; Luk et al. 2008; Imai 2009). This salvage pathway is predominantly used by mammals for NAD+ biosynthesis, thus PBEF plays a central role in regulation of NAD+ production and energy metabolism. In this study, we have provided several lines of evidence demonstrating that PBEF functions as a NAD+ biosynthetic enzyme and exerts a neuronal protective effect in ischemia using in vitro ischemic models. First, the treatments of NAD+ and NAM ameliorated OGD- and glutamate-induced neuronal death; Second, FK866, an inhibitor of PBEF aggravated OGD-induced neuronal death and reduced intracellular NAD+ level in neurons; Third, overexpression of WT hPBEF in neurons reduced glutamate-induced neuronal death, while mutant hPBEF without enzymatic activity do not have beneficial effect on neuronal death; Fourth, replenishment of NAD+ and NAM suppressed OGD-induced mitochondrial loss; Lastly, our results further showed that overexpression of WT hPBEF reduced MMP depolarization after excitotoxic glutamate stimulation while hPBEF mutants lacking enzymatic activity did not improve mitochondrial function.
Our study can explain that ischemic injury results from energy depletion and a compensation for an energy deficit can ameliorate acute neuronal death and brain damage through reduced glutamate excitotoxicity, a common mechanism of acute neuronal damage in the mouse model of ischemia (Dirnagl et al. 1999). Our results also showed that neurons are crucially dependent on PBEF for their function and survival as they face massive NAD+ depletion and cell demise when this enzymatic activity is inhibited by FK866. The consequences of PBEF inhibition in neurons appeared to be more deleterious in OGD injury than neurons without PBEF inhibition. This fact is in line with previous study that NAD+ levels change in response to biological stress or diet and impact on cell survival and metabolism (Viswanathan et al. 2005; Porcu and Chiarugi 2005), indicating that retaining NAD+ storage is necessary to ensure neuronal survival. Interestingly, we also found that NAM supplementation rescues NAD+ levels when PBEF is inhibited by FK866. There are two possible interpretations. First, the enzymatic activity of PBEF is not completely inhibited, and thus the presence of high concentration of NAM will produce sufficient NAD+. Secondly, although salvage pathway is a predominant pathway for NAD+ synthesis in mammals, it can not be excluded that neurons can convert NAM into nicotinic acid by nicotinamidase coupling to de nova pathway for NAD+ synthesis for compensation especially when the predominant pathway is blocked (Balan et al. 2008). Nevertheless, these data in combination indicate that NAD+ levels are raised through enhancing PBEF enzymatic reaction by administering substrate. Consistent with this notion, the reductions of NAD+ levels induced by OGD were increased via administration of NAM in a time dependent manner.
Neuronal death due to NAD+ depletion also involves ATP shortage leading to cellular energy depletion (Liu et al. 2008). In keeping with depletion of NAD+, OGD also triggered a significant reduction of ATP, while NAD+ replenishment preserved intracellular ATP content at almost normal levels, suggesting the maintenance of cellular energy homeostasis and NAD+ levels is of critical importance in supporting the neuronal survival. Interestingly, both NAD+ and NAM could increase ATP content when there is not any stimulation. We reasoned that NAM administration might accelerate NAD+ resynthesis by PBEF as the enzymatic reaction rate is increased with the high substrate concentration, and this mediation of NAD+ is a potent and indirect way of rescuing energy failure.
NAD+ is known as an important energy substrate and cofactor involved in multiple metabolic reactions (Brennan et al. 2006), including glycolysis, DNA repair processes, and the function of several NAD+-dependent enzymes, such as the histodeacetylase SIRT1 and poly (ADPribose) polymerase 1 (PARP1). In ischemic condition, those NAD+-consuming enzymes might have harmful effect on neuronal viability through the depletion of NAD+ and ATP pool (Eliasson et al. 1997; Ha and Snyder 1999; Liu et al. 2009a; Kauppinen et al. 2009; Hamby et al. 2007). Our previous study showed that PBEF knockout mice have a reduced level of NAD+ as compared with WT mice, so it will be important to test whether the neuronal protective effect in ischemia in vivo by the overexpression of PBEF is through the regulation of the activities and expression levels of PARP-1 and SIRT1. Since DNA transfection in primary neuronal culture has very low efficiency, transgenic mice or viral transduction that can efficiently overexpress PBEF in neurons in vivo are required for those studies.
Mitochondrial oxidative phosphorylation is the primary source of high-energy compounds in the cell. Dysfunction of mitochondrial energy metabolism leads to impaired calcium buffering and generation of ROS (Beal 2005). Further, impaired mitochondria (e.g., in neurodegenerative disease and aging) also may diminish ATP production, thereby impairing the synthesis and secretion of neurotransmitters that serve as signals in CNS. Since PBEF is a rate-limiting enzyme that synthesizes NAD+, we postulate it will reduce mitochondrial bioenergetic failure after ischemia. Using MitoTracker, we found NAD+ and NAM can also prevent OGD-induced mitochondrial loss (Fig 5A–C) which is also confirmed by measuring the mtDNA and nucDNA. The results indicate PBEF is critical in maintaining mitochondrial homeostasis and biogenesis, therefore neuronal viability in health and disease. Our results corroborated with the report that prolonged focal cerebral ischemia causes permanent loss of mtDNA (Chen et al. 2001a), an indication of the failure of mitochondrial renewal mechanisms.
NAD+ depletion is also thought to suppress mitochondrial function, and impaired mitochondria result in ATP depletion and depolarization of MMP which leads to mitochondrial permeability transition (MPT), and subsequently triggers downstream events of apoptosis (Lemasters et al. 1998). Previous studies have indicated that central to maintaining neuronal survival is the regulation of MMP, and maintenance of MMP is an ATP facilitated process (La Piana et al. 2003). Moreover, ischemia limits the delivery of oxygen and glucose to cells and disturbs the maintenance of MMP (Chong et al. 2004). Thus, MMP is an important parameter in determining the fate of neurons. Glutamate-induced excitotoxicity is known lead to a reduction in NAD+ levels and MMP depolarization. In this study we showed neurons with overexpression of hPBEF had much slower reduction rate in MMP depolarization than neurons without overexpression of PBEF during excitotoxic stimulation of glutamate, while overexpression of mutant hPBEF without enzymatic activity in neurons did not affect MMP loss. Since inhibition of PBEF can reduce NAD+ levels, our results thus demonstrate PBEF can maintain mitochondrial integrity under ischemic condition via synthesis of NAD+. Because loss of MMP can initiate apoptotic cell death, our results also suggest that PBEF can ameliorate apoptotic neuronal death after ischemia, yet further study on apoptosis needs to be done. The fact that mutant hPBEF can not protect MMP loss suggests a close biochemical link between NAD+ depletion and mitochondrial failure. Our recent study showed that knockout of PBEF exacerbates ischemic brain injury. Thus our findings from in vitro and in vivo ischemia studies demonstrate the neuronal protective effect of PBEF after ischemia is through the prevention of MMP depolarization that requires its enzymatic activity.
PBEF was first identified as a secreted protein that stimulates Pre-B-cell formation, and is highly conserved in living species including humans (Samal et al. 1994). PBEF is released by a variety of cells as a proinflammatory cytokine by inflammatory stimuli such as LPS, TNFα, IL-1α and IL-6 in cells involving innate immunity(Liu et al. 2009c; Ognjanovic et al. 2001). Although whether PBEF exists in extracellular space in the brain is unknown, it will be interesting to test whether knockout and overexpression of PBEF will affect long term outcomes of ischemia through inflammatory process.
In summary, our current study found a novel role of PBEF in ischemia. PBEF can protect neurons through maintaining energy metabolism homeostasis and diminishing of mitochondrial dysfunction. Such protective effect requires its enzymatic activity. Since some NAD+-consuming enzymes such as poly(ADP-ribose) polymerases (PARPs) and deacetylase sirtuins might also involved in ischemic injury, further study is necessary to find whether overexpression of PBEF in neurons will regulate the activity and the expression levels of those enzymes. Given the possibility of its cytokine nature, it is also important to test whether PBEF contributes to neuronal protection through the regulation of inflammation.
Supplementary Material
Acknowledgments
The study was provided by grants from the American Heart Association (0735133N), NIH R01NS069726, startup funds from University of Missouri, Research Board Award from University of Missouri System to SD, and NIH1R01HL080042 and 3RO1 HL080042-04S1 to SQY. We thank China Scholarship Council for financial support to JB. Confocal images were acquired with an Olympus FV1000 confocal microscope purchased by NIH shared instrument grant (RR022578).
Footnotes
The authors declare no conflict of interest.
The following additional supporting information may be found in the online version of this article:
Methods of Immunocytochemistry, Intracellular NAD+ and ATP assay, and Transient transfection of neuronal cell cultures.
References
- Andrabi SA, Kang HC, Haince JF, Lee YI, Zhang J, Chi Z, West AB, Koehler RC, Poirier GG, Dawson TM, Dawson VL. Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly(ADP-ribose) polymer-induced cell death. Nat Med. 2011;17:692–699. doi: 10.1038/nm.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased Nuclear NAD+ Biosynthesis and SIRT1 Activation Prevent Axonal Degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, Kaplun A, VanBerkum MFA, Arking R, Freeman DC, Maiese K, Tzivion G. Life Span Extension and Neuronal Cell Protection by Drosophila Nicotinamidase. J Biol Chem. 2008;283:27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber PA, Demchuk AMH. Biochemistry Ischemic Stroke. Advances in Neurology. 2003;92:151–164. [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of Silencing and Accelerated Aging by Nicotinamide, a Putative Negative Regulator of Yeast Sir2 and Human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J Cereb Blood Flow Metab. 2006;26:1389–1406. doi: 10.1038/sj.jcbfm.9600292. [DOI] [PubMed] [Google Scholar]
- Cao G, Clark RSB, Pei W, Yin W, Zhang F, Sun FY, Graham SH, Chen J. Translocation of Apoptosis-Inducing Factor in Vulnerable Neurons After Transient Cerebral Ischemia and in Neuronal Cultures After Oxygen-Glucose Deprivation. J Cereb Blood Flow Metab. 2003;23:1137–1150. doi: 10.1097/01.WCB.0000087090.01171.E7. [DOI] [PubMed] [Google Scholar]
- Chen H, Hu CJ, He YY, Yang DI, Xu J, Hsu CY. Reduction and Restoration of Mitochondrial DNA Content After Focal Cerebral Ischemia/Reperfusion. Stroke. 2001b;32:2382–2387. doi: 10.1161/hs1001.097099. [DOI] [PubMed] [Google Scholar]
- Chen H, Hu CJ, He YY, Yang DI, Xu J, Hsu CY. Reduction and Restoration of Mitochondrial DNA Content After Focal Cerebral Ischemia/Reperfusion. Stroke. 2001a;32:2382–2387. doi: 10.1161/hs1001.097099. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JYJ. Shear stress, SIRT1, and vascular homeostasis. PNAS. 2010;107:10268–10273. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. The NAD+ Precursor Nicotinamide Governs Neuronal Survival During Oxidative Stress Through Protein Kinase B Coupled to FOXO3a and Mitochondrial Membrane Potential. J Cereb Blood Flow Metab. 2004;24:728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in Neurosciences. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly (ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nature Medicine. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- Ha HC, Snyder SH. Poly (ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. PNAS. 1999;96:13978–13982. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby AM, Suh SW, Kauppinen TM, Swanson RA. Use of a Poly (ADP-Ribose) Polymerase Inhibitor to Suppress Inflammation and Neuronal Death After Cerebral Ischemia-Reperfusion. Stroke. 2007;38:632–636. doi: 10.1161/01.STR.0000250742.61241.79. [DOI] [PubMed] [Google Scholar]
- He Y, Cui J, Lee JC, Ding S, Chalimoniuk M, Simonyi A, Sun AY, Gu Z, Weisman GA, Gibson Wood W, Sun GY. Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (−)-epigallocatechin-3-gallate. ASN NEURO. 2011;3:e00050. doi: 10.1042/AN20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide Phosphoribosyltransferase Regulates Cell Survival Through NAD+ Synthesis in Cardiac Myocytes. Circ Res. 2009;105:481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai SI. The NAD World: a new systemic regulatory network for metabolism and aging--Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochemistry & Biophysics. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen TM, Suh SW, Berman AE, Hamby AM, Swanson RA. Inhibition of poly (ADP-ribose) polymerase suppresses inflammation and promotes recovery after ischemic injury. J Cereb Blood Flow Metab. 2009;29:820–829. doi: 10.1038/jcbfm.2009.9. [DOI] [PubMed] [Google Scholar]
- La Piana G, Marzulli D, Irno Consalvo M, Lofrumento NE. Cytochrome c-induced cytosolic nicotinamide adenine dinucleotide oxidation, mitochondrial permeability transition, and apoptosis. Archives of Biochemistry and Biophysics. 2003;410:201–211. doi: 10.1016/s0003-9861(02)00687-2. [DOI] [PubMed] [Google Scholar]
- Marie Lagouge, Carmen Argmann, Zachary Gerhart-Hines, Hamid Meziane, Carles Lerin, Frederic Daussin, Nadia Messadeq, Jill Milne, Philip Lambert, Peter Elliott, Bernard Geny, Markku Laakso, Pere Puigserver, Johan Auwerx. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochimica et Biophysica Acta (BBA)- Bioenergetics. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- Li H, Liu P, Cepeda J, Fang D, Easley RB, Simon B, Zhang L, Ye S. Augmentation of Pulmonary Epithelial Cell IL-8 Expression and Permeability by Pre-B-cell Colony Enhancing Factor. Journal of Inflammation. 2008a;5:15. doi: 10.1186/1476-9255-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, Brunkan CS, Wolberger C, Imai SI, Tabas I. Extracellular Nampt Promotes Macrophage Survival via a Nonenzymatic Interleukin-6/STAT3 Signaling Mechanism. J Biol Chem. 2008b;283:34833–34843. doi: 10.1074/jbc.M805866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP, Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. NeuroMolecular Medicine. 2009a;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Pitta M, Mattson MP. Preventing NAD+ Depletion Protects Neurons against Excitotoxicity. Annals of the New York Academy of Sciences. 2008;1147:275–282. doi: 10.1196/annals.1427.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Li H, Cepeda J, Xia Y, Kempf JA, Ye H, Zhang LQ, Ye SQ. Regulation of Inflammatory Cytokine Expression in Pulmonary Epithelial Cells by Pre-B-cell Colony-enhancing Factor via a Nonenzymatic and AP-1-dependent Mechanism. J Biol Chem. 2009b;284:27344–27351. doi: 10.1074/jbc.M109.002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Li H, Cepeda J, Zhang LQ, Cui X, Garcia JGN, Ye SQ. Critical role of PBEF expression in pulmonary cell inflammation and permeability. Cell Biology International. 2009c;33:19–30. doi: 10.1016/j.cellbi.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA, Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nature Reviews Neuroscience. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83:804–816. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S. Enzymology of NAD+ synthesis. Advances in Enzymology & Related Areas of Molecular Biology. 1999;73:135–182. doi: 10.1002/9780470123195.ch5. [DOI] [PubMed] [Google Scholar]
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nature Medicine. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends in Pharmacological Sciences. 2005;26:94–103. doi: 10.1016/j.tips.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai SI. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- Grimm AA, Imai SI. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Current Opinion in Gastroenterology. 2007;23:164–170. doi: 10.1097/MOG.0b013e32801b3c8f. [DOI] [PubMed] [Google Scholar]
- Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF., Jr Suppression of the JNK Pathway by Induction of a Metabolic Stress Response Prevents Vascular Injury and Dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova MM, Maki-Hokkonen AMJ, Cao J, Komarovski V, Forsberg KM, Koistinaho M, Coffey ET, Courtney MJ. Rho mediates calcium-dependent activation of p38[alpha] and subsequent excitotoxic cell death. Nat Neurosci. 2007;10:436–443. doi: 10.1038/nn1869. [DOI] [PubMed] [Google Scholar]
- Skokowa J, Lan D, Thakur BK, Wang F, Gupta K, Cario G, Brechlin AM, Schambach A, Hinrichsen L, Meyer G, Gaestel M, Stanulla M, Tong Q, Welte K. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD+-sirtuin-1-dependent pathway. Nat Med. 2009;15:151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Current Molecular Medicine. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- Taghibiglou C, Martin HGS, Lai TW, Cho T, Prasad S, Kojic L, Lu J, Liu Y, Lo E, Zhang S, Wu JZZ, Li YP, Wen YH, Imm JH, Cynader MS, Wang YT. Role of NMDA receptor-dependent activation of SREBP1 in excitotoxic and ischemic neuronal injuries. Nat Med. 2009;15:1399–1406. doi: 10.1038/nm.2064. [DOI] [PubMed] [Google Scholar]
- Valerio A, Bertolotti P, Delbarba A, Perego C, Dossena M, Ragni M, Spano P, Carruba MO, De Simoni MG, Nisoli E. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. Journal of Neurochemistry. 2011;116(6):1148–59. doi: 10.1111/j.1471-4159.2011.07171.x. [DOI] [PubMed] [Google Scholar]
- Kim Viswanathan Mohan, Stuart K, Ala Berdichevsky, Leonard Guarente. A Role for SIR-2.1 Regulation of ER Stress Response Genes in Determining C. elegans Life Span. Developmental Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, de Cabo R, Sauve AA, Sinclair DA. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Signore AP, Iwai M, Cao G, Gao Y, Chen J. Rapidly Increased Neuronal Mitochondrial Biogenesis After Hypoxic-Ischemic Brain Injury. Stroke. 2008;39:3057–3063. doi: 10.1161/STROKEAHA.108.520114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W. NAD+ and NADH in cellular functions and cell death. Frontiers in Bioscience. 2006;11:3129–3148. doi: 10.2741/2038. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xie Y, Wang T, Bi J, Li H, Zhang LQ, Ye SQ, Ding S. Neuronal protective role of PBEF in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1962–1971. doi: 10.1038/jcbfm.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.