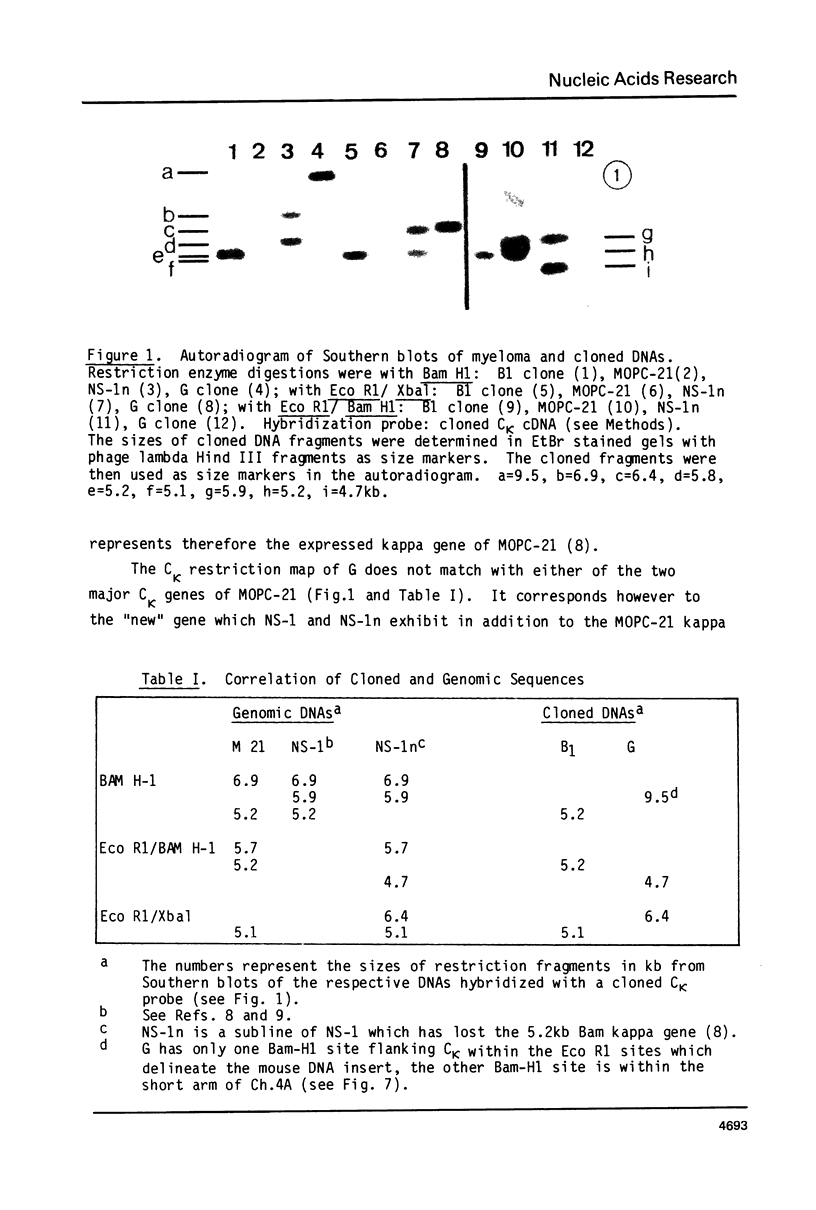
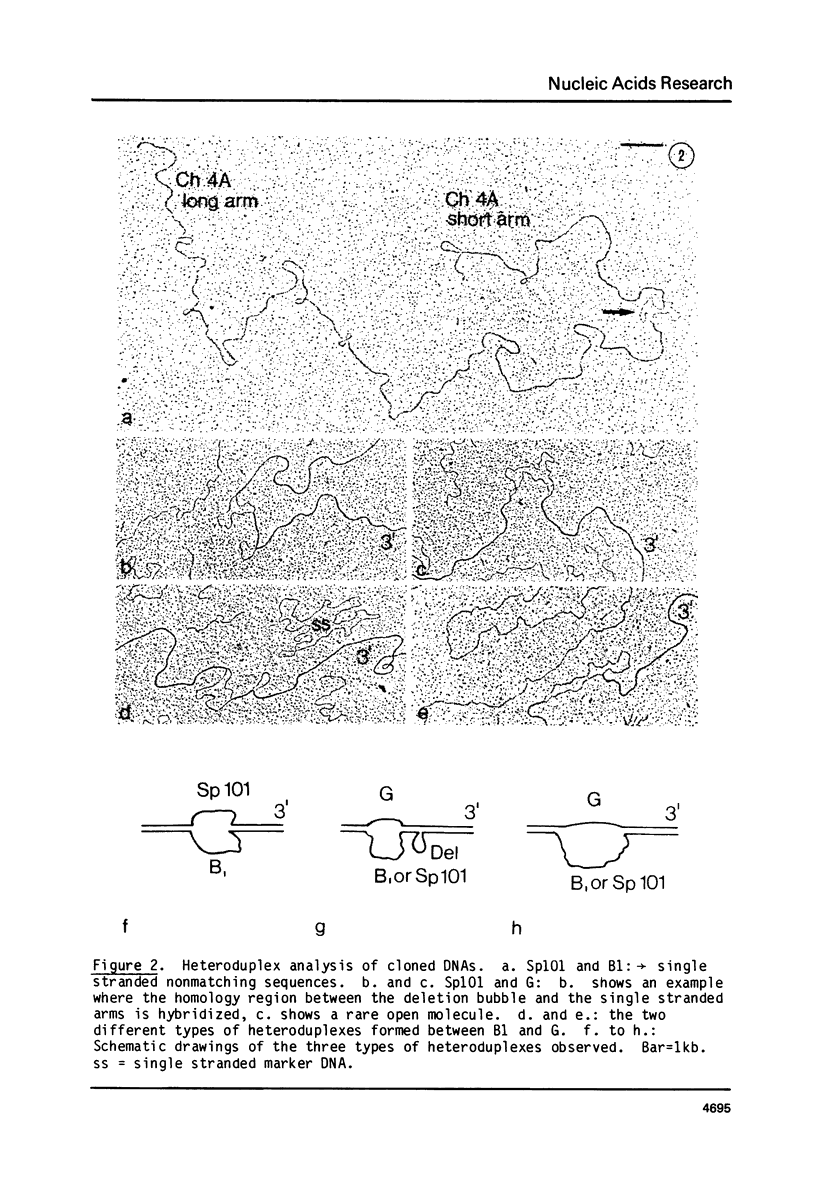
Abstract
We have studied the organization and function of different rearranged kappa genes in a myeloma, MOPC-21. Two kappa genes were cloned into Charon 4A and compared with each other and with a cloned germline CK gene by restriction mapping and electron microscopy. One MOPC-21 clone corresponds to the gene coding for the MOPC-21 kappa chain polypeptide; it has the V21 gene joined with the CK gene at the J2 sequence. The other MOPC-21 clone corresponds to a nonfunctional rearranged MOPC-21 kappa gene, except for a lkb deletion, 3' of J4. A similar deletion is also found in a "new" kappa gene present in NS-1, a cellular subclone of MOPC-21. The clone of the "nonfunctional" kappa gene has a V gene which is distinct from V21 which is joined to CK in the vicinity of J2. The undeleted form of this gene codes for a KRNA having the size of mature KmRNA which, however, is not translated into kappa chains. Thus the defect of the "nonfunctional" gene manifests itself at a late step of gene expression. The basis for "allelic exclusion" of antibody genes may simply be the complexity of the processes between genes and gene products, resulting in the expression of only one gene.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Gray W. R., Dreyer W. J., Hood L. Mechanism of antibody synthesis: size differences between mouse kappa chains. Science. 1967 Jan 27;155(3761):465–467. doi: 10.1126/science.155.3761.465. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R., Neugebauer K., Zentgraf H., Schaller H. Transposition of a DNA sequence determining kanamycin resistance into the single-stranded genome of bacteriophage fd. Mol Gen Genet. 1978 Feb 16;159(2):171–178. doi: 10.1007/BF00270890. [DOI] [PubMed] [Google Scholar]
- Hood L., McKean D., Farnsworth V., Potter M. Mouse immunoglobulin chains. A survey of the amino-terminal sequences of kappa chains. Biochemistry. 1973 Feb;12(4):741–749. doi: 10.1021/bi00728a026. [DOI] [PubMed] [Google Scholar]
- Joho R., Weissman I. L., Early P., Cole J., Hood L. Organization of kappa light chain genes in germ-line and somatic tissue. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1106–1110. doi: 10.1073/pnas.77.2.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Lenhard-Schuller R., Hohn B., Brack C., Hirama M., Tonegawa S. DNA clones containing mouse immunoglobulin kappa chain genes isolated by in vitro packaging into phage lambda coats. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4709–4713. doi: 10.1073/pnas.75.10.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near R. I., Storb U. RNA sequences homologous to the 3' portion of immunoglobulin alpha-chain mRNA in thymus-derived lymphocytes. Biochemistry. 1979 Mar 20;18(6):964–972. doi: 10.1021/bi00573a005. [DOI] [PubMed] [Google Scholar]
- Potter M. Antigen-binding myeloma proteins of mice. Adv Immunol. 1977;25:141–211. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Potter M. kappa Chain variable region from M167, a phosphorylcholine binding myeloma protein. Biochemistry. 1978 Jul 11;17(14):2703–2707. doi: 10.1021/bi00607a001. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Wells R. D. Preparation of triple-block DNA polymers using recombinant DNA techniques. Nucleic Acids Res. 1979 Jul 11;6(9):3025–3040. doi: 10.1093/nar/6.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Zachau H. G. Two rearranged immunoglobulin kappa light chain genes in one mouse myeloma. Nucleic Acids Res. 1980 Apr 25;8(8):1693–1707. doi: 10.1093/nar/8.8.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb U. Direct demonstration of immunoglobulin kappa chain RNA in thymus T cells by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2905–2908. doi: 10.1073/pnas.75.6.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svasti J., Milstein C. The complete amino acid sequence of a mouse kappa light chain. Biochem J. 1972 Jun;128(2):427–444. doi: 10.1042/bj1280427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M., Gatmaitan L., Loh E., Schilling J., Hood L. Rearrangement of genetic information may produce immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):785–790. doi: 10.1038/276785a0. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Wilson R., Miller J., Storb U. Rearrangement of immunoglobulin genes. Biochemistry. 1979 Oct 30;18(22):5013–5021. doi: 10.1021/bi00589a032. [DOI] [PubMed] [Google Scholar]
- Wilson R., Storb U., Arp B. Immunoglobulin gene rearrangements in hybridoma cells. J Immunol. 1980 May;124(5):2071–2076. [PubMed] [Google Scholar]