Abstract
Objective
To determine the cost-effectiveness of two different vascular access strategies among incident dialysis patients.
Summary Background Data
Vascular access is a principal cause of morbidity and cost in hemodialysis patients. Recent guidelines and initiatives are intended to increase the proportion of patients with a fistula. However, there is growing awareness of the high prevalence of fistula failures and attendant complications.
Methods
A decision analysis using a Markov model was implemented to compare two different vascular access strategies among incident dialysis patients: a) placing an arteriovenous fistula (AVF1st) as the initial access followed by a synthetic vascular access if the AVF did not mature compared to b) placing a synthetic vascular access (SVA1st) as the initial access device. The cost-utility was evaluated across a range of the risk of complications from temporary catheters and SVA.
Results
Under base case assumptions, the AVF1st strategy yielded 2.19 QALYs compared to 2.06 QALYs from the SVA1st strategy. The incremental cost-effectiveness was $9389/QALY for AVF1st compared to SVA1st and was less than $50,000 per QALY as long as the probability of maturation is 36% or greater. AVF1st was the dominant strategy when the AVF maturation rate was 69% or greater.
Conclusion
The high risk of complications of temporary catheters and the overall low AVF maturation rate explain why a universal policy of AVF 1st for all incident dialysis patients may not optimize clinical outcomes. Strong consideration should be given to a more patient-centered approach taking into account the likelihood of AVF maturation.
Introduction
Vascular access costs for hemodialysis (HD) are high, and their complications have been associated with as 10-15% of all inpatient stays among hemodialysis patients.1 Arteriovenous fistulas (AVF) are the preferred vascular access for hemodialysis2. Mature AVFs require many fewer interventions to maintain their long-term patency than do arteriovenous grafts3 and have been associated with a survival advantage 4, 5.
Disadvantages of AVFs include the inability to be used in the acute setting because of the time needed for their full maturation is three to four months. Fistula maturation indicates dilatation, augmented blood flow, and vessel wall thickening sufficient for use in hemodialysis. The probability of maturation is difficult to predict with up to 60% of AVFs failing to mature adequately 6-12. As a result of these attributes of AVFs and the common need for dialytic therapy by the time an AVF is placed, temporary catheter placement frequently occurs at the time of AVF surgical construction. Indeed, the large proportion of end-stage renal disease (ESRD) patients who require dialysis at the time of initial clinical presentation has been cited as a partial explanation for the predominance of synthetic vascular access13,14. Approximately 40% of patients present to the nephrologist three or fewer months before needing dialysis treatment, making it difficult to establish a mature AVF prior to initiation of chronic dialysis treatments13 .
Synthetic vascular accesses (SVA) are frequently placed in patients who fail AVF placement or in whom rapid availability of vascular access is desired, because they can be used one to two weeks after placement. Synthetic vascular accesses have higher failure/complication rates than those associated with AVFs due primarily to the development of stenosis at the venous anastomosis resulting from intimal hyperplasia.
The reported cumulative patency rates of synthetic vascular access are 42-60% at three years compared to 80% for maturing AVFs 15, 16. The complication rate for AVF is at least five times lower that for synthetic grafts 9, 17.
In 1997, The National Kidney Foundation published the Dialysis Outcome and Quality Initiative (DOQI), which included the goal of increasing the use of AVF as new accesses are placed 18. This was followed by the KDOQI vascular access guidelines and a Centers for Medicare and Medicaid Services initiative (Fistula First) to increase the proportion of patients who undergo dialysis with a fistula to 66%19, 20.
Competing issues contribute to the decision about which hemodialysis vascular access strategy to pursue. SVAs can be cannulated and used for hemodialysis much sooner than AVFs, greatly reducing the exposure to central venous catheter complications, a benefit of SVAs at least partially offset by their higher rate of complications and failure. We undertook a decision analysis using a Markov model to examine the factors influencing the risk-reward balance between AVFs and SVAs as the initial approach to vascular access placement.
Methods
Model Design
A state-transition Markov model was constructed to compare two potential vascular access management strategies: synthetic vascular access as the initial vascular access (SVA1st) vs. AVF as the initial vascular access backed by synthetic vascular access (AVF1st). Using a Markov model, a mathematical simulation in which patients move among various states of health and disease over time21, 22, permits examination of various treatment options on health state transitions as well as net treatment effects measurable in terms of both health outcomes and costs. In addition, each potential health state outcome can be modified by its value or utility. The source and value of probabilities of different clinical outcomes for each decision strategy are presented in Table 1. Hypothetical patients were transitioned through the different health states in cycles one year in duration. The model was fit across a total of five years. Costs were examined in dollars and effects in quality-adjusted life years (QALY). Costs were evaluated from the perspective Medicare the primary payer for 85% of hemodialysis patients during the study period23. Therefore, all payments made by Medicare are easily identified and represent national data. An annual discount rate of 3% was incorporated into the models.
Table 1.
Probabilities incorporated into the decision model
| Type of Access | AVF | SVA |
|---|---|---|
| Maturation rate | 66% | |
| Patency at 1 year | 97% | 73% |
| Complications (% of access) | 3%* | 27% |
| Declotting Success Surgical | 98% | 85% |
| Declotting Success Radiological | N/A | 85% |
Includes pseudoaneurysm, inflammation, ischemia, steal syndrome, hemorrhage, venous stenosis, low flow, tears and others.
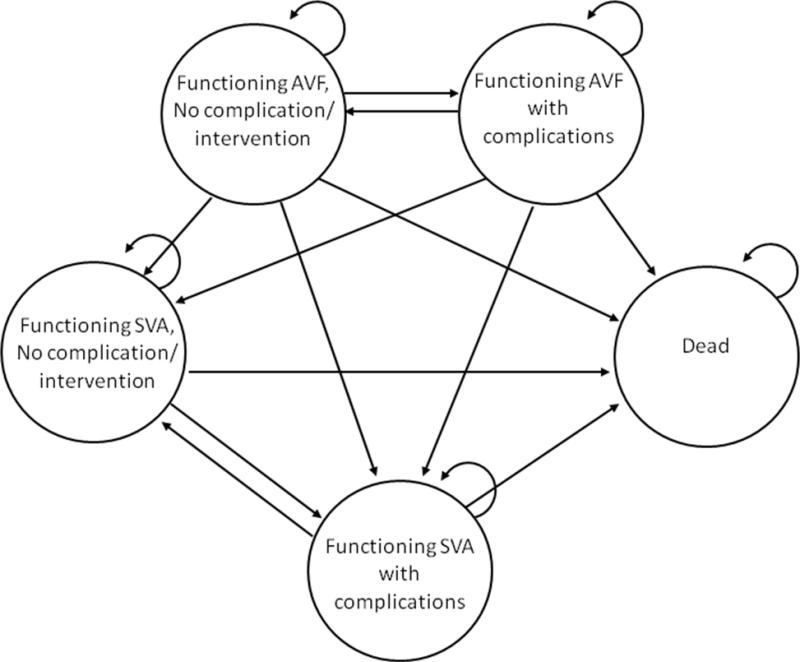
Under the SVA1st strategy, five possible clinical states were assumed: a) uncomplicated synthetic vascular access, b) an infective episode resolved with medical management, c) an occlusive event resolved with medical management, d) abandonment of access due to infection of thrombotic complication requiring a new access, or e) death. In our model, patients could transition to any of the other clinical states during the next transition period according to the assumed probabilities of change between one state and another (Figure 1). For example, subjects could progress from an episode of thrombosis that required intervention to a no complication state the following cycle.
Figure 1.
Markov decision tree model for comparison of strategies
Under the AVF1st strategy, an initial maturation probability of AVF was defined. If the AVF matured, it could remain patent with or without intervention. Only one AVF was placed in an individual. If the AVF failed to mature after twelve weeks, the patient were assumed to undergo SVA placement with subsequent health transitions occurring analogous to those experienced by patients receiving an SVA as their initial access (SVA1st). The need for longer periods of temporary catheter use and a second surgical procedure were incorporated into the decision tree. Death was an absorbing state.
The following assumptions were incorporated into the decision models:
Patients had temporary dialysis catheters in place, on average, for two weeks following SVA placement and for twelve weeks following AVF placement.
Patients may have had one access complication per year-long cycle.
The probability of an access complication was 20% higher among subjects who experienced a prior complication.
Catheters and the vascular access were placed at the same time.
Vascular access placement was guided by venous mapping.
If an AVF failed a synthetic vascular access was placed during the same cycle.
The decision model was developed using DATA 3.5 (TreeAge Software, Inc. Williamstown, MA)
Summary of available data
a) Probabilities of events
The probabilities of events were obtained from a review of the English language literature in MEDLIINE from 1966 to 2008 using keywords: vascular access, PTFE, arteriovenous fistula, hemodialysis, and temporary catheters. Data were included only from original articles written in English describing research in humans. Articles were excluded if AVF and SVA were not examined separately. Table 1 includes a summary of the probabilities used for AVF and SVA in this decision analysis.
b) Utility measures
There are no published data on the reduction in quality of life associated with vascular access complications. Therefore, we obtained estimated the magnitude of these reductions by asking individuals undergoing hemodialysis to score complications from all types of vascular access using a Likert scale. An interval scale was constructed in which optimal health in an ESRD patient with a functional hemodialysis access was assigned a value of ten and death a value of zero. All utilities assigned were divided by ten and used in the modeling process to generate quality-adjusted survival data. The dysutility of an access complication was applied only to the cycle in which the patient had the complication. Therefore, a patient who experienced a complication during one cycle could have a different utility score for the following cycle. In a sensitivity analysis, this utility measure was varied, specifically, first doubled and then halved (Table 2).
Table 2.
Utilities assigned to the different health states
| Health State | SVA | AVF | CVC |
| No complications | 1 | 1 | 1 |
| Death | 0 | 0 | 0 |
| Vascular access thrombosed corrected using radiological intervention | .66 | .66 | .58 |
| Vascular access thrombosed corrected using surgical intervention | .48 | .48 | .72 |
| Infection/Antibiotics | .46 | .46 | .42 |
| Infection/Excision Access | .38 | .44 | N/A |
| SVA placement | .44 | N/A | N/A |
| AVF placement | N/A | .48 | N/A |
| Use of Urokinase | N/A | N/A | .54 |
CVC= Central venous catheter
SVA=Synthetic vascular access
AVF=Arteriovenous fistula
c) Costs
Direct costs for access placement included costs of physician and hospital care. Costs of complications such as infected grafts, revision, and thrombectomy were based on Medicare payments to hospitals and physicians for the relevant DRG and CPT. The frequencies and costs of hospitalization secondary to vascular procedures during 2004 were obtained from Centers for Medicare & Medicaid Services. Institutional inpatient and outpatient costs were taken from USRDS13 and adjusted to 2004 dollars. All pertinent DRG, ICD-9 and CPT codes are listed in Table 3. When there was more than one possible code for a procedure, a weighted average was used to derive the cost for that procedure.
Table 3.
Costs used for vascular access procedures
| PROCEDURE | REIMBURSEMENT*(USD) |
|---|---|
| Outpatient Hospital cost39 | 1246.80 |
| Inpatient Hospital cost39 | 11008.10 |
| Adjusted hospital cost * | 6127.45 |
| Placement AVF | 762.70 |
| Placement SVA | 703.03 |
| Surgical Revision AVF | 563 |
| Surgical Revision SVA | 557 |
| Septicemia cost ×12 weeks40 | 20,067 |
| Placement tunneled dialysis catheter | 238.00 |
| Femoral catheter placement | 160.71 |
| Thrombectomy, open | 453.63 |
| Anesthesia | 170.83 |
| Use of Permacath (12 wks) | 5,774.18 |
| Excision infected SVA | 538.72 |
Unless otherwise specified, all costs are derived from data provided by CMS for 2004.
**Adjusted Hospital Cost= 1/2 outpatient + 1/2 inpatient costs
For example, the cost of replacement of an infected SVA was estimated to be the cost of the SVA excision added to the cost of a femoral line placement for hemodialysis at the time the infection was recognized, the cost of placement and use of a dialysis catheter for two weeks, a course of antibiotic treatment for two weeks, and the cost of a new SVA placement including anesthesia costs. These costs summed to $14,506.
Costs of catheter complications for two weeks were assumed to be those associated with placement, and catheter complications such as thrombosis and infection (bacteremia and septicemia). The average cost of placing AVFs and SVAs was the average cost of any such procedure at any site. For example, to estimate costs for AVFs, codes 36821 (Cimino type), 36825 (autogenous graft), 36819 (basilic vein transposition), and 36820 (forearm vein transposition) were included.
Since half the vascular access procedures are performed on an outpatient basis, an adjusted hospital cost was estimated from the average of the hospital outpatient and inpatient costs.
The frequency of use and cost of emergency dialysis through a femoral temporary catheter placed acutely secondary to a non-functioning permanent vascular access were estimated from billing data from the Inpatient Dialysis Unit of the University of Pennsylvania Medical Center during a six-month period (unpublished data).
d) Sensitivity and Threshold Analyses
We modified all probabilities in one- and two-way sensitivity analyses to explore how assumptions built into our base-case estimates influenced the interpretation of our model. In particular we targeted the impact of utilities, AVF maturation rate, cost of AVF placement, and complication rate of central venous catheters.
Results
Utilities
Utility questionnaires were completed by ten hemodialysis patients. Five were male and five were African American. Their mean age was 54. Half had a native arteriovenous fistula at the time they completed the questionnaire. Only one was using a central venous catheter as a hemodialysis vascular access. Five subjects stated they had never had any episode of infection or thrombosis with their current vascular access while three stated they had never had an episode of infection or thrombosis with any previous hemodialysis vascular access. Results are presented in Table 2.
Decision Tree
Using our baseline case with a maturation rate of AVF of 66% as this is the goal of the Fistula First program, the cost for the AVF1st strategy was $16,151 compared to $14,930 for the SVA1st strategy. The AVF1st strategy generated a total of 2.19 QALYs compared to 2.06 from the SVA1st strategy. SVA1st strategy had a better cost-utility ratio [$7,248 vs. $7,375]. In addition, the incremental cost-effectiveness was $9,389 for each QALY gained from AVF1st strategy compared to SVA1st strategy.
Table 4 depicts the proportion of the cohort in each Markov state at the end of five years. The majority of our cohort (70.8%) in either strategy had died. Under the AVF1st strategy, owing to assumed placement of an SVA after AVF failure, 11.9% had a functioning SVA.
Table 4.
Proportion of the cohort in each Markov state at the end of the fifth cycle
| Markov States | AVF 1st strategy (%) | SVA1st strategy (%) |
|---|---|---|
| Death | 70.8 | 70.8 |
| AVF | 17.3 | n/a |
| SVA states | ||
| No complications | 0.17 | 0.10 |
| Thrombotic complications | 7.98 | 19.8 |
| Infective complications | 1.39 | 3.50 |
| New SVA | 2.37 | 5.8 |
Sensitivity and Threshold Analysis
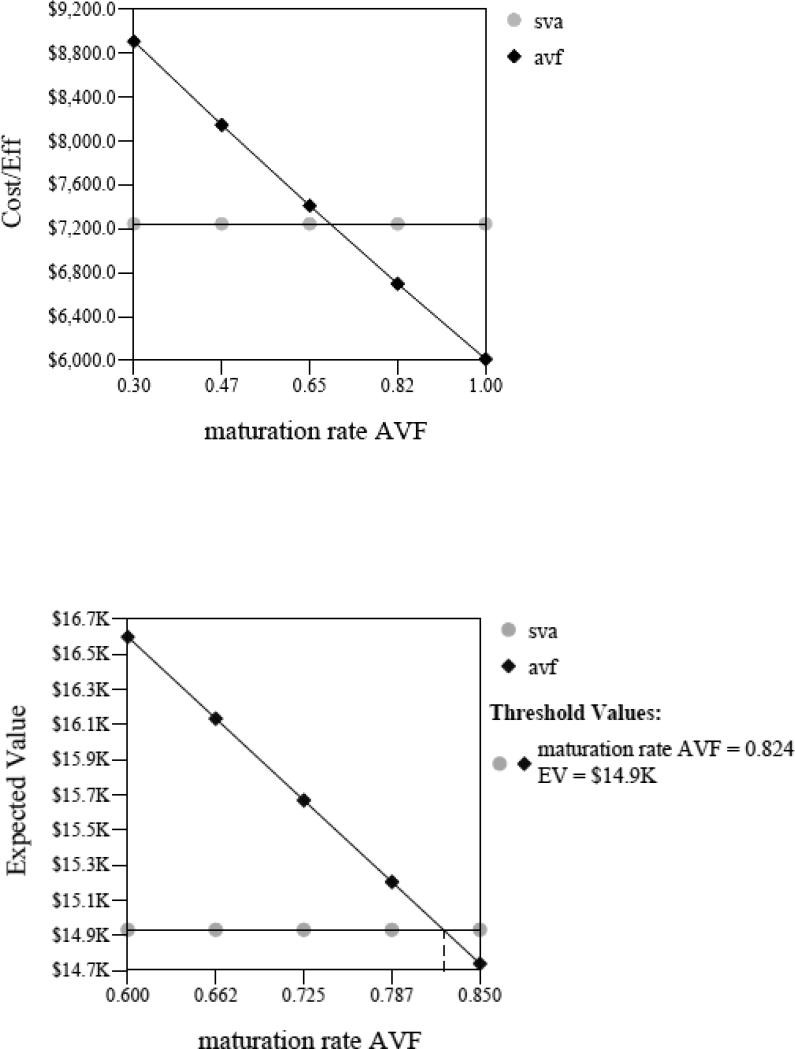
We evaluated the maturation rate at which the AVF1st strategy had; a) a better average cost-utility, b) was less costly, or c) produced more QALYs compared to the SVA1st strategy. The AVF1st strategy had a better average cost-utility than SVA1st as long as the AVF maturation rate was greater than 69%. Further, as presented in Figure 2, the AVF1st strategy became less costly than SVA1st only if the AVF maturation rate was greater than or equal to 82%. In addition, the AVF1st strategy yielded more QALYs if the AVF maturation rate was only 5% or greater. The incremental cost-effectiveness ratio (cost/utilities) comparing AVF1st vs. SVA1st exceeded $50,000 per QALY for AVF maturation rates of 36% or below. A threshold of $50,000 per QALY has been suggested as the incremental cost-effectiveness ratio below which an intervention is considered cost-effective.
Figure 2.
Sensitivity analysis of AVF maturation rate on cost-utility (above) and sensitivity analysis of AVF maturation rate on cost (below)
An increase in reimbursement for AVF placement has been suggested as an option to increase placement of AVF. Even if the reimbursement for AVF placement would triple compared to SVA placement, the incremental cost-effectiveness ratio of AVF1st vs. SVA1st strategy would only rise from $9,389 to $20,000.
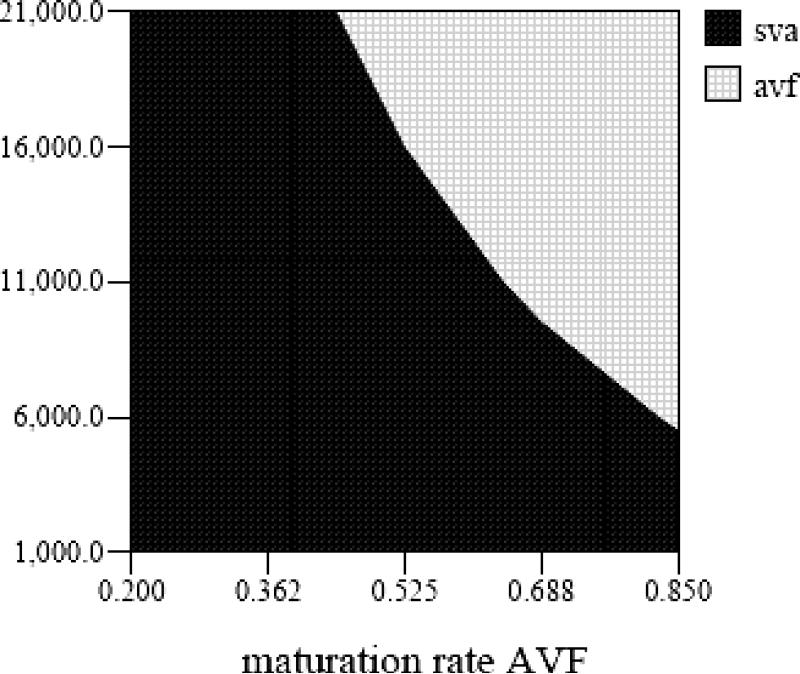
Over the years a larger proportion of hemodialysis access placement is done in the outpatient setting and, therefore, the reimbursed hospital costs per procedure have decreased over time. In Figure 3, we present a two way sensitivity on how changes in AVF maturation rate and adjusted hospital costs, an average of the hospital outpatient and inpatient costs for vascular access, affect which strategy is less costly. Using our base case of AVF maturation rate of 66%, if the adjusted hospital costs were greater than $10,472, the AVF1st strategy would be less costly.
Figure 3.
Two way sensitivity analysis of AVF maturation rate and adjusted hospital cost
We evaluated the effect of varying utilities on the different strategies. If the utility estimates for all SVA estimates were 50% lower, at any maturation rate, AVF1st yielded more QALYs. If the utility estimates were decreased by 50% for all AVF estimates, at a maturation rate of 50%, the incremental cost-effectiveness ratio fell to less than $50,000 comparing AVF1st to SVA1st.
Discussion
Vascular access failure causes substantial morbidity for patients and high costs for the ESRD program15,1. The National Kidney Foundation's Dialysis Outcome Quality Initiative (DOQI) clinical practice guidelines on vascular access recommend that AVF be the preferred vascular access for chronic hemodialysis patients 18. The frequent need for immediate dialysis has been cited as a reason for placing SVA in incident dialysis patients 24, 25. Using our baseline case the AVF1st strategy generated 0.13 additional QALYs compared to the SVA1st strategy. The incremental cost-effectiveness was $9,389/QALY for AVF1st compared to SVA1st. These findings were not significantly changed by variations in utility values. The AVF1st strategy had a better average cost-utility than SVA1st when the AVF maturation rate was greater than 69%. The rate of maturation would need to be unrealistically high at 82% for AVF1st strategy to be less costly. However, the incremental cost-effectiveness of AVF1st versus SVA1st fell below $50,000 per QALY only when the AVF maturation rate was at or below 36%. In light of the high probability of AVF maturation failure, often reported to be 50% or higher10, the AVF1st strategy may be most appropriate only for a subset of hemodialysis patients whose risk of AVF maturation failure is relatively low. These findings were stable across a range of assumptions.
Outside of the US, the majority of hemodialysis patients use an AVF as their vascular access. The wide regional variation in type of vascular access suggests AVF utilization could be increased substantially17,26. AVF in 1990 incident dialysis subjects ranged from a low of only 1% in the southeastern central US to a high of 77% in New England (p<0.0001). Therefore, a great majority of patients undergo synthetic vascular access placement despite the fact that a many may have been able to undergo successful placement of an AVF. Despite national practice guidelines, a recent national study reveals the same geographic variability in AVF placement along with known gender and racial disparities 26. The reasons for use of SVA as the first access placed in hemodialysis patients are unclear, but it may include increased reimbursement for SVA placement compared to AVF, decreased availability of surgeons with expertise for AVF placement27, and ease of placement of SVA compared to AVF 28.
Our estimates are very conservative in that assumptions favored the use of SVA. We assumed that all SVA that were placed were functional. Initial successful placement of an SVA has been estimated to be between 90 to 98%8, 29, 30 with primary unassisted patency at one year of 23%31. We allowed for the placement of only one AVF per patient in our model. Several recent reports suggest that AVFs that do not mature initially may be salvaged after a combination of angioplasty and accessory vein ligation 32. Others suggest the use of vein transposition33, using upper arm brachiocephalic or brachiobasilic fistulas in an effort to increase the number of AVFs in the hemodialysis population. In addition, maturation rates of 97.5% have been reported using three major surgical approaches according to the preoperative evaluation: forearm AVF, perforating vein fistula at the elbow and non-perforating vein fistula at the elbow 34. We also only allowed one complication per cycle. We did not include costs of performing procedures in non-thrombosed SVA detected by flow monitoring devices, which would increase the cost of SVA.
In our base model, AVF 1st strategy meets the $50,000 per QALY threshold as long as the probability of maturation is 36% or greater. This rate of maturation of AVF is similar to some described in the literature and is not always easy to predict in an individual patient. Therefore, there is a need for diagnostic tests and predictive rules that will provide risk stratification of AVF maturation.
A functioning AVF is associated with patient survival. AVF use 90 days after dialysis initiation is associated with lower cardiovascular mortality35. Several authors have demonstrated that increasing the use of AVF in incident dialysis patients is possible in the US 36, 37. Gibson et al, with the adoption of a multidisciplinary approach, increased their AVF placements from 41.3 to 73.7% during a seven year period. A recent economic analysis found that the use of AV fistula according to Fistula First Program's goal would prolong survival of the average patient by an estimated 0.38 yr38.
Our study has several limitations including the possibility that our assumptions were not a depiction of the “real world”. However, we used sensitivity analyses to examine the influence of assumptions regarding the impact of utilities, AVF maturation rate, cost of AVF placement, and complication rate of central venous catheters on our decision model. As with many Markov models, ours also incorporated the assumption that the probability of moving from one state to another is independent of the history of the individual before arriving in that state. We addressed this in the model by increasing the probability of a future SVA complication by 20% if a complication had occurred in the previous cycle. There is no published information on patient utilities for hemodialysis vascular access. Therefore, utilities for the different types of vascular access procedures were directly measured in a small group of patients. However, sensitivity analysis showed that even under extreme assumptions regarding high SVA utilities, the AVF1st strategy was more cost-effective if the maturation probability was greater than 50%.
Conclusions
The incremental cost-effectiveness of AVF 1st vs. SVA1st strategies was less than $50,000 per QALY as long as the probability of AVF maturation was set at 36% or greater. Given the observation from recent trials that the average AVF maturation rate is very close to this threshold figure,10 the current emphasis of placing AVFs in all dialysis patients may not be optimal. Initiatives to develop better predictive models of AVF maturation that would permit stratification of patients with regard to their probability of fistula maturation are now underway at the NIH and promise to provide tools enabling more targeted use of fistulas to optimize clinical outcomes.
Acknowledgements
We appreciate the assistance of Paul W. Eggers, PhD, National Institute of Diabetes and Digestive and Kidney Diseases, for provision of data used in the analysis
Supported in part by NIH Grant R01-DK-044994-04S1
Dr. Rosas receives salary support from NIDDK R01 080033
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Feldman H, Held P, Hutchinson J, Stoiber E, Hartigan M, Berlin J. Hemodialysis vascular access morbidity in the United States. Kidney International. 1993;43:1091–6. doi: 10.1038/ki.1993.153. [DOI] [PubMed] [Google Scholar]
- 2.III. NKF-K/DOQI Clinical Practice Guidelines for Vascular Access: update 2000. Am J Kidney Dis. 2001;37:S137–81. doi: 10.1016/s0272-6386(01)70007-8. [DOI] [PubMed] [Google Scholar]
- 3.Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney International. 2002;62:1109–24. doi: 10.1111/j.1523-1755.2002.kid551.x. [DOI] [PubMed] [Google Scholar]
- 4.Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001;60:1443–51. doi: 10.1046/j.1523-1755.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 5.Astor BC, Eustace JA, Powe NR, Klag MJ, Fink NE, Coresh J. Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol. 2005;16:1449–55. doi: 10.1681/ASN.2004090748. [DOI] [PubMed] [Google Scholar]
- 6.Feldman HI, Joffe M, Rosas SE, Burns JE, Knauss J, Brayman K. Predictors of successful arteriovenous fistula maturation. Am J Kidney Dis. 2003;42:1000–12. doi: 10.1016/j.ajkd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Field M, MacNamara K, Bailey G, Jaipersad A, Morgan RH, Pherwani AD. Primary patency rates of AV fistulas and the effect of patient variables. J Vasc Access. 2008;9:45–50. [PubMed] [Google Scholar]
- 8.Palder SB, Kirkman RL, Whittemore AD, Hakim RM, Lazarus JM, Tilney NL. Vascular access for hemodialysis. Patency rates and results of revision. Annals of Surgery. 1985;202:235–9. doi: 10.1097/00000658-198508000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winsett OE, Wolma FJ. Complications of vascular access for hemodialysis. Southern Medical Journal. 1985;78:513–7. doi: 10.1097/00007611-198505000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Dember LM, Beck GJ, Allon M, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA. 2008;299:2164–71. doi: 10.1001/jama.299.18.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huijbregts HJ, Bots ML, Wittens CH, Schrama YC, Moll FL, Blankestijn PJ. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol. 2008;3:714–9. doi: 10.2215/CJN.02950707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TH, Bui TD, Gordon IL, Wilson SE. Functional patency of autogenous AV fistulas for hemodialysis. J Vasc Access. 2007;8:275–80. [PubMed] [Google Scholar]
- 13.USRDS . Annual Data Report. National Institutes of Health; Bethesda: 1997. [Google Scholar]
- 14.Avorn J, Winkelmayer WC, Bohn RL, et al. Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. Journal of Clinical Epidemiology. 2002;55:711–6. doi: 10.1016/s0895-4356(02)00415-8. [DOI] [PubMed] [Google Scholar]
- 15.Feldman HI, Kobrin S, Wasserstein A. Hemodialysis vascular access morbidity [editorial]. Journal of the American Society of Nephrology. 1996;7:523–35. doi: 10.1681/ASN.V74523. [DOI] [PubMed] [Google Scholar]
- 16.Rosas SE, Joffe M, Burns JE, Knauss J, Brayman K, Feldman HI. Determinants of successful synthetic hemodialysis vascular access graft placement. J Vasc Surg. 2003;37:1036–42. doi: 10.1067/mva.2003.257. [DOI] [PubMed] [Google Scholar]
- 17.Hirth RA, Turenne MN, Woods JD, et al. Predictors of type of vascular access in hemodialysis patients [see comments]. Jama. 1996;276:1303–8. [PubMed] [Google Scholar]
- 18.National Kidney Foundation-Dialysis Outcomes Quality Initiative NKF-DOQI clinical practice guidelines for vascular access. American Journal of Kidney Diseases. 1997;30:S150–91. [PubMed] [Google Scholar]
- 19.Centers for Medicare & Medicaid Services CMS Launches Breakthrough Initiative for Major Improvement in care for Kidney Patients: Safe Vascular Access through Collaborative Fistula First Initiative. 2005 Press Release. [Google Scholar]
- 20.Tonnessen BH, Money SR. Embracing the fistula first national vascular access improvement initiative. J Vasc Surg. 2005;42:585–6. doi: 10.1016/j.jvs.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 21.Beck JR, Pauker SG. The Markov process in medical prognosis. Medical Decision Making. 1983;3:419–58. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 22.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Medical Decision Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 23.USRDS . Atlas of End-Stage Renal Disease in the United States. National Institutes of Health; 2007. [Google Scholar]
- 24.Jungers P, Zingraff J, Page B, Albouze G, Hannedouche T, Man NK. Detrimental effects of late referral in patients with chronic renal failure: a case-control study. Kidney International - Supplement. 1993;41:S170–3. [PubMed] [Google Scholar]
- 25.Sands JJ. Increasing AV fistulas: revisiting a time-tested solution. Seminars in Dialysis. 2000;13:351–3. doi: 10.1046/j.1525-139x.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- 26.Reddan D, Klassen P, Frankenfield DL, et al. National profile of practice patterns for hemodialysis vascular access in the United States. Journal of the American Society of Nephrology. 2002;13:2117–24. doi: 10.1097/01.asn.0000022422.79790.a8. [DOI] [PubMed] [Google Scholar]
- 27.Saran R, Elder SJ, Goodkin DA, et al. Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study. Ann Surg. 2008;247:885–91. doi: 10.1097/SLA.0b013e31816c4044. [DOI] [PubMed] [Google Scholar]
- 28.Chertow GM. Grafts vs fistulas for hemodialysis patients: equal access for all? [editorial; comment]. Jama. 1996;276:1343–4. [PubMed] [Google Scholar]
- 29.Tordoir JH, Herman JM, Kwan TS, Diderich PM. Long-term follow-up of the polytetrafluoroethylene (PTFE) prosthesis as an arteriovenous fistula for haemodialysis. European Journal of Vascular Surgery. 1987;2:3–7. doi: 10.1016/s0950-821x(88)80099-9. [DOI] [PubMed] [Google Scholar]
- 30.Rizzuti RP, Hale JC, Burkart TE. Extended patency of expanded polytetrafluoroethylene grafts for vascular access using optimal configuration and revisions. Surgery, Gynecology & Obstetrics. 1988;166:23–7. [PubMed] [Google Scholar]
- 31.Dixon BS, Beck GJ, Vazquez MA, et al. Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med. 2009;360:2191–201. doi: 10.1056/NEJMoa0805840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beathard GA, Settle SM, Shields MW. Salvage of the nonfunctioning arteriovenous fistula. American Journal of Kidney Diseases. 1999;33:910–6. doi: 10.1016/s0272-6386(99)70425-7. [DOI] [PubMed] [Google Scholar]
- 33.Silva MB, Jr., Hobson RW, 2nd, Pappas PJ, et al. Vein transposition in the forearm for autogenous hemodialysis access.[comment]. Journal of Vascular Surgery. 1997;26:981–6. doi: 10.1016/s0741-5214(97)70010-7. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 34.Konner K, Hulbert-Shearon TE, Roys EC, Port FK. Tailoring the initial vascular access for dialysis patients. Kidney International. 2002;62:329–38. doi: 10.1046/j.1523-1755.2002.00436.x. [DOI] [PubMed] [Google Scholar]
- 35.Wasse H, Speckman RA, McClellan WM. Arteriovenous fistula use is associated with lower cardiovascular mortality compared with catheter use among ESRD patients. Semin Dial. 2008;21:483–9. doi: 10.1111/j.1525-139X.2008.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sands J, Miranda CL. Increasing numbers of AV fistulas for hemodialysis access. Clinical Nephrology. 1997;48:114–7. [PubMed] [Google Scholar]
- 37.Gibson KD, Caps MT, Kohler TR, et al. Assessment of a policy to reduce placement of prosthetic hemodialysis access. Kidney International. 2001;59:2335–45. doi: 10.1046/j.1523-1755.2001.00751.x. [DOI] [PubMed] [Google Scholar]
- 38.Schon D, Blume SW, Niebauer K, Hollenbeak CS, de Lissovoy G. Increasing the use of arteriovenous fistula in hemodialysis: economic benefits and economic barriers. Clin J Am Soc Nephrol. 2007;2:268–76. doi: 10.2215/CJN.01880606. [DOI] [PubMed] [Google Scholar]
- 39.The economic cost of ESRD, vascular access procedures, and Medicare spending for alternative modalities of treatment. USRDS. United States Renal Data System. American Journal of Kidney Diseases. 1997;30:S160–77. doi: 10.1016/s0272-6386(97)90187-6. Anonymous. [DOI] [PubMed] [Google Scholar]
- 40.Nissenson AR, Dylan ML, Griffiths RI, et al. Clinical and economic outcomes of Staphylococcus aureus septicemia in ESRD patients receiving hemodialysis. Am J Kidney Dis. 2005;46:301–8. doi: 10.1053/j.ajkd.2005.04.019. [DOI] [PubMed] [Google Scholar]