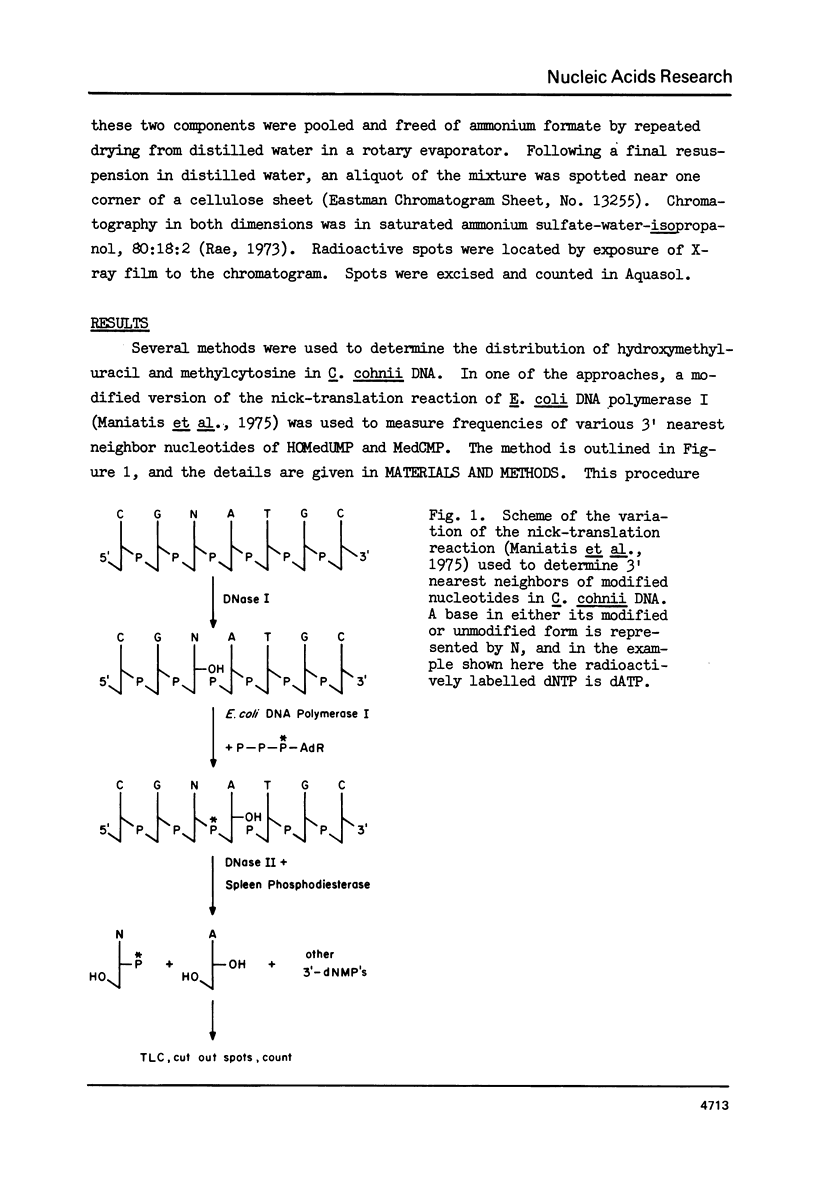
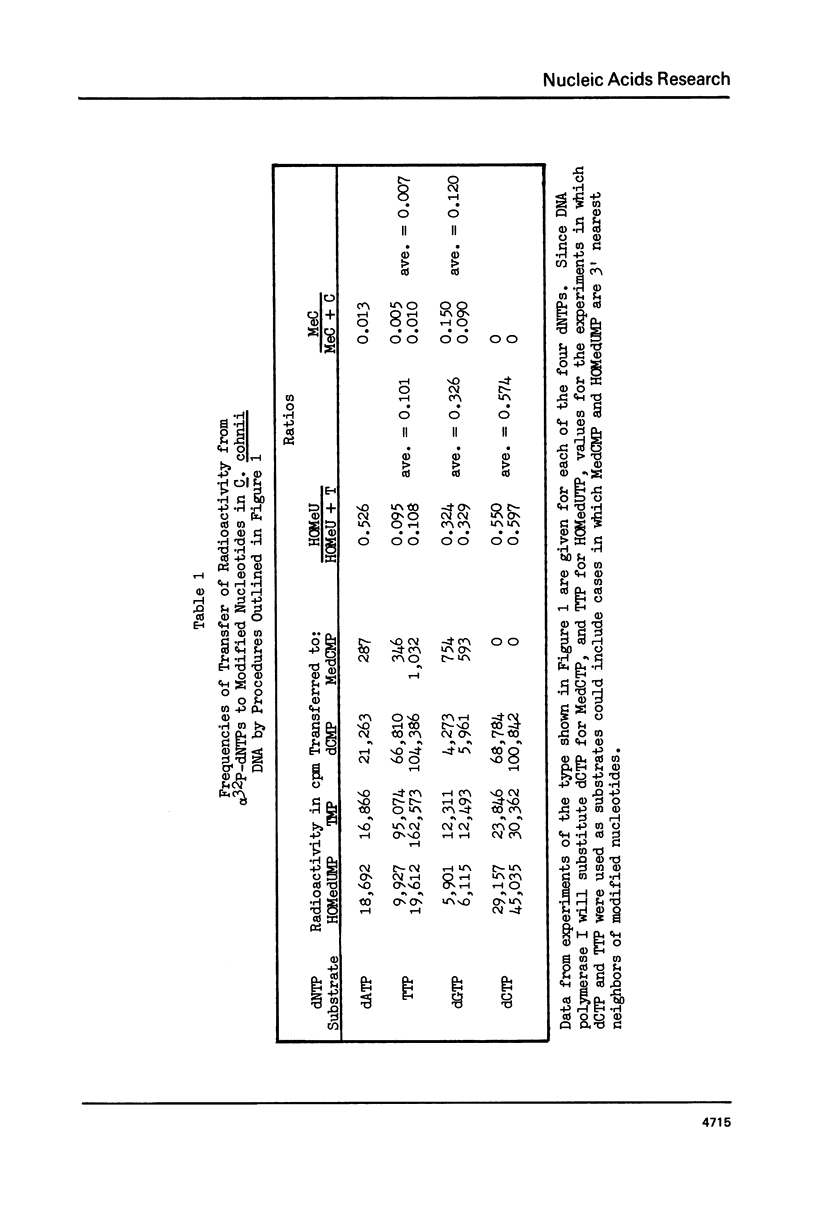
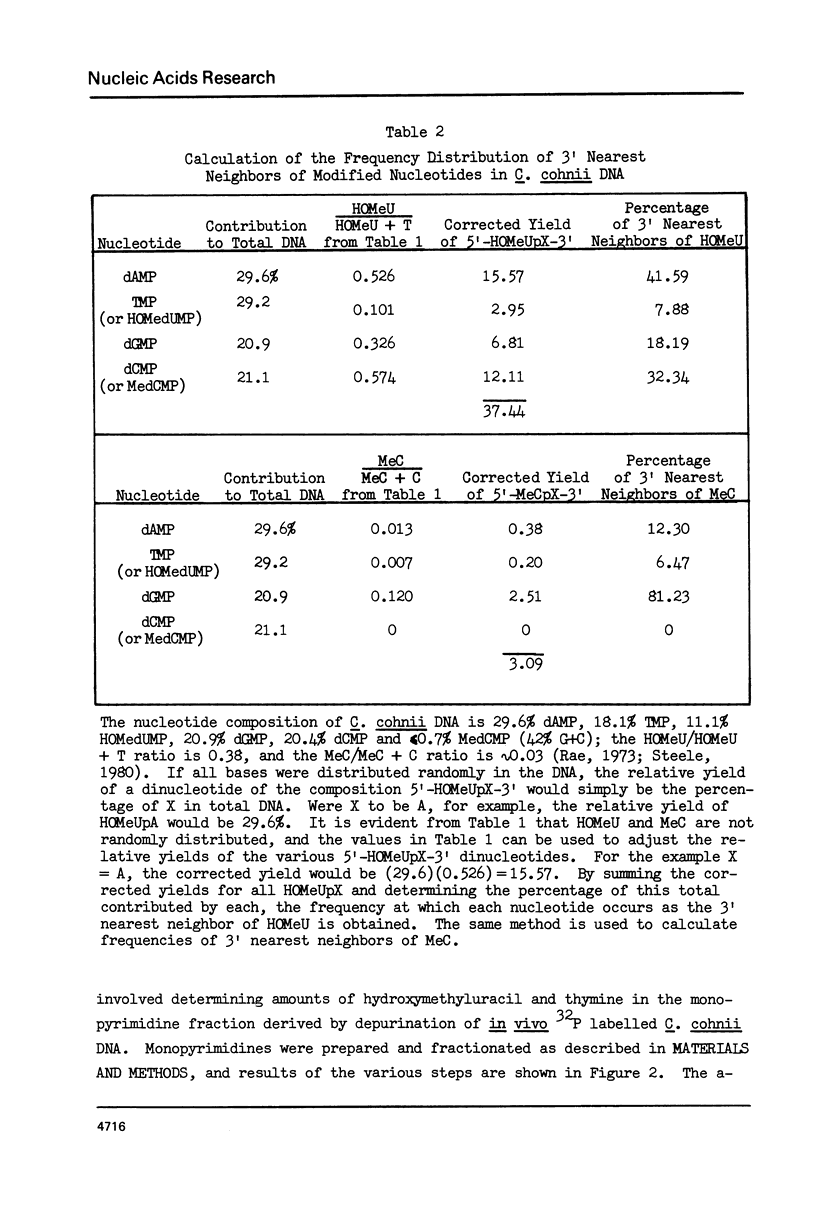
Abstract
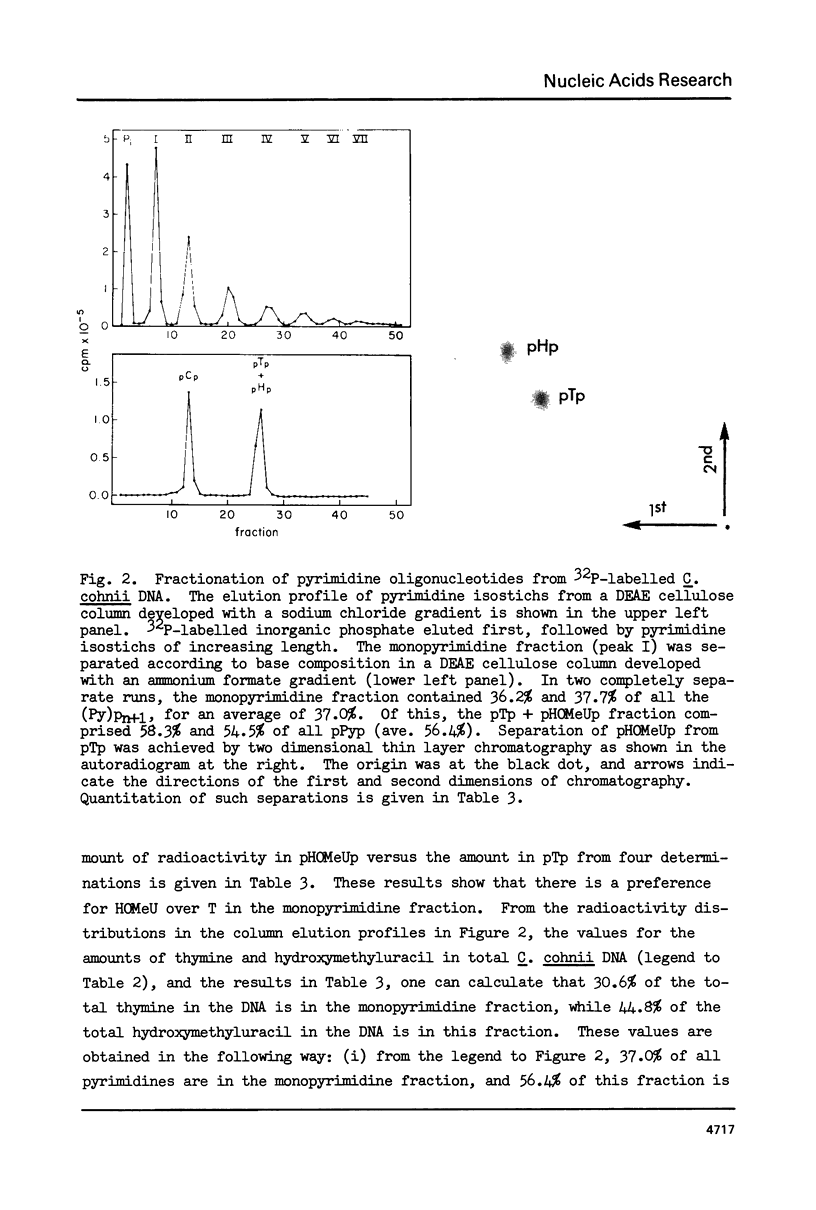
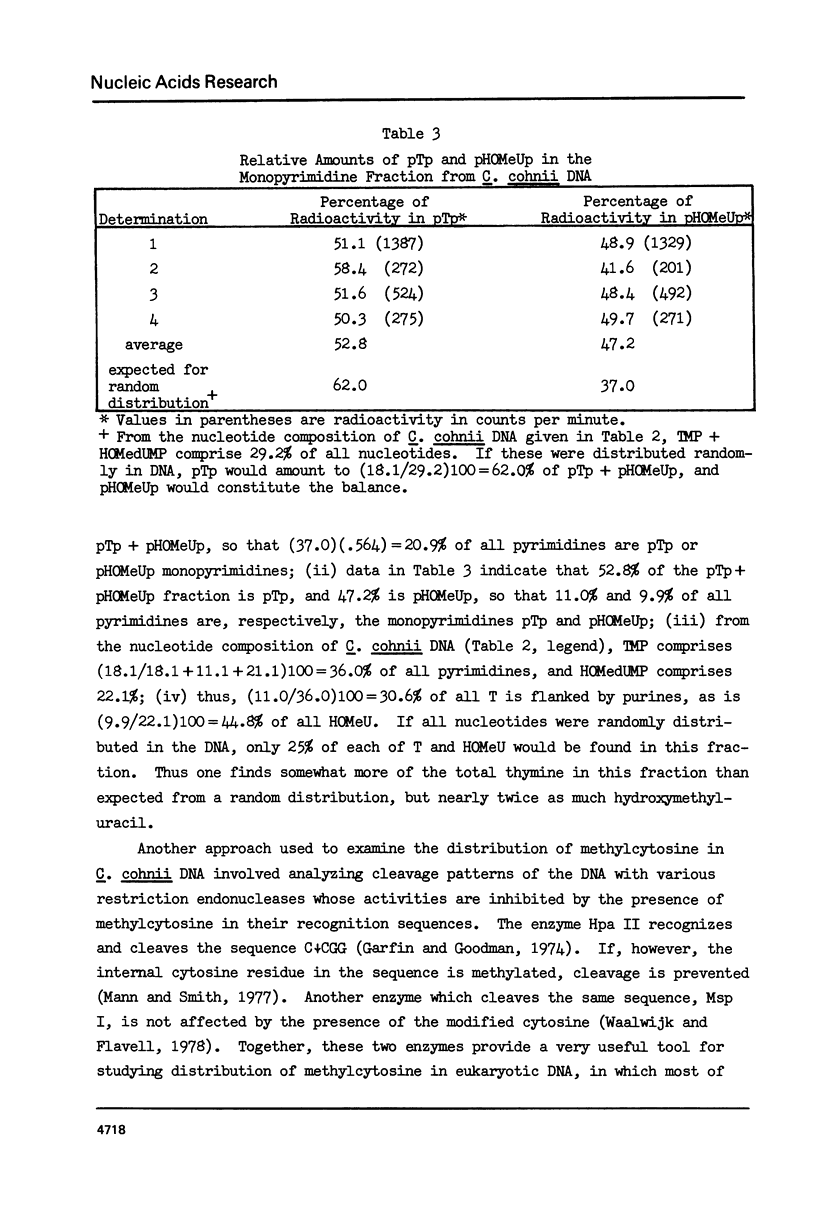
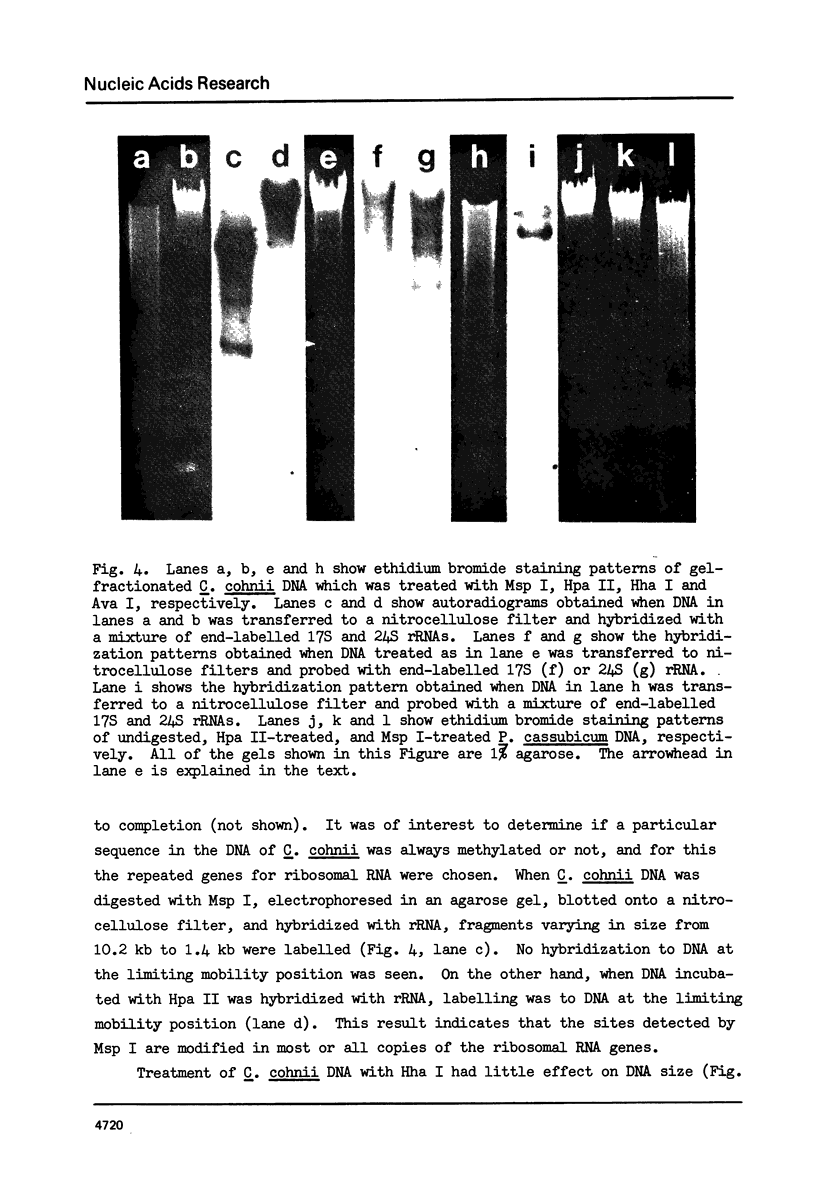
In DNA of the dinoflagellate Crypthecodinium cohnii, 38% of the thymine is replaced by the modified base 5-hydroxymethyluracil, and approximately 3% of the cytosine is replaced by 5-methylcytosine. Both of the modified bases are non-randomly distributed in the DNA. Determinations of 3' nearest neighbors show that HOMeU is preferentially located in the dinucleotides HOMeUpA and HOMeUpC. Pyrimidine tract analysis shows that HOMeU is also greatly enriched in the trinucleotide purine-HOMeU-purine. As in other eukaryotes, methylcytosine in C. cohnii DNA occurs predominantly in the dinucleotide MeCpG. By analysis of restriction endonuclease digestion patterns of C. cohnii total DNA and ribosomal DNA, we have found that the central CpG dinucleotides in the sites for the enzymes Hpa II (CCGG) and Hha I (GCGC) are extensively methylated in both total DNA and ribosomal DNA. Results of digestion with Ava I, however, indicated that not all CpG dinucleotides in the sequence CCTCGGAG are methylated in C. cohnii DNA.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K., PETERSEN G. B. The frequencies of certain sequences of nucleotides in deoxyribonucleic acid. Biochem J. 1960 Apr;75:17–27. doi: 10.1042/bj0750017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett T., Rae P. M. A 9.6 kb intervening sequence in D. virilis rDNA, and sequence homology in rDNA interruptions of diverse species of Drosophila and other diptera. Cell. 1979 Apr;16(4):763–775. doi: 10.1016/0092-8674(79)90092-8. [DOI] [PubMed] [Google Scholar]
- Bernardi G., Ehrlich S. D., Thiery J. P. The specificity of deoxyribonucleases and their use in nucleotide sequence studies. Nat New Biol. 1973 Nov 14;246(150):36–40. doi: 10.1038/newbio246036a0. [DOI] [PubMed] [Google Scholar]
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Bird A. P., Taggart M. H. Variable patterns of total DNA and rDNA methylation in animals. Nucleic Acids Res. 1980 Apr 11;8(7):1485–1497. doi: 10.1093/nar/8.7.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., McKenna G., Sharp P. A. Cleavage of mouse DNA by a restriction enzyme as a clue to the arrangement of genes. Cold Spring Harb Symp Quant Biol. 1974;38:383–395. doi: 10.1101/sqb.1974.038.01.041. [DOI] [PubMed] [Google Scholar]
- Burdon R. H., Adams R. L. The in vivo methylation of DNA in mouse fibroblasts. Biochim Biophys Acta. 1969 Jan 21;174(1):322–329. doi: 10.1016/0005-2787(69)90257-3. [DOI] [PubMed] [Google Scholar]
- Cerný R., Mushynski W. E., Spencer J. H. Nucleotide clusters in deoxyribonucleic acids. 3. Separation of pyrimidine isostichs according to base composition. Biochim Biophys Acta. 1968 Dec 17;169(2):439–450. doi: 10.1016/0005-2787(68)90052-x. [DOI] [PubMed] [Google Scholar]
- Dodge J. D. A dinoflagellate with both a mesocaryotic and a eucaryotic nucleus. I. Fine structure of the nuclei. Protoplasma. 1971;73(2):145–157. doi: 10.1007/BF01275591. [DOI] [PubMed] [Google Scholar]
- Garfin D. E., Goodman H. M. Nucleotide sequences at the cleavage sites of two restriction endonucleases from Hemophilus parainfluenzae. Biochem Biophys Res Commun. 1974 Jul 10;59(1):108–116. doi: 10.1016/s0006-291x(74)80181-6. [DOI] [PubMed] [Google Scholar]
- Gautier F., Mayer H., Goebel W. Cloning of calf thymus satellite I DNA in Escherichia coli. Mol Gen Genet. 1976 Nov 24;149(1):23–31. doi: 10.1007/BF00275957. [DOI] [PubMed] [Google Scholar]
- Grippo P., Iaccarino M., Parisi E., Scarano E. Methylation of DNA in developing sea urchin embryos. J Mol Biol. 1968 Sep 14;36(2):195–208. doi: 10.1016/0022-2836(68)90375-6. [DOI] [PubMed] [Google Scholar]
- Hemphill H. E., Whiteley H. R. Bacteriophages of Bacillus subtilis. Bacteriol Rev. 1975 Sep;39(3):257–315. doi: 10.1128/br.39.3.257-315.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. G., Murray K. The nucleotide sequences recognized by endonucleases AvaI and AvaII from Anabaena variabilis. Biochem J. 1980 Jan 1;185(1):65–75. doi: 10.1042/bj1850065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeblich A. R., 3rd Dinoflagellate evolution: speculation and evidence. J Protozool. 1976 Feb;23(1):13–28. doi: 10.1111/j.1550-7408.1976.tb05241.x. [DOI] [PubMed] [Google Scholar]
- Maizels N. Dictyostelium 17S, 25S, and 5S rDNAs lie within a 38,000 base pair repeated unit. Cell. 1976 Nov;9(3):431–438. doi: 10.1016/0092-8674(76)90088-x. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M. B., Smith H. O. Specificity of Hpa II and Hae III DNA methylases. Nucleic Acids Res. 1977 Dec;4(12):4211–4221. doi: 10.1093/nar/4.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R., Heywood J. Restriction and modification of DNA. Annu Rev Biochem. 1972;41:447–466. doi: 10.1146/annurev.bi.41.070172.002311. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Maltman K. L., Warren R. A. Bacteriophage phi W-14-infected Pseudomonas acidovorans synthesizes hydroxymethyldeoxyuridine triphosphate. J Virol. 1980 May;34(2):347–353. doi: 10.1128/jvi.34.2.347-353.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. M. 5-Hydroxymethyluracil in the DNA of a dinoflagellate. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1141–1145. doi: 10.1073/pnas.70.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. M. Hydroxymethyluracil in eukaryote DNA: a natural feature of the pyrrophyta (dinoflagellates). Science. 1976 Dec 3;194(4269):1062–1064. doi: 10.1126/science.988637. [DOI] [PubMed] [Google Scholar]
- Rae P. M., Steele R. E. Absence of cytosine methylation at C-C-G-G and G-C-G-C sites in the rDNA coding regions and intervening sequences of Drosophila and the rDNA of other insects. Nucleic Acids Res. 1979 Jul 11;6(9):2987–2995. doi: 10.1093/nar/6.9.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. M., Steele R. E. Modified bases in the DNAs of unicellular eukaryotes: an examination of distributions and possible roles, with emphasis on hydroxymethyluracil in dinoflagellates. Biosystems. 1978 Apr;10(1-2):37–53. doi: 10.1016/0303-2647(78)90027-8. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Myers P. A., Morrison A., Murray K. A specific endonuclease from Haemophilus haemolyticus. J Mol Biol. 1976 May 5;103(1):199–208. doi: 10.1016/0022-2836(76)90060-7. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Restriction and modification enzymes and their recognition sequences. Nucleic Acids Res. 1980 Jan 11;8(1):r63–r80. doi: 10.1093/nar/8.1.197-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H., Tucker R. G. The biosynthesis of 5-hydroxymethyldeoxyuridylic acid in bacteriophage-infected Bacillus subtilis. Virology. 1966 May;29(1):157–166. doi: 10.1016/0042-6822(66)90205-4. [DOI] [PubMed] [Google Scholar]
- SINSHEIMER R. L. The action of pancreatic desoxyribonuclease. I. Isolation of mono- and dinucleotides. J Biol Chem. 1954 May;208(1):445–459. [PubMed] [Google Scholar]
- Sneider T. W., Potter V. R. Methylation of mammalian DNA: studies on Novikoff hepatoma cells in tissue culture. J Mol Biol. 1969 Jun 14;42(2):271–284. doi: 10.1016/0022-2836(69)90043-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]