Abstract
Rupture of ACL is a common injury. While the current surgical treatments are effective, many patients still suffer from precocious osteoarthritis, and there is an increasing interest in bioengineering approaches to improve ACL repair. Bovine collagen is a material currently in use for tissue engineering of ligaments. The alpha-gal epitopes found on bovine cells are a source of immunogenic stimulus for human cells. In this study, we wished to determine if those epitopes could be removed sufficiently to mitigate an immunogenic response using either a decellularization protocol or decellularization followed by alpha-galactosidase treatment. Bovine ACLs were treated with Triton-X, sodium deoxycholate, ribonuclease, and deoxyribonuclease to remove cells. A subset of the decellularized tissues was further treated with alpha-galactosidase. Human peripheral blood mononuclear cells (PBMCs) were exposed to untreated, decellularized, and alpha-galactosidase-treated tissues, and PBMC migration and IL-6 release were measured. PBMCs were significantly more attracted to untreated ACL compared to decellularized or alpha-galactosidase-treated tissue, but no difference was seen between the two treatment groups. PBMCs also released significantly more IL-6 when exposed to untreated tissue compared to decellularized ACL or alpha-galactosidase-treated ACL, but no difference was seen between the two treatment groups. Immunohistochemistry using anti-alpha-gal antibody detected the epitopes throughout the untreated ACL, but similar areas of reaction were not seen on decellularized or alpha-galactosidase-treated ACL. These results suggest that our decellularization protocol minimizes the immunogenic reactions of human PBMCs to bovine ACL tissue. Therefore, decellularized bovine ACL tissue may be a safe, effective biomaterial for ACL injury treatments.
Keywords: Tissue Engineering, Anterior Cruciate Ligament, Graft Rejection, Inflammation, Wound Healing
INTRODUCTION
The anterior cruciate ligament (ACL) is a commonly injured knee ligament. In the United States alone, there are an estimated 400,000 ACL ruptures per year (1). In addition to causing pain, discomfort, instability, and limited mobility, ACL rupture increases the risk of precocious osteoarthritis (2). ACL reconstruction with patellar tendon graft is currently the most common surgical treatment for this injury (3), but even this treatment does not decrease the risk of joint degeneration (4). Thus, there is much interest in improving the treatment of patients with ACL injuries.
Preliminary studies of tissue-engineered treatments have shown promising results. In particular, the use of collagen scaffolds loaded with platelet-rich-plasma (PRP) has been shown to improve the strength of both the repaired ACL and grafts used for ACL reconstruction in animal models (5–10). Platelets are known to stimulate angiogenesis and wound repair (11), and have been studied and utilized in a variety of surgical fields (12). It is also known that ACL fibroblasts can attach, migrate, and colonize collagen scaffolds (13). However, although pepsin-digested collagen serves as a good scaffold for ACL healing, it does not mimic the precise collagenous organization and structure of the ACL.
With an appropriate decellularization procedure, native tissues can become a useful biomaterial particularly as these tissues have the structural composition of the targeted tissue. Decellularization of meniscus (14), heart valves (15), skin (16), blood vessels (17), bladder (18) and nerves (19) have been studied and shown promising results. Previously, we have developed an effective protocol for ACL decellularization, which greatly reduced DNA content with minimal effects on collagen and total protein content (20). Furthermore, the decellularized ACL could be successfully reseeded with human ACL fibroblasts (20). Decellularized ACLs from animals would be readily available, would not carry diseases such as HIV, and would be very similar in structure to human ACLs.
However, one key concern when using a xenograft is its immunogenicity. A thorough decellularization protocol should remove immunogenic donor cells from the tissue, but a small number of remaining cells or non-cellular components of the tissue may induce immunogenic reactions. One such cellular component is the alpha-gal (Galalpha1-3Galbeta1-(3)4GlcNAc-R) epitope. The alpha-gal epitope is a carbohydrate structure that is absent in humans but present in non-primate mammals including pigs and cows (21). Interaction between human anti-alpha-gal antibodies and alpha-gal epitopes is an important obstacle for using xenografts in humans (22). This problem can be avoided by treating tissues with alpha-galactosidase to remove the epitope or using knockout pigs lacking alpha-gal epitopes (23).
In this study, we hypothesized that alpha-gal epitope would be present in a bovine xenograft ACL when treated only with Triton-X for decellularization, but that the immunogenic components of the xenograft would be removed by treatment with alpha-galactosidase. We tested this hypothesis using two techniques: first, to see whether each group of xenografts attracted human peripheral blood mononuclear cells (PBMCs), and second to see if exposure to each group of xenografts would activate human PBMCs.
METHODS
Experimental design
Untreated, decellularized, and alpha-galactosidase treated bovine ACLs (n=8 for each group) were prepared. Then, three experiments were performed using these ACLs: (1) migration of PBMCs toward each group of ACLs, (2) activation of PBMCs exposed to each group of ACLs, and (3) immunohistochemistry for alpha-gal epitopes.
Tissue collection
Eight bovine ACLs were aseptically harvested. One third of the fascicles of each ligament (1 cm by 0.5 cm each) were removed from each ACL, placed in PBS and frozen for later use as “untreated ACL tissue”.
Decellularization with Triton X
The remaining fascicles of the ACLs were decellularized as follows. Specimens were incubated in Triton-X solution (PBS with 0.25% Triton-X and 0.25% NA-deoxycholate) for 24 hours at 37°C. This was followed by a wash with DMEM for 72 hours at 4°C. Finally, the tissue was incubated in PBS containing 100 μg/ml RNase and 150 IU/ml DNase for 24 hours at 37°C, and then washed with PBS containing 0.02% EDTA for 24 hours at 4°C (20).
Alpha-gal epitope digestion
The decellularized ACL fascicles were split into two equal sections. For each ligament, one set of fascicles was frozen for later use as “decellularized ACL tissue”, while the second set of fascicles was treated with alpha-galactosidase as follows. First, the specimens were incubated in phosphate citrate sodium chloride buffer containing 100 U/ml alpha-galactosidase (P0731, New England Biolabs, Ipswich, MA) for 4 hours at 26°C with gentle shaking. Then, each specimen was washed five times with 40 ml of PBS for 30 minutes each time with gentle shaking. Finally, they were washed with 50 ml of saline at 4°C overnight with gentle shaking. The saline was discarded, and specimens were frozen for use as “alpha-galactosidase-treated ACLs”.
PBMC migration test
Using sterile technique, untreated ACLs, decellularized ACLs, and alpha-galactosidase-treated ACLs (n=8 for each group) were ground with mortar and pestle and resuspended in DMEM. The final protein concentrations were adjusted to 100 μg/ml. PBMCs were purified from human whole blood using Histopaque®-1077 (Sigma Chemicals, St. Louis, MO). QCM™ Chemotaxis 96-Well Cell Migration Assay with 3 μm pore size (Millipore, Billerica, MA) was used to measure the migration of PBMCs toward ground ACLs. The assay was conducted according to the manual provided by the manufacturer. Briefly, 2.0 × 105 PBMCs were placed into each well of a 96 well plate and 100 μl of ground ACL was placed in each migration chamber. After 24 hours at 37°C, migrated cells were mixed with lysis buffer/dye solution provided in the kit. The fluorescence of the lysate-dye mixture was read using 480/520 nm filter set.
PBMC Monocyte Activation Test
PBMCs were obtained from human whole blood obtained from volunteers with no history of inflammatory disease (Research Blood Components, Brighton, MA). The blood was collected into bags containing 10% acid-citrate dextrose and the PBMCs isolated on a Ficoll column using Histopaque®-1077 (Sigma Chemicals, St. Louis, MO), according to the protocol outlined in the Histopaque®-1077 manual. Approximately 5 × 107 PBMCs were obtained from each of three donors and resuspended in DMEM. 50 μl of 100 μg/ml ground ACLs (n=8 for each group) in DMEM were added to 1 × 106 PBMCS in 450 μl of DMEM and incubated for 24 hours at 37°C. Levels of IL-6 in the media were measured for all samples using the Human IL-6 Colorimetric ELISA Kit (Pierce Protein Research Products, Rockford, IL) according to the manufacturer’s manual.
Histology
Immunohistochemistry was performed with anti-alpha-gal antibody on untreated ACL fascicles, decellularized ACL fascicles, and alpha-galactosidase-treated ACL fascicles. Each fascicle was fixed in formalin, embedded in paraffin and sectioned at 7 micrometers. Sections were stained with hematoxylin and eosin and immunohistochemistry for the alpha-gal epitope was performed. For the immunohistochemistry, the slides were incubated with monoclonal anti-alpha-gal epitope M86 antibody (Alexis Biochemicals, Plymouth Meeting, PA) at a 1:100 dilution for 2 hours at room temperature. The slides were washed with PBS and incubated with a secondary antibody (horse anti-mouse IgG, Standard Elite ABC kit; Vector, Burlingame, CA), diluted 1:200, and then incubated with an avidin–biotincomplex agent for 30 minutes at room temperature and with 3,30-diaminobenzidine (Sigma Chemicals, St. Louis, MO) for 15 minutes at room temperature. Four sections, each 25 mm2, from each sample were analyzed. Numbers of alpha-gal positive and alpha-gal negative cells in each section were counted.
Statistical Analyses
All results are given as mean SEM. Groups were compared using ANOVA with post-hoc testing with Bonferroni adjustment. An alpha of 5% was considered significant. All calculations were done using intercooled STATA 10 (StataCorp, College Station, TX).
RESULTS
Human PBMC migration
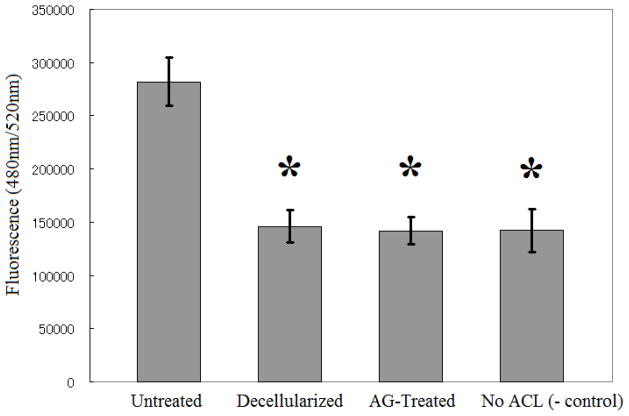
Overall, there was a significant difference in PBMC migration across all groups (Figure 1, p<0.001). Untreated ACLs attracted more PBMCs than decellularized ACLs (p<0.001) and alpha-galactosidase-treated ACLs (p<0.001). There was no statistically significant difference between decellularized ACLs and alpha-galactosidase-treated ACLs (p=0.8347).
Figure 1.
PBMC attraction by untreated and treated ACL tissue. There was no significant difference between the decellularized ACL group and the group undergoing both decellularization and treatment with alpha-galactosidase (AG-treated group). The PBMCs migrated at a higher rate towards the untreated ACL tissue when compared with the decellularized (p<0.001) or AG-treated group (p<0.001). PBMC migration was measured using QCM™ Chemotaxis 96-Well Cell Migration Assay. The results are shown as mean ± SEM (n=8 for each group). *Significantly different from the untreated group (p<0.001).
IL-6 release from human PBMCs
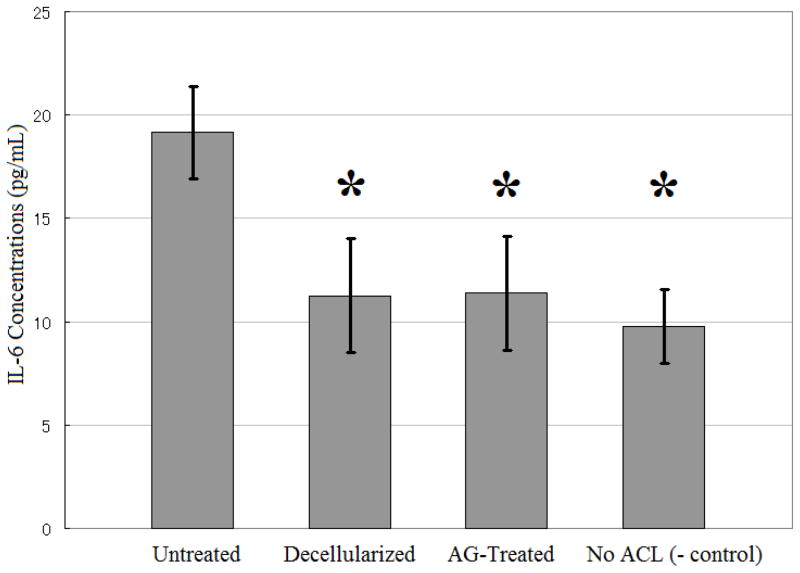
The release of IL-6 from human PBMCs exposed to the ACL fascicles was also significantly different across groups (Figure 2, p<0.001). Media from PBMCs exposed to untreated ACLs contained higher levels of IL-6 than media from PBMCs exposed to either decellularized ACLs (p=0.0434) or alpha-galactosidase-treated ACLs (p=0.0454). There were no statistically significant differences in the IL-6 levels in the media for the decellularized ACLs and alpha-galactosidase-treated groups (p=0.9755).
Figure 2.
IL-6 release by PBMCs. While there was a higher release of IL-6 by PBMCs exposed to untreated ACL tissue, there was no difference in IL-6 release between the decellularized ACL group and the group undergoing both decellularization and treatment with alpha-galactosidase (AG-treated group). The results are shown as mean ± SEM (n=8 for each group). *Significantly different from the untreated group (p<0.05).
Anti-alpha-gal immunohistochemistry
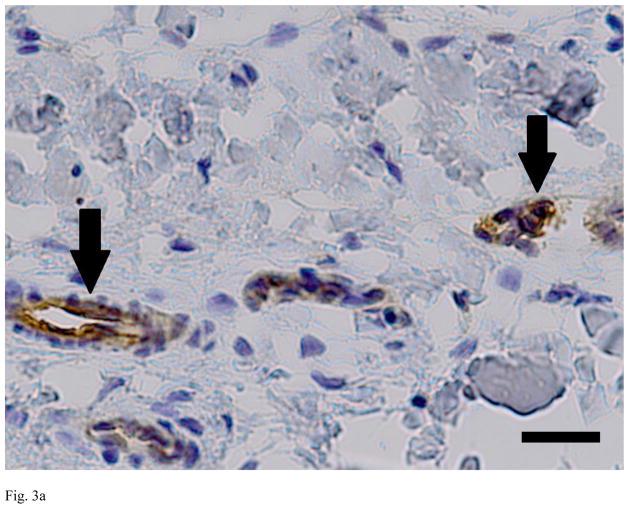
Evaluation of the sections from the untreated, decellularized and alpha-galactosidase-treated ACLs demonstrated clear differences between the groups. The untreated ACLs had alpha-gal epitopes detected throughout the tissue (461 areas of positive stain). In the decellularized and alpha-galactosidase treated tissues, there were a smaller number of positively stained areas (less than 10 in each group), Figure 3).
Figure 3.
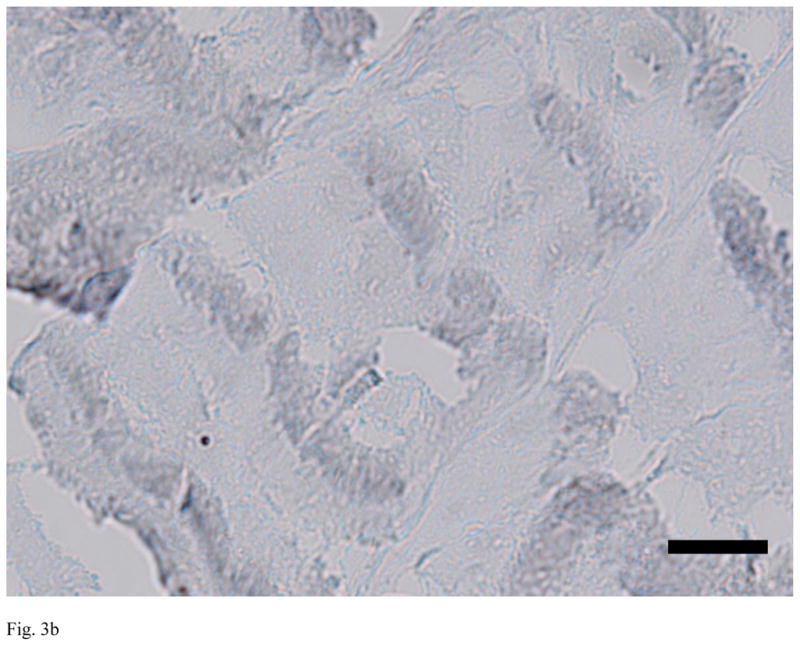
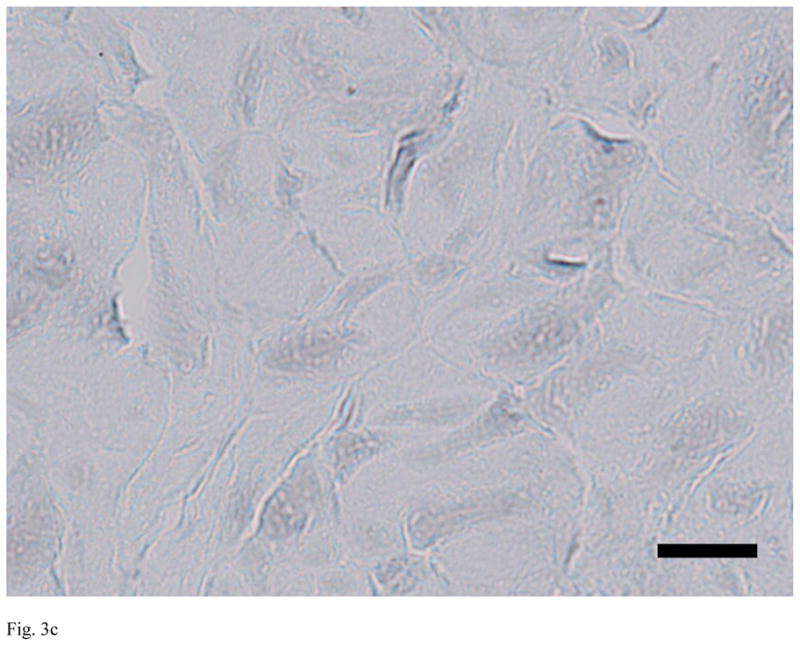
Immunohistochemistry for alpha-gal on representative sections of each group of ACL tissue, where red is a positive stain for alpha-gal. Note the strong positive staining in the untreated bovine ACL tissue (A), and the lack of staining in the decellularized specimen (B) and the alpha-galactosidase-treated specimen (C). Scale bars: 20 μm.
DISCUSSION
ACL rupture is a common injury that is difficult to treat. In addition to the pain and instability it causes, the long-term consequence of joint degeneration, even after surgical treatment, is a serious problem. Use of biomaterials, such as decellularized bovine ACL, may be an effective approach to improve ACL rupture treatment.
In our previous study (20), we had shown that a decellularization protocol using Triton-X is highly effective while causing minimal changes in tissue biochemistry. We had also shown that the decellularized bovine ACL could be successfully reseeded with human ACL fibroblasts. However, a safe and effective graft must not induce significant immunogenic reaction. Our current study showed that decellularized bovine ACLs do not attract PBMCs or activate monocytes. Furthermore, in both tests, no significant difference was seen between alpha-galactosidase -treated and decellularized bovine ACLs, which also suggests that the decellularization process itself may have removed the alpha-gal epitopes. While such decellularization protocols may not effectively remove alpha-gal epitopes from other glycoprotein-rich tissues, our protocol seems to effectively eliminate alpha-gal epitopes from the bovine ACL xenograft tissue.
Both PBMC migration and IL-6 release results suggest that the decellularization of bovine ACL minimized the immunogenic reactions by the human PBMCs. PBMCs are any blood cell with a round nucleus, including lymphocytes, monocytes, and macrophages. These cells play a crucial role in xenograft rejection (24), and therefore the study of these human cells and their lack of response to the decellularized bovine material is reassuring. Second, the monocyte activation test, which measures interleukin-6 release from PBMCs, is a sensitive test used in the pharmaceutical industry to detect pro-inflammatory and pyrogenic components (25). The lack of human monocyte activation by the decellularized ACL tissue suggests that the decellularization process removed such components. Our data suggest that decellularization alone is sufficient to prevent immunogenic reaction by human PBMCs to bovine xenografts.
Antibodies against the alpha-gal epitope are abundantly present in humans as IgG, IgM, and IgA isotypes in humans and primates, so these antibodies can bind to xenografts from non-primate mammals and induce graft rejection (26). Enzymatic removal of the epitope or the use of knockout animal has been used to avoid this problem (23). However, our immunohistochemistry results suggest that the decellularization protocol removes over 98% of the alpha-gal epitopes from the bovine ACL fascicles. A possible explanation may be that the alpha-gal epitopes are present on cells in ACL, but decellularization and multiple washes eliminates those cells and the epitopes. This explanation is consistent with the finding that the epitope is present in high densities as cell surface molecules in the untreated ACL tissue (27).
In conclusion, our findings suggest that our decellularization protocol greatly reduces the immunogenicity of bovine ACL. Future studies to assess the in-vivo efficacy of decellularized ACL xenografts, particularly in comparison with other graft material choices, would be of great interest. Nevertheless, combined with the results of our previous study, which showed that the decellularized ACL tissue can be reseeded with human ACL fibroblasts, our current study suggests that the decellularized bovine ACL xenografts may be a safe and effective biomaterial for the treatment of human ACL injuries.
Acknowledgments
This study was supported by the NIH-NIAMS grant 052772 (MMM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kibler WB. Orthopaedic Knowledge Update 4: Sports Medicine. Rosemont IL: American Academy of Orthopaedic Surgeons; 2009. p. 135. [Google Scholar]
- 2.Neuman P, Englund M, Kostogiannis I, et al. Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am J Sports Med. 2008;36:1717–25. doi: 10.1177/0363546508316770. [DOI] [PubMed] [Google Scholar]
- 3.Miller SL, Gladstone JN. Graft selection in anterior cruciate ligament reconstruction. Orthop Clin North Am. 2002;33:675. doi: 10.1016/s0030-5898(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 4.Liden M, Sernert N, Rostgard-Christensen L, et al. Osteoarthritic changes after anterior cruciate ligament reconstruction using bone-patellar tendon-bone or hamstring tendon autografts: a retrospective, 7-year radiographic and clinical follow-up study. Arthroscopy. 2008;24:899–908. doi: 10.1016/j.arthro.2008.04.066. [DOI] [PubMed] [Google Scholar]
- 5.Murray MM, Spindler KP, Abreu E, et al. Collagen platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 6.Murray MM, Spindler KP, Ballard P, et al. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–17. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 7.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820–30. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 8.Spindler KP, Murray MM, Carey JL, et al. The use of platelets to affect functional healing of an anterior cruciate ligament (ACL) autograft in a caprine ACL reconstruction model. J Orthop Res. 2009;27:631–8. doi: 10.1002/jor.20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fufa D, Shealy B, Jacobson M, et al. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–90. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming BC, Spindler KP, Palmer MP, et al. Collagen-platelet composites improve the biomechanical properties of healing ACL grafts in a porcine model. Am J Sports Med. 2009;37:1554–63. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pietramaggiori G, Scherer SS, Mathews JC, et al. Quiescent platelets stimulate angiogenesis and diabetic wound repair. J Surg Res. 2010;160:169–77. doi: 10.1016/j.jss.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 13.Robayo LM, Mouin VJ, Tremblay P, et al. New ligament healing model based on tissue-engineered collagen scaffolds. Wound Repair Regen. 2011;19:38–48. doi: 10.1111/j.1524-475X.2010.00640.x. [DOI] [PubMed] [Google Scholar]
- 14.Sandmann GH, Eichhorn S, Vogt S, et al. Generation and characterization of a human acellular meniscus scaffold for tissue engineering. J Biomed Mater Res A. 2009;91:567–74. doi: 10.1002/jbm.a.32269. [DOI] [PubMed] [Google Scholar]
- 15.Rieder E, Seebacher G, Kasimir MT, et al. Tissue engineering of heart valves: decellularized porcine and human valve scaffolds differ importantly in residual potential to attract monocytic cells. Circulation. 2005;111:2792–97. doi: 10.1161/CIRCULATIONAHA.104.473629. [DOI] [PubMed] [Google Scholar]
- 16.Chen RN, Ho HO, Tsai YT, et al. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials. 2004;25:2679–86. doi: 10.1016/j.biomaterials.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 17.Courtman DW, Pereira CA, Kashef V, et al. Development of a pericardial acellular matrix biomaterial: biochemical and mechanical effects of cell extraction. J Biomed Mater Res. 1994;28:655–66. doi: 10.1002/jbm.820280602. [DOI] [PubMed] [Google Scholar]
- 18.Farhat WA, Chen J, Haig J, et al. Porcine bladder acellular matrix (ACM): protein expression, mechanical properties. Biomed Mater. 2008;3:025015. doi: 10.1088/1748-6041/3/2/025015. [DOI] [PubMed] [Google Scholar]
- 19.Dumont CE, Hentz VR. Enhancement of axon growth by detergent-extracted nerve grafts. Transplantation. 1997;63:1210–5. doi: 10.1097/00007890-199705150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Vavken P, Joshi S, Murray MM. TRITON-X is most effective among three decellularization agents for ACL tissue engineering. J Orthop Res. 2009;27:1612–8. doi: 10.1002/jor.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–86. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 22.Galili U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: A major obstacle for xenotransplantation in humans. Immunol Today. 1993;14:480. doi: 10.1016/0167-5699(93)90261-i. [DOI] [PubMed] [Google Scholar]
- 23.Stone KR, Abdel-Motal UM, Walgenbach AW, et al. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83:211–9. doi: 10.1097/01.tp.0000250598.29377.13. [DOI] [PubMed] [Google Scholar]
- 24.Friedman T, Shimizu A, Smith RN, et al. Human CD4+ T cells mediate rejection of porcine xenografts. J Immunol. 1999;162:5256–62. [PubMed] [Google Scholar]
- 25.Gaines Das RE, Brügger P, Patel M, et al. Monocyte activation test for pro-inflammatory and pyrogenic contaminants of parenteral drugs: test design and data analysis. J Immunol Methods. 2004;288:165–77. doi: 10.1016/j.jim.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Galili U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol. 2005;83:674–86. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 27.Badylak SF, Gilbert TW. Immune Response to Biologic Scaffold Materials. Semin Immunol. 2008;20:109–16. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]