Abstract
Purpose
Malignant peripheral nerve sheath tumor (MPNST) is a rare sarcoma that lacks effective therapeutic strategies. We gain insight into the most recurrent genetically altered pathways with the purpose of scanning possible therapeutic targets.
Experimental design
We performed a microarray based-comparative genomic hybridization (aCGH) profiling of two cohorts of primary MPNST tissue samples including 25 patients treated at The University of Texas MD Anderson Cancer Center and 26 patients from Tianjin Cancer Hospital. IHC and cell biology detection and validation were performed on human MPNST tissues and cell lines.
Results
Genomic characterization of 51 MPNST tissue samples identified several frequently amplified regions harboring 2,599 genes and regions of deletion including 4,901 genes. At the pathway level, we identified a significant enrichment of copy number–altering events in the insulin-like growth factor 1 receptor (IGF1R) pathway, including frequent amplifications of the IGF1R gene itself. To validate the IGF1R pathway as a potential target in MPNSTs, we first confirmed that high IGF1R protein correlated with worse tumor-free survival in an independent set of samples using immunohistochemistry. Two MPNST cell lines (ST88-14 and STS26T) were used to determine the effect of attenuating IGF1R. Inhibition of IGF1R in ST88-14 cells using small interfering RNAs or an IGF1R inhibitor, MK-0646, led to significant decreases in cell proliferation, invasion, and migration accompanied by attenuation of the PI3K/AKT and MAPK pathways.
Conclusion
These integrated genomic and molecular studies provide evidence that the IGF1R pathway is a potential therapeutic target for patients with MPNST.
Keywords: malignant peripheral nerve sheath tumor, insulin-like growth factor 1 receptor, genomic characterization, targeted therapy, microarray-based comparative genomic hybridization, gene amplification, MK-0646, epidermal growth factor receptor, Gefitinib
Introduction
Malignant peripheral nerve sheath tumors (MPNSTs), a subtype of soft-tissue sarcomas of neural crest origin (1), are highly malignant and account for approximately 5–10% of all soft-tissue sarcomas (2). Currently, the 5-year survival rates of MPNST patients are still only 30–50%, even with multidisciplinary treatments such as aggressive surgery, high-dose adjuvant chemotherapy, and radiotherapy (1). The dismal outcome not only points to the urgent need to establish better therapeutic strategies for patients harboring MPNSTs but also highlights the importance of exploring the genomic basis of the disease to identify recurrent oncogenic events for targeted therapy.
A number of large cancer genome characterization efforts have already proven the value of the genomic approach by identifying several new therapeutic targets and giving insights into general cancer biology(3). However, such large projects concentrate on common cancers that have a high incidence and prevalence. For rare types of cancers, collecting large enough amounts of samples is a major challenge even to multinational consortia. Therefore, there remains a pressing need to characterize the genomes of rare cancers such as MPNST, albeit at a relatively smaller scale. Microarray-based comparative genomic hybridization (aCGH) is a well-established method for detecting chromosomal gains and losses of DNA segments. Recent advances in this technology have enabled genome-wide characterization of the common genetic alterations in many different cancers (4). In MPNSTs, some genetic aberrations have already been associated with prognosis, while other oncogenic events have been implicated in the pathogenesis and development of the disease (5–7).
Candidate regions with potentially relevant proto-oncogenes include chromosomal bands 17q24-q25, 7p11-p13, 5p15, 8q22-q24, and 12q21-q24 (6, 7). Regions with putative tumor-suppressor genes have been identified in 9p21-p24, 13q14-q22, and 1p (7). Frequent gains affect several putative target genes such as BIRC5, CCNE2, DAB2, DDX15, EGFR, DAB2, MSH2, CDK6, HGF, ITGB4, KCNK12, LAMA3, LOXL2, MET, and PDGFRA. Genes that have been suggested as targets of common deletions include CDH1, GLTSCR2, EGR1, CTSB, GATA3, SULT2A1, GLTSCR2, HMMR/RHAMM, LICAM2, MMP13, p16/INK4a, RASSF2, NM-23H1, and TP53 (5–9). Recent reports show that alterations of TOP2A, CDK4, and FOXM1 not only are associated with survival but also are potential therapeutic targets (4, 5, 9).
Even though there are studies characterizing genetic abnormalities in MPNSTs, many reports have based their observations on small patient cohorts, given the rarity of the disease. In this study, we present a comprehensive characterization of a large cohort of 51 primary tumors using aCGH technology. The depth of our material results in a map of the MPNST genome. Furthermore, we applied pathway-level analyses that resulted in a unique view into the aberrant signaling networks in MPNST, which we then proceeded to validate with IHC and molecular approaches in tissue culture in two cell lines. These integrated genomic and molecular studies provided evidence that IGF1R is a promising therapeutic target in MPNST patients.
Materials and Methods
Ethics Statement
All of the tissue and information collection took place at Tianjin Medical University Cancer Hospital and MD Anderson Cancer Center with Institutional Review Board (IRB) approved protocols and the patients’ consent.
Primary tumors
Archived MPNST samples and matching patient records were acquired from The University of Texas MD Anderson Cancer Center (25 formalin-fixed, paraffin-embedded [FFPE] tumor samples) and Tianjin Cancer Hospital of China (26 fresh-frozen tumor samples) (Supplemental table 1). All samples had at least 90% tumor content. In addition, we acquired 56 FFPE tumor samples for immunohistochemical validations (Supplemental table 2). All samples were obtained with the approval of the institutional review boards of the two institutions. Patient records included age, sex, tumor location, tumor size (largest diameter of the tumor), American Joint Committee on Cancer (AJCC) stage of the tumor, time to recurrence, metastatic status, treatments administered, and follow-up outcomes. The presence or absence of NF1 syndrome was determined on the basis of established NIH criteria (10). MPNST patients received chemotherapy after the primary tumor excision using a regimen of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID). When indicated, 30–60 Gy of radiotherapy was administered to the surgical region and/or metastatic lesions. The range of surgical operations included wide and subtotal (including subtotal wide, marginal, and intralesional) resections.
Array CGH hybridization
Genome-wide copy number measurements were made for 51 primary tumor samples. Commercially available normal genomic DNAs were used as control (Clontech Laboratories, Inc., Mountain View, CA). All the surgical samples were collected before radiation treatment. Genomic DNA was isolated according to standard procedures. Labeled genomic DNA was hybridized using an Agilent Human Genome CGH Microarray kit (4×44k; Agilent Technologies, Palo Alto, CA). These arrays represent over 43,000 coding and non-coding human sequences yielding an average of 35-kbp oligonucleotide probe spatial resolution. At least one target sequence was analyzed for every well-characterized gene, and at least two target sequences were analyzed for every known cancer gene. The probes were designed based on the University of California Santa Cruz hg17 human genome (National Center for Biotechnology build 35, May 2004).
The processing of the aCGH data and the frequency analyses were performed as described previously (11). Briefly, the ratios of intensity values from tumor and normal tissues were transformed to log2-space. Log ratio data were then subjected to a circular binary segmentation (CBS) algorithm to reduce the effect of noise. After that, the CGHcall algorithm was used to give each segment an aberration label: normal, deletion, or amplification. An aberration frequency for each probe was established by combining the labels from individual samples.
IHC methods
Fifty-six FFPE tissues from Tianjin Cancer Hospital were cut into 4-μm sections and mounted on charged glass slides (ProbeOn Plus; Fisher Scientific, Pittsburgh, PA) for IHC analyses according to published methods (12–14). IGFIR antibody was used in 1:75 dilutions (Santa Cruz Biotechnology, Santa Cruz, CA). The same concentrations of nonimmune rabbit or goat serum were used as negative controls. The expression levels of IGF1R (Santa Cruz Biotechnology) were estimated according to the criteria previously reported (12–14). Scoring was performed according to the percentage of positive cells: <5% was classified as negative (−), 6–30% was classified as a weak positive (+), 31–60% as a moderate positive (++), and >60% as a strong positive (+++).
Cell culture and compounds
The MPNST cell lines ST88-14 and STS26T were maintained in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin solution. Cells were incubated at 37°C in a humidified atmosphere of 7.5% CO2. Authentication of these two MPNST cell lines was conducted utilizing short tandem repeat (STR) DNA fingerprinting. The ST-8814 line is NF1−/−, whereas STS26T is NF1+/+. IGF1R monoclonal antibody MK-0646 (obtained from Merck, Whitehouse Station, NJ) was dissolved in sterile water at a concentration of 20 mg/mL and stored at −20°C. Gefitinib was stored as a 20 mM stock solution in dimethyl sulfoxide (DMSO).
SiRNA and plasmid transfections
For the siRNA studies, a smart pool of three double-stranded siRNAs against IGF-1R (IGF1R-NM-000875) was obtained from Dharmacon Tech (Lafayette, CO) and used according to the manufacturer’s instructions and this siRNA smart pool has been proven specific and effective in previous reports (12–16). Because some reports have reported crosstalk between the IGF1R and EGFR signal pathways (17–19), a previously proven specific and effective EGFR siRNA (sc-29301, Santa Cruz Biotechnology) was also used both individually and combined with siRNA for IGF-1R as described previously (12–16). Nonspecific siRNA (D-001206-01-05) were obtained from Dharmacon Tech (Lafayette, CO) was used as a control in all experiments (16). To generate IGF1R expression vectors, the IGF1R cDNA insert was digested by EcoR1 and then ligated to the pCDNA3.1(+). Positive clones were verified by sequencing. The transfection of plasmid DNA were performed as described previously (13, 14, 20, 21).
Western blot analysis and cell proliferation, invasion, and migration assays
Western blot analysis was performed according to standard procedures as described previously (22). The antibodies for EGFR, AKT, PI3K, IRS-1, ERK, and their phosphorylated forms were purchased from Abcam, Santa Cruz, Cell Signaling, and Sigma and were used according to the manufacturers’ instructions. Cell proliferation was analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, and cell invasion and migration were analyzed by Transwell migration assays (EMD Biosciences, San Diego, CA) as previously described (22).
Statistical analyses
Clinical and pathologic characteristics of the 26 Chinese and 25 American MPNST patients were compared using Chi-Square test (Supplemental table 1). The associations between clinicopathological and molecular characteristics and the survival were analyzed with Cox multiple regression models (Supplemental table 2). The associations between CNAs and survival were computed with Mantel-Cox test of difference of Kaplan-Meier survival estimators (Supplemental data 2). Associations between CNAs and other clinical variables (Supplemental data 2) were computed using the Fisher’s exact test. This test was also used in comparing the differences (in gene level) between aberration profiles categorized by different clinical variables. Pathway enrichment analysis, using a standard hypergeometric test, was performed on the genes that were either amplified or deleted in at least 25% of the samples. Enrichment p-values were computed for all signaling pathways available in Biocarta (http://www.biocarta.com/). A P-value of 0.05 was considered the threshold of statistical significance in all tests.
Results
MPNSTs exhibit recurrent genetic aberrations that significantly alter multiple signaling pathways
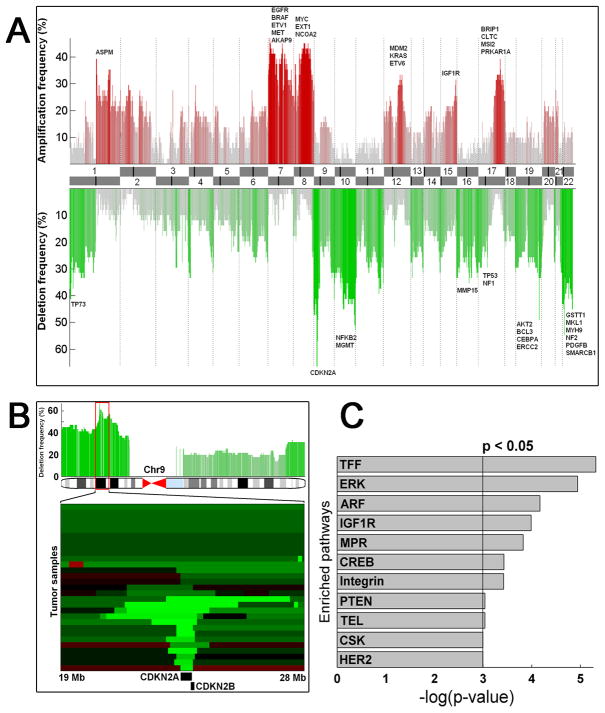
Integration of copy number profiles of the individual samples resulted in the discovery of several major regions of frequent deletions and amplifications in the 51 primary MPNST tissue samples (Fig. 1A). With approximately 65% of patients affected, we identified focal deletion of 9p21.3 (harboring tumor suppressors CDKN2A and CDKN2B) as the most recurrent genomic event in our data, consistent with a previous study (9) (Fig. 1B). Highly recurrent amplifications in 7p harboring EGFR, BRAF, ETV1, MET, AKAP9, 8q harboring MYC, EXT1, NCOA2, and 17q harboring BRIP1, CLTC, MSI2, PRKAR1A were also prominent (30–40% frequency), as observed previously (Fig. 1A) (6–8). More novel chromosomal abnormalities included deletions of 1p, containing TP73 and MIIP, 10q26 containing MGMT, 16p containing MMP15, chromosome 19 with several cancer-related genes including AKT2, BCL3, CEBPA, and ERCC2, and 22q containing GSTT1, MKL1, MYH9, NF2, PDGFB, SMARCB1. Previously unreported amplifications were identified in chromosomes 1q (with ASPM), 12q (with MDM2, KRAS, ETV6), and 15q (with IGF1R) (Fig. 1A). In total, frequently amplified and deleted regions (aberrated in at least 25% of the samples) harbored 2,599 and 4,901 genes, respectively (Supplementary data 1).
Figure 1. Global characterization of the MPNST genome.
A, Recurrent gene copy alterations in the MPNST genome. Frequent gains (red) and deletions (green) are plotted with chromosome ideograms at the center. Genes of interest are indicated for the most frequently aberrant regions. B, The most frequently deleted (over 65% recurrence) locus harbors two tumor suppressors in 9p21.3: cyclin-dependent kinase inhibitors 2A and 2B (CDKN2A and CDKN2B). A minimal common region of ~30 kb is targeted by chromosomal losses spanning the entire 9p (dark green) and focal high-amplitude deletions (light green). C, The most significantly altered signaling pathways in MPNST.
Subsequently, we investigated the translational relevance of these genes by correlating the loci with several clinical parameters such as AJCC, tumor size, local recurrence, and metastasis (Supplemental data 2). For example, the amplification of MYC was significantly associated with tumor recurrence, and the deletion of AKT1 was associated with the presence of tumor metastases. Interestingly, we could not associate any individual aberration with patient survival, suggesting that multiple events might co-occur to affect survival. However, correlating the overall frequency of CNAs with survival did not implicate increased genomic instability in inducing statistically significant survival effects. To investigate the genomic relevance of the clinical variables, we compared the effect of several parameters to the global aberration profiles. First, the samples were divided based on patient ethnicity. The most significant difference in the aberration profiles was the decreased overall aberration rate in the Chinese patients, yet the overall pattern of aberrations remained fairly similar. Second, comparisons of aberration profiles with respect to other clinical parameters such as tumors extracted from the trunk vs. extremity resulted in a low number of loci that were aberrated with a significantly different rate.
To investigate the alterations at the signaling pathway level, we computed pathway enrichment scores in pathways described in Biocarta. This analysis resulted in 11 statistically significantly altered pathways (Fig. 1C). The most enriched pathway, TFF, is a mucosal healing pathway that contains parts of the ERK (the second most significant) and the EGFR pathways, both of which have been linked previously to MPNST (23). The third pathway, ARF, is the tumor-suppressor pathway in which CDKN2A (p16) plays a central role; this has been reported to be involved in the pathogenesis of MPNSTs (9, 24, 25). The fourth most significantly altered signaling pathway, the insulin-like growth factor 1 receptor (IGF1R) signaling pathway, a major cell survival pathway, has not been previously reported in MPNST.
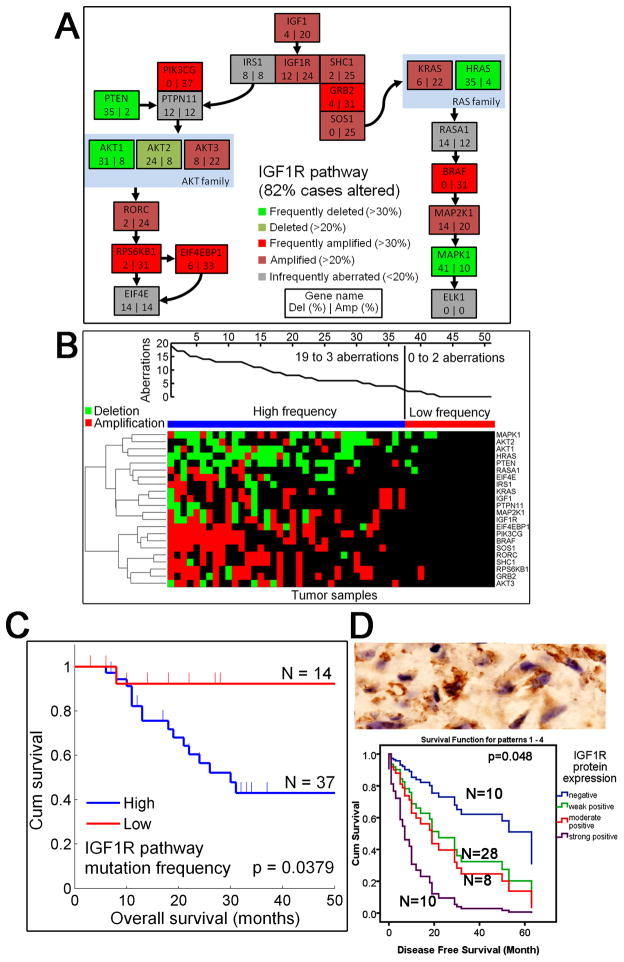
Extensive IGF1R pathway alterations and increased IGF1R protein expression correlate with patient survival
IGF1R amplification, which was amplified in 24% of our samples, is an attractive therapeutic target that has not been reported in MPNSTs (14, 26). Therefore, we investigated in greater depth the frequency and pattern of gene alterations in IGF1R signaling pathway(Fig 2, A and B). In addition to IGF1R amplifications, at least one gene in the IGF1R pathway was altered in 82% of the cases making the pathway highly significant. Frequent deletions included MAPK1 (41%), H-RAS (35%), and PTEN (35%). Notably, the PTEN signaling pathway was also significantly altered in MPNSTs (Fig 2C). The most commonly amplified genes in the IGF1R pathway were BRAF (31%), GRB2 (31%), PIK3CG (37%), RPS6KB1 (31%), and EIF4EBP1 (33%).
Figure 2. Genetic aberrations in the IGF1R pathway and IGF1R protein expression.
A, Alteration frequencies (deletion rate (%) on the left side of the box, and amplification rate (%) on the right) for the IGF1R pathway are shown for each gene individually. Green genes are significantly often deleted (bright green >30% of the cases; dark green >20%), and red genes are amplified (bright red >30% of the cases; dark red >20%). Copy numbers of the gray genes are infrequently altered (in less than 20% of the cases). At least one gene in this pathway was altered in 82% of cases. B, IGF1R pathway alterations are shown for each sample. The upper plot shows the number of aberrations. The samples in the heatmap are sorted based on the aberration frequency of the pathway genes. Both amplifications (red) and deletions (green) are shown. Samples have been divided into two groups (blue and red group) based on the frequency of mutations in the IGF1R pathway. C, Patients with high frequency of IGF1R pathway aberrations (blue group) have a significantly worse overall survival than patients with only few aberrations (red group) (P = 0.0379). D, Chinese MPNST patients with higher IGF1R protein expression have increasingly worse disease-free survival. Upper panel: As shown by immunohistochemical analysis (Envision+, ×400), IGF1R protein is frequently expressed. Lower panel: Survival estimators for patients with increasing levels of IGF1R protein expression are color-coded in the Kaplan-Meier plot with case number shown. The patients with higher IGF1R protein expression have increasingly worse disease-free survival.
Because IGF1R copy number status itself was not correlated with survival, we sought to determine whether there was a survival effect at the pathway level. We divided the samples into two groups based on the extent of IGF1R pathway alterations. One group was characterized by more than 10% of pathway genes altered, and the other group with less than 10% of altered genes (Fig 2C). Interestingly, we found that the patients in the group with less alterations had a significantly better prognosis than the patients in the other group (P = 0.0379) (Fig 2C). The alteration frequency in the IGF1R pathway correlated with the overall frequency of CNAs, but overall CNAs did not confer a significant survival effect. The two groups were also independent of the clinical parameter with prognostic significance identified in our analysis, the tumor size (Supplemental table 2).
To further study the clinical importance of IGF1R, we analyzed the extent of IGF1R protein expression in an independent set of 56 FFPE MPNST tissue samples with an immunohistochemical assay (Supplemental table 2). The protein expression of IGF1R exhibited various patterns, from negative and weak positives to moderate and strong positives, with a total positive rate of 82.1% (46/56) (Fig. 2D, upper panel). Clinically, patients with an increased IGF1R protein expression had significantly worse tumor-free survival rates and a higher risk of tumor progression (Fig. 2D, lower panel).
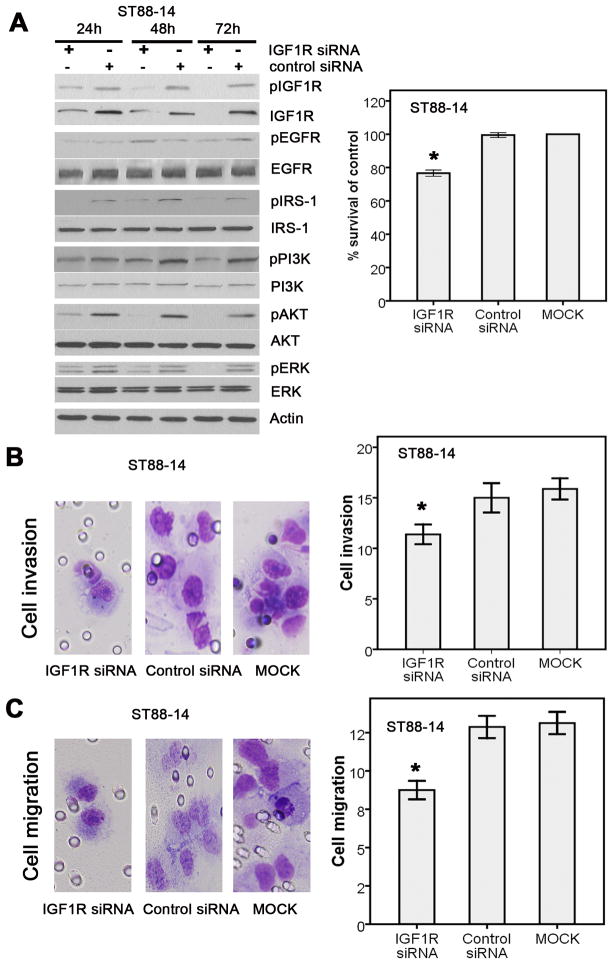
IGF1R activation contributes to MPNST cell proliferation, migration, and invasion by the activation of PI3K and AKT pathway signaling
Several lines of evidence indicate that IGF1R may potentially be a very interesting clinical target in MPNST: the IGF1R gene is frequently amplified, the IGF1R protein expression correlates with survival, there are significant alterations in the signaling pathway that also correlate with survival, and there are successful IGF1R inhibitors already available to treat other cancers (13, 26–31). To determine whether IGF1R is a potential therapeutic target in MPNST, we evaluated the effect of its inhibition using two in vitro cell culture systems, the ST88-14 and STS26T MPNST cell lines. Western blotting indicated that IGF1R was readily detectable in the ST88-14 cell line, but the STS26T cells showed no detectable IGF1R expression. In ST88-14 cells, the decrease in IGF1R expression caused by IGF1R siRNA significantly reduced expression of pIGF1R and other AKT/PI3K signaling pathway activators (Fig. 3A, left panel). Accordingly, IGF1R siRNA effectively blocked tumor cell proliferation (Fig. 3A, right panel), invasion (Fig. 3B), and migration (Fig. 3C). We next evaluated the effect of anti-IGF1R (MK-0646) agents that are being used clinically. In ST88-14 cells, treatment with MK-0646 led to a decrease in the activated form of IGF1R as well as a decrease in cell proliferation relative to control (Fig. 4, A and B). These results suggested that IGF1R is potential therapeutic target in MPNST.
Figure 3. IGF1R downregulated by IGF1R siRNAs significantly decreased tumor cell proliferation, invasion, and migration by blocking PI3K/AKT and MAPK pathways in ST88-14 cells.
A, A pool of three IGF1R siRNAs decreased IGF1R expression, IGF1R activation, and tumor cell proliferation compared with the nonspecific control siRNA. Left panel: Inactivatation of PI3K/AKT and MAPK pathway factors with IGF1R inhibition. Right panel: the decrease of tumor cell proliferation. B, Apool of three IGF1R siRNAs significantly decreased tumor cell invasion compared with the nonspecific control siRNA. left panel, Cell invasion. Right panel, Cell count. C, Apool of three IGF1R siRNA significantly decreased tumor cell migration compared with the nonspecific control siRNA. Left panel, Cell migration. Right panel, Cell count.
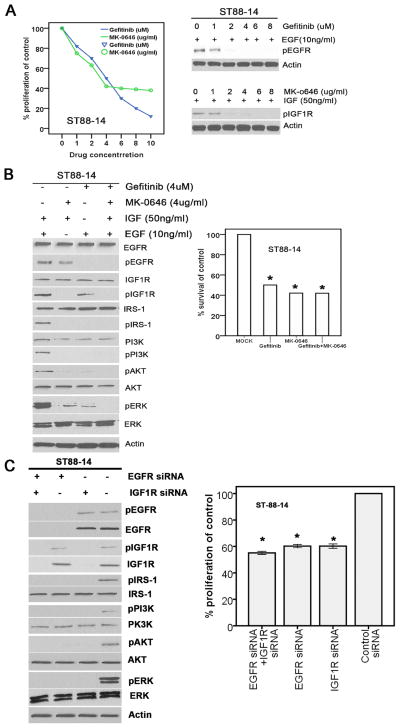
Figure 4. Attenuated IGF1R and/or EGFR significantly inhibited cell proliferation in MPNST ST88-14 cells by blocking the PI3K/AKT and MAPK pathways.
A, Gefitinib and MK-0646 significantly decreased the activation of EGFR and IGF1R. Left panel: IC50 of MK-0646 and gefitinib in MPNST ST88-14 cells. Right panel: the inactivation of EGFR and IGF1R. B, Gefitinib and/or MK-0646 blocked activation of the PI3K/AKT and MAPK pathways and the MPNST ST88-14 tumor cell proliferation. Left panel: the inactivation of the PI3K/AKT and MAPK pathways. Right panel: the inhabitation of the MPNST ST88-14 tumor cell proliferation. C, The IGF1R and/or EGFR siRNAs inhibited the activation of PI3K/AKT and MAPK pathways and tumor cell proliferation in ST88-14 MPNST cells. Left panel: the inhabitation of the activation of PI3K/AKT and MAPK pathways. Right panel: the inhabitation of ST88-14 tumor cell proliferation.
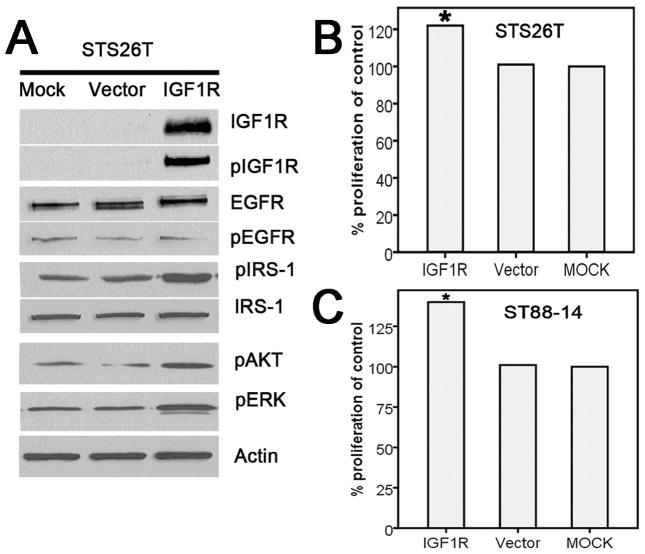
The lack of IGF1R expression in STS26T cells provided us with an opportunity to evaluate whether IGF1R expression exerted a stimulating effect on MPNST cell proliferation. We transfected the cells with an IGF1R expression vector and found a marked increase in the levels of phosphorylated IGF1R (pIGF1R), pAKT, phosphorylated IRS-1 (pIRS-1), and pERK in these cells. The cell proliferation assay showed an increased rate of cell growth after the addition of the IGF1R expression vector (Fig. 5, A and B). Similarly, the effect of augmented IGF1R expression levels was also observed in transfected ST88-14 cells, which had detectable levels of endogenous IGF1R expression (Fig. 5C). Combining all these data, we suggest IGF1R is targetable because its activation contributes to MPNST cell proliferation, migration, and invasion by the activation of PI3K and AKT pathway signaling.
Figure 5. Increases in IGF1R expression caused by the IGF1R vector induced increases in MPNST tumor cell proliferation, invasion, and migration through activation of the PI3K/AKT and MAKP pathways.
A, Increase in the expression of IGF1R resulted in the activation of the PI3K/AKT and MAPK pathways. B, Increase in IGF1R expression induced tumor cell proliferation in the STS26T cell line. C, Increase in IGF1R expression induced tumor cell proliferation in the ST88-14 cell line.
Targeting IGF1R and EGFR in combination does not result in additive anti-MPNST effects compared with using each agent alone
Because cross-talk between the IGF1R and EGFR signaling pathways has been detected in other types of cancers (12, 17–19, 32), we wanted to evaluate the possibility of synergistic or antagonistic effects resulting from simultaneously blocking both IGF1R and EGFR in MPNSTs. Because ST88-14 cells expressed both IGF1R and EGFR, we investigated the effect of inhibiting these two signaling molecules individually and in combination. Similar to the effect of IGF1R blocking, the decreased EGFR expression caused by EGFR siRNA had an inhibitory effect on AKT/PI3K signaling activators as well as on cell proliferation (Fig. 4C). Notably, attenuation of IGF1R and EGFR by combined siRNAs in ST88-14 cells significantly decreased cell proliferation without noticeable addictive or combinational synergistic effects (Fig. 4C). By using EGFR and IGF1R inhibitors, we noticed that treatment with gefitinib in ST88-14 cells led to a decrease in the activated forms of EGFR as well as a decrease in cell proliferation relative to control without the activation of IGF1R signal pathways (Fig. 4, A and B). Most importantly, combined treatment with both inhibitors did not result in a stronger inhibition of cell proliferation even though the combined treatment led to a larger decrease in pAKT and pERK at the molecular level (Fig 4B).
Discussion
MPNST poses significant clinical challenges because it is a highly malignant tumor characterized by a high rate of local recurrence and a strong tendency to metastasize (33). The dismal prognosis highlights the importance of identifying new clinicopathologic and molecular factors that affect MPNST outcome and the urgent need to establish better therapeutic strategies for patients with MPNST. In the current study, we performed genomic and molecular studies of MPNST samples and found evidence that IGF1R protein overexpression is an important molecular marker for tumor-free survival in MPNST patients and that IGF1R is a promising therapeutic target in this disease.
A major contribution of this study is the extensive characterization of IGF1R as a potential therapeutic target for MPNST patients by genomic, IHC, and cellular biologic approaches. Several lines of evidence implicate IGF1R as a potential therapeutic target in MPNST: the IGF1R gene is frequently amplified; the IGF1R protein expression correlates with survival; and there are significant alterations in the signaling pathway that also correlate with survival. IGF1R inhibitors have already been successfully used to treat some types of cancers (13, 26–31). IGF1R is a multifunctional tyrosine kinase receptor involved in several biologic processes, including cell proliferation, differentiation, DNA repair, and cell survival (14, 31, 34, 35). Aberrant activation of the IGF1/IGF1R axis has been associated with a worse prognosis in many tumors, including breast, gastric, and prostate cancers (36, 37). Furthermore, in pancreatic cancer and anaplastic thyroid carcinomas, IGF1R inhibitors were shown to also reduce vascularization and VEGF expression (38, 39). Therefore, IGF1R is a logical potential molecular target in several types of cancer including breast, cervical, non-small cell lung, and prostate cancers (23, 32, 40–48). However, IGF1R-targeted therapies for sarcomas lag behind those for other cancers at present. Inhibition of IGF1R activity by its tyrosine kinase inhibitor NVP-AEW541 or its siRNA led to cytotoxicity and apoptosis in GIST cell lines by blocking the AKT and mitogen-activated protein kinase (MAPK) pathway signaling. Furthermore, the combination of NVP-AEW541 and imatinib in GIST cell lines induced a strong cytotoxic response (13, 14). In MPNST, however, information about IGF1R expression, its prognostic significance, and the cytotoxic potential of IGF1R inhibition is still lacking. In the current study, the aCGH profile characterized the significant genetic amplifications of IGF1R signaling pathway genes including IGF1R itself. The deregulation of expression of IGF1R is an independent prognostic factor for this type of sarcoma. In the cell line studies, IGF1R siRNA and monoclonal antibody MK-0646 inhibited MPNST proliferation, invasion, and migration by blocking the AKT and PI3K pathways.
The introduction of anti-IGF1R antibodies in clinical trials and the dramatic single-agent anti-IGF1R activity observed in sarcoma patients provided the initial excitement in the sarcoma community (49, 50). However, the benefit of this therapeutic approach does not extend to all patients, with Phase II studies demonstrating less promising responses than initially anticipated (50). A major mechanism of resistance to highly specific inhibitors of IGF-1R, either antibodies or tyrosine kinase inhibitors may involve enhanced insulin receptor (IR)-A homodimer formation and IGF-2 production (50). Furthermore, the sensitivity to IGF1R targeted therapy might be sarcoma-type dependent because our preclinical study shows IGF1R targeted therapy might be effective in treating MPNST patients. One possible explanation is different compensatory responses in different sarcoma types in response to IGF1R inhibition. For example, different from the results in other tumors (17–19), in our study the inhibition of IGF1R did not result in the activation of EGFR pathways in MPNST and the combined inhibition of IGF1R and EGFR did not show additive antitumor effects at the cellular level, suggesting lack of cross-talk between IGF1R and EGFR pathways in MPNSTs. These indicate that targeting IGF1R in MPNST might be more efficient than in other cancer types. Thus, despite the disappointing Phase II data in some sarcoma types, this novel class of drugs may constitute an active treatment in a proportion of sarcoma patients, especially MPNSTs.
Our aCGH profile with a large MPNST cohort revealed several important genetic aberrations with clinical relevance. We found that many genetic aberration events had significant correlation with clinical parameters of MPNST patients, including AJCC staging, tumor size, local recurrence, and metastasis. Extensive investigations of these genetic events and these correlations would shed light on MPNST pathogenesis. Furthermore, the pathway analyses revealed several signaling pathway genes harboring frequent genetic aberrations such as the TFF, ERK, ARF and other signaling pathways. This is the first time that the genetic aberrations of important signaling pathways have been investigated with the purpose of scanning possible therapeutic targets in MPNST. Extensive investigations of these pathways might give added confidence to move translational research results to the clinic to benefit patients with MPNST.
Supplementary Material
Translational Relevance.
A major contribution of our study is the characterization of IGF1R as a potential therapeutic target for MPNST patients by genomic, IHC, and cell biology approaches. We present a comprehensive characterization of a large cohort of 51 primary tumors using aCGH technology resulting in a map of the MPNST genome. Furthermore, we apply pathway-level analyses that provide a unique view into the aberrant signaling networks in MPNST, which we then proceed to validate with IHC and cell biology approaches in MPNST cell lines. These integrated genomic and molecular studies provide evidence that IGF1R is a promising therapeutic target in MPNST patients. This is the first time that the genetic aberrations of important signaling pathways have been investigated with the purpose of scanning possible therapeutic targets in MPNST. Extensive investigations of these pathways might give added confidence to move translational research results to the clinics to benefit patients with MPNST.
Acknowledgments
This work was supported by the Hope Fund of the National Foundation for Cancer Research (W. Zhang), a grant from the Liddy Shriver Sarcoma Initiatives (W. Zhang and J Yang), program for Changjiang Scholars and Innovative Research Team in University(PCSIRT) in China (IRT1076) and National Key Scientific and Technological Project (2011ZX09307-001-04) (K. Chen), the Academy of Finland project no. 132877 (A. Ylipää and M. Nykter), and the Finnish Funding Agency for Technology and Innovation Finland Distinguished Professor Program (A. Ylipää and M. Nykter). The genomic studies were supported in part by the Cancer Genomics Core Laboratory and by the National Institutes of Health through The University of Texas MD Anderson’s Cancer Center Support Grant CA016672. J. Yang was jointly supported by postdoctoral fellowships from Tianjin Cancer Institute and Hospital and The University of Texas MD Anderson Cancer Center. J. Yang was a recipient of the Connie & Jim Walter Fellowship in Sarcoma Research and the US China Anti-Cancer Association Fellowship.
We would like to thank Limei Hu and David Cogdell for performing the aCGH experiments. We thank Maude Veech and Michael Worley at the Department of Scientific Publications of MD Anderson Cancer Center for editing this manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lin CT, Huang TW, Nieh S, Lee SC. Treatment of a malignant peripheral nerve sheath tumor. Onkologie. 2009;32:503–5. doi: 10.1159/000226591. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs B, Spinner RJ, Rock MG. Malignant peripheral nerve sheath tumors: an update. J Surg Orthop Adv. 2005;14:168–74. [PubMed] [Google Scholar]
- 3.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu J, Deshmukh H, Payton JE, Dunham C, Scheithauer BW, Tihan T, et al. Array-Based Comparative Genomic Hybridization Identifies CDK4 and FOXM1 Alterations as Independent Predictors of Survival in Malignant Peripheral Nerve Sheath Tumor. Clin Cancer Res. 17:1924–34. doi: 10.1158/1078-0432.CCR-10-1551. [DOI] [PubMed] [Google Scholar]
- 5.Kresse SH, Skarn M, Ohnstad HO, Namlos HM, Bjerkehagen B, Myklebost O, et al. DNA copy number changes in high-grade malignant peripheral nerve sheath tumors by array CGH. Mol Cancer. 2008;7:48. doi: 10.1186/1476-4598-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantripragada KK, Spurlock G, Kluwe L, Chuzhanova N, Ferner RE, Frayling IM, et al. High-resolution DNA copy number profiling of malignant peripheral nerve sheath tumors using targeted microarray-based comparative genomic hybridization. Clin Cancer Res. 2008;14:1015–24. doi: 10.1158/1078-0432.CCR-07-1305. [DOI] [PubMed] [Google Scholar]
- 7.Mechtersheimer G, Otano-Joos M, Ohl S, Benner A, Lehnert T, Willeke F, et al. Analysis of chromosomal imbalances in sporadic and NF1-associated peripheral nerve sheath tumors by comparative genomic hybridization. Genes Chromosomes Cancer. 1999;25:362–9. [PubMed] [Google Scholar]
- 8.Schmidt H, Wurl P, Taubert H, Meye A, Bache M, Holzhausen HJ, et al. Genomic imbalances of 7p and 17q in malignant peripheral nerve sheath tumors are clinically relevant. Genes Chromosomes Cancer. 1999;25:205–11. [PubMed] [Google Scholar]
- 9.Endo M, Kobayashi C, Setsu N, Takahashi Y, Kohashi K, Yamamoto H, et al. Prognostic significance of p14ARF, p15INK4b and p16INK4a inactivation in malignant peripheral nerve sheath tumors. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-2393. [DOI] [PubMed] [Google Scholar]
- 10.Anghileri M, Miceli R, Fiore M, Mariani L, Ferrari A, Mussi C, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107:1065–74. doi: 10.1002/cncr.22098. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Cogdell D, Yang D, Hu L, Li H, Zheng H, et al. Deletion of the WWOX gene and frequent loss of its protein expression in human osteosarcoma. Cancer Lett. 291:31–8. doi: 10.1016/j.canlet.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Kaulfuss S, Burfeind P, Gaedcke J, Scharf JG. Dual silencing of insulin-like growth factor-I receptor and epidermal growth factor receptor in colorectal cancer cells is associated with decreased proliferation and enhanced apoptosis. Mol Cancer Ther. 2009;8:821–33. doi: 10.1158/1535-7163.MCT-09-0058. [DOI] [PubMed] [Google Scholar]
- 13.Pantaleo MA, Astolfi A, Di Battista M, Heinrich MC, Paterini P, Scotlandi K, et al. Insulin-like growth factor 1 receptor expression in wild-type GISTs: a potential novel therapeutic target. Int J Cancer. 2009;125:2991–4. doi: 10.1002/ijc.24595. [DOI] [PubMed] [Google Scholar]
- 14.Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A. 2008;105:8387–92. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metalli D, Lovat F, Tripodi F, Genua M, Xu SQ, Spinelli M, et al. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am J Pathol. 176:2997–3006. doi: 10.2353/ajpath.2010.090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101:2076–81. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueda S, Hatsuse K, Tsuda H, Ogata S, Kawarabayashi N, Takigawa T, et al. Potential crosstalk between insulin-like growth factor receptor type 1 and epidermal growth factor receptor in progression and metastasis of pancreatic cancer. Mod Pathol. 2006;19:788–96. doi: 10.1038/modpathol.3800582. [DOI] [PubMed] [Google Scholar]
- 18.Riedemann J, Takiguchi M, Sohail M, Macaulay VM. The EGF receptor interacts with the type 1 IGF receptor and regulates its stability. Biochem Biophys Res Commun. 2007;355:707–14. doi: 10.1016/j.bbrc.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Hu YP, Patil SB, Panasiewicz M, Li W, Hauser J, Humphrey LE, et al. Heterogeneity of receptor function in colon carcinoma cells determined by cross-talk between type I insulin-like growth factor receptor and epidermal growth factor receptor. Cancer Res. 2008;68:8004–13. doi: 10.1158/0008-5472.CAN-08-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang MY, Lu KV, Zhu S, Dia EQ, Vivanco I, Shackleford GM, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–9. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 21.Johns TG, Perera RM, Vernes SC, Vitali AA, Cao DX, Cavenee WK, et al. The efficacy of epidermal growth factor receptor-specific antibodies against glioma xenografts is influenced by receptor levels, activation status, and heterodimerization. Clin Cancer Res. 2007;13:1911–25. doi: 10.1158/1078-0432.CCR-06-1453. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Eddy JA, Pan Y, Hategan A, Tabus I, Wang Y, et al. Integrated proteomics and genomics analysis reveals a novel mesenchymal to epithelial reverting transition in leiomyosarcoma through regulation of slug. Mol Cell Proteomics. 9:2405–13. doi: 10.1074/mcp.M110.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrone F, Da Riva L, Orsenigo M, Losa M, Jocolle G, Millefanti C, et al. PDGFRA, PDGFRB, EGFR, and downstream signaling activation in malignant peripheral nerve sheath tumor. Neuro Oncol. 2009;11:725–36. doi: 10.1215/15228517-2009-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller SJ, Rangwala F, Williams J, Ackerman P, Kong S, Jegga AG, et al. Large-scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res. 2006;66:2584–91. doi: 10.1158/0008-5472.CAN-05-3330. [DOI] [PubMed] [Google Scholar]
- 25.Perrone F, Tabano S, Colombo F, Dagrada G, Birindelli S, Gronchi A, et al. p15INK4b, p14ARF, and p16INK4a inactivation in sporadic and neurofibromatosis type 1-related malignant peripheral nerve sheath tumors. Clin Cancer Res. 2003;9:4132–8. [PubMed] [Google Scholar]
- 26.Cappuzzo F, Tallini G, Finocchiaro G, Wilson RS, Ligorio C, Giordano L, et al. Insulin-like growth factor receptor 1 (IGF1R) expression and survival in surgically resected non-small-cell lung cancer (NSCLC) patients. Ann Oncol. 21:562–7. doi: 10.1093/annonc/mdp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewing GP, Goff LW. The insulin-like growth factor signaling pathway as a target for treatment of colorectal carcinoma. Clin Colorectal Cancer. 9:219–23. doi: 10.3816/CCC.2010.n.032. [DOI] [PubMed] [Google Scholar]
- 28.Janeway KA, Zhu MJ, Barretina J, Perez-Atayde A, Demetri GD, Fletcher JA. Strong expression of IGF1R in pediatric gastrointestinal stromal tumors without IGF1R genomic amplification. Int J Cancer. 127:2718–22. doi: 10.1002/ijc.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayeenuddin LH, Yu Y, Kang Z, Helman LJ, Cao L. Insulin-like growth factor 1 receptor antibody induces rhabdomyosarcoma cell death via a process involving AKT and Bcl-x(L) Oncogene. doi: 10.1038/onc.2010.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins AS, Mackintosh C, Martin DH, Campos M, Hernandez T, Ordonez JL, et al. Insulin-like growth factor I receptor pathway inhibition by ADW742, alone or in combination with imatinib, doxorubicin, or vincristine, is a novel therapeutic approach in Ewing tumor. Clin Cancer Res. 2006;12:3532–40. doi: 10.1158/1078-0432.CCR-05-1778. [DOI] [PubMed] [Google Scholar]
- 31.Li R, Pourpak A, Morris SW. Inhibition of the insulin-like growth factor-1 receptor (IGF1R) tyrosine kinase as a novel cancer therapy approach. J Med Chem. 2009;52:4981–5004. doi: 10.1021/jm9002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludovini V, Bellezza G, Pistola L, Bianconi F, Di Carlo L, Sidoni A, et al. High coexpression of both insulin-like growth factor receptor-1 (IGFR-1) and epidermal growth factor receptor (EGFR) is associated with shorter disease-free survival in resected non-small-cell lung cancer patients. Ann Oncol. 2009;20:842–9. doi: 10.1093/annonc/mdn727. [DOI] [PubMed] [Google Scholar]
- 33.Grobmyer SR, Reith JD, Shahlaee A, Bush CH, Hochwald SN. Malignant Peripheral Nerve Sheath Tumor: molecular pathogenesis and current management considerations. J Surg Oncol. 2008;97:340–9. doi: 10.1002/jso.20971. [DOI] [PubMed] [Google Scholar]
- 34.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–70. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 35.Bruchim I, Attias Z, Werner H. Targeting the IGF1 axis in cancer proliferation. Expert Opin Ther Targets. 2009;13:1179–92. doi: 10.1517/14728220903201702. [DOI] [PubMed] [Google Scholar]
- 36.Liao Y, Abel U, Grobholz R, Hermani A, Trojan L, Angel P, et al. Up-regulation of insulin-like growth factor axis components in human primary prostate cancer correlates with tumor grade. Hum Pathol. 2005;36:1186–96. doi: 10.1016/j.humpath.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Y, Wang L, Gong W, Wei D, Le X, Yao J, et al. A high expression level of insulin-like growth factor I receptor is associated with increased expression of transcription factor Sp1 and regional lymph node metastasis of human gastric cancer. Clin Exp Metastasis. 2004;21:755–64. doi: 10.1007/s10585-005-1198-2. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Chakravarty G, Kim S, Yazici YD, Younes MN, Jasser SA, et al. Growth-inhibitory effects of human anti-insulin-like growth factor-I receptor antibody (A12) in an orthotopic nude mouse model of anaplastic thyroid carcinoma. Clin Cancer Res. 2006;12:4755–65. doi: 10.1158/1078-0432.CCR-05-2691. [DOI] [PubMed] [Google Scholar]
- 39.Moser C, Schachtschneider P, Lang SA, Gaumann A, Mori A, Zimmermann J, et al. Inhibition of insulin-like growth factor-I receptor (IGF-IR) using NVP-AEW541, a small molecule kinase inhibitor, reduces orthotopic pancreatic cancer growth and angiogenesis. Eur J Cancer. 2008;44:1577–86. doi: 10.1016/j.ejca.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Klinakis A, Szabolcs M, Chen G, Xuan S, Hibshoosh H, Efstratiadis A. Igf1r as a therapeutic target in a mouse model of basal-like breast cancer. Proc Natl Acad Sci U S A. 2009;106:2359–64. doi: 10.1073/pnas.0810221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pappano WN, Jung PM, Meulbroek JA, Wang YC, Hubbard RD, Zhang Q, et al. Reversal of oncogene transformation and suppression of tumor growth by the novel IGF1R kinase inhibitor A-928605. BMC Cancer. 2009;9:314. doi: 10.1186/1471-2407-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon J, Stephan S, Mukhopadhyay A, Muders MH, Dutta SK, Lau JS, et al. Insulin receptor substrate-2 mediated insulin-like growth factor-I receptor overexpression in pancreatic adenocarcinoma through protein kinase Cdelta. Cancer Res. 2009;69:1350–7. doi: 10.1158/0008-5472.CAN-08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–46. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura K, Hongo A, Kodama J, Miyagi Y, Yoshinouchi M, Kudo T. Down-regulation of the insulin-like growth factor I receptor by antisense RNA can reverse the transformed phenotype of human cervical cancer cell lines. Cancer Res. 2000;60:760–5. [PubMed] [Google Scholar]
- 45.Ji QS, Mulvihill MJ, Rosenfeld-Franklin M, Cooke A, Feng L, Mak G, et al. A novel, potent, and selective insulin-like growth factor-I receptor kinase inhibitor blocks insulin-like growth factor-I receptor signaling in vitro and inhibits insulin-like growth factor-I receptor dependent tumor growth in vivo. Mol Cancer Ther. 2007;6:2158–67. doi: 10.1158/1535-7163.MCT-07-0070. [DOI] [PubMed] [Google Scholar]
- 46.Sabbatini P, Rowand JL, Groy A, Korenchuk S, Liu Q, Atkins C, et al. Antitumor activity of GSK1904529A, a small-molecule inhibitor of the insulin-like growth factor-I receptor tyrosine kinase. Clin Cancer Res. 2009;15:3058–67. doi: 10.1158/1078-0432.CCR-08-2530. [DOI] [PubMed] [Google Scholar]
- 47.Hewish M, Chau I, Cunningham D. Insulin-like growth factor 1 receptor targeted therapeutics: novel compounds and novel treatment strategies for cancer medicine. Recent Pat Anticancer Drug Discov. 2009;4:54–72. doi: 10.2174/157489209787002515. [DOI] [PubMed] [Google Scholar]
- 48.Imsumran A, Adachi Y, Yamamoto H, Li R, Wang Y, Min Y, et al. Insulin-like growth factor-I receptor as a marker for prognosis and a therapeutic target in human esophageal squamous cell carcinoma. Carcinogenesis. 2007;28:947–56. doi: 10.1093/carcin/bgl247. [DOI] [PubMed] [Google Scholar]
- 49.Olmos D, Postel-Vinay S, Molife LR, Okuno SH, Schuetze SM, Paccagnella ML, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 11:129–35. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garofalo C, Manara MC, Nicoletti G, Marino MT, Lollini PL, Astolfi A, et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing’s sarcoma is dependent on insulin receptor signaling. Oncogene. 30:2730–40. doi: 10.1038/onc.2010.640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.