Abstract
Messenger RNAs (mRNAs) are emerging as prime targets for small-molecule drugs. They afford an opportunity to assert control over an enormous range of biological processes: mRNAs regulate protein synthesis rates, have specific 3-D regulatory structures, and, in nucleated cells, are separated from DNA in space and time. All of the many steps between DNA copying (transcription) and ribosome binding (translation) represent potential control points. Messenger RNAs can fold into complex, 3-D shapes, such as transfer RNAs and ribosomal RNAs, providing an added dimension to the 2-D RNA structure (base pairing) targeted in many mRNA interference approaches. In this Account, we describe the structural and functional properties of the IRE (iron-responsive element) family, one of the few 3-D mRNA regulatory elements with known 3-D structure. This family of related base sequences regulates the mRNAs that encode proteins for iron metabolism.
We begin by considering the IRE-RNA structure, which consists of a short (~30-nucleotide) RNA helix. Nature tuned the structure by combining a conserved AGU pseudotriloop, a closing C-G base pair, and a bulge C with various RNA helix base pairs. The result is a set of IRE-mRNAs with individual iron responses. The physiological iron signal is hexahydrated ferrous ion; in vivo iron responses vary over 10-fold depending on the individual IRE-RNA structure.
We then discuss the interaction between the IRE-RNA structure and the proteins associated with it. IRE-RNA structures, which are usually noncoding, tightly bind specific proteins called IRPs. These repressor proteins are bound to IRE-RNA through C-bulge and AGU contacts that flip out a loop AG and a bulge C, bending the RNA helix. After binding, the exposed RNA surface then invites further interactions, such as with iron and other proteins. Binding of the IRE-RNA and the IRP also changes the IRP conformation. IRP binding stabilities vary 10-fold within the IRE family, reflecting individual IRE-RNA paired and unpaired bases. This variation contributes to the graded (hierarchical) iron responses in vivo.
We also consider the mechanisms of IRE-mRNA control. The binding of Fe2+ to IRE-RNA facilitates IRP release and the binding of eukaryotic initiation factors (eIFs), which are proteins that assemble mRNA, ribosomes, and tRNA for translation. IRE-RNAs are riboregulators for the inorganic metabolic signal, Fe2+; they control protein synthesis rates by changing the distribution of the iron metabolic mRNAs between complexes with enhancing eIFs and inhibitory IRPs.
The regulation of mRNA in the cytoplasm of eukaryotic cells is a burgeoning frontier in biomedicine. The evolutionarily refined IRE-RNAs, although absent in plants and bacteria, constitute a model system for 3-D mRNAs in all organisms. IRE-mRNAs have yielded “proof of principle” data for small-molecule targeting of mRNA structures, demonstrating tremendous potential for chemical manipulation of mRNA and protein synthesis in living systems.
1. Overview of messenger RNA regulation
Messenger RNA regulation complements DNA regulation to control the amount of specific proteins synthesized by living cells. The multiple sizes, shapes, domain functions and metabolic histories of mRNAs contrast with the relatively constant sizes and overall shapes of tRNAs and ribosomal RNAs. In prokaryotic cells (without nuclei), DNA transcription to form mRNA and mRNA translation (protein biosynthesis) are coupled. By contrast, in nucleated cells transcription and translation are separated in space and time, by the nuclear membrane and by RNA “processing” (e.g. editing, splicing, 5′ “capping” and 3′ “polyA tailing”) and transport to ribosomes in the cytoplasm, providing many opportunities to regulate mRNA translation. Subcellular compartments such as mitochondria and plant plastids, which contain their own DNA, and transcribe their own mRNAs for localized protein synthesis, have novel features not considered here.
The variety of mRNAs in each cell type, e.g. skin cells vs. muscle cells, is specific. Thus, RNA provides a very rich set of targets that are more cell-specific than DNA. The rapid growth in RNA interference methods underscore the potential of RNA targeting, although selectivity with RNAi is based on primary sequence and secondary structure.
RNA regulators, primarily described in bacteria1, are a heterogeneous group of molecules that modulate a wide range of physiological responses. Of most relevance here are riboswitches, noncoding, structured mRNA regions that undergo conformational changes upon binding small metabolites, creating an RNA molecular sensor for cellular and/or environmental changes in metabolite concentrations. The RNA structural changes lead to changes in gene expression (protein accumulation). In addition to bacteria, a thiamine-pyrophosphate-binding riboswitch occurs in plants and fungi 2. Riboregulator RNA contrasts with riboswitches by the absence of changed helix base pairs. An intensely studied set of mRNA regulatory structures, the internal ribosome entry site, is found in viruses and normal cells 3. Here we describe a family of riboregulators in animal mRNAs that bind an inorganic metabolite, iron, to regulate gene expression; there are no base pair changes but RNA helix bending is important.
Generally, mRNA regulatory structures are non-coding, and in the untranslated region (UTR) of mRNAs, either before (5′UTR) or after (3′UTR) the coding region. The most extensively characterized animal (including humans) mRNA regulatory structures are a family of related sequences named IRE (iron responsive element) because of the response to changes in environmental iron4. IRE-containing mRNAs code for a variety of proteins involved in iron metabolism and transport. The vast majority of studies are about the extensively reviewed iron repressor proteins, IRP1/2, e.g.,4–8. Only recently, the the iron metabolite signal is identified as a ferrous ion/RNA interaction9.
IRE-RNA sequences fold into 3D structures selectively recognized by the specific repressor proteins (IRPs) and possibly by translation initiation proteins10. They occur in both the 5′UTR to control ribosome binding/translation initiation, responding to elevated iron, and in the 3′UTR to control mRNA stability during iron deprivation. Much more is known about 5′UTR IRE structures, the focus of this discussion. The evolutionarily oldest IRE in the 5′ UTR, is in ferritin mRNAs 11 and it has been studied by chemical and nuclease probing, secondary structure prediction, mutagenesis, and NMR spectroscopy as well as X-ray diffraction of IRE-RNA/protein repressor crystals 4,6,12–15. Chemical nucleases, models for selective, small RNA-targeting molecules, confirmed constant IRE structure between native ferritin mRNA in solution and cells 16, full length in vitro transcripts and short oligoribonucleotides 17,18.
2. IRE-RNA –the conserved core structure
Repressor protein (IRP) recognition of IRE-RNAs depends on the conserved IRE-RNA core structure (30 nt) described below (Fig. 1,2). The protein repressors (IRP1 and 2) bind IRE-mRNA subdomains (Figure 3) to inhibit protein synthesis (5′UTR) or mRNA degradation (3′ UTR)4–6. IRP1 and 2 are degraded by iron-dependent processes 7,8. IRP1 is converted to c-aconitase by iron-sulfur cluster insertion at the RNA site6,14,15. IRE sequence is more than 90% conserved for IRE-mRNAs coding for the same protein in different animals; but sequence conservation of the element in IRE–mRNAs coding for different proteins of the same animal varies as much as 40% 13,15,19.
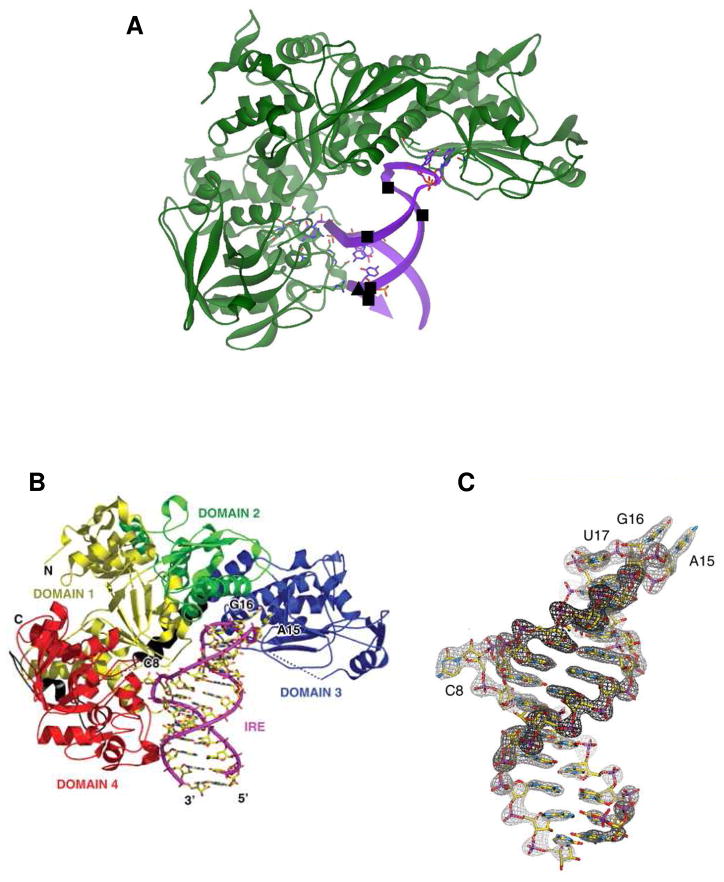
Figure 1. IRP1 binding to FRT IRE-RNA.
The RNA helix bends and contact bases C8 and triloop bases A15 and G16 are flipped out. Panel A: ■, hydrated Mg2+, determined by solution NMR; ▲, Cu1+-1.10-phenanthroline, determined by RNA cleavage in O2. The figure prepared from Protein Data Bank file 2IPY. (From ref. 12, with permission.) Panel B: IRE:IRP complex showing IRP domains (1 yellow; 2 green; 3 blue; 4 red) and contacts with IRE-RNA. Panel C: Electron density of IRE in IRE:IRP complex. (From Ref. 12, see for details).
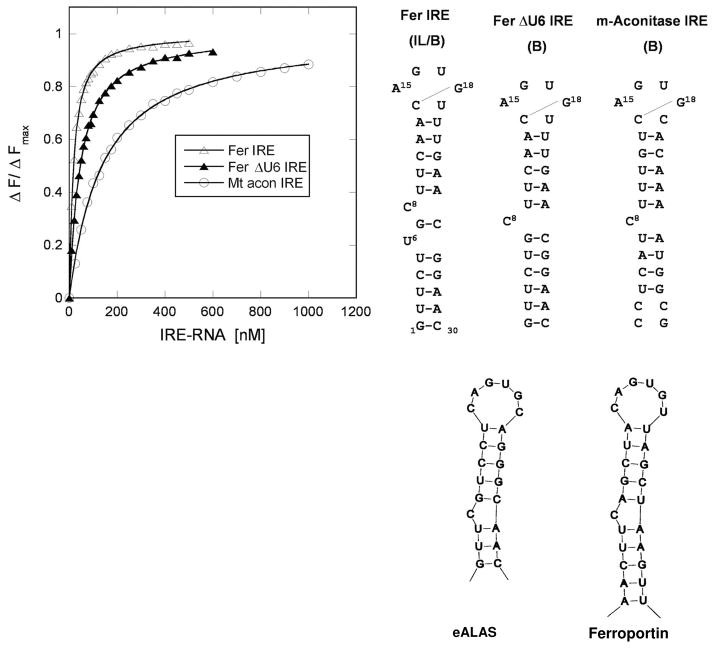
Figure 2. IRP1/IRE-RNA solution binding compared for three IRE-RNAs.
IRP1 preferentially binds to the FRT IRE-RNA compared with ACO2 IRE-RNA with nM affinity: binding curve (protein fluorescence quenching) on the left, conserved IRE secondary structures on the right (From ref. 9).
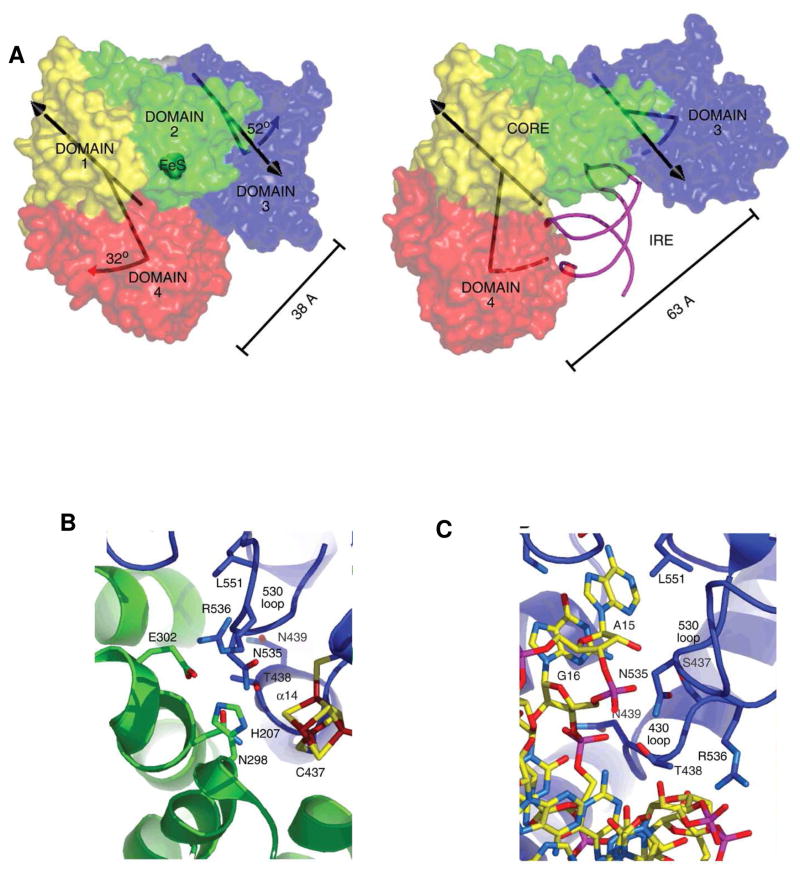
Figure 3. Crystal structure of IRP1 as c-aconitase and complexes with IRE-RNA.
A. Differences in protein domain positions between c-aconitase and IRP1:IRE-RNA complex. Left: FeS-apo-IRP; Right: IRE-RNA/IRP. RNA-magenta; protein- blue, green, red, yellow. B. Close up of the protein–RNA contacts at the RNA triloop; C. Close-up of the protein–RNA contacts at the RNA bulge C8. (From ref. 12, with permission).
NMR studies of an IRE-RNA 30 base oligoribonucleotide, representing the IRP1 “footprint” 20,21, showed: 1. Both well-defined and conformationally disordered regions; 2. Well defined terminal loop residues C14, A15 and G18 and the bases in helix pairs below and above the C8 bulge; 3. Base pairs were folded into the RNA A helix conformation; 4. The orientation of helix sections flanking the C8 bulge was not fixed with respect to each other, allowing conformational changes during IRP binding that are indicated by differences between the IRE-RNA solution NMR structure and in X-ray diffraction of IRP/IRE crystals 12.
All IRE-RNAs have five base pairs in the upper stem 22. While the constant number of base pairs may seem like a “molecular ruler” for spacing of the two protein contact sites at the bulge C8 residue and the upper pseudotriloop, the identity of the base pairs in the upper stem vary in an IRE-mRNA specific way. Moreover, IRE-RNAs of at least 30 nucleotides, the RNA length protected by IRP binding 23, show that the natural variations in base pairs of the upper stem influences IRP binding 24–26. In the IRE-RNA/IRP complex, the RNA helix is bent as much as 30° from the helix axis 12 contrasting with IRE-RNA in solution 20,27. Likely, the individual sets of five base pairs in the upper RNA stem control the kinetics of IRE-RNA helix bending during IRP binding.
The apical loop of all IRE-RNAs, one of the two IRP contact sites (Fig. 1,2 3B), has a C14-G18 base pair that creates an AGU pseudotriloop 12,20,21,27,28. G16 and U17 are disordered. In the IRP/RNA protein complex A15 and G16 are flipped out of the helix penetrating deep into the IRP structure12. The C14-G18 base pair is crucial for loop structure/stability since G18A IRE-RNA has a lower Tm; an IRE loop with U14-A18 retains high affinity for IRP121,29. Apparently variable base 19 cannot be G, since an alternate base pair to C14-G19 would occur.
Binding specificity in the IRP1/H ferritin IRE-RNA complex is achieved with only two, widely separated, contact sites 12. In the complex, the RNA is inserted between protein domains I–II and IV (Fig 3), using the numbering of aconitases. Aconitases are globular structures with close interactions among all four domains, whereas in the IRE-RNA/IRP complex, IRP protein is L-shaped and the RNA is bent. Twenty two bonds hold the RNA and protein together: 10 contacts are formed between A15 and G16 in the pseudotriloop at the RNA terminus (Figure 3B) and amino acids, such as residues 371 and 379 in a pocket generated in domain III at a site that is blocked by domains I and II in the globular form (Fig. 3B). Eight bonds are formed between IRE-RNA C8 and amino acids in Domain IV of IRP1; four additional bonds occur between amino acids in domain IV of IRP1 and the IRE stem below C8 (Figure 3C). In addition to flipping out of terminal loop bases A15 and G16 and helix bulge C8 20, in the protein/RNA complex, the RNA backbone is distorted by a sharp mid-helix turn, unpaired U19 in the hexaloop is flipped out of the RNA helix and unpaired U6 is tucked into the minor groove (Fig. 1,3). The differences between the solution structure of free IRE-RNA and protein bound IRE-RNA require conformational changes in the RNA, and likely of the unliganded protein; while the structure of apo-IRP is not yet known, it is more disordered than in either the RNA or [4Fe-4S] complexes30. In the protein/RNA crystal structure 12, a large surface of the IRE-RNA is exposed free for interactions with other proteins, metal ions, or RNA.
3. IRE-RNA Structure- natural variations and protein repressor (IRP) binding
The conserved structure of the IRP contact sites in IRE-RNAs, the terminal loop and bulge C, means that differences in RNA/IRP complex stability must depend on structural differences in helices. IRP binding affinity is altered by increasing the length of the upper stem or by disrupting the helices above and below the C bulge 22,23,31,32. Natural variations, which occur in helix base pairs of IRE-containing mRNAs coding for different proteins coincide with quantitative differences in IRP binding affinity and the magnitude of the iron response in vivo. It is as if Nature has created a set of graded “dimmer” switches in the IRE family, using helix sequences that vary as much as 36%, rather than an on/off switch, so that IRE-mRNAs can have a range of sensitivities to iron.
Ferritin H and MT-aconitase (ACO2) IRE-mRNAs 9,26 differ by at least an order of magnitude in the in vivo response to iron levels and show the largest differences in IRP1 binding in solution 24,33 (Fig. 2). The relative affinity of ferritin IRE-RNA and ACO2 IRE-RNA with IRP1 differ by 9-fold whether by mobility shift in gel electrophoresis or fluorescence quenching in solution; the pmolar binding constants from gel shifts contrast with nmolar binding constants for solution fluorescence; this difference may be caused by an adsorptive component in the gels. Ferritin IRE-RNA with the additional U6 bulge (Fig 2) binds IRP the most tightly, and may reflect faster kinetics of helix bending during protein binding. When large numbers of IRE-RNAs were compared, a binding hierarchy was defined by Goforth et al. 26, also described earlier as combinatorial regulation 34, since different combinations of variable elements (base pairs, helix bulges) and constant elements (terminal loop, C bulge) are combined in each phylogenetically conserved IRE-RNA. IRE-RNA base pairs can change protein/RNA stability as much as 14-fold 26, even though there is little or no contact between IRP protein and most of the helix base pairs (Fig. 2), likely reflecting the contributions of helix pitch, flexibility and binding dynamics.
The array of similar but distinct structures among IRE-RNA family members, selectively recognized by IRP binding suggest an RNA scaffold, similar to tRNAs, presenting key residues for interaction with recognition proteins. Recognition of tRNA by tRNA synthetase depends on core elements, as does IRE recognition by IRP1; tRNAs also contain minor elements that fine tune the interactions35–37. Like the tRNAs, IRE-RNAs have both nonconserved sequences, particularly in the stem region that contributes to IRP binding11, and core elements. However, the binding affinities do not have the range of discrimination or energetics observed in tRNAs 35,37,38 which have to be recognized by individual synthetases. Different IREs are recognized by the same IRP proteins.
IRE-RNAs were originally associated with iron and oxygen metabolism. Recently, however, IRE-mRNAs have been identified that encode a number of other proteins such as hypoxia inducible transcription factor 39, cell cycle phosphatase 40, α-hemoglobin stabilizing protein 41, MT- p75 protein42 and Alzheimer amyloid precursor (AAP) protein 43,44. The AAP IRE-mRNA secondary structure is predicted to contain the pseudo-triloop and also contains the conserved C8 residue. While predicted structure for APP IRE-RNA does not predict the C8 residue to be a bulge base, G7 is predicted to be a bulge base43. The binding stability of the complex appears to be similar to the ferritin IRE-RNA/IRP1 complex. However, AAP IRE does not bind IRP243. The greater selectivity of IRP2/IRE-RNA interactions has also been observed for the IRE-mRNAs involved in iron metabolism 24,45. It is likely that additional IRE structures differentially binding IRP1 and IRP2 will be identified.
4. IRE-mRNA translation
Effects of changes in environmental iron (solutions of inorganic salts) on ferritin mRNA function and protein synthesis in animals and cells were first observed in the 1970’s and 80’s 5,46, long before cloning, recombinant mRNAs and discovery of the conserved IRE sequences 47,48. The effect was later extended to many other IRE-mRNAs in iron metabolic pathways; the environmental signals were also extended to include heme, oxygen (anoxia), and oxidants (hydrogen peroxide) as reviewed in 4,6,15. Because IRE-RNAs are located in two different locations in mRNA, iron can have opposite effects on different IRE-mRNAs. In Type1 IRE regulation, the IRE structure is in the 5′UTR and regulates ribosome binding. Iron signals release IRP to allow ribosome binding and translation of the mRNA increases. In Type 2 IRE regulation, the IRE is in the 3′UTR, to regulate nuclease binding and mRNA degradation. Most IRE-RNAs that have been characterized to date regulate ribosome binding rather than nuclease binding.
How cellular iron signals change the affinity of IRP for IRE-RNA and remove translational repression was not understood until very recently 9. Moreover, ribosome binding is a very complex process requiring the binding of many protein “factors” to form an initiation complex of mRNA, initiator tRNA and both ribosomal subunits. The exact sequence of events in releasing IRP and assembling an initiation complex with an IRE-RNA remains unclear. Some features suggest IRE structures function synergistically with other elements of the mRNA structure based on a short distance between the beginning of the mRNA and the IRE 10,49; in some IRE-RNAs such as ACO2, the initiator AUG is embedded in the IRE-RNA; the functional significance of initiation at the IRE is not known. The initiation of protein synthesis begins with the binding of a large protein, eukaryotic initiation factor (eIF) 4F, to the 5′ cap of mRNA; eIF4F is actually a supramolecular complex of a helicase (4A), RNA binding protein (4B) and scaffolding protein (4G) that may also contact IRP 50. eIF4F binding can facilitate rapid responses to cellular iron levels since it binds competitively with IRP to IRE-RNA indicating the two proteins occupy the same or overlapping binding sites(Ma, Khan, Merrick, Haldar, Theil, Goss, to be published). Many of the details of the assembly of an active protein synthesis initiation complex remain to be elucidated. The IRE-RNA binds metal ions, which include physiologically relevant Fe2+, to decrease IRE-RNA/IRP stability, indicating a direct role for metal ions in dissociation of the IRP1/IRE complex.
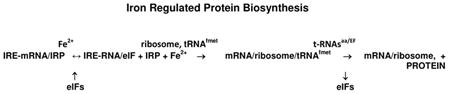 |
[1] |
The impact of Fe2+, the physiological iron signal on IRE-mRNA translation, i.e., protein biosynthesis, is shown in [1]. The reaction, a series of sequential, supramolecular interactions between IRE-mRNA with IRP(iron regulatory protein), Fe2+, eIFs (initiation factor proteins), rRNA/protein complexes (ribosome) and tRNA/protein (EF, elongation factor) complexes, is required for Iron-induced protein biosynthesis as shown in equation [1]. Not shown is the consumption of GTP at every tRNAaminoacid binding step, which makes ribosome–dependent protein biosynthesis the most bioenergetcially expensive reaction in growing cells.
5. Metal ions and IRE-RNA/IRP binding interactions
The addition of iron chelators, exemplified by a recent study of the Alzheimer amyloid precursor protein (AAP) IRE, illustrate the effects of changing iron concentrations: cells exposed to the Fe(III) chelator desferrioxamine increased binding of IRP1 to the IRE-RNA 43, as observed for many IRE-RNAs in a variety of cultured cell types32,51–53. The molecular mechanism was not clear until the recent observations that low concentrations of ferrous ions (anaerobic) weakened IRE-RNA/IRP1 complexes; other metal ions have selective effects9. The magnitude of the metal ion effects varies with individual IRE-RNA sequences/structures. For example, ferritin IRE/IRP1 binding affinity decreased 5–10 times while ACO2 IRE/IRP1 binding decreased only 2–5 times over the concentration ranges used9 indicating the impact of Nature’s modulation of riboregulation among IRE-RNAs.
Metal ions bind directly to the IRE-RNA based on ethidium bromide displacement, effects on NMR spectra, binding of metal complexes 9,17,27,54, and the absence of predicted metal ion binding sites on IRP beyond the [4Fe-4S] cluster insertion site. Moreover, eIF4F binding to IRE-RNA is metal ion sensitive (Ma, Khan, Merrick, Haldar, Theil and Goss to be published). Finally, a number of RNA protein bonds involve sites that are hypersensitive to cleavage by Fe2+-EDTA23, suggesting specific interactions with Fe2+-EDTA, solvent or both. The fact that metal ion effects were greatly reduced when the conserved ferritin IRE-RNA bulge U6 was deleted9 and the earlier studies of Cu-1,10-phenanthroline and modeling of Co(III)hexamine binding 27,54, support the idea of the IRE-RNA mid-helix bulge as the binding site of regulatory metal ions. Bulge bases are metal ion binding sites in other RNAs 55,56. The metal selectivity of Fe2+ on IRP binding to IRE-RNAs, which is 200x more effective than Mg2+ 9, indicates that Fe2+ is the physiological signal that targets the IRE-RNA riboregulator to decrease IRP binding and increase translation of the IRE-mRNA.
6. IRE-mRNA as Fe2+ riboregulator
Riboswitches and riboregulators regulate gene expression with target molecules, usually small metabolites that cause RNA conformational changes upon binding. Often a polymerase or ribosome binding site is exposed as a result. In the IRE-RNAs, based on solution structures of free RNA and a crystal structure of RNA bound to repressor proteins 12,20,27, structural changes induced by Fe2+ binding involve changes in the orientation of unpaired bases and helix bending with retention of base pairing. Riboregulator activity can depend on kinetics, thermodynamics (steady state binding stability) or a combination of both, depending on ligand concentrations. In IRE-RNAs, the conformational changes caused by Fe2+ binding results in the release of the IRP repressor protein. In addition, Fe2+ - induced RNA conformational changes enhance binding of the eukaryotic initiation factor 4F to increase translation. If only equilibrium effects are considered, a change in ligand concentration of ~ 80-fold is required for a change in the fraction of an RNA/protein complex from 10 to 90%. However, many riboswitches fine tune ligand sensitivity by changing binding kinetics57. Riboregulators that are kinetically controlled rely on the occupancy of the RNA site; gene expression will depend on the dissociation rate of the ligand, or for the Fe2+-IRE-RNA/IRP, dissociation rate of the IRP, association rate of eIF-4F and ribosomes, and dissociation rate of Fe2+. If initiation factor binding is rapid, expression (protein synthesis of IRE-encoded proteins) will be sensitive to small changes in Fe2+ concentrations and regulation will be dominated by kinetics of IRP release and not by equilibrium binding because eIF binding will occur before equilibrium is established.
7. Kinetics and mechanisms of IRP binding to ferritin and ACO2 IRE-RNAs
The role of IRP/IRE-mRNA binding kinetics and direct Fe2+/RNA interactions in controlling downstream gene expression, i.e. rates of mRNA translation, have been little considered. However, a characteristic of a kinetically operated riboregulator, and indeed of life itself, is genetic decisions made under nonequilibrium conditions for RNA and ligand. Rate constants for association and dissociation are, thus, more important than the equilibrium constants, albeit experimentally more difficult to measure in complex biological systems. When we investigated the rates of association and dissociation of IRP1 to ferritin and ACO2 IRE (Ma, Khan, Haldar, Merrick, Theil, and Goss, to be published), we found that the smaller Kd for ferritin IRE-RNA/IRP1 binding, compared to ACO2, depended on a much faster association rate; the dissociation rate constants were comparable. In the presence of Fe2+ (anaerobic) or the oxygen-resistant, Fe2+- surrogate, Mn2+ in air, both the kon and koff rates were changed, more so for the ferritin IRE-RNA than for ACO2 IRE-RNA; whether Mn2+/IRE-RNA interactions are physiologically relevant is not known. The lifetime of the IRP-IRE complex (1/koff), in the presence of Mn2+, decreased for both the ferritin IRE-RNA/IRP and ACO2 IRE-RNA/IRP complex. Since the concentrations of IRE-mRNAs and IRP repressor proteins vary in different types of cells, kinetic contributions to the concentration of IRE-RNA/IRP complexes are predicted to be large and cell specific.
The interaction of IRE-mRNAs with translation initiation factors (eIFs) and/or ribosomes is a second potential set of targets for riboregulator-Fe2+ control. The association of eIF4F increases approximately 5-fold in the presence of 50 μM Mn2+ or Fe2+, an effect opposite that for the IRP (Ma, Khan, Haldar, Merrick, Theil, and Goss, to be published). Thus, when cellular concentrations of free or loosely bound Fe2+ increase, IRE-RNA-Fe2+ complexes are in a conformation unfavorable to IRP repressor binding and favorable to eIF4F binding. The IRE riboregulator in the presence of metals increases protein synthesis by two mechanisms: facilitating IRP repressor release and eIF4F binding, explaining earlier observations that the presence of an IRE-RNA had positive effect on protein synthesis rates 10. Nature has tuned the IRE-RNA riboregulator in different IRE-mRNAs by changing the base pairs, which results in changes in IRP binding stability. 9,24,26,33.
8. Small molecule drug targeting of IRE –RNA and other mRNA regulatory structures
Current development of RNA drugs, e.g. based on RNAi, depends heavily on RNA secondary structure whereas for proteins, 3D structure is the more common drug target. RNA therapies are advantageous because of the smaller target size (number of molecules/cell) compared to proteins. Small molecules, e.g., Cu-1,10-phenathroline and yohimbine bind to selective sites in the IRE-RNA in solution altering mRNA function in soluiton and in cultured, human cells 16,17,58. Such results showed that the small, RNA binding molecules can enter living cells and bind to folded, target RNA structures with the same selectivity as in solution. In one case, such as IRP-inactivated ferritn mRNA, a small molecule could activate the fraction of mRNA repressed in iron overload, and decrease the accumulation of damaged ferritin (toxic hemosiderin). In another case, small molecules could target, riboreuglators in oncogene mRNAs or mRNAs important in metabolic diseases. All that is needed is identification of more riboreuglatory mRNA structures and the expansion of small molecule targeting of 3D structures of mRNAs.
9. Summary and Perspective
MRNA regulation in the cytoplasm of eukaryotic (nucleated cells) is a burgeoning frontier in the understanding of Chemistry and Biology. Here we describe an example of a group of three dimensional RNA structures, iron responsive elements (IRE-RNAs) in normal cellular mRNAs. These structures selectively bind iron to regulate the stability of a protein repressor complex that impedes ribosome binding and protein synthesis (mRNA translation). Positive regulation occurs through binding of protein synthesis initiation factor eIF4F. The IRE-RNA is described in terms of the IRE-RNA loop and bulge common to each IRE-mRNA that forms the separated protein binding sites. Variations in the sequence of individual IRE–RNA helix base pairs modulate both repressor protein (IRP) binding and the metabolite Fe2+ bindng, tuning protein synthesis to in vivo environmental iron. Future studies to characterize the Fe2+ binding site on the IRE will identify target regions of the IRE-RNA for future drug design. IRE-RNAs, evolutionarily old in animals, are absent in plants and bacteria, but form a model system for other 3D mRNAs in any organism. IRE-mRNAs have yielded “proof of principle” data for small molecule targeting of mRNA structures that illustrate the untapped potential for chemical manipulation of messenger RNA and protein synthesis in living systems.
Acknowledgments
Research in the Goss and Theil labs is supported by National Institutes of Health Grant DK20251 (E.C.T. and D.J. G.) and National Science Foundation Grant MCB 0814051 (D.J.G.).
Biographies
Dixie J. Goss is a biophysicist/biochemist, educated in a one-room schoolhouse in rural Nebraska, Nebraska Wesleyan University and the University of Nebraska. Her research has focused on the role of RNA structure in regulation of protein synthesis and protein interactions. Currently she is Professor and Gertrude Elion Endowed Scholar of Chemistry at Hunter College City University of New York.
Elizabeth C. Theil is a biochemist/bioinorganic chemist, educated in the public schools of New York City, and Cornell and Columbia Universities. Her work, spanning DNA, mRNA and protein structure/ function has relevance to iron nutrition and medicine and is focused on the ferritin family of protein nanocages. Currently she is Senior Scientist at CHORI: Children’s Hospital Oakland Research Institute and Prof. Nutr. Sci. & Tox. (adj) UC-Berkeley
References
- 1.Waters L, Storz G. Regulatory RNAs in Bacteria. Cell. 2009;136:615–628. doi: 10.1016/j.cell.2009.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bocobza S, Aharoni A. Switching the light on plant riboswitches. Trends Plant Sci. 2008;13:526–533. doi: 10.1016/j.tplants.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Filbin M, Kieft J. Toward a structural understanding of IRES RNA function. Curr Opinion Structural Biology. 2009;19:267–276. doi: 10.1016/j.sbi.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theil EC, Goss DJ. Living with iron (and oxygen): questions and answers about iron homeostasis. Chem Rev. 2009;109:4568–79. doi: 10.1021/cr900052g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zahringer J, Baliga BS, Munro HN. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci US A. 1976;73:857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muckenthaler M, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- 7.Salahudeen AA, Thompson JW, Ma HW, Kinch LN, Li Q, Grishin NV, Bruick RK. An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science. 2009;326:722–6. doi: 10.1126/science.1176326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vashisht AA, Zumbrennen K, Huang X, Powers D, Durazo A, Sun D, Bhaskaran N, Persson A, Uhlen M, Sangfelt O, Spruck C, Leibold EA, Wohlschlegel JA. Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science. 2009;326:718–21. doi: 10.1126/science.1176333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MA, Walden WE, Goss DJ, Theil EC. Direct Fe2+ sensing by iron-reponsive messenger RNA:repressor complexes weakens binding. J Biol Chem. 2009;284:30122–8. doi: 10.1074/jbc.M109.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dix DJ, Lin PN, Kimata Y, Theil EC. The iron regulatory region of ferritin mRNA is also a positive control element for iron-independent translation. Biochemistry. 1992;31:2818–2822. doi: 10.1021/bi00125a024. [DOI] [PubMed] [Google Scholar]
- 11.Piccinelli P, Samuelsson T. Evolution of the iron-responsive element. RNA. 2007;13:952–966. doi: 10.1261/rna.464807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walden WE, Selezneva AI, Dupuy J, Volbeda A, Fontecilla-Camps JC, Theil EC, Volz K. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science. 2006;314:1903–1908. doi: 10.1126/science.1133116. [DOI] [PubMed] [Google Scholar]
- 13.Volz K. The functional duality of iron regulatory proein 1. Curr Opin Struct Biol. 2008;18:106–111. doi: 10.1016/j.sbi.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallander ML, Leibold EA, Eisenstein RS. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim Biophys Acta. 2006;1763:668–689. doi: 10.1016/j.bbamcr.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leipuviene R, Theil EC. The family of iron responsive RNA structures regulated by changes in cellular iron and oxygen. Cell Mol Life Sci. 2007;64:2945–2955. doi: 10.1007/s00018-007-7198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke Y, Theil EC. An mRNA loop/bulge in the ferritin iron responsive element (IRE) formed in vivo, and detected by radical probing with Cu-phen and protein (IRP) footprinting. J Biol Chem. 2002;277:2373–2376. doi: 10.1074/jbc.C100614200. [DOI] [PubMed] [Google Scholar]
- 17.Wang YH, Sczekan SR, Theil EC. Structure of the 5′ untranslated regulatory region of ferritin mRNA studied in solution. Nucleic Acids Res. 1990;18:4463–4468. doi: 10.1093/nar/18.15.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorp HH, McKenzie RA, Lin PN, Walden WE, Theil EC. Cleavage of functionally relevant sites in ferritin mRNA by oxidizing metal complexes. Inorg Chem. 1996;35:2773–2779. [Google Scholar]
- 19.Dandekar T, Stripecke R, Gray NK, Goossen B, Constable A, Johannson HE, Hentze MW. Identification of a novel iron-resonsive element in murine and human erthroid delta-aminolevulinic acid synthase mRNA. EMBO J. 1991;10:1903–1909. doi: 10.1002/j.1460-2075.1991.tb07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Addess KJ, Basilion JP, Klausner RD, Rouault TA, Pardi A. Structure and dynamics of the iron responsive element RNA: implications for binding of the RNA by iron regulatory binding proteins. J Mol Biol. 1997;274:72–83. doi: 10.1006/jmbi.1997.1377. [DOI] [PubMed] [Google Scholar]
- 21.Sierzputowska-Gracz H, McKenzie RA, Theil EC. The importance of a single G in the hairpin loop of the iron responsive element (IRE) in ferritin mRNA for structure: an NMR spectroscopy study. Nucleic Acids Res. 1995;23:145–153. doi: 10.1093/nar/23.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kikinis Z, Eisenstein RS, Bettany AJE, Munro HN. Role of RNA secondary structure of the IRE in translational regulation of ferritin synthesis. Nucleic Acids Res. 1995;23:4190–4195. doi: 10.1093/nar/23.20.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrell CM, McKenzie AR, Patino MM, Walden WE, Theil EC. Ferritin mRNA: interactions of iron regulatory element with translational regulator protein P-90 and the effect on base-paired flanking regions. Proc Natl Acad Sci US A. 1991;88:4166–4170. doi: 10.1073/pnas.88.10.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ke Y, Wu J, Leibold EA, Walden WE, Theil EC. Loops and bulge/loops in iron-responsive element isoforms influence iron regulatory protein binding. J Biol Chem. 1998;273:23637–23640. doi: 10.1074/jbc.273.37.23637. [DOI] [PubMed] [Google Scholar]
- 25.Khan M, Yumak H, Goss DJ. Kinetic mechanism for the binding of eIF4F and tobacco etch virus internal ribosome entry site RNA: effects of eIF4B and poly(A)-binding protein. J Biol Chem. 2009;284:35461–70. doi: 10.1074/jbc.M109.038463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goforth JB, Anderson SA, Nizzi CP, Eisenstein RS. Multiple determinants within iron-reponsive elements dictate iron regulatory protein binding and regulatory hierarchy. RNA. 2010;16:154–169. doi: 10.1261/rna.1857210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gdaniec Z, Sierzputowska-Gracz H, Theil EC. Iron regulatory element and internal loop/bulge structure for ferritin mRNA studied by cobalt(III) hexamine binding, molecular modeling, and NMR spectroscopy. Biochemistry. 1998;37:1505–1512. doi: 10.1021/bi9719814. [DOI] [PubMed] [Google Scholar]
- 28.Henderson BR, Menotti E, Bonnard C, Kühn LC. Optimal sequence and structure of iron-responsive elements: selection of RNA stem-loops with high affinity for iron regulatory factor. J Biol Chem. 1994;269:17481–17489. [PubMed] [Google Scholar]
- 29.Laing LG, Hall KB. A model of the iron responsive element RNA hairpin loop structure determined from NMR and thermodynamic data. Biochemistry. 1996;35:13586–13596. doi: 10.1021/bi961310q. [DOI] [PubMed] [Google Scholar]
- 30.Brazzolotto X, Timmins P, Dupont Y, Moulis JM. Structural changes associated with switching activites of human iron regulatory protein 1. J Biol Chem. 2002;277:11995–2000. doi: 10.1074/jbc.M110938200. [DOI] [PubMed] [Google Scholar]
- 31.Barton HA, Eisenstein RS, Bomford A, Munro HN. Determinants of the interaction of the iron responsive element bidning protein with its binding site in rat L-ferritin mRNA. J Biol Chem. 1990;265:7000–7008. [PubMed] [Google Scholar]
- 32.Leibold EA, Laudano A, Yu Y. Structural requirements of iron-responsive elements for binding of the protein involved in both transferrin receptor and ferritin mRNA post-transcriptional regulation. Nucleic Acids Res. 1990;18:1819–1824. doi: 10.1093/nar/18.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gunshin H, Allerson CR, Polycarpou-Schwarz M, Rofts A, Rogers JT, Kishi F, Hentze MW, Rouault TA, Andrews NC, Hediger MA. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001;509:309–316. doi: 10.1016/s0014-5793(01)03189-1. [DOI] [PubMed] [Google Scholar]
- 34.Theil EC, Eisenstein RS. Combinatorial mRNA regulation: Iron regulatory proteins and Iso-iron responsive elements (iso-IREs) J Biol Chem. 2000;275:40659–40662. doi: 10.1074/jbc.R000019200. [DOI] [PubMed] [Google Scholar]
- 35.Beuning PJ, Musier-Forsyth K. Transfer RNA recognition by aminoacyl-tRNA synthetases. Biopolymers. 1999;52:1–28. doi: 10.1002/(SICI)1097-0282(1999)52:1<1::AID-BIP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 36.Frugier M, Helm M, Felden B, Giege R, Florentz C. Sequences outside recognition sets are not neutral for tRNA aminoacylation. Evidence for nonpermissive combinations of nucleotides in acceptro stem of yeast tRNAPhe. J Biol Chem. 1998;273:11605–11610. doi: 10.1074/jbc.273.19.11605. [DOI] [PubMed] [Google Scholar]
- 37.Schrader JM, Chapman SJ, Uhlenbeck O. Understanding the sequence specificity of tRNA binding to elongation factor Tu using tRNA mutagenesis. J Mol Biol. 2009;386:1255–64. doi: 10.1016/j.jmb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Opperman L, Hook B, DeFino M, Bernstein DS, Wickens M. A single spacer nucleotide determines the specificities of two mRNA regulatory proteins. Nat Struct Mol Biol. 2005;12:945–951. doi: 10.1038/nsmb1010. [DOI] [PubMed] [Google Scholar]
- 39.Sanchez M, Galy B, Dandekar T, Bengert P, Vainshtein Y, Stolte J, Muckenthaler MU, Hentze MW. Iron regulation and the cell cycle: identification of an iron-responsive element in the 3′-untranslated region of human cell division cycle 14A mRNA by a refined microarray-based screening strategy. J Biol Chem. 2006;281:22865–22874. doi: 10.1074/jbc.M603876200. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nat Struct Mol Biol. 2007;14:420–426. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- 41.dos Santos CO, Dore LC, Valentine E, Shelat SG, Hardison RC, Ghosh M, Wang W, Eisenstein RS, Costa FF, Weiss MJ. An IRE-like stem-loop regulates alpha hemoglobin-stabilizing protein mRNA. J Biol Chem. 2008;283:26956–26964. doi: 10.1074/jbc.M802421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin E, Graziano JH, Freyer GA. J Biol Chem. 2001;276:27685–27692. doi: 10.1074/jbc.M100941200. [DOI] [PubMed] [Google Scholar]
- 43.Cho HH, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X, Rogers JT. Selective Translational Control of the Alzheimer Amyloid Precursor Protein Transcript by Iron Regulatory Protein1. J Biol Chem. 2010;285:31217–31232. doi: 10.1074/jbc.M110.149161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H, Leiter L, McPhee J, Sarang SS, Utsuki T, Greig NH, Lahiri DK, Tanzi RE, Bush AI, Giordano T, Gullans SR. An iron-responsive element type II in the 5′ untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem. 2002;277:45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- 45.Erlitzki R, Long JC, Theil EC. Multiple, conserved iron responsive elements in the 3′ untranslated region of transferrin receptor mRNA enhance binding of iron regulatory protein 2. J Biol Chem. 2002;277:42579–42587. doi: 10.1074/jbc.M207918200. [DOI] [PubMed] [Google Scholar]
- 46.Schaefer FV, Theil EC. The effect of iron on the synthesis and amount of ferritin in red blood cells during ontogeny. J Biol Chem. 1981;256:1711–1715. [PubMed] [Google Scholar]
- 47.Leibold EA, Munro HN. Characterization and evolution of the expressed rat ferritin light subunit gene and its pseudogene family. Conservation of sequences with in oncoding regions of ferritin genes. J Biol Chem. 1987;262:7335–41. [PubMed] [Google Scholar]
- 48.Casey JL, Hentze MW, Keoller DM, Caughman SW, Rouault T, Klausner RD, Harford JB. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988;240:924–8. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- 49.Goossen B, Hentze MW. Position is the critical determinant for function of iron-responsive elements as translational regulators. Mol Cell Biol. 1992:1959–1966. doi: 10.1128/mcb.12.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muckenthaler M, Gray NK, Hentze MW. IRP-1 binding to ferritin mRNA prevents the recruitment of the small ribosomal subunit by the cap-binding complex eIF4F. Mol Cell. 1998;2:383–388. doi: 10.1016/s1097-2765(00)80282-8. [DOI] [PubMed] [Google Scholar]
- 51.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 52.Hentze MW, Muckenthaler M, Galy B, Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 53.Eisenstein RS. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu Rev Nutr. 2000;20:627–662. doi: 10.1146/annurev.nutr.20.1.627. [DOI] [PubMed] [Google Scholar]
- 54.Ke Y, HSG, Gdaniec Z, Theil EC. Internal loop/bulge and hairpin loop of the iron-responsive element of ferritin mRNA contribute to maximal iron regulatory protein 2 binding and translational regulation in the iso-iron-responsive element/iso-iron regulatory protein family. Biochemistry. 2000;39:6235–6242. doi: 10.1021/bi9924765. [DOI] [PubMed] [Google Scholar]
- 55.Shenvi CL, Dong KC, Friedman EM, Hanson JA, Cate JH. Accessibility of 18S rRNA in human 40S subunits and 80S ribosomes at physiological magnesium ion concentrations--implications for the study of ribosome dynamics. Rna. 2005;11:1898–908. doi: 10.1261/rna.2192805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martick M, Horan LH, Noller HF, Scott WG. A discontinuous hammerhead ribozyme embedded in a mammalian messenger RNA. Nature. 2008;454:899–902. doi: 10.1038/nature07117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wickiser JK, Cheah MT, Breaker RR, Crothers DM. The kinetics of ligand binding by an Adenine-sensing riboswitch. Biochemistry. 2005;44:13404–13414. doi: 10.1021/bi051008u. [DOI] [PubMed] [Google Scholar]
- 58.Tibodeau JD, Fox PM, PAR, Theil EC, Thorp HH. The upregulation of ferritin expression using a small molecule ligand to the native mRNA. Proc Natl Acad Sci USA. 2006;103:253–257. doi: 10.1073/pnas.0509744102. [DOI] [PMC free article] [PubMed] [Google Scholar]