Abstract
Objective
C-type natriuretic peptide (CNP) and thyroid hormone (TH) are essential for normal skeletal growth. Plasma CNP peptides correlate with growth velocity, but the relationship between thyroid status and CNP production is unknown. This study examined the impact of restoring normal TH levels on CNP and height velocity (HV) in children with acquired hypo- and hyperthyroidism.
Design
We performed a prospective, observational study in prepubertal children with acquired hypothyroidism (n=15) and hyperthyroidism (n=12).
Measurements
Blood levels of CNP, amino-terminal proCNP (NTproCNP), bone-specific alkaline phosphatase (BSAP), IGF-I, and TH levels were measured before and during the first six months of standard treatment for hypo- and hyperthyroidism, and correlations were determined.
Results
At baseline, HV, CNP, NTproCNP, and BSAP were significantly higher in hyper- than in hypothyroid subjects. Changes in TH after treatment were closely coupled to change in CNP and NTproCNP in hyper-, but not in hypothyroid children. In addition, a positive association of HV with CNP peptides was found during treatment of hyperthyroidism. Normalizing TH did not correlate with changes in BSAP or IGF-I in either group.
Conclusions
Plasma CNP peptides are higher in children with hyper- than in hypothyroidism at diagnosis, and, in hyperthyroid children, change concordantly with TH and HV during treatment. Differential responses of CNP in the two groups suggest CNP production is dependent on growth plate activity and not a direct effect of TH on CNP gene expression. Our findings suggest novel mechanisms underlying changes in skeletal response during treatment in children with acquired thyroid disease.
Keywords: C-type natriuretic peptide, amino-terminal proCNP, bone-specific alkaline phosphatase, hypothyroidism, hyperthyroidism, height velocity
Introduction
C-type natriuretic peptide (CNP) is a member of a family of peptides best known for their actions in cardiac and vascular tissues (1), and is expressed in a range of tissues (2) where it is thought to act locally in regulating cell proliferation and maturation. Recent studies employing genetic modification and observations from spontaneous mutations in animals and humans indicate that the CNP signaling pathway is essential to normal growth plate expansion and postnatal linear growth (3). Normal growth of the skeleton results from a highly coordinated process involving a range of local growth factors within the growth plate of long bones which is further regulated by circulating hormones at different phases of development. The amino-terminal propeptide of CNP (NTproCNP) is a stable product of the gene coding for CNP (natriuretic peptide precursor C, NPPC) (4). Recent studies in humans have shown that the plasma concentration of NTproCNP correlates with linear growth velocity across all phases of childhood growth (5-7). In addition, NTproCNP reflects changes in bone growth during interventions known to affect growth velocity in growing lambs (5,8) and children (7). Furthermore, multiple other hormones have concordant effects on linear growth and CNP secretion, including growth hormone and testosterone (stimulatory), and glucocorticoids (inhibitory).
Thyroid hormones (TH) are known to have profound effects on height velocity (HV) in children. Marked slowing or augmented skeletal growth occurs in profoundly hypo- and hyperthyroid states, respectively (9). Whether these changes involve the participation of CNP is unknown. Postulating that plasma CNP forms (i.e., NTproCNP and CNP) will reflect linear growth before and during restoration of euthyroid status, we studied serial changes in TH, plasma CNP forms, bone-specific alkaline phosphatase (BSAP), and insulin-like growth factor-I (IGF-I) in children presenting with acquired hypo- and hyperthyroidism.
Subjects and Methods
We performed a prospective, observational study in prepubertal children with acquired autoimmune thyroid disease to monitor HV and TH levels in relation to CNP, NTproCNP, and traditional markers of bone formation, including BSAP and IGF-I. Children with newly diagnosed hypo- and hyperthyroidism (93% and 75% female, respectively) were consecutively enrolled in this study and followed from 2007 to 2010 either at Children’s Hospital Los Angeles or at Nemours Children’s Clinic in Jacksonville, FL. Subjects were identified at the time of initial referral by their primary care provider for endocrine consultation regarding abnormal thyroid function tests. The study protocol was approved by the Institutional Review Boards of both institutions and written informed consent and assent were obtained from all families/subjects (as appropriate) prior to study participation.
Subjects were pre-screened as potential candidates based on age (≥3 yr) and initial thyroid function tests. In order to participate in the entire study, subjects with primary acquired hypothyroidism had to have a baseline elevation in thyroid-stimulating hormone (TSH) of >20 mIU/L and a low circulating thyroxine (T4 or free T4) level. Children with hyperthyroidism were included if laboratory evidence showed suppressed TSH (<0.1 mIU/L), and elevation of total or free T4 and/or total or free tri-iodothyronine (T3) levels.
Subjects were excluded if they were deemed to have congenital hypothyroidism or were on treatment for a thyroid disorder at the time of the first visit. In addition, children were required to be prepubertal on initial physical examination, defined as Tanner 1 breasts in females and prepubertal testicular volumes (<3 mL) in males. Subjects with any chronic disease or major illness or injury in the 6 mo prior to diagnosis were also excluded.
Outpatient visits occurred at regular intervals over the 6-mo study period. The baseline visit (#1) was followed by successive visits (#2-#8) occurring at approximately 2 wk, 4 wk, and 6 wk, and then monthly in hyperthyroid subjects and at 3 mo and 6 mo in hypothyroid subjects. During this time, subjects received standard treatment with levothyroxine (starting dose of 25 μg/m2/day for hypothyroid subjects) or methimazole (starting dose of 0.4-0.7 mg/kg/day divided into three daily doses for hyperthyroid subjects). Medication doses were subsequently adjusted according to the TH response. At each study visit, anthropometric measures were performed. Height was measured in triplicate using a wall-mounted stadiometer, accurate to 0.1 cm, and the average of the measurements was used. Height standard deviation scores (SDS) were derived from reference growth standards (10). Weight was measured on an electronic scale accurate to 0.1 kg. HVs were annualized (cm/yr) using measurements from previous visits. Baseline HV was calculated using growth records provided by referring physicians. Because dates of height measurements after enrollment did not always coincide with those of blood sampling, quadratic curves were fitted to each individual’s serial height data so that HV could be derived for any single time point. This approach also has the potential to reduce imprecision arising from variations in posture, diurnal effects, etc. A quadratic function was chosen on the a priori assumption that HV would de-accelerate (convex curve) or accelerate (concave curve) once treatment started in hyper- and hypothyroid children, respectively. Bone age radiographs were performed at baseline. Each film was read separately by two pediatric endocrinologists blinded to diagnosis, using the method of Greulich and Pyle (11), and the readings averaged. Differences between the two evaluators were less than 1 year in 92% of the readings. Blood samples were drawn for the following laboratory studies: total T3, total T4, and third-generation TSH by chemiluminescent assay with a Vitros ECi analyzer (Ortho Clinical Diagnostics, Rochester NY) or by immunoenzymatic assay with a Unicel DXI 800 Access® analyzer (Beckman Coulter, Inc., Brea CA). BSAP was also processed by a Unicel DXI 800 Access® analyzer via a chemiluminescent immunoassay (Beckman Coulter, Inc., Brea CA), and IGF-I by competitive binding radioimmunoassay (Esoterix/LabCorp Endocrine Sciences, Calabasas Hills CA). EDTA plasma from all samples was stored at −80 C and then processed for radioimmunoassay of CNP and NTproCNP in the laboratories of the Christchurch Cardioendocrine Research Group (Christchurch School of Medicine, University of Otago, Christchurch New Zealand) as previously described (4,5). The limit of detection of the assays for human NTproCNP and CNP are 0.4 pmol/L and 0.2 pmol/L, respectively. Cross-reactivity with either T3 or T4 was <0.002%. Within- and between-assay coefficients of variation were 6.5% and 7.5%, respectively, at 15 pmol/L for NTproCNP, and 2.6% and 5.9%, respectively, at 8 pmol/L for CNP. SDS for CNP and NTproCNP were calculated using a reference range compiled from healthy normal children as previously described (12).
Statistical Methods
Basic descriptive statistical approaches were used to describe the demographic characteristics of the study sample. Continuous variables were summarized using mean and SD for normally distributed variables or median with interquartile range as appropriate. The targeted sample size of 18 evaluable subjects per group would have 80% power with Type I error=0.05 to detect a mean within-group z-score that differs from zero by 0.61 or more with 1-sided paired t-test, a between group difference of at least 0.85 with a 1-sided independent sample t-test, and a Pearson correlation coefficient of 0.41 or greater.
The hypo- and hyperthyroid groups were compared at baseline with t-tests or Wilcoxon rank sum test for continuous variables and Fisher’s exact test for categorical variables. The associations between variables at baseline and change in variables, from baseline to the mean of values drawn between 2.5 - 6 mo, were quantified by Spearman correlation analysis. All tests were two-sided with statistical significance set at a p-value of 0.05. Patterns of change in analyte concentrations and HV across the treatment period were assessed by repeated measures ANOVA. General linear models were used to test associations (correlations) between variables using data obtained from visits 1-8. Statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary NC) and Intercooled Stata version 10.1 (StataCorp LP, College Station TX).
Results
Baseline data
Twenty -seven prepubertal subjects (85% female) with newly diagnosed acquired hypothyroidism (n=15) and hyperthyroidism (n=12) participated in this study. Anti-thyroid antibodies were measured and were positive in all but one of the subjects. That subject also had a thyroid nodule and pathology after excision demonstrated Hashimoto thyroiditis. Except for one hyperthyroid female who showed early breast-budding (T2) at 2 mo, all subjects remained prepubertal for the initial 5 mo of treatment. At the last visit (i.e., at 6 mo), 8 females (4 hypo- and 4 hyperthyroid) subjects showed T2 development.
Baseline characteristics are shown in Table 1. At the initial visit, chronological age and gender distributions of hypo- and hyperthyroid subjects were similar. As expected, mean bone age was younger in those with hypothyroidism compared to those with hyperthyroidism (6.8 ± 2.2 yr vs. 9.5 ± 1.9 yr, p=0.002). Also as expected, mean height SDS was lower in the hypothyroid group compared to that of the hyperthyroid group (−0.61 ± 1.09 vs. 1.58 ± 0.84, p<0.0001) and mean HV was significantly lower at baseline in those with hypothyroidism vs. those with hyperthyroidism (3.5 ± 2.0 cm/yr vs. 10.0 ± 3.2 cm/yr, p<0.0001).
Table 1.
Baseline Characteristics
| Hypothyroidism | Hyperthyroidism | p-value | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Number of subjects | 15 | 12 | |
| Age (yr) | 7.9 ± 2.6 | 8.0 ± 2.2 | 0.90 |
| Bone age (yr) | 6.8 ± 2.2 | 9.5 ± 1.9 | 0.002 |
| Gender (% female) | 93% | 75% | 0.29 |
| Height SDS | −0.61 ± 1.09 | 1.58 ± 0.84 | <0.0001 |
| Height velocity (cm/yr) | 3.5 ± 2.0 | 10.0 ± 3.2 | <0.0001 |
| TSH (mU/L) | 107 (31.3,150)* | 0.03 (0.02, 0.03)* | 0.0002 |
| T3 (nmol/L) | 1.61 ± 0.83 | 9.1 ± 3.61 | <0.0001 |
| T4 (nmol/L) | 38.6 ± 29.6 | 248.4 ± 63.1 | <0.0001 |
| CNP (pmol/L) | 1.1 (0.9,1.6)* | 1.8 (1.7, 2.2)* | 0.02 |
| CNP SDS | −1.08 (−1.48-0.10)* | 0.65 (0.15,1.31)* | 0.02 |
| NTproCNP (pmol/L) | 35.3 (29.2,48.1)* | 58.1 (37.8, 76.7)* | 0.02 |
| NTproCNP SDS | 0.03 (−0.67, 0.90)* | 2.28 (1.21,3.51)* | 0.03 |
| NTproCNP:CNP | 32.2 ± 8.6 | 31.3 ± 8.7 | 0.81 |
| BSAP (mcg/L) | 59.6 ± 29.3 | 102.9 ± 21.3 | 0.0003 |
| IGF-I (nmol/L) | 16.6 ± 6.0 | 18.2 ± 7.0 | 0.54 |
Note: Numbers are mean ± SD, except those marked with an asterix, which are median with interquartile range. Significant values in are in bold.
Laboratory studies at baseline showed that CNP and NTproCNP levels were positively correlated (r=0.76, p<0.0001). Subjects with hypothyroidism had a significantly lower mean CNP SDS (p=0.02) and lower mean NTproCNP SDS (p=0.03) compared to those with hyperthyroidism. The mean ratio of NTproCNP to CNP (a measure of CNP clearance) was not different between groups (p=0.81). Mean BSAP at baseline was significantly lower in hypothyroid children (p=0.0003). There was no difference in mean IGF-I (p=0.54) (Table 1).
Correlations between CNP forms and analytes at baseline in hypo- and hyperthyroid groups are shown in Table 2. In hyperthyroid subjects, significant associations of CNP with TH (T4 and T3) and of NTproCNP with BSAP were observed. In hypothyroidism, the only significant association was that of NTproCNP with BSAP. There was no association of IGF-I with CNP peptides in either group (Table 2).
Table 2.
Baseline Correlation of CNP and NTproCNP to Variables
| Analyte | CNP | NTproCNP | ||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| A. Hyperthyroidism | ||||
| Total T3 (nmol/L) | 0.76 | 0.004 | 0.50 | 0.09 |
| Total T4 (nmol/L) | 0.64 | 0.03 | 0.50 | 0.10 |
| BSAP (mcg/L) | 0.54 | 0.07 | 0.70 | 0.01 |
| IGF-I (nmol/L) | −0.20 | 0.52 | −0.47 | 0.12 |
| B. Hypothyroidism | ||||
| Total T3 (nmol/L) | 0.30 | 0.30 | 0.27 | 0.34 |
| Total T4 (nmol/L) | 0.16 | 0.56 | 0.02 | 0.95 |
| BSAP (mcg/L) | 0.35 | 0.22 | 0.59 | 0.03 |
| IGF-I (nmol/L) | 0.29 | 0.31 | −0.16 | 0.59 |
Note: Significant values in are in bold.
Response to treatment
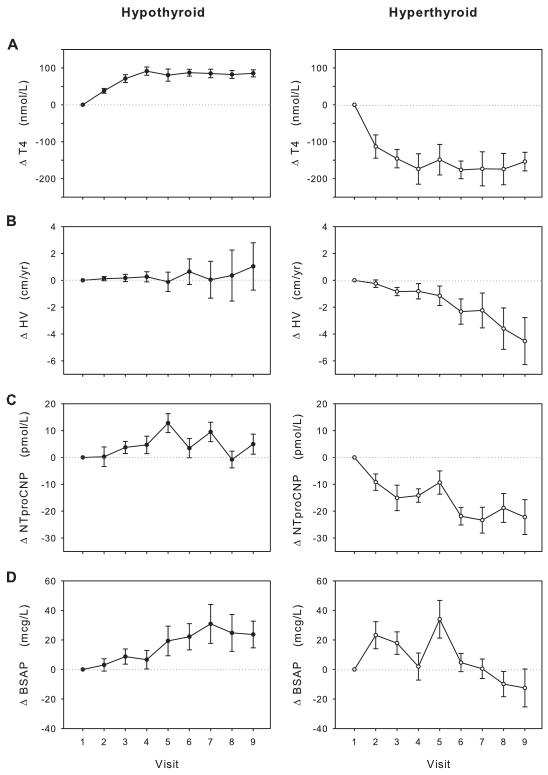
Serial changes in HV and plasma analytes during treatment are shown separately for hypo- and hyperthyroid groups in Figure 1. Overall, euthyroid status as judged by absolute concentrations of T4 (130.0 + 27.0 and 104.2 + 92.7 nmol/L) in the hypo and hyperthyroid groups, respectively. This was attained in both groups by approximately 6 to 8 weeks after initiation of treatment (Fig 1A). Mean changes in HV and mean plasma NTproCNP, while concordant in both groups, were greater during treatment in the hyperthyroid group (Fig 1B and 1C) compared to the hypothyroid group. A similar profile was observed for plasma CNP (data not shown). In contrast, mean changes in BSAP (Fig 1D) differed. Despite the marked declines in mean HV (F=3.7, p=0.002) and mean NTproCNP (F=6.9, p<0.001) and CNP (F=2.3, p=0.04) in hyperthyroid children, mean BSAP did not decrease significantly over time (F=1.5, p=0.17) and was greater than at baseline until about 13 weeks of treatment. In the hypothyroid children, although directional changes in BSAP (F=5.3, p=<0.001), NTproCNP (F=1.3, p=0.24) and HV (F=0.2, p=0.99) were similar, only those for BSAP were significant. No significant difference in serum IGF-I levels were observed between the hypo- and hyperthyroid groups (data not shown).
Figure 1.
Change (Δ) of total T4 (A), height velocity (HV, B), plasma NTproCNP (C), and BSAP (D) from baseline in response to treatment of subjects with hypo- (left) and hyperthyroidism (right). Visit 4 corresponds to 4-6 wk and visit 9 to approximately 24 wk after commencing treatment.
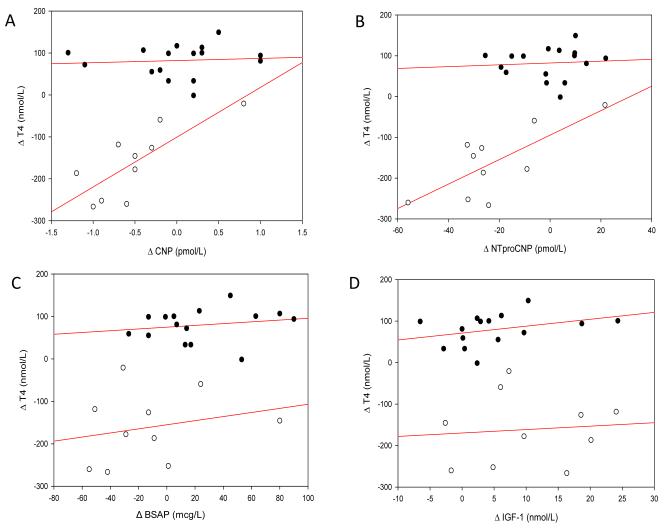
In order to assess the effects of changing concentrations of T4, we compared changes (from baseline to mean of values drawn between 2.5-6 mo) in T4 with those of CNP (Fig 2A), NTproCNP (Fig 2B), BSAP (Fig 2C), and IGF-I (Fig 2D). Highly significant correlations were observed between change in T4 and change in CNP peptides in hyperthyroidism (CNP, r=0.78, p=0.007; NTproCNP r=0.73, p=0.02), but there was no association of T4 with either BSAP or IGF-I. In the hypothyroid group, no significant associations were observed between changing T4 concentration and any one of the four analytes studied (Fig 2, A-D). Consistent with the above, a significant and positive association of HV (fall) was noted with plasma NTproCNP during treatment of hyperthyroid children (r=0.40, p=0.002). Conversely, the relationship between BSAP and HV was not significant in hyperthyroidism (r=0.21, p=0.13), but was highly significant in hypothyroidism (r=0.34, p=0.001). There was no association between IGF-I and HV in either group.
Figure 2.
Correlation of change in T4 during treatment of hypo- (filled symbols) and hyperthyroid (open symbols) subjects, from baseline to mean at 2.5-6 mo, with
(A) Δ CNP (Hypo: r=0.07, p=0.80, Hyper: r=0.78, p=0.007),
(B) Δ NTproCNP (Hypo: r=0.08, p=0.78, Hyper: r=0.73, p=0.02),
(C) Δ BSAP (Hypo: r=0.17, p=0.55, Hyper: r=0.23, p=0.52), and
(D) Δ IGF-I (Hypo: r=0.28, p=0.33, Hyper: r=0.09, p=0.80).
Regression lines were derived by the method of least squares.
Effects of bone maturation on NTproCNP responses to treatment
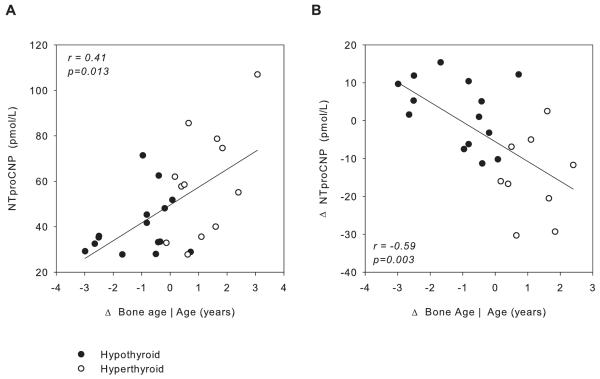
Because change in skeletal maturation is likely to indicate the duration and severity of the disturbed thyroid function and, hence, the amount of catch-up or catch-down growth expected early in the treatment period, the relation between deviation of bone age from chronological age and plasma NTproCNP was examined. As shown in Fig 3A, plasma NTproCNP was more perturbed (lower in hypothyroidism and higher in hyperthyroidism) at baseline in children exhibiting more profound change in bone age (r=0.41, p=0.013). Similarly, as shown in Fig 3B, when NTproCNP was measured after 1 month of treatment, the change in NTproCNP values from baseline was strongly correlated with deviation in baseline bone age from chronological age (r=−0.59, p=0.003).
Figure 3.
A. Relation between plasma NTproCNP at baseline and deviation in bone age from chronological age (Δ bone age) in hypo- (filled symbols) and hyperthyroid subjects (open symbols). B. Relation between change from baseline in plasma NTproCNP at 1 mo of treatment (Δ) and deviation in bone age from chronological age in hypo- (filled symbols) and hyperthyroid subjects (open symbols). Regression lines were derived by the method of least squares.
Discussion
This is the first report to document the changes in plasma CNP peptides in children presenting with acquired disorders of thyroid function. In keeping with the known effects of TH on skeletal growth, we found at presentation highly significant differences in HV, CNP peptides, and bone turnover (BSAP), but not in IGF-I, in hyperthyroid children when compared to those with hypothyroidism. During treatment restoring euthyroidism, changes in HV and CNP peptides were greater in hyperthyroid children where T4 and CNP/NTproCNP were closely coupled. Since equally strong relationships were found for both CNP and bio-inactive NTproCNP and the ratios of NTproCNP to CNP were unaltered, it is reasonable to assume that these relationships are based on changes in CNP secretion rather than a response to changes in peptide clearance. Taken together, these novel findings suggest important interactions of TH and CNP signaling within growth plates and show that TH can be added to the previously documented factors (which include GH, testosterone, glucocorticoids, and nutrients) impacting growth and circulating CNP forms in juveniles.
Whereas other reports document the medium- (beyond 6 mo) and long-term response of linear growth to treatment in hypothyroid children, there are few detailed prospective studies of the early response. There are none reporting the growth responses of hyperthyroid children. Our observations of the responses of the two groups reveal several novel findings. First, although height SDS (and bone age) were similarly perturbed at baseline and consistent with the well-recognized effects of excess and deficiency of TH on the skeleton, change in HV in response to treatment differed in the two groups. Decrease in both HV and plasma CNP forms was more prompt and greater in subjects with hyperthyroidism than the changes observed in subjects with hypothyroidism. Proportionate change (i.e., at 6-wk compared to basal) in TH values (3-fold rise in hypothyroidism and 4-fold fall in hyperthyroidism), while greater in the latter group, seem insufficient to account for these different responses in HV and plasma CNP forms during treatment. Why the skeletal response of the hypothyroid group was less sensitive to change in T3 is unclear, but could relate to dysregulated signaling pathways and the disorganized appearance of the hypothyroid growth plate as reported in rodents (13). Although the condition of the growth plate in hypothyroid children is largely unknown, there are likely to be structural changes with increased deposition of glycosaminoglycans (14) which may reduce access of CNP to chondrocytes. Furthermore, TSH is reported to impair CNP signalling in cultured rat thyroid follicular cells (15), raising the possibility that the bioactivity of CNP is reduced in primary hypothyroidism or at least until TSH concentrations are restored to normal. In contrast, in hyperthyroidism, the structural integrity of the growth plate is retained (13,14). Since some 70% of linear growth results from the increase in cell volume of hypertrophic chondrocytes, reducing drive from T3 at this late stage of endochondral growth would be expected to have a major impact on HV and, for the reasons stated below, on plasma CNP forms as well, as shown in the current study. The failure to show a significant correlation of CNP forms with HV in the hypothyroid group likely follows from the small (and temporally variable) response in HV in these children, features which do not apply in the hyperthyroid children.
While we cannot exclude contributions to circulating concentrations of CNP forms from extra-skeletal sources, prior evidence from studies in lambs (16) and children (5), and more recently from GH-deficient rodent pups (17), strongly supports the view that the growth plate or a closely related tissue is a major source. Since in vitro and ex vivo studies show that the integrity of T3 signaling is essential to endochondral bone growth (9) and that lack or excess of T3 strongly impacts HV in children, the current findings are consistent with the hypothesis that T3 is one of the factors driving the production of CNP by growth plate chondrocytes. Moreover, an effect of duration and severity of the disturbed T3 production on CNP secretion is evidenced by the links between plasma NTproCNP and the degree of change in skeletal maturation. Several in vitro studies show that T3 acts principally to promote differentiation of proliferating cells to hypertrophic chondrocytes and facilitates their progression (9,13). A key pathway mediating these actions of T3 in growth plate tissues includes BMP/Wnt 4 ß-catenin activation (18) modulated by IGF-I/P13K/Akt signaling (19). To our knowledge, the role of CNP in the trophic action of T3 within growth plate tissues has not been examined. In this context, it is intriguing to note that the principal growth-promoting locus of CNP is also the hypertrophic chondrocyte (20) and that the molecular events underpinning this action involve some of the same mediators, e.g., PI3K/Akt (21) and Wnt ß-catenin (22). Our findings that changes in CNP forms correlate highly with T4 and HV in hyper-, but not in hypothyroidism, suggest that TH do not directly increase CNP gene expression, but act rather by increasing the supply of pre-hypertrophic chondrocytes [the putative dominant site of NPPC gene expression in the growth plate (23)]. However, the dynamic changes we observed during treatment strongly suggest that CNP responds to T3 drive and is downstream of T3 signaling.
Another novel observation is the differential pattern of response in BSAP shown by the two groups during treatment. Previous studies (5,8) in growing lambs have shown a concordant (but slightly delayed) response of BSAP with plasma CNP forms in keeping with the close coupling of growth plate expansion and mineralization of newly formed osteoid. This pattern is evident in the response of the hypothyroid group. However, the temporal (and directional) response to treatment in hyperthyroid children was quite different, with discordant changes in plasma NTproCNP (fall) and BSAP (rise) as euthyroid status was restored over approximately 6 wk. As well as indicating that these analytes are biomarkers of separate and distinct processes within the skeleton, the findings suggest that the BSAP response (as observed in serum) is the sum of changing activity in several different tissues, including those of bone remodeling units and membranous bone, in addition to the growth plate and newly formed osteoid (9). In hyperthyroid adults (where growth plates are atrophic), BSAP is increased at presentation in keeping with increased bone turnover (24) and rises further in the first 6-8 wk as normal levels of T3 are restored. Since bone density also increases (25), this rise in BSAP (a marker of bone formation) likely reflects increasing mineralization of an osteopenic skeleton. In contrast, in hypothyroid adults, markers of bone formation appear to be less affected or unchanged (26). Hyperthyroid children will also have varying degrees of under-mineralized bone remodeling units. Seen in this light, the rise in BSAP is not unexpected and, relative to the mass of tissue involved (osteopenic trabeculae versus growth plate-related tissue), would be expected to mask any fall in BSAP related to reduction in endochondral bone growth. While it is tempting to view the consistent fall observed in plasma NTproCNP in hyperthyroid children as largely a response to reduced activity of growth plates, change in CNP gene expression in osteoblasts (27) or osteoclasts cannot be excluded until more is known of the role of CNP, if any, in bone remodeling. In this context, it will be important to study changes in plasma CNP forms accompanying disorders of bone turnover in adults, including the responses of those with thyrotoxicosis.
Consistent with previous findings in childhood hypothyroidism (28), we found no association of serum IGF-I with T3 across the whole spectrum of TH concentrations in serum and, similarly, we found no correlation of IGF-I with HV or BSAP in either group. Since circulating IGF-I is largely produced in the liver (29) and does not necessarily correlate with events at the growth plate, the above observations are not unexpected. In contrast, compared to the liver and other organs, the skeleton appears to make significant contributions to plasma CNP forms in youth and plasma levels are positively associated with HV. Based on previous data showing an increase of NTproCNP in response to recombinant human growth hormone therapy (7), we conclude that CNP expression is also downstream of the IGF-I effect within growth plate chondrocytes. These unique features of CNP suggest that measurements of plasma CNP forms could provide a useful index of growth plate function in children receiving growth-promoting therapies. Thus, in the context of thyroid-based disorders of growth, a significant and sustained rise in plasma NTproCNP during treatment of hypothyroidism is likely to herald an increase in HV. Similarly, in hyperthyroid children with accelerating HV, the finding of a fall in NTproCNP could be useful in predicting an expected slowing of HV well before formal measurements of HV become available.
In conclusion, plasma NTproCNP and CNP levels in children presenting with hyperthyroidism are higher than those of hypothyroid children of similar age. Directional changes in TH, HV, and NTproCNP are concordant in response to treatment, whereas plasma IGF-I is unaffected. Links between skeletal maturation and plasma NTproCNP at presentation, and the early NTproCNP response to treatment, provide further evidence of the participation of CNP in growth plate activity and linear growth. Together with results from previous work, these findings suggest that NTproCNP may be a useful biomarker for linear growth in children with abnormal growth patterns.
Acknowledgments
This project was funded in part by an investigator-initiated grant from Pfizer and the General Clinical Research Center (GCRC) at Children’s Hospital Los Angeles (M01-RR00043-45) and Children’s Hospital Los Angeles Clinical Translational Science Institute (1UL1RR031986), with funds provided by the National Center for Research Resources (NCRR).
Footnotes
Disclosure summary: TCP and EAE have a patent pending for NTproCNP peptides and uses thereof. MEG receives funding from Pfizer for institutional participation in KIGS (Pfizer International Growth Database); for research subject participation in the multi-center clinical trial: “A Four-Year Open-Label Multi-Center Randomized Two-Arm Study of Genotropin in Idiopathic Short Stature Patients: Comparing an Individualized, Target-Driven Treatment Regimen to Standard Dosing of Genotropin”; and for serving as a member of US and international KIGS advisory boards.
References
- 1.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 2.Minamino N, Aburaya M, Kojima M, et al. Distribution of C-type natriuretic peptide and its messenger RNA in rat central nervous system and peripheral tissue. Biochem Biophys Res Commun. 1993;197:326–335. doi: 10.1006/bbrc.1993.2479. [DOI] [PubMed] [Google Scholar]
- 3.Olney RC. C-type natriuretic peptide in growth: a new paradigm. Growth Horm IGF Res. 2006;16(Suppl A):S6–14. doi: 10.1016/j.ghir.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Prickett TC, Yandle TG, Nicholls MG, et al. Identification of amino-terminal pro-C-type natriuretic peptide in human plasma. Biochem Biophys Res Commun. 2001;286:513–517. doi: 10.1006/bbrc.2001.5419. [DOI] [PubMed] [Google Scholar]
- 5.Prickett TC, Lynn AM, Barrell GK, et al. Amino-terminal proCNP: a putative marker of cartilage activity in postnatal growth. Pediatr Res. 2005;58:334–340. doi: 10.1203/01.PDR.0000169964.66260.4B. [DOI] [PubMed] [Google Scholar]
- 6.Prickett TC, Dixon B, Frampton C, et al. Plasma amino-terminal pro C-type natriuretic Peptide in the neonate: relation to gestational age and postnatal linear growth. J Clin Endocrinol Metab. 2008;93:225–232. doi: 10.1210/jc.2007-1815. [DOI] [PubMed] [Google Scholar]
- 7.Olney RC, Prickett TC, Yandle TG, et al. Amino-terminal propeptide of C-type natriuretic peptide and linear growth in children: effects of puberty, testosterone, and growth hormone. J Clin Endocrinol Metab. 2007;92:4294–4298. doi: 10.1210/jc.2007-0567. [DOI] [PubMed] [Google Scholar]
- 8.Prickett TC, Barrell GK, Wellby M, et al. Response of plasma CNP forms to acute anabolic and catabolic interventions in growing lambs. Am J Physiol Endocrinol Metab. 2007;292:E1395–E1400. doi: 10.1152/ajpendo.00469.2006. [DOI] [PubMed] [Google Scholar]
- 9.Gogakos AI, Bassett JH Duncan, Williams GR. Thyroid and bone. Arch Biochem Biophys. 2010;503:129–136. doi: 10.1016/j.abb.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Prader A, Largo RH, Molinari L, et al. Physical growth of Swiss children from birth to 20 years of age. First Zurich longitudinal study of growth and development. Helv Paediatr Acta Suppl. 1989;52:1–125. [PubMed] [Google Scholar]
- 11.Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd edition Stanford University Press; Stanford, CA: 1959. [Google Scholar]
- 12.Olney RC, Permuy JW, Warde MK, et al. Amino-terminal propeptide of c-type natriuretic peptide (NTproCNP): reference range and levels in children with idiopathic short stature treated with growth hormone. Growth Horm IGF Res. 2010;20(Suppl.1):S45. (Abstract P16) [Google Scholar]
- 13.Stevens DA, Hasserjian RP, Robson H, et al. Thyroid hormones regulate hypertrophic chondrocyte differentiation and expression of parathyroid hormone-related peptide and its receptor during endochondral bone formation. J Bone Miner Res. 2000;15:2431–2442. doi: 10.1359/jbmr.2000.15.12.2431. [DOI] [PubMed] [Google Scholar]
- 14.Bassett JH, Swinhoe R, Chassande O, et al. Thyroid hormone regulates heparan sulfate proteoglycan expression in the growth plate. Endocrinology. 2006;147:295–305. doi: 10.1210/en.2005-0485. [DOI] [PubMed] [Google Scholar]
- 15.Sellitti DF, Puggina E, Lagranha C, et al. cAMP inhibits natriuretic peptide receptor-B activity and increases C-type natriuretic peptide in FRTL-5 rat thyroid cells. J Endocrinol. 2004;180:23–34. doi: 10.1677/joe.0.1800023. [DOI] [PubMed] [Google Scholar]
- 16.Prickett TC, Charles CJ, Yandle TG, et al. Skeletal contributions to plasma CNP forms: evidence from regional sampling in growing lambs. Peptides. 2009;30:2343–2347. doi: 10.1016/j.peptides.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Prickett TCR, Bothwell JC, Yandle TG, et al. Pharmacodynamic responses of c-type natriuretic peptide to GH in growth hormone deficient rats: correlation with linear growth. The 92th Annual Meeting of the Endocrine Society; San Diego, CA. 2010. (Abstract P3-234) [Google Scholar]
- 18.Lassova L, Niu Z, Golden EB, et al. Thyroid hormone treatment of cultured chondrocytes mimics in vivo stimulation of collagen X mRNA by increasing BMP 4 expression. J Cell Physiol. 2009;219:595–605. doi: 10.1002/jcp.21704. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Shao YY, Ballock RT. Thyroid hormone-mediated growth and differentiation of growth plate chondrocytes involves IGF-1 modulation of beta-catenin signaling. J Bone Miner Res. 2010;25:1138–1146. doi: 10.1002/jbmr.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasoda A, Komatsu Y, Chusho H, et al. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004;10:80–86. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- 21.Ulici V, Hoenselaar KD, Gillespie JR, et al. The PI3K pathway regulates endochondral bone growth through control of hypertrophic chondrocyte differentiation. BMC Dev Biol. 2008;8:40. doi: 10.1186/1471-213X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasaki Y, Kugimiya F, Chikuda H, et al. Phosphorylation of GSK-3beta by cGMP-dependent protein kinase II promotes hypertrophic differentiation of murine chondrocytes. J Clin Invest. 2008;118:2506–2515. doi: 10.1172/JCI35243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chusho H, Tamura N, Ogawa Y, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci U S A. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pantazi H, Papapetrou PD. Changes in parameters of bone and mineral metabolism during therapy for hyperthyroidism. J Clin Endocrinol Metab. 2000;85:1099–1106. doi: 10.1210/jcem.85.3.6457. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqi A, Burrin JM, Noonan K, et al. A longitudinal study of markers of bone turnover in Graves’ disease and their value in predicting bone mineral density. J Clin Endocrinol Metab. 1997;82:753–759. doi: 10.1210/jcem.82.3.3804. [DOI] [PubMed] [Google Scholar]
- 26.Sabuncu T, Aksoy N, Arikan E, et al. Early changes in parameters of bone and mineral metabolism during therapy for hyper- and hypothyroidism. Endocr.Res. 2001;27:203–213. doi: 10.1081/erc-100107181. [DOI] [PubMed] [Google Scholar]
- 27.Suda M, Tanaka K, Fukushima M, et al. C-type natriuretic peptide as an autocrine/paracrine regulator of osteoblast. Evidence for possible presence of bone natriuretic peptide system. Biochem Biophys Res Commun. 1996;223:1–6. doi: 10.1006/bbrc.1996.0836. [DOI] [PubMed] [Google Scholar]
- 28.Purandare A, Co NL, Godil M, et al. Effect of hypothyroidism and its treatment on the IGF system in infants and children. J.Pediatr Endocrinol Metab. 2003;16:35–42. doi: 10.1515/jpem.2003.16.1.35. [DOI] [PubMed] [Google Scholar]
- 29.Ohlsson C, Mohan S, Sjogren K, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]