Abstract
Background
We sought to prospectively duplicate our retrospective findings showing prestorage leukoreduction (PS-LR) blunts the detrimental effect of aging on banked packed red blood cells (PRBCs) transfused after injury.
Methods
Over 19 months, trauma patients transfused > 4 units PRBCs and surviving at least 24 hours were followed. The age of each unit was collected.
Results
The cohort consisted of 153 patients. All models showed no association between advancing blood age and the likelihood of developing multiorgan dysfunction syndrome (MODS) or infections regardless of whether mean age of blood was analyzed as a continuous variable, as a percentage of blood received which was <14 days old, or as a dichotomized value above or below 14 days old.
Conclusions
This prospective study duplicates our retrospective findings of an abrogation of the detrimental effects of advancing mean PRBC age on outcomes after trauma by performing PS-LR.
WORDS FOR INDEXING: Trauma, leukoreduction, outcomes, transfusion
INTRODUCTION
The decision to transfuse a patient with packed red blood cells (PRBCs) should balance the beneficial effects of increasing oxygen carrying capacity against the well known immunomodulatory effects that accompany this intervention [1–8]. A strong body of evidence implicates the length of time that a given unit of blood sits in storage after donation as a cause of these immune-modulating properties. Purdy et al demonstrated that septic patients who died had received blood which was a median of 8 days “older” (ie, PRBC units that had in the blood bank for 8 days longer) than a comparison cohort of survivors [9]. Further, Zalen and colleagues showed that trauma patients who developed multiple organ failure received blood that was an average of 6 days older than similarly injured patients in whom the syndrome was not seen [10]. Finally, Offner and colleagues demonstrated that each unit of blood greater than 14 days old received increased the odds of an infectious complication by 12%[11].
One hypothesis for the mechanism behind this storage lesion centers on the role of leukocytes in the donor units. Previous work has suggested that these leukocytes exert a variety of immunomodulatory effects on the recipient in a magnitude that is proportional to the length of time the donor unit is stored [12–16]. The process of prestorage leukoreduction (PS-LR) removes 99.9% of donor white blood cells, and would presumably abrogate any immunomodulatory effect resulting from donor leukocyte storage and transfusion. Earlier efforts by our group demonstrated that PS-LR did not impact mortality rates in trauma patients, but it and others examining the effects of PS-LR on outcomes in transfused trauma patients did not control for the age of the units transfused [17–19]. Given the importance of this confounder, our group subsequently examined the relationship between PS-LR and the age effect of transfused blood in trauma patients in a retrospective fashion [20]. We found that PS-LR blunted the detrimental effect of aging on banked PRBCs, and that the receipt of PS-LR blood with an older mean age was associated with a protective effect on survival in some analyses. These results were at odds with another retrospective study of this question in which the effect of older blood was examined on a unit-by-unit basis [21]. We therefore sought to duplicate our results with a prospective cohort study in which we would perform both analyses. To our knowledge, this effort is the first prospective investigation in trauma patients to examine this issue.
METHODS
Study Design and Patients
This prospective cohort study was approved by the Institutional Review Board, and due to its observational nature a waiver of consent was granted. Our institution practiced universal PS-LR on all blood products throughout the time period of the study. Patient accrual took place at our urban Level I trauma center for a total of 19 months. Inclusion criteria were all trauma patients who: 1) were transfused at least four units packed red blood cells (PRBCs) within their first 24 hours in the hospital, 2) survived 24 hrs post-injury, and 3) were >18 yrs old. Pregnant patients and prisoners were excluded as they constituted vulnerable populations. Demographic and physiologic data were collected, as were the total amount of PRBCs transfused in the first 24 hrs, age in days of each unit transfused, and outcomes.
Dependent Variables
The primary outcomes were development of multiple organ dysfunction syndrome (MODS) and infectious complications. Each outcome was a binomial variable operationally defined as the presence or absence of the condition. In this study, we modeled the probability of the presence of the outcome (death, MODS, and infectious complications, respectively).
A modified Marshall score [22] identical to that used by the National Institute of General Medical Science’s “Inflammation and the Host Response to Injury” project (the “Glue Grant”) was used to calculate the rate of MODS. This modification consists of dropping the neurologic component of the score, starting to calculate scores 48 hours post-injury, and using a score of six or greater as diagnostic of MODS.
Infectious complications were defined as follows: ventilator associated pneumonia (VAP) was diagnosed using quantitative bronchoalveolar lavage specimens of ≥104 colony forming units (CFU) and urinary tract infection (UTI) was diagnosed with a positive culture of ≥105 CFU. Wound infections were defined as cellulitis, erythema, or drainage requiring opening of a skin incision and/or the initiation of antibiotic therapy. Cavitary abscess, empyema, and mediastinitis were diagnosed after radiographic findings were clinically correlated with reoperative findings or percutaneous drainage. Catheter-related blood stream infections were diagnosed by peripheral blood cultures and a catheter tip growing an identical organism, while bacteremia was diagnosed by a non-S. epidermidis positive peripheral blood culture.
Independent Variables and Covariates
The primary independent variables were measured in four ways. First, mean age of blood was measured in days as a continuous variable. Second, the absolute number of PRBC units <14 days old as well as the total number of PRBC units transfused were counted for each patient. The percentage of blood transfused which was <14 days old was entered into the model as a continuous independent variable for each patient. Mean age of blood was also dichotomized as ≥ or < 14 days old (with <14 days as the reference group). Finally, mean age of blood for each patient was dichotomized as ≥ or < 21 days old (with <21 days as the reference group). Prospectively maintained covariates included total units of PRBCs transfused in the first 24 hours, patient age, Injury Severity Score (ISS) and Head Abbreviated Injury Score (AIS).
Data Analysis
Multiple logistic regression was used to estimate the adjusted odds of each outcome (MODS and infectious complications, respectively) with all covariates included in each model along with each respective independent variable. In model 1, mean age of blood was entered as a continuous independent variable. In model 2, the percentage of all PRBC units transfused which were <14 days old was entered as a continuous independent variable. In model 3, mean age of blood was entered after being dichotomized at 14 days as per the conventions of the field. The MODS and infectious complications analyses were also run with an additional analysis in which the age of blood was entered after dichotomization at 21 days. Models 2 and 3 were also repeated using mean age of blood as a binary indicator dichotomized at 21 days. Models 1–3 were conducted separately for each outcome and the 95% profile likelihood ratio confidence intervals (CI) were calculated for the Odds Ratios (OR). The likelihood ratio Chi-square statistic was used to test for a significant association between each independent variable/risk factor and each outcome. The area under the curve (AUC) for each multiple logistic regression model was calculated.
The Kaplan-Meier method of survival analysis was used to compare the time-to-first infection for patients with MODS vs. those without MODS. The hazard ratio was also estimated. The Logrank test was used to compare the two curves. As part of the Kaplan-Meier analysis, right-censoring was used in the current study. Right-censoring occurred when a patient completed the study by dying or being discharged without an infection.
We performed all statistical analyses using SAS 9.2 (SAS Institute, Inc., Cary, NC). The level of significance for all tests was set at α = .05 (two-tailed) and p values were left unadjusted for multiple testing.
RESULTS
Table 1 demonstrates the demographic characteristics for the 153 patient cohort.. The cohort was severely injured with a mean ISS of 25.9 ± 15.4 and a mean transfusion burden of 9.9 ± 9.0 units of PRBCs. Patients in whom MODS developed were found to be significantly more severely injured, had longer ICU and hospital stays, required higher volumes of transfusion in the first 24 hours, and had higher mortality (Table 2). Similarly, patients who developed at least one infectious complication were found to be more severely injured with longer ICU and hospital stays (Table 3). Of the 57 patients developing an infectious complication, 40.4% developed more than one. VAP and UTI were the most common infectious complication seen (Table 4).
Table 1.
Demographic characteristics of the cohort.
| n | 153 |
| Age (years) | 40.0 ± 16.4 |
| Male/female (%) | 78.4/21.6 |
| Blunt/penetrating (%) | 69.9/30.1 |
| ISS | 25.9 ± 15.4 |
| PRBC (units) | 9.9 ± 9.0 |
| Mortality | 11% |
| MODS | 18% |
| Infectious complications | 37% |
ISS= Injury Severity Score; PRBC= Packed Red Blood Cell; AIS= Abbreviated Injury Score; MODS= Multiple Organ Dysfunction Score. All values are means ± SD
Table 2.
Demographic characteristics of patients with and without MODS.
| MODS (n=28) | No MODS (n=125) | p value | |
|---|---|---|---|
| Age | 44.7 ± 17.1 | 39.1 ± 15.8 | 0.10 |
| Blunt/penetrating (%) | 85.7/14.3 | 67.9/32.1 | 0.06 |
| Male/Female (%) | 78.6/21.4 | 79.4/20.6 | 0.92 |
| Injury severity score | 32.9 ± 14.6 | 25.1 ± 15.1 | 0.01 |
| Head AIS | 1.4 ± 1.9 | 1.5 ± 1.9 | 0.81 |
| Chest AIS | 3.1 ± 1.5 | 2.1 ± 1.9 | 0.01 |
| Abdominal AIS | 2.2 ± 1.4 | 1.8 ± 1.6 | 0.22 |
| First base excess | −9.7 ± 5.4 | −8.2 ± 5.4 | 0.19 |
| First lactate | 4.4 ± 1.9 | 4.0 ± 2.5 | 0.44 |
| ICU Length of stay | 14.8 ± 9.6 | 8.1 ± 11.0 | 0.003 |
| Hospital Length of stay | 37.5 ± 43.7 | 21.9 ± 20.4 | 0.005 |
| Mortality (%) | 32.1 | 8.4 | 0.0003 |
| PRBCs in first 24 hrs | 13.5 ± 8.5 | 9.4 ± 9.0 | 0.03 |
AIS=Abbreviated Injury Score; ICU=Intensive Care Unit; PRBCs=Packed Red Blood Cells
Table 3.
Demographic characteristics of patients with and without infectious complications.
| Infection (n=57) | No Infection (n=96) | p value | |
|---|---|---|---|
| Age | 41.5 ± 15.8 | 39.6 ± 17.1 | 0.50 |
| Blunt/penetrating (%) | 82.5/17.5 | 63.5/36.5 | 0.01 |
| Male/Female (%) | 75.4/24.6 | 80.2/19.8 | 0.49 |
| Injury severity score | 32.3 ± 14.3 | 22.7 ± 14.9 | 0.0003 |
| Head AIS | 1.9 ± 2.0 | 1.1 ± 1.8 | 0.002 |
| Chest AIS | 2.7 ± 1.7 | 1.9 ± 1.9 | 0.002 |
| Abd AIS | 2.3 ± 1.6 | 1.6 ± 1.5 | 0.01 |
| First base excess | −7.6 ± 4.3 | −9.0 ± 6.1 | 0.13 |
| First lactate | 3.9 ± 1.7 | 4.2 ± 2.8 | 0.55 |
| ICU Length of stay | 16.2 ± 14.7 | 5.1 ± 5.0 | <0.0001 |
| Hosp Length of stay | 40.5 ± 34.5 | 15.2 ± 14.7 | <0.0001 |
| Mortality (%) | 8.8 | 13.5 | 0.38 |
| PRBCs in first 24 hrs | 9.6 ± 6.8 | 10.1 ± 9.9 | 0.72 |
AIS=Abbreviated Injury Score; ICU=Intensive Care Unit; PRBCs=Packed Red Blood Cells
Table 4.
Type and frequency of infectious complications.
| Infection type | Infectious episode | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | |
| Ventilator associated pneumonia | 24 | 5 | 5 | 2 | 2 | 1 | 1 | - | - |
| Urinary tract infection | 19 | 9 | 5 | 2 | 1 | 1 | 1 | 2 | - |
| Wound infection | 9 | 6 | 2 | 1 | 2 | 1 | 1 | - | - |
| intraabdominal abscess | - | 1 | 1 | - | - | - | - | - | - |
| Line infection | 1 | 1 | - | - | - | - | - | - | 1 |
| Other soft tissue | 2 | - | 1 | - | - | - | - | - | - |
| Sepsis | 2 | 2 | - | 1 | - | - | - | - | - |
| Other | - | 1 | 1 | - | - | - | - | - | - |
Overall, 63.4% of the patients in the current study were right-censored. About 87.6% of the patients without MODS were right-censored, whereas about 12.4% of those with MODS were right-censored.
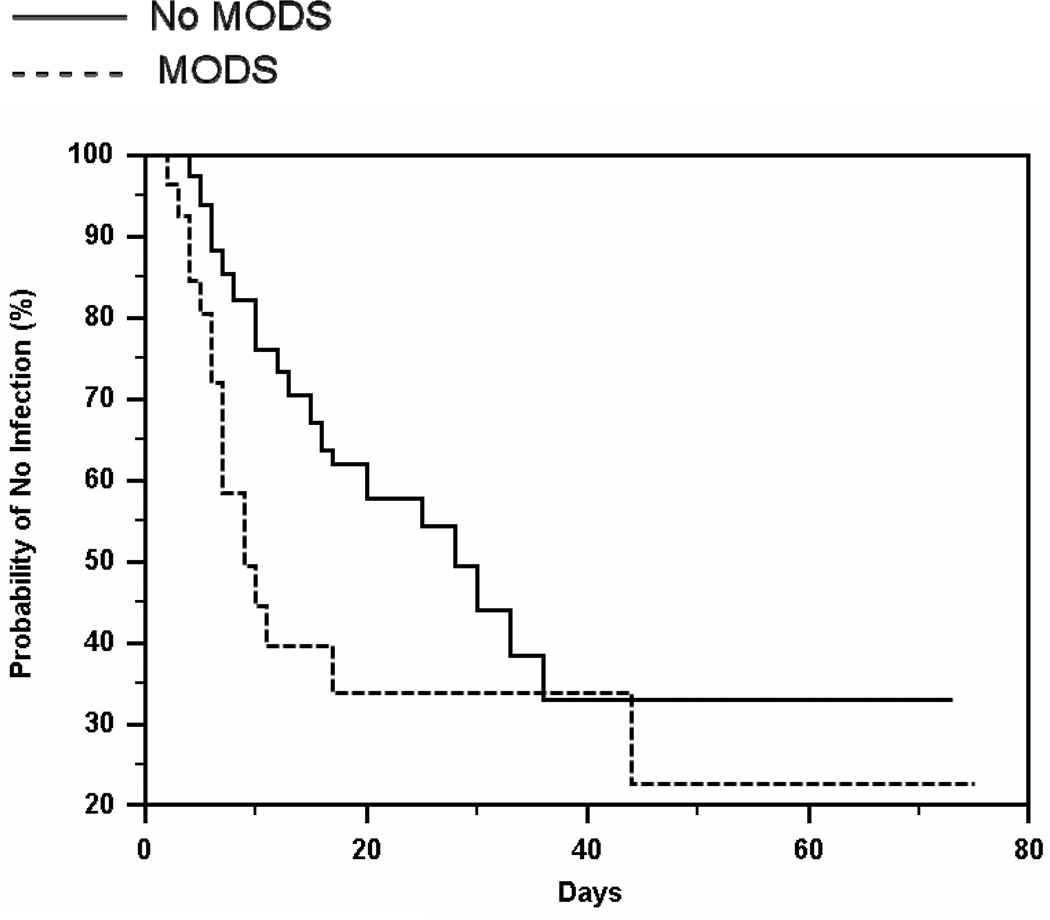
The Kaplan-Meier analysis revealed that the two curves of time-to-infection differed significantly between patients with MODS vs. those without MODS [χ2(1) = 6.35, p=.0118]. Figure 1 displays the survival curves and indicated that the probability of no infection was greater across the study for patients without MODS than for those with MODS. The median time to first infection was 28 days for patients without MODS and 9 days for those with MODS.
Fig 1. Time to infection for patients with and without MODS.
MODS patients had a significantly higher probability of infection (p=0.012) with a risk of slightly more than twice that of patients without MODS.
The Hazard Ratio indicated that patients with MODS had a significantly greater risk of infection than those without MODS (hazard ratio = 2.04, 95% CI = 1.00 to 4.13, p<.05). The hazard of infection for those with MODS was about 2 times greater than the hazard of infection for those without MODS.
For the MODS analysis, increasing ISS was found to be significantly associated with a higher likelihood of developing MODS through all analyses, as was increasing total amount of blood transfused in the analysis of dichotomization at 14 days and the analysis of the percentage of transfused blood <14 days old was (Table 5). Similarly, when conducting the analysis for infectious complications increasing ISS was found to be significantly associated with higher rates of infectious complications through all analyses (Table 6).
Table 5.
MODS analysis by model type.
| Variable | Odds ratio | 95% Confidence Interval |
|---|---|---|
| Mean PRBC age as a continuous variable | 0.969 | 0.910 – 1.030 |
| Number of transfused PRBC units | 1.044 | 0.997 – 1.095 |
| Patient age | 1.028 | 0.999 – 1.058 |
| ISS | 1.046 | 1.011 – 1.085 |
| Head AIS | 0.868 | 0.644 – 1.147 |
| Percentage of PRBC units <14 days old | 0.999 | 0.984 – 1.017 |
| Number of transfused PRBC units | 1.048 | 1.001 – 1.100 |
| Patient age | 1.026 | 0.998 – 1.056 |
| ISS | 1.044 | 1.010 – 1.083 |
| Head AIS | 0.863 | 0.643 – 1.137 |
| Percentage of PRBC units <21 days old | 0.997 | 0.985 – 1.008 |
| Number of transfused PRBC units | 1.046 | 0.999 – 1.097 |
| Patient age | 1.027 | 0.999 – 1.057 |
| ISS | 1.045 | 1.010 – 1.083 |
| Head AIS | 0.869 | 0.645 – 1.146 |
| PRBC age dichotomized at 14 days old | 0.621 | 0.159 – 3.076 |
| Number of transfused PRBC units | 1.048 | 1.002 – 1.099 |
| Patient age | 1.027 | 0.998 – 1.057 |
| ISS | 1.046 | 1.011 – 1.085 |
| Head AIS | 0.858 | 0.639 – 1.131 |
ISS= Injury Severity Score; PRBC= Packed Red Blood Cell; AIS= Abbreviated Injury Score; MODS= Multiple Organ Dysfunction Score.
Table 6.
Infectious complications analysis by model type.
| Variable | Odds ratio | 95% Confidence Interval |
|---|---|---|
| Mean PRBC age as a continuous variable | 0.976 | 0.927 – 1.026 |
| Number of transfused PRBC units | 0.981 | 0.932 – 1.025 |
| Patient age | 1.013 | 0.990 – 1.038 |
| ISS | 1.043 | 1.013 – 1.077 |
| Head AIS | 1.087 | 0.864 – 1.361 |
| Percentage of PRBC units <14 days old | 0.992 | 0.979 – 1.006 |
| Number of transfused PRBC units | 0.981 | 0.933 – 1.024 |
| Patient age | 1.012 | 0.989 – 1.036 |
| ISS | 1.044 | 1.014 – 1.077 |
| Head AIS | 1.086 | 0.865 – 1.358 |
| Percentage of PRBC units <21 days old | 0.995 | 0.986 – 1.005 |
| Number of transfused PRBC units | 0.980 | 0.931 – 1.024 |
| Patient age | 1.013 | 0.990 – 1.038 |
| ISS | 1.043 | 1.013 – 1.077 |
| Head AIS | 1.092 | 0.867 – 1.368 |
| PRBC age dichotomized at 14 days old | 0.612 | 0.189 – 2.070 |
| Number of transfused PRBC units | 0.984 | 0.935 – 1.027 |
| Patient age | 1.012 | 0.989 – 1.036 |
| ISS | 1.043 | 1.013 – 1.076 |
| Head AIS | 1.076 | 0.855 – 1.345 |
ISS= Injury Severity Score; PRBC= Packed Red Blood Cell; AIS= Abbreviated Injury Score; MODS= Multiple Organ Dysfunction Score.
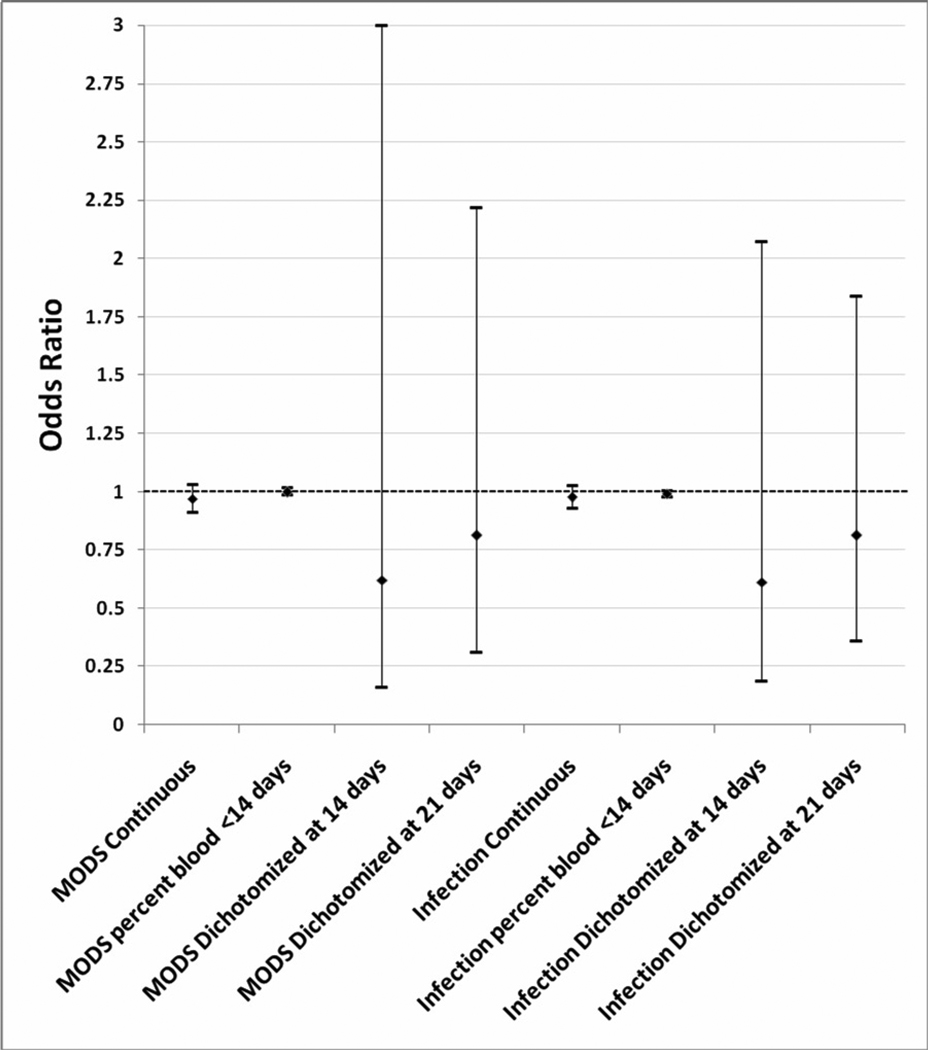
No significant relationship between age of blood and the development of MODS or infectious complications were seen in any of the analyses (Figure 2). The AUCs were 0.75 for the MODS model, and 0.72 for the infectious complications model.
Fig 2. Odds ratios of MODS and infectious complications with 95% Confidence Intervals in the logistic regression models.
PRBC= Packed Red Blood; ISS= Injury Severity Score; AIS= Abbreviated Injury Score
DISCUSSION
Our results suggest that PS-LR seems to blunt the detrimental effects of the known PRBC storage lesion on MODS and infectious complications in transfused trauma patients. Further, the direction of most of the signals implies that the transfusion of older PS-LR products may actually be protective for these end points.
Of the many confounders that have muddled efforts to ascertain the true interaction between the process of PS-LR and the effects of aging on transfused blood, two have been the most problematic: whether there is a threshold for an effect, and if so how to separate that threshold from the overall transfusion burden[21]. The following example will illustrate this point. Let us suppose that PS-LR has no impact on the detrimental effects of storage time on banked PRBCs. If two injured patients are simultaneously transfused, and one patient received 10 units of younger, fresher PS-LR blood while the other received 9 units of young blood and 1 unit of older blood, would the age effect manifest itself? Skeptics of PS-LR’s effects would argue that single unit of older blood “primes” the immune system, and exerts an effect that would independently contribute to a poorer outcome than the patient who received 10 units of all younger, fresher blood. Further, they would argue that using the mean age of blood is inappropriate because it diminishes the importance of this one outlier unit, and that the analysis should be conducted looking at the absolute number of older units that are transfused. What makes this method of analysis problematic in its own right is that it in turn discounts the importance of the overall transfusion burden since it treats a patient who received three units of blood, all of which were old, no differently from a patient who received three older units out of a total of 10. Our investigation sought to address this conundrum by performing an analysis in which the number of older units was expressed as a percent of the total transfusion burden, and then entering this value into the logistic regression model as a continuous variable (maintaining its continuous metric properties). By doing so, we feel that we have optimally controlled for any potential threshold effect as well as the overall transfusion burden in the best manner possible short of a randomized controlled trial. Unfortunately, neither our study nor the other large investigation dealing with this question [21] had large enough numbers of patients who received transfusions of exclusively young or old blood to be able to draw meaningful conclusions about the presence or absence of a threshold effect. Given blood bank practices of releasing older blood first to prevent wastage, it is unlikely that these studies will be done outside the auspices of a large, funded, multi-institutional endeavor. Short of that strength of evidence, however, we feel that investigations centered around a unit-by-unit analysis must be buttressed by some effort to control for the effect of the overall transfusion burden.
The mechanism behind an abrogation of the detrimental effect of aging by the process of PS-LR is straightforward if true. Given the evidence supporting the harmful effects of donor leukocytes that have been allowed to age [12–15], their removal followed by a resultant decline in risk to baseline makes intuitive mechanistic sense. Interestingly, the direction of most of the signals point to that of a protective effect to older blood after the process of PS-LR (albeit in a nonsignificant manner). This direction was also seen in our retrospective study of this same topic, and the previously stated explanation would not account for PS-LR conferring a protective effect on outcomes rather than simply bringing an increased risk back down to baseline. It is possible that the process of PS-LR causes an activation of donor leukocytes or other cellular elements exposed to the filter which in turn causes a release of inflammatory mediators into the donor unit. If these elements were to degrade over time, one would theoretically see patients transfused with fresher PS-LR blood be exposed to higher concentrations of these immunomodulatory mediators and consequently have higher rates of immune-based complications. Patients transfused with older blood in which these mediators have denatured in a time-dependent manner would be transfused with lower concentrations of these agents and consequently have better outcomes. If true, the corollary to this mechanism is that the optimal transfusion product would be fresh, non-leukoreduced blood as this would avoid the known problems of prolonged storage on donor leukocytes as well as our hypothesized proinflammatory effect of filtration.
This study is not without its limitations. First, transfusions of fresh frozen plasma (FFP) and platelets were not tracked. The ability of these products to have their own immunomodulatory properties is coming to be appreciated, and this is a potential confounder. Further, we chose not to analyze all-cause deaths. This was felt to be too problematic methodologically as neurologic deaths lie outside the putative immunologic pathways that PSLR and the age effect exert their influence. Finally, our limited sample size precluded us from being able to analyze patients who received exclusively young blood in order to further investigate the threshold effect more rigorously.
CONCLUSION
This prospective study duplicates our group’s retrospective findings of an abrogation of the detrimental effects of advancing mean PRBC age on MODS and infectious complications. Given that the direction of the signal across most analyses suggests that a small protective effect may be conferred on advancing mean PRBC age by PS-LR as well, further studies are justified.
ACKNOWLEDGEMENTS
The authors would like to thank Kendra Armijo, Josh Hill MD, Jennifer Barillas RN, and James McMurtry for their assistance and support in the production of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Herb A. Phelan, University of Texas-Southwestern Medical Center, Parkland Memorial Hospital, Department of Surgery, Division of Burns/Trauma/Critical Care, Dallas, Texas USA, herb.phelan@utsouthwestern.edu.
Alexander L. Eastman, University of Texas-Southwestern Medical Center, Parkland Memorial Hospital, Department of Surgery, Division of Burns/Trauma/Critical Care, Dallas, Texas USA alexander.eastman@utsouthwestern.edu.
Kim Aldy, University of Texas-Southwestern Medical Center, Department of Surgery, Division of Burns/Trauma/Critical Care, Dallas, Texas USA, kim.aldy@utsouthwestern.edu.
Elizabeth A. Carroll, University of Texas-Southwestern Medical Center, Department of Surgery, Division of Burns/Trauma/Critical Care, Dallas, Texas USA elizabeth.carroll@utsouthwestern.edu.
Paul A. Nakonezny, University of Texas-Southwestern Medical Center, Department of Clinical Sciences, Division of Biostatistics, Dallas, Texas USA, paul.nakonezny@utsouthwestern.edu.
Tiffany Jan, University of Texas-Southwestern Medical Center, Dallas, Texas USA, tiffany.jan@utsouthwestern.edu.
Jessi L. Howard, LSU-New Orleans Health Sciences Center, New Orleans, Louisiana USA, jhowa2@lsuhsc.edu.
Yixiao Chen, University of Texas-Southwestern Medical Center, Dallas, Texas USA, yixiao.chen@utsouthwestern.edu.
Randall S. Friese, University of Arizona Health Sciences Center, Department of Surgery, Division of Trauma, Critical Care, and Emergency Surgery, Tucson, Arizona USA, rfriese@surgery.arizona.edu.
Joseph P. Minei, University of Texas-Southwestern Medical Center, Parkland Memorial Hospital, Department of Surgery, Division of Burns/Trauma/Critical Care, Dallas, Texas USA, joseph.minei@utsouthwestern.edu.
REFERENCES
- 1.Opelz G, Sengar DP, Mickey MR, Teraski PI. Effect of blood transfusions on subsequent kidney transplants. Transplantation Proceedings. 1973;5:253–259. [PubMed] [Google Scholar]
- 2.Opelz G, Teraski J. Improvement of kidney-graft survival with increased numbers of blood transfusions. NEJM. 1978;299:799–803. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 3.Foster RS, Jr, Costanza MC, Foster JC, et al. Adverse relationship between blood transfusions and survival after colectomy for colon cancer. Cancer. 1985;55:1195–1201. doi: 10.1002/1097-0142(19850315)55:6<1195::aid-cncr2820550610>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Creasy TS, Veitch PS, Bell PR. A relationship between perioperative blood transfusion and recurrence of carcinoma of the sigmoid colon following potentially curative surgery. Ann of Royal Coll Surg of England. 1987;69:100–103. [PMC free article] [PubMed] [Google Scholar]
- 5.Blumberg N, Heal J, Chuang C, et al. Further evidence supporting a cause and effect relationship between blood transfusion and earlier cancer recurrence. Ann Surg. 1988;207:410–415. doi: 10.1097/00000658-198804000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung M, Steinmetz OK, Gordon PH. Perioperative blood transfusion and outcome after resection for colorectal carcinoma. BJS. 1993;80:427–432. doi: 10.1002/bjs.1800800407. [DOI] [PubMed] [Google Scholar]
- 7.Edna TH, Bjerkeset T. Association between transfusion of stored blood and infective bacterial complications after resection for colorectal cancer. European J Surg. 1998;164:449–456. doi: 10.1080/110241598750004265. [DOI] [PubMed] [Google Scholar]
- 8.Chiarugi M, Buccianti P, Disarli M, et al. Effect of blood transfusions on disease-free interval after rectal cancer surgery. Hepato-Gastroenterology. 2000;47:1002–1005. [PubMed] [Google Scholar]
- 9.Purdy FR, Tweeddale MG, Merrick PM. Association of mortality with age of blood transfused in septic ICU patients. Canadian J Anesthesia. 1997;44:1256–1261. doi: 10.1007/BF03012772. [DOI] [PubMed] [Google Scholar]
- 10.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–572. doi: 10.1016/s0002-9610(99)00239-1. [DOI] [PubMed] [Google Scholar]
- 11.Offner PJ, Moore EE, Biffl WL, et al. Increased rate of infection associated with transfusion of old blood after severe injury. Arch Surg. 2002;137:711–717. doi: 10.1001/archsurg.137.6.711. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen HJ, Reimert CM, Pedersen AN, et al. Time-dependent, spontaneous release of white cell- and platelet-derived bioactive substances from stored human blood. Transfusion. 1996;36:960–965. doi: 10.1046/j.1537-2995.1996.36111297091738.x. [DOI] [PubMed] [Google Scholar]
- 13.Bordin JO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1703–1721. [PubMed] [Google Scholar]
- 14.Kristiansson M, Soop M, Saraste L, Sundqvist KG. Cytokines in stored red blood cell concentrates: promoters of systemic inflammation and simulators of acute transfusion reactions? Acta Anaesthesiologica Scandinavica. 1996;40:496–501. doi: 10.1111/j.1399-6576.1996.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 15.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- 16.Spinella PC, Carroll CL, Staff I, et al. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Critical Care (London, England) 1999;13(5):R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phelan HA, Sperry JL, Friese RS. Leukoreduction before red cell transfusion has no impact on mortality in trauma patients. Journal of Surgical Research. 2007;138:32–36. doi: 10.1016/j.jss.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 18.Nathens AB, Nester TA, Rubenfeld GD, et al. The effects of leukoreduced blood transfusion on infection risk following injury: A randomized controlled trial. Shock. 2006;26:342–347. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- 19.Friese RS, Sperry JL, Phelan HA, et al. The use of leukoreduced red cell products is associated with fewer infectious complications in trauma patients. Am J Surg. 2008;196(1):56–61. doi: 10.1016/j.amjsurg.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 20.Phelan HA, Gonzalez RP, Patel H, et al. Prestorage leukoreduction ameliorates the detrimental effects of age on stored blood. J Trauma. 2010;69(2):330–337. doi: 10.1097/TA.0b013e3181e0b253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinberg JA, McGwin G, Jr, Griffin RL, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008;65:279–284. doi: 10.1097/TA.0b013e31817c9687. [DOI] [PubMed] [Google Scholar]
- 22.Marshall JC, Cook DJ, Christou NV, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]