Summary
CD8 T cells are strongly induced in response to certain strains of vaccinia virus (VACV) and the generation of this population are tightly regulated by two Tumor Necrosis Factor (TNF)/TNFR superfamily members, OX40 (CD134) and CD27. In this study, we examined the role of another member of the TNFR superfamily, 4-1BB (CD137, TNFRSF9), and its ligand (4-1BBL, CD137L, TNFSF9), that have been described to control the generation of memory CD8 T cell populations elicited by other viruses such as influenza. Expression of 4-1BB and 4-1BBL was observed in wild-type mice during the primary infection, but we found that both 4-1BB and 4-1BBL deficient mice generated normal numbers of VACV-specific effector CD8 T cells that produced IFN- and TNF. Additionally, CD8 T cells deficient in 4-1BB were able to expand and persist comparably to wild-type T cells in response to VACV infection. Furthermore, the knockout mice also showed no defect in development of VACV-specific CD8 memory T cell populations. Lastly, showing alternate control mechanisms were not active in the gene-deficient environments that masked any activity, blocking 4-1BB/4-1BBL interactions using neutralizing antibody also had no effect on the number of VACV-specific memory CD8 T cells induced. Thus, our data demonstrate that 4-1BB and 4-1BBL do not play a strong or dominant role in driving the generation of high frequencies of VACV-specific CD8 T cells.
Keywords: Vaccinia Virus, 4-1BB, 4-1BBL, CD8 T cells
Introduction
Infection with all viruses results in the generation of CD8 T cell responses, but it is becoming clear that the molecular pathways that lead to development and persistence of these pools of CD8 T cells might vary with the nature of the virus and/or the level of infection. Vaccinia virus (VACV) infects acutely but might not be equivalent to other well-studied acute viruses such as influenza or LCMV Armstrong. The level of immunity induced by VACV strains and how immunity is generated to VACV is of significance as this virus and variant vectors are being used in vaccines.
In humans and mice, immunization with some strains of VACV induces a strong and long-lasting CD8 T cell response that can be protective against re-infection [1-6]. However, until recently, the molecular interactions that drive the generation of protective pools of anti-VACV CD8 T cells was not clear. It has been known for some time that members of the Tumor Necrosis Factor (TNF)/TNFR superfamily are important mediators of survival signaling in the immune system, and it has been hypothesized that a number of these molecules might play roles at several stages of an immune response [7,8]. Our lab and others recently found that the TNFR superfamily members OX40 (CD134) [9] and CD27 [10,11] drive the development of high frequencies of both primary effector and memory CD8 T cells following infection with the virulent strain of VACV, Western Reserve. However, whether other TNFR interactions also control anti-VACV responses is not clear, including whether they might be needed at alternate stages of the T cell response, or whether they are overlapping in activity, or possibly redundant.
In particular, the interaction of 4-1BB (CD137) with its ligand, 4-1BBL, might be of particular significance to the generation of memory T cells to viruses. 4-1BBL-/- mice have been found to display reduced CD8 T cell memory/recall responses to both influenza virus and LCMV Armstrong [12-16]. Endogenous 4-1BB/4-1BBL interactions might act late in these responses, after normal development of acute responses, to promote influenza-specific CD8 T cell memory formation, and also participate in either the maintenance and/or reactivation of these persisting cells [15,17]. In another LCMV model, viral peptide-immunized 4-1BBL-/- mice also had fewer epitope-specific CD8 T cells and were impaired in their ability to resolve the infection [14]. An analysis of LCMV-specific CD8 memory cells further suggested that the memory state was maintained via 4-1BB as anti-4-1BBL antibody reduced RNA levels and certain characteristics in memory T cells cultured with dendritic cells [18]. 4-1BBL-/- mice were additionally found to generate impaired functional CD8 T cells during latent mouse gammaherpesvirus-68 (MHV-68) infection, although in this case their numbers were not affected [19]. Moreover, 4-1BB-/- mice, and wild-type mice injected with a neutralizing antibody to 4-1BBL, generated lower CD8 T cell responses to MCMV inflationary epitopes that arise after the acute infection and are characteristic of the chronic/latent phase of MCMV infection [20].
These data show that 4-1BB/4-1BBL interactions are prominent positive regulators of memory CD8 T cell development and/or reactivity in a number of viral infections. Hence, we investigated the role of 4-1BB and 4-1BBL during VACV infection by analyzing gene deficient mice, tracking adoptively transferred 4-1BB-deficient CD8 T cells responding to VACV-OVA, and following blocking of 4-1BBL. Despite low level or transient expression of both 4-1BBL and 4-1BB during acute infection with VACV, we found no major role for these molecules in regulating CD8 T cell priming, strongly contrasting with our prior results for OX40 and CD27. Our data suggest that although 4-1BB is important in modulating CD8 T cell responses to some infectious pathogens, other TNFR superfamily members may provide a dominant activity during the response to VACV.
Results
Priming of VACV-specific CD8 T cells during acute infection is independent of 4-1BB and 4-1BBL
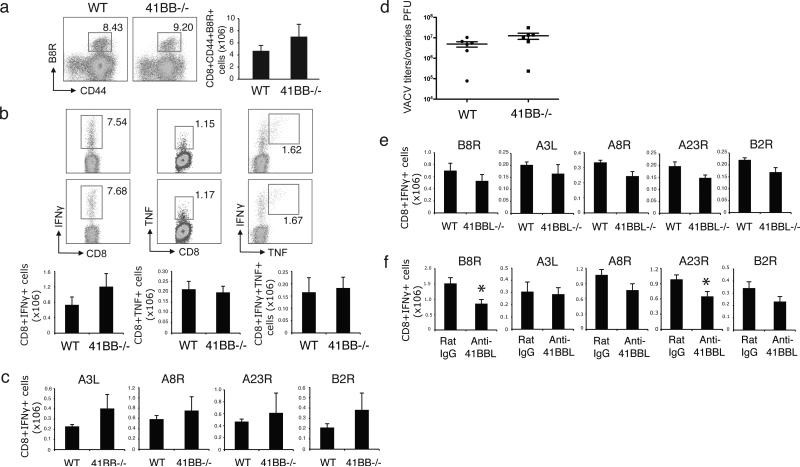
WT C57BL/6 (B6) and 4-1BB-deficient (4-1BB-/-) mice were infected with VACV-WR. Seven days after infection, the number of VACV-specific CD8 T cells in the spleen were quantified using a tetramer of B8R, the immunodominant class I epitope of VACV. In contrast to two other TNFR superfamily members, OX40 and CD27, which we showed control the size of primary effector CD8 T cell populations after VACV-WR infection [9], the absence of 4-1BB had no appreciable effect at this stage. Both the percentage and total numbers of VACV-specific CD8 T cells were comparable in WT and 4-1BB-/- mice (Fig. 1a). Furthermore, epitope-reactive CD8 T cells that made IFN- and TNF following in vitro stimulation with both dominant (B8R) (Fig. 1b) and subdominant (A3L, A8R, A23R, B2R) (Fig. 1c) VACV peptides were not significantly diminished or enhanced in the absence of 4-1BB. A similar result was also observed with VACV-WR given via dermal scarification, mimicking the route of vaccination against smallpox (data not shown). In addition, virus titers in the ovaries were comparable between WT and 4-1BB deficient mice, either at day 7 at the peak of replication (Fig. 1d), or at day 14 when titers were strongly reduced (data not shown).
Figure 1.
4-1BB-/- and 4-1BBL-/- mice have normal VACV-specific CD8 effector responses. (a-d) WT and 4-1BB-/- mice were infected i.p. with VACV-WR (2 × 105 PFU/mouse) for 7 days. (a) Splenocytes were stained with anti-CD8, -CD44, and B8R-Tetramer. Left: Representative dot plots of gated CD8+ cells staining for CD44 and B8R tetramer. The numbers indicate the percentage of CD8+CD44+B8R-tetramer positive cells. Right: Total number of CD8+CD44+B8R-tetramer positive cells. (b) Splenocytes were stimulated with B8R peptide for intracellular IFN- and TNF staining. Top: Representative plots of IFN- (left), TNF (middle), and IFN- /TNF (right), gating on CD8+CD62L- cells. Percentages positive are indicated. Bottom: Total numbers of CD8+IFN- + (left), CD8+TNF+ (middle), and CD8+IFN- +TNF+ cells (right). (c) Splenocytes were stimulated with A3L, A8R, A23R or B2R peptides. Total numbers of CD8+IFN- + cells were calculated. (d) On day 7 post-infection, VACV titers were determined in the ovaries. (e) WT and 4-1BBL-/- mice were infected i.p. with VACV-WR (2 × 105 PFU/mouse). Seven days after infection, splenocytes were stimulated with B8R, A3L, A8R, A23R or B2R peptides. Total numbers of CD8+IFN- + cells were calculated. (f) WT B6 mice were infected i.p. with VACVWR (2 × 105 PFU/mouse) and treated with IgG or 4-1BBL neutralizing antibody (19H3) on days 0, 2, 5 post infection. Seven days after infection, splenocytes were stimulated with B8R, A3L, A8R, A23R or B2R peptides. Total numbers of CD8+IFN- + cells were calculated. Data represent mean value ± sem from n=4 to 6 mice. Similar results were reproduced in two separate experiments.
In previous studies of another virus, MCMV, we found alternate regulation in 4-1BB-/-versus 4-1BBL-deficient (4-1BBL-/-) mice during acute infection [20]. We therefore assessed response to VACV in 4-1BBL-/- mice. This also showed that 4-1BBL appeared to be dispensable for primary CD8 T cell responses to VACV-WR. Infected WT and 4-1BBL-/- mice generated comparable numbers of virus-specific CD8 T cells that produced IFN- 7 days post infection (Fig. 1e). Neutralizing 4-1BBL at the time of infection in WT mice, with a blocking antibody (19H3) used previously [20], partially impaired the expansion of certain VACV-epitope reactive IFN- producing CD8 T cells, however, the effect was not universal (Fig. 1f). This strongly contrasted with globally reduced responses in OX40 or CD27-deficient mice [10], again suggesting that 4-1BB/4-1BBL interactions are not be a major driving force in determining the priming of VACV-specific CD8 T cells.
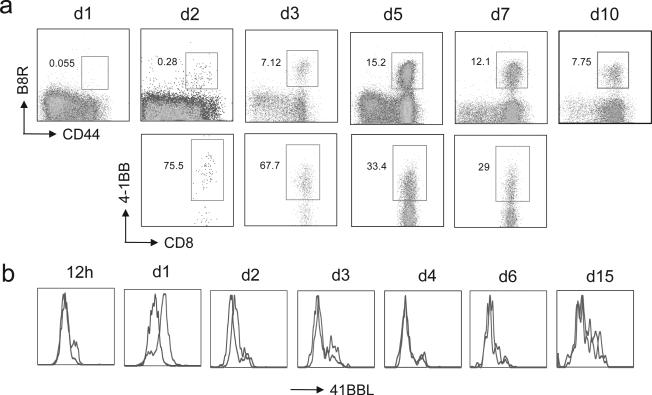
4-1BB and 4-1BBL are both inducible molecules, and so these results also questioned whether 4-1BB or 4-1BBL were in fact available during the response to VACV. By analyzing the immunodominant CD8 population, visualized by B8R-peptide tetramer staining, which is first detectable within 2 days of infection, we found a high level of expression of 4-1BB. We could not determine if 4-1BB was expressed after 1 day due to the very low frequency of tetramer positive T cells. 4-1BB was maintained on day 3 and then the percentage of VACV-reactive CD8 T cells bearing 4-1BB was gradually reduced through to day 7 (Fig. 2a). 4-1BBL has traditionally been hard to detect, however we found that 4-1BBL was strongly expressed on CD8+CD11c+ splenic dendritic cells within 1 day post infection, still evident on day 2 at lower levels, but with no detectable expression seen after 3 days (Fig. 2b). CD8+CD11c+ dendritic cells are the APC thought largely responsible for priming VACV-specific CD8 T cells [21,22]. Analysis of other potential APC populations, including CD8-CD11c+ dendritic cells, revealed no expression during the time period examined providing an internal control for the 4-1BBL staining (data not shown). Therefore these data suggest that 4-1BB interactions with 4-1BBL were a possibility early during infection, although peak expression of 4-1BB on the CD8 T cells may not have coincided with peak expression of 4-1BBL.
Figure 2.
4-1BB and 4-1BBL could be detected on VACV-specific CD8 T cells and DC respectively following infection. a-b) WT mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). a) On indicated days post-infection splenocytes were harvested and stained for CD8, CD44, B8R-tetramer, and 4-1BB. Top panel, Percentage of CD44-high expressing B8R-specific CD8 T cells. Bottom panel, Percentage of 4-1BB+ cells gating on CD8+ CD44-high B8R-tetramer positive cells. Quadrant settings based on isotype controls. b) On indicated days post-infection splenocytes were harvested and stained for CD8, CD11c, CD11b, and 4-1BBL. Representative histogram of 4-1BBL expression gated on CD8+CD11c+ cells.
Generation of VACV-specific memory CD8 T cells is independent of 4-1BB and 4-1BBL
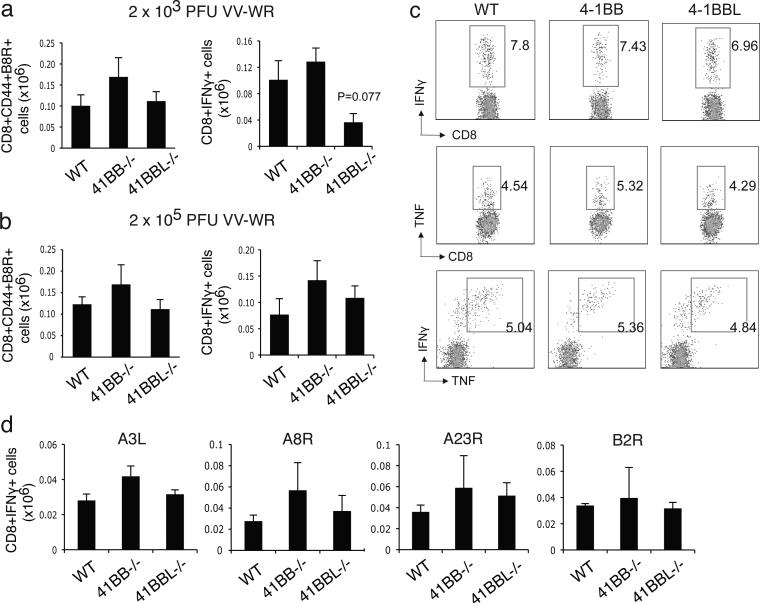
Although no apparent strong activity was found during effector T cell priming, it was possible that either early or late 4-1BB interactions would dictate the generation of memory. Prior results with influenza found that while T cell responses were essentially normal during the first week of infection in 4-1BBL-/- mice, diminished accumulation of CD8 T cells was evident from day 21 onwards [12]. Similarly, defective accumulation of CD8 T cells reactive with MCMV were only evident when assessed 2-4 weeks after infection of 4-1BB-/- mice [20]. Using the same infection protocol for assessing the acute response, we found no significant role for 4-1BB at a late time after VACV infection when CD8 T cell responses were measured at 120 days. 4-1BB-/- mice displayed a comparable percentage and total number of B8R-specific memory CD8 T cells that produced the cytokines IFN- and TNF compared to WT mice following either a low dose (Fig. 3a) or a high dose of infection (Fig. 3b and c). A similar size of the B8R-reactive memory CD8 T cell pool was also found in 4-1BBL-/- mice after VACV infection (Fig. 3a and 3b). There were less IFN-γ producing CD8 T cells with the lower dose of VACV in 4-1BBL-/- mice but no difference with high dose infection. A comparable intensity of staining for IFN-γ or TNF was observed after in vitro stimulation with B8R peptide (Fig. 3c), suggesting that the reactivity of the memory CD8 T cells induced in the absence of 4-1BB or 4-1BBL was normal. Lastly, analysis of reactivity to alternate VACV peptides, representing a sampling of subdominant CD8 T cell populations, also showed no apparent difference in IFN-γ production or the number of IFN-γ-producing cells in 4-1BB or 4-1BBL-deficient mice (Fig. 3d). This suggested that 4-1BB/4-1BBL did not alter the diversity of CD8 memory generated to VACV.
Figure 3.
VACV-specific memory CD8 T cell generation is independent of 4-1BB and 4-1BBL. WT, 4-1BB-/-, and 4-1BBL-/- mice were infected i.p. with VACV-WR (a: 2 × 103 PFU/mouse, or b-d: 2 × 105 PFU/mouse). 120 days after infection, splenocytes were stimulated with B8R peptide (a, b, c) or VACV subdominant peptides, A3L, A8R, A23R and B2R (d). Total numbers of CD8+CD44+B8R-tetramer positive cells (a, b) and CD8+IFN- + cells (c) were enumerated. (c) Representative plots of IFN- (left), TNF (middle), and IFN- /TNF (right), gating on CD8+CD62L- cells. Percentages positive are indicated. Data represent mean value ± sem from n=4 to 6 mice. Similar results were reproduced in two separate experiments.
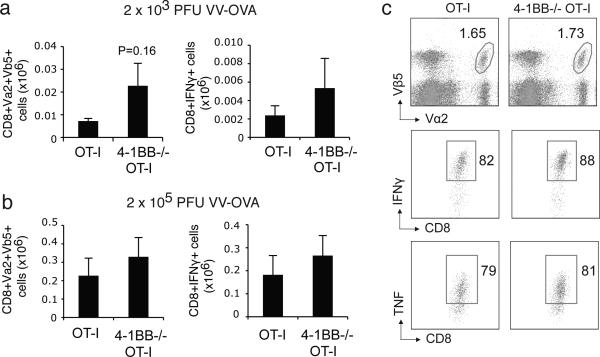
As 4-1BB can be expressed on many different cell types, the lack of 4-1BB/4-1BBL signaling on other cell types in the local environment, such as regulatory T cells, might indirectly have affected our ability to detect an activity of 4-1BB on the CD8 T cell response. Therefore, we sought to address the intrinsic effect of 4-1BB on CD8 T cells during VACV infection by using an adoptive transfer model in which 4-1BB is only absent on the responding CD8 T cells. Naïve 4-1BB-deficient OVA-specific OT-I CD8 T cells, when transferred into wild-type mice, still generated equivalent numbers of total memory cells and memory cells producing IFN-γ in response to either low or high dose VACV-OVA infection (Fig. 4a and 4b). There was a trend toward enhanced numbers, but this was not statistically significant. Analysis of IFN-γ and TNF staining after in vitro restimulation with OVA peptide showed an equivalent intensity in expression in 4-1BB-deficient memory cells, again implying their reactivity was normal (Fig. 4c).
Figure 4.
4-1BB is not required on antigen-reactive CD8 T cells responding during VACV infection. (a-b) 5 × 104 naive WT or 4-1BB-/- OT-I CD8 T cells were adoptively transferred into congenic B6 mice. One day later, mice were infected i.p. with recombinant VACV expressing full-length OVA (VACV-OVA; a: 2 × 103 PFU/mouse, or b-c: 2 × 105 PFU/mouse). 45 days after infection, splenocytes were stimulated with OVA (SINFEKL) peptide. Total numbers of donor CD8+Vα2+Vβ5+ cells (a, b) and CD8+IFN- + cells (c) were enumerated. (c) Representative plots of IFN- and TNF, gating on CD8+Vα+Vβ+ cells. Percentages positive are indicated. Data represent mean value ± sem from n=4 to 6 mice. Similar results were reproduced in two separate experiments.
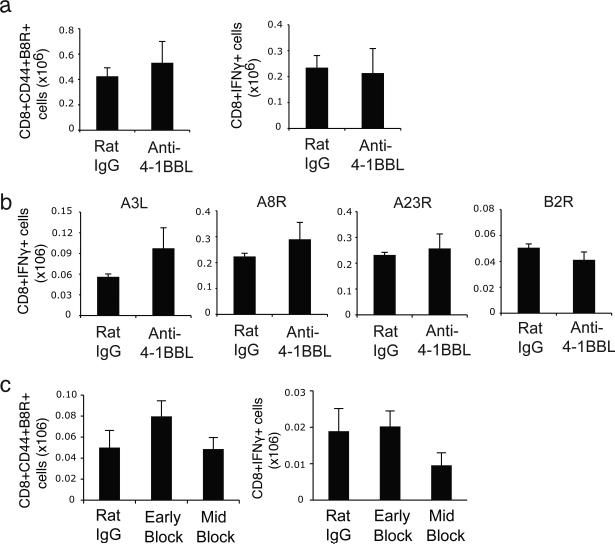
Lastly, we confirmed our data by treating WT mice with a blocking antibody against 4-1BBL, given either on days 0-5 (Early block) or days 7-13 (Mid block) post-infection. Neutralizing 4-1BBL in infected B6 WT mice also did not significantly impair the generation of total B8R-specific memory CD8 T cells or IFN-γ-producing B8R-reactive memory cells that were observed either 35 days (Fig. 5a) or 120 days (Fig. 5c) after infection. Similarly, reactivity to subdominant VACV epitopes was equivalent in mice injected with anti-4-1BBL (Fig. 5b), again reinforcing the notion that 4-1BBL interactions did not control the clonal diversity of the CD8 T cell response. Overall, our data show that the 4-1BB/4-1BBL pathway does not have a major role in regulating the generation of VACV-specific primary effector or memory CD8 T cells, and that 4-1BB is intrinsically not required on CD8 T cells for development of high frequencies of memory cells during infection with VACV.
Figure 5.
Blocking 4-1BB and 4-1BBL interaction does not impair generation of VACV-specific memory CD8 T cells. (a-b) WT B6 mice were infected i.p. with VACV-WR (2 × 105 PFU/mouse) and treated with IgG or 4-1BBL neutralizing antibody (19H3) on days 0, 2, and 5 post infection. (c) WT B6 mice were infected i.p. with VACV-WR (2 × 105 PFU/mouse) and treated with IgG or 4-1BBL neutralizing antibody (19H3) on days 0, 2 and 5 (early block) or 7, 10 and 13 (late block). 35 days (a, b) or 120 days (c) after infection, splenocytes were stimulated with B8R peptide (a, c) or VACV subdominant peptides, A3L, A8R, A23R and B2R (b) to measure CD8+IFN- + cells (a-c), or CD8+ T cells were stained with B8R-tetramer (a, c). Data represent mean total numbers ± sem from n=4 to 6 mice. Similar results were reproduced in two separate experiments.
Discussion
CD8+ T cells are critical mediators of immunity to VACV infection, as high frequency memory T cell populations can provide protective immunity against re-infection in the absence of antibodies [9]. We have previously identified several key molecules in OX40 and CD27 from the TNFR superfamily that play a major role in driving CD8 T cell priming and memory against VACV, and provided data that suggested that these molecules might work in a temporal manner during the course of infection. As 4-1BBL has previously been shown to be active relatively late post-infection with certain viruses such as influenza, LCMV Armstrong, and MCMV, this implied that 4-1BB/4-1BBL interactions may also form part of the complex network of molecules that drive memory formation to VACV. Although we found that both 4-1BB and 4-1BBL were induced during infection with VACV, our data show no strong role for these molecules in determining the number of IFN-γ or TNF producing effector or memory CD8 T cells that develop.
4-1BB is highly expressed by CD8 and CD4 T cells following activation [23]. The known TNF related ligand, 4-1BBL, is mainly expressed by activated APC [24,25]. Consistent with our and others prior observations, this suggests these molecules may mediate cross-talk between T cells and APC and provide costimulatory signals to enhance T cell responsiveness. We were able to detect transient upregulation of 4-1BBL on dendritic cells 24-48 hours after VACV infection. However, interestingly, this was not prolonged and we did not detect significant expression of 4-1BBL at late times. We did detect 4-1BB on responding VACV-specific CD8 T cells two or more days after infection, but directly infecting 4-1BB- and 4-1BBL-deficient mice revealed that although these molecules were available, they were neither required for generating optimal anti-viral effector CD8 T cells nor development of the VACV-specific memory CD8 T cell pool. To exclude the possibility that the level of antigen stimulation might overcome the need for these costimulatory molecules in regulating the T cell response, we also infected the mice with lower doses of VACV, but this also did not reveal any significant activity of 4-1BB and 4-1BBL. Although our data imply that these molecules are therefore redundant during infection with this virus, this statement has to be qualified in that we cannot prove that any 4-1BB/4-1BBL interactions did take place, which is the true definition of redundancy.
In addition to activated CD4 and CD8 T cells, 4-1BB has also been found on a number of cell types including NK cells, NKT cells, and dendritic cells. In particular, some data suggest 4-1BB may positively impact Foxp3+ Treg division, survival, or function [26,27], possibly complicating the interpretation of studies in knockout animals. To exclude the possibility that the lack of 4-1BB or 4-1BBL signaling on other cell types in the local environment, such as Treg, was indirectly affecting our ability to understand the significance of 4-1BB, we used adoptive transfer of TCR transgenic CD8 T cells. These experiments further confirmed the redundant/non-essential role of 4-1BB in controlling the number of T cells that were induced over time to VACV.
Emerging evidence suggests that there is a strong role for 4-1BB/4-1BBL interactions in inducing priming and/or long-lasting memory of virus-specific CD8 T cells to certain viruses including MCMV [20], influenza virus [12,15], LCMV [14,16], MHV-68 [16,19], and HSV [28]. However, our data are the first report finding no significant role for these molecules in driving the development of a T cell response to a virus, suggesting that the requirement for the 4-1BB/4-1BBL pathway in modulating virus specific immunity might not be universal. This may be explained by the fact that the TNFR superfamily members OX40 and CD27 are strongly engaged after infection with VACV and their activity compensate for any requirement for 4-1BB/4-1BBL interactions that are seen with other viruses. Alternatively VACV may not induce a similar inflammatory environment compared to other viruses, such that expression of 4-1BB and 4-1BBL are not optimal nor concordant. Although we found no substantial role in dictating the frequency of VACV-specific memory CD8 T cells, we did not specifically address a role for 4-1BB/4-1BBL in controlling the recall activity of the memory populations elicited. Based on intracellular staining for IFN-γ and TNF after in vitro stimulation with VACV peptide, there was apparent defect in activity of the T cells formed in the absence of 4-1BB or 4-1BBL. However, it is possible that other recall activities, such as the ability to secrete IL-2, or to proliferate and re-expand, might be influenced by these molecules, and this will need to be investigated in the future.
Obviously, because viruses replicate to varying degrees, and also exhibit differential tropism for alternate cell types, this presents numerous scenarios whereby the driving forces governing the CD8 T cell response might not coincide. As mentioned earlier, OX40 has been shown to play a crucial role in the generation of CD8 T cell memory to VACV [9] and MCMV [29], while in other virus infections such as with LCMV and influenza virus, it was found to either not play a role, or not a dominant role [30]. Similarly, reports of the dependency for CD27 signaling to generate virus-specific CD8 T cells also diverge considerably [11,17,31,32]. The accumulation of virus-specific CD8 T cells in the secondary response to influenza (A/NT/60/68) infection was critically dependent on the collective contributions of CD27, 4–1BBL, and OX40L [17]. In contrast, another study of intranasal influenza A/HKX31 (HKX31, H3N2) infection revealed that CD27 was not required for the generation of virus-specific memory CD8 T cells, but its activity in the priming phase led to reduced secondary expansion and reduced functionality [32]. CD27 is required for optimal priming of effector CD8 T cells following VACV and VSV [11] but not LCMV infection [33]. However, ligation of CD27 on existing LCMV-specific memory CTLs strongly enhanced autocrine IL-2 production and thereby promoted secondary expansion and protection from re-infection [34]. Thus, these data suggest that co-stimulation through TNFR family members can play critical roles at several phases during the generation of virus-specific effector and memory CD8 T cells, but the use and activity of these molecules may differ depending on either the infection studied or possibly other factors that are not readily apparent at present.
Materials and Methods
Mice
Eight to twelve wk-old female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). 4-1BB-/- and 4-1BBL-/- mice on a B6 background, originally provided by Byoung Kwon and Amgen, respectively, were bred in-house. OT-I TCR-transgenic mice were used as a source of Vβ5/Vα2 CD8+ T cells responsive to OVA-derived SIINFEKL peptide. 4-1BB-deficient OT-I TCR transgenic mice were generated in house by crossing OT-I mice with 4-1BB-/- mice. All experiments used age and sex-matched mice. The studies reported here conform to the animal Welfare Act and the NIH guidelines for the care and use of animals in biomedical research. Studies were conducted following the guidelines of the La Jolla Institute for Allergy and Immunology's Institutional Animal Care and Use Committee.
Viruses
The VACV-WR strain was purchased from the American Type Culture Collection, grown in HeLa cells, and titered on VeroE6 cells (28).
Immunization protocols
For most experiments, mice were infected i.p. with 2 × 105 or 2 × 103 PFU of VACV. In some experiments, mice were also injected with 250 μg rat IgG (Chemicon) or anti-4-1BBL (clone 19H3) on the days stated in the figure legends.
For adoptive transfer experiments, 5 × 104 naive wild-type (WT) or 4-1BB-/- OT-I CD8 T cells were transferred into WT non-transgenic B6 mice. One day later, mice were infected i.p. with recombinant VACV expressing full-length OVA protein (VACV-OVA; 2 × 105 or 2 × 103 PFU/mouse) or PBS as indicated. OT-I expansion and memory formation were detected by FACS staining of transgenic TCR - and -chains after gating on CD8 T cells and in some cases after restimulating in vitro with OVA (SINFEKL) peptide.
Peptides and Tetramers
Vaccinia virus peptide epitopes used in this study were predicted and synthesized as described previously [35,36]. B8R (20-27; TSYKFESV), A3L (270-227; KSYNYMLL), A8R (189-196; ITYRFYLI), B2R (54-62; YSQVNKRYI), A23R (297-305; IGMFNLTFI). MHC/peptide tetramers for the VACV-WR epitope B8R (20-27; TSYKFESV)/H-2Kb, which were conjugated to allophycocyanin, were obtained from the National Institutes of Health Tetramer Core facility (Emory University, Atlanta, GA).
Immunofluorescence labeling
For staining of cell surface 4-1BB and 4-1BBL, splenocytes from infected mice were lysed with red blood cells (RBC) and then 1-2 × 106 cells were labeled with following Abs: anti-CD44 (FITC), anti-CD8 (PerCP) from BD Biosciences and biotin labeled anti-4-1BB from Biolegend followed by PE-labeled streptavidin (Molecular Probes) and followed by MHC/B8R-tetramer (APC); or anti-CD8 (PerCP), anti-CD11c (APC), anti-CD11b (PE) from BD Biosciences and biotin labeled anti-4-1BBL from Biolegend followed by PE-labeled streptavidin (Molecular Probes).
Intracellular staining for cytokine production in T cells was performed as previously described [9], with some modifications. Briefly, after lysing RBC, splenocytes from infected mice were resuspended in RPMI-1640 medium (Gibco) supplemented with 10% FCS (Omega Scientific), 1% L-glutamine (Invitrogen), 100 mg/ml streptomycin, 100 U/ml penicillin and 50 mM 2-mercaptoethanol (Sigma). 1-2 × 106 cells were plated in round-bottomed 96-well microtiter plates in 200 μl with medium or the indicated VACV peptides at 1 μg/ml for 1 hr at 37°C. GolgiPlug (BD Biosciences) was then added to the cultures according to the manufacture's instructions and the incubation continued for 6-9 hrs. Cells were stained with anti-CD8 (PerCP) and CD62L (PE), followed by fixation with cytofix-cytoperm (BD Biosciences) for 20 min at 4 °C. Fixed cells were subjected to intracellular cytokine staining in BD Perm/Wash buffer for 30 min at 4°C. Anti-TNF (FITC) and IFN- (APC) were obtained from e-Biosience and used at a 1:100 dilution. Samples were analyzed for their proportion of cytoplasmic cytokines after gating on CD8+CD62Llow T cells by FACSCaliburTM flow cytometer using CellQuest (BD Biosciences) and FlowJo software (Tree Star, san Carlos, CA).
VACV-titer assay
Tissues from individual mice were homogenized and sonicated for 1 min with a pause every half minute using an ultrasonic cleaner 1210 Branson (Danbury, CT). Serial dilutions were made and the virus titers were then determined by plaque assay on confluent VeroE6 cells.
Statistical analysis
Statistical significance was determined by two-tailed Student's t test. Unless otherwise indicated, data represent the means ± SEM. P < 0.05 was considered significant and is indicated by an asterisk.
 4-1BB drives CD8 T cell responses to several viruses
4-1BB drives CD8 T cell responses to several viruses We determined if 4-1BB was required for CD8 T cell development to vaccinia virus
We determined if 4-1BB was required for CD8 T cell development to vaccinia virus No major role for 4-1BB was found during priming of vaccinia-specific CD8 T cells
No major role for 4-1BB was found during priming of vaccinia-specific CD8 T cells
Acknowledgements
We thank Y. Adam and X. Tang for excellent technical assistances, Dr. S. Salek-ardakani and Dr. S. Lee for valuable discussions. This work was supported by NIH grants AI67341, AI42944, and AI089624 to M.C., and F32AI091323 to Y. Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicting financial interests.
References
- 1.Miller JD, van der Most RG, Akondy RS, Glidewell JT, Albott S, Masopust D, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–722. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 3.Harrington LE, Most Rv R, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. Journal of virology. 2002;76:3329–3337. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amanna IJ, Slifka MK, Crotty S. Immunity and immunological memory following smallpox vaccination. Immunological reviews. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 5.Amara RR, Nigam P, Sharma S, Liu J, Bostik V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. Journal of virology. 2004;78:3811–3816. doi: 10.1128/JVI.78.8.3811-3816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 7.Croft M. Co-stimulatory members of the TNFR family: Keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 8.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salek-Ardakani S, Moutaftsi M, Crotty S, Sette A, Croft M. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181:7969–7976. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salek-Ardakani S, Flynn R, Arens R, Yagita H, Smith GL, Borst J, et al. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J Clin Invest. 2011;121:296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schildknecht A, Miescher I, Yagita H, van den Broek M. Priming of CD8+ T cell responses by pathogens typically depends on CD70-mediated interactions with dendritic cells. Eur J Immunol. 2007;37:716–728. doi: 10.1002/eji.200636824. [DOI] [PubMed] [Google Scholar]
- 12.Bertram EM, Lau P, Watts TH. Temporal Segregation of 4-1BB Versus CD28-Mediated Costimulation: 4-1BB Ligand Influences T Cell Numbers Late in the Primary Response and Regulates the Size of the T Cell Memory Response Following Influenza Infection. J Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 13.DeBenedette MA, Wen T, Bachmann MF, Ohashi PS, Barber BH, Stocking KL, et al. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J Immunol. 1999;163:4833–4841. [PubMed] [Google Scholar]
- 14.Tan JT, Whitmire JK, Murali-Krishna K, Ahmed R, Altman JD, Mittler RS, et al. 4-1BB costimulation is required for protective anti-viral immunity after peptide vaccination. J Immunol. 2000;164:2320–2325. doi: 10.4049/jimmunol.164.5.2320. [DOI] [PubMed] [Google Scholar]
- 15.Bertram EM, Dawicki W, Sedgmen B, Bramson JL, Lynch DH, Watts TH. A switch in costimulation from CD28 to 4-1BB during primary versus secondary CD8 T cell response to influenza in vivo. J Immunol. 2004;172:981–988. doi: 10.4049/jimmunol.172.2.981. [DOI] [PubMed] [Google Scholar]
- 16.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- 17.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, et al. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 18.Allam A, Conze DB, Giardino Torchia ML, Munitic I, Yagita H, Sowell RT, et al. The CD8+ memory T-cell state of readiness is actively maintained and reversible. Blood. 2009;114:2121–2130. doi: 10.1182/blood-2009-05-220087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuse S, Bellfy S, Yagita H, Usherwood EJ. CD8+ T cell dysfunction and increase in murine gammaherpesvirus latent viral burden in the absence of 4-1BB ligand. J Immunol. 2007;178:5227–5236. doi: 10.4049/jimmunol.178.8.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humphreys IR, Lee SW, Jones M, Loewendorf A, Gostick E, Price DA, et al. Biphasic role of 4-1BB in the regulation of mouse cytomegalovirus-specific CD8(+) T cells. Eur J Immunol. 2010;40:2762–2768. doi: 10.1002/eji.200940256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belz GT, Smith CM, Eichner D, Shortman K, Karupiah G, Carbone FR, et al. Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J Immunol. 2004;172:1996–2000. doi: 10.4049/jimmunol.172.4.1996. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, De Trez C, Flynn R, Ware CF, Croft M, Salek-Ardakani S. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J Immunol. 2009;182:6278–6286. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon BS, Kestler DP, Eshhar Z, Oh KO, Wakulchik M. Expression characteristics of two potential T cell mediator genes. Cell Immunol. 1989;121:414–422. doi: 10.1016/0008-8749(89)90040-3. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin RG, Din WS, Davis ST, Anderson DM, Gimpel SD, Sato TA, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz H. Biological activities of reverse signal transduction through CD137 ligand. J Leukoc Biol. 2005;77:281–286. doi: 10.1189/jlb.0904558. [DOI] [PubMed] [Google Scholar]
- 26.Zheng G, Wang B, Chen A. The 4-1BB costimulation augments the proliferation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:2428–2434. doi: 10.4049/jimmunol.173.4.2428. [DOI] [PubMed] [Google Scholar]
- 27.Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H. Ex Vivo Expansion of CD4+CD25+FoxP3+ T Regulatory Cells Based on Synergy between IL-2 and 4-1BB Signaling. J Immunol. 2007;179:7295–7304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
- 28.Seo SK, Park HY, Choi JH, Kim WY, Kim YH, Jung HW, et al. Blocking 4-1BB/4-1BB ligand interactions prevents herpetic stromal keratitis. J Immunol. 2003;171:576–583. doi: 10.4049/jimmunol.171.2.576. [DOI] [PubMed] [Google Scholar]
- 29.Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW, et al. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T cells: A CD4-dependent mechanism. J Immunol. 2007;179:2195–2202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 30.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, et al. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 31.Salek-Ardakani S, Flynn R, Arens R, Yagita H, Smith GL, Borst J, et al. The TNFR family members OX40 and CD27 link viral virulence to protective T cell vaccines in mice. J Clin Invest. 2011;121:296–307. doi: 10.1172/JCI42056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolfi DV, Boesteanu AC, Petrovas C, Xia D, Butz EA, Katsikis PD. Late signals from CD27 prevent Fas-dependent apoptosis of primary CD8+ T cells. J Immunol. 2008;180:2912–2921. doi: 10.4049/jimmunol.180.5.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matter M, Mumprecht S, Pinschewer DD, Pavelic V, Yagita H, Krautwald S, et al. Virus-induced polyclonal B cell activation improves protective CTL memory via retained CD27 expression on memory CTL. Eur J Immunol. 2005;35:3229–3239. doi: 10.1002/eji.200535179. [DOI] [PubMed] [Google Scholar]
- 34.Matter MS, Claus C, Ochsenbein AF. CD4(+) T cell help improves CD8(+) T cell memory by retained CD27 expression. Eur J Immunol. 2008;38:1847–1856. doi: 10.1002/eji.200737824. [DOI] [PubMed] [Google Scholar]
- 35.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, et al. Identification of poxvirus CD8+ T cell determinants to enable rational design and characterization of smallpox vaccines. The Journal of experimental medicine. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moutaftsi M, Peters B, Pasquetto V, Tscharke DC, Sidney J, Bui HH, et al. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nature biotechnology. 2006;24:817–819. doi: 10.1038/nbt1215. [DOI] [PubMed] [Google Scholar]