Abstract
Trop2, an oncogenic cell-surface protein under investigation as a therapeutic target, is commonly overexpressed in several epithelial tumor types yet its function in tumor biology remains relatively unexplored. To investigate the role of Trop2 in epithelial carcinogenesis, we generated Trop2−/− mice, which are viable and possess a normal lifespan. Contrary to expectations, Trop2 loss fails to suppress keratinocyte transformation. Instead, ras-transformed Trop2−/− keratinocytes preferentially pass through an epithelial to mesenchymal transition (EMT) and form tumors with spindle cell histology. Furthermore, Trop2 loss renders Arf null mice susceptible to the formation of biphasic sarcomatoid carcinomas containing both squamous and spindle cell components upon carcinogen exposure in an otherwise skin-cancer resistant strain (C57Bl/6). Immortalized keratinocytes derived from Trop2−/−Arf−/− mice exhibit enhanced proliferative and migratory capacity as well as increased activation of MAPK and Src prior to transformation. The clinical relevance of these findings was supported by studying the molecular epidemiology of Trop2 in primary head and neck squamous cell carcinomas. This analysis revealed that Trop2 mRNA levels are decreased in a subset of tumors with features of EMT, and total loss of Trop2 protein expression is observed in the spindle cell component of sarcomatoid carcinomas. Therefore, while previous studies have emphasized the potential importance of Trop2 gain-of-function, these results uncover a role for Trop2 loss in tumorigenesis and the mesenchymal transdifferentiation observed in a subset of squamous cell carcinomas.
Keywords: Trop2, E-cadherin, Epithelial to Mesenchymal Transition, Squamous Cell Carcinoma
Introduction
Trop2 (TACSTD2, M1S1) is a single-pass transmembrane protein expressed primarily in epithelial cells (1) and is often overexpressed in various epithelial tumor types (2–6). We previously reported that Trop2 overexpression has transforming activity whereas loss-of-function in colon cancer cells suppresses their tumorigenicity (7). More recently, Trop2 overexpression was demonstrated to strongly induce MAPK activity and metastasis in pancreatic cancer cells (8). As such, anti-Trop2 antibodies are under investigation as cancer therapeutics in the pre-clinical setting (9, 10). Intriguingly, Trop2 expression was found to mark prostate stem cells that are preferentially susceptible to transformation (11). These diverse epidemiologic and functional (12) data point to an important role for Trop2 in tumorigenesis and as a possible treatment target, yet its function(s) remains obscure. Studies on gelatinous drop-like corneal dystrophy (GDLD), a rare form of congenital blindness caused by Trop2 loss-of-function mutations (1, 13, 14), have revealed a role for Trop2 in modulating cell adhesion in the cornea. However, despite this increasing interest in Trop2 as a therapeutic target, its role in normal biology or neoplastic progression is poorly understood.
The epithelial to mesenchymal transition (EMT) consists of a series of cell biological and biochemical changes that endow normal epithelial cells with the plasticity required for body patterning and tissue differentiation. Acquisition of a mesenchymal phenotype by epithelial cells is regulated at multiple levels and is characterized by the loss of E-cadherin-mediated cell-cell adhesion, decreased cell-matrix adhesion, and gain of mesenchymal markers such as vimentin and fibronectin (15–18). An EMT-like profile is considered to be a characteristic of aggressive cancers. However, definitive identification of EMT in primary human tumors has been difficult, possibly because the transition may be restricted - both spatially and temporally - to a subset of cells within the tumor mass (19). Notably, some epithelial tumors contain areas of pure mesenchymal histology and possess the molecular features of EMT more uniformly. Sarcomatoid carcinoma is one specific example. These tumors are a distinct variant of squamous cell carcinoma that frequently originate in the epithelia of the upper aerodigestive tract (12, 20), display spindle cell histology, and typically lose epithelial gene expression consistent with mesenchymal transdifferentiation. Notably, sarcomatoid carcinoma can be biphasic and exhibit discreet regions of squamous and spindle cell histology. Additionally, microarray analyses have resulted in the identification of subtypes of epithelial tumors that do not histologically appear as spindle cells yet possess a gene expression signature suggestive of an epithelial to mesenchymal transition (21–23).
Given the reported overexpression of Trop2 in various epithelial tumors, we sought to examine its role in epithelial carcinogenesis by deriving Trop2 knockout mice and report the generation of a viable Trop2 null strain. Contrary to expectations, we show that Trop2 loss promotes the development of EMT during ras-mediated transformation of primary keratinocytes. Additionally, Trop2 loss increases susceptibility to the development of biphasic sarcomatoid carcinomas of the skin in response to carcinogens. Accordingly, in head and neck squamous cell carcinomas (HNSCC) we find that Trop2 expression is decreased in tumors possessing an EMT-like signature and completely absent in sarcomatoid variants. Collectively, these data implicate Trop2 loss in tumorigenesis in keratinocytes and in the promotion of EMT in a subset of squamous cell carcinomas.
Materials and Methods
Generation of Trop2−/− mice
BAC DNA clone RP23-23I10 containing trop-2 locus was obtained from CHORI, and was used to construct targeting vector by PCR amplification. The primer sequences used for 5′ homology arm of the targeting construct were 5′-GAT TGA GGA TCC AAT CTC TCC CAG GTC ATA AAC-3′ and 5′-GAT TGA GGA TCC GGT GGG GTG GAG TAG AAT GGA-3′. The primer sequences used for 3′ homology arm of the targeting construct were 5′-GAT TGA GAT ATC CCA TGG CCA AAG ACT TAA ACG GTT TGA AAT G-3′ and 5′-GAT TGA GCG GCC GCA GAG CCC CTC CTG CTT CTC ACA-3′. The amplified 5′ homology arm (2.6 kb) and 3′ homology arm (3.0 kb) were cloned into the p1338 vector. The targeting vector was linearized with NotI, and gene targeting was conducted following standard procedures in C57Bl6/SJ embryonic stem cell line B6/Blu, which was injected into a blastocyst. A pseudopregnant C57Bl6/SJ mother was used as the recipient. Embryonic stem cell clones that underwent the correct integration were identified by southern blotting with a 5′ external probe and 3′ external probe. The primer sequences used for the 5′ external probe were 5′-CTC TAC TCC TAA CCC TAA TCT GCC-3′ and 5′-TAC CAT CCC TAC CTT AGT CCA CC-3′, The primer sequences used for the 3′ external probe were 5′-GTC CCT ACT CAA CTC CTT CTC CC-3′ and 5′-TAG CCA ATT ACA AAA CAG TCA TTC C-3′. One embryonic stem cell clone was used to generate chimeras by Washington University Murine Embryonic Stem Cell Core.
Skin tumor induction
The backs of 8-wk-old male and female mice were shaved and treated with a single application of DMBA (20 μg in 200 μl of acetone; Sigma, St. Louis, Missouri, United States) followed a week later by twice weekly applications of TPA (12.5 μg in 200 μl of acetone). The number and size of papillomas on each mouse were recorded every week for 36 weeks. Animals were handled according to protocols approved by the Washington University Animal Studies Committee.
Mouse keratinocyte isolation and manipulation
Primary epidermal keratinocytes populations were harvested from newborn mice (1 day old). Skin was floated dermis down at 4°C overnight in dispase I (Invitrogen). Afterwards, skin pieces were placed with the dermis-side up, and the dermis was peeled off with a forceps. Keratinocytes were isolated by trypsinizing the tissues for an additional 15 min at 37°C. Cells were collected, washed in PBS containing 0.5% FBS and were resuspended and seeded in EMEM media (Lonza).
Antibodies and immunoblot analysis
Cells were lysed in 50 mM Tris, pH 7.4; 150 mM NaCl; 0.5% DOC; 0.5% NP-40. 50 ug of protein was resolved on a 4–15% Tris-HCl gel and transferred to PVDF membranes. Antibodies used in this study were against Trop2 A650 and AF1122 (against human and mouse, respectively, R&D Systems), E-cadherin (BD Transduction Laboratories), Vimentin (BD Pharmingen), p-Src (Tyr416, Calbiochem and Cell Signaling), Tubulin (Abcam), Erk 1/2 and p-ERK 1/2 (Thr202/Tyr204), p-FAK (Tyr397), and p-p130Cas (Tyr165) from Cell Signaling, H-Ras (Santa Cruz Biotechnology). Image quantification was performed with ImageJ software (National Institutes of Health).
Lentiviral gene transfer and tumorigenicity assays
Lentiviruses expressing H-rasV12 or mouse Trop2 were produced as previously described (7). Filtered supernatant supplemented with 4 μg/ml polybrene was used to infect freshly isolated mouse skin keratinocytes within 48 hr. postplating. Two days after infection, lentivirally transduced cells were harvested, and either 2×106 cells were injected s.c. into the flanks of nude mice. Tumor growth was monitored every 3 days.
Immunohistochemical analysis
The slides were deparaffinized and hydrated, treated in citrate buffer (0.1 M sodium citrate and citric acid, pH 3.0) for 10 minutes in a pressure cooker and quenched in 3% hydrogen peroxide solution for 10 minutes. Sections were blocked with Avidin solution and Biotin solution after washing with TBST thoroughly. Sections were incubated with anti-Trop2 antibody for 1 hour at room temperature after TBST washing. Sections were incubated with LSAB+System-HRP solutions (Dako) per the manufacturer’s instructions. Color was developed by adding DAB substrate solution and stopped by washing with water. Mouse tumor slides were counterstained in hematoxylin, dehydrated, cleared, and mounted in Cytoseal. The histology of keratinocyte tumors was quantified by the Olympus Microsuite 5 software package.
Statistical Analysis
All statistical tests were two-sided and P values of less than 0.05 were considered statistically significant. Additional methods can be found in supplementary material.
Results
Trop2 loss does not result in embryonic lethality and is not required for development in mice
To gain insight into the effects of Trop2 loss of function, we generated a Trop2 null mouse strain by deleting the single exon that encodes the open reading frame using homologous recombination in embryonic stem cells (Figs. 1A–E). Trop2 has been proposed to play a role in development because its expression is high in certain epithelial stem cell compartments (24, 25) and the levels fluctuate widely in response to developmental cues (26). Additionally, EpCAM, the gene most closely related to Trop2 (forty-nine percent homology) is essential in mice (27). However, we found that homozygous null mice are born at the normal expected Mendelian frequencies, display no overt developmental abnormalities, and have a normal life expectancy (Supplementary Figs. 1 and 2 and data not shown).
Figure 1.
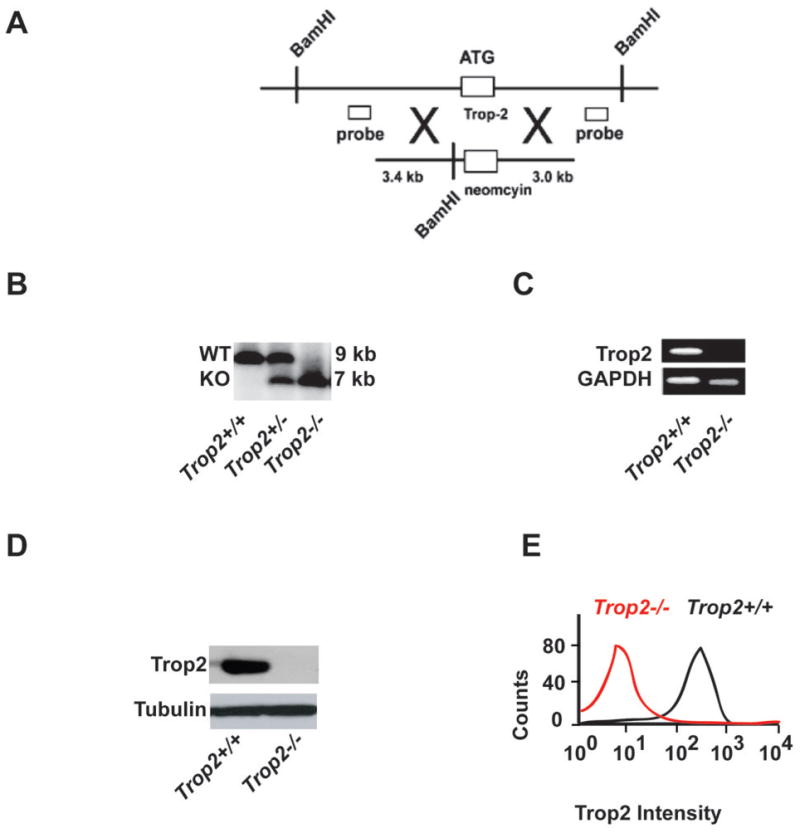
Strategy for the generation and validation of a Trop2 knockout mouse strain. A, Schematic depicting the knockout vector and probes for Southern analysis. B, Southern analysis showing knockout of the Trop2 locus in F1 offspring. C, Reverse-transcriptase polymerase chain reaction of Trop2 transcripts from RNA isolated from tails of the denoted genotypes. D, Immunoblot analysis using anti-Trop2 antibodies on lysates from primary mouse keratinocytes. E, Flow cytometric analysis of Trop2 on primary mouse keratinocytes.
Induction of tumors with spindle cell histology upon transformation of Trop2−/− null keratinocytes
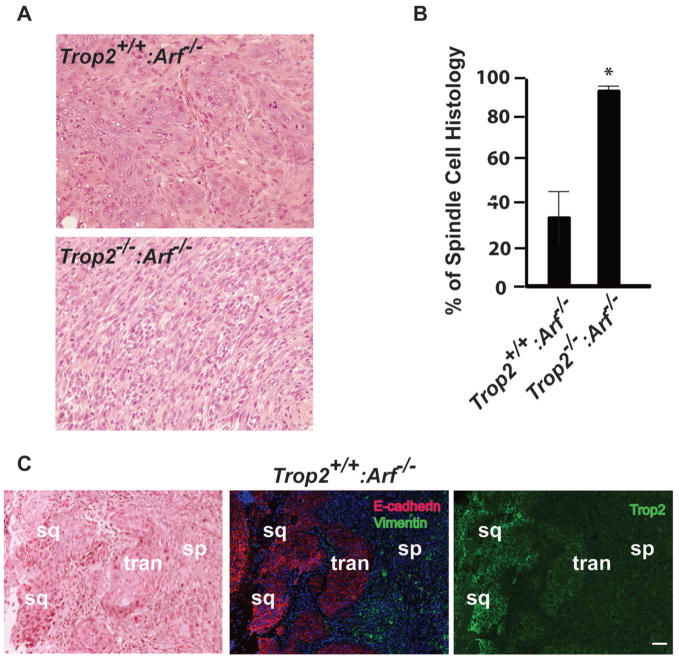
To model the effects of Trop2 loss of function on epithelial transformation, we employed the two-hit transformation assay of primary mouse keratinocytes (28) in which ras pathway activation in the context of Arf deletion drives the development of squamous cell carcinomas (29). Primary keratinocytes were isolated from Trop2+/+:Arf−/− and Trop2−/−:Arf−/− mice, infected in vitro with an H-RasV12 expressing lentivirus (Supplementary Fig. 3), and then grafted subcutaneously into the flanks of nude mice. Palpable tumors were noted in both genetic backgrounds after two weeks when a cell dosage of 2×106 was injected. However, histopathological examination of the tumors that arose revealed that keratinocytes from Trop2−/−:Arf−/− mice displayed predominantly spindle with almost no squamous cell histology. In contrast tumors from Trop2+/+:Arf−/− mice exhibited predominantly squamous cell histology and some spindle cell components (Fig. 2A). Quantification of the percentages of spindle and squamous histology in each tumor using image analysis software confirmed an increase in spindle cell conversion in tumors derived from Trop2−/−:Arf−/− cells compared to those arising from Trop2+/+:Arf−/− cells (Fig. 2B). Analysis of E-cadherin and vimentin in tumors arising from both genotypes revealed the retention of E-cadherin and the absence of vimentin expression in areas of squamous cell histology, while spindle cells displayed the opposite pattern, consistent with EMT (Fig. 2C and Supplementary Fig. 4). Strikingly, in areas of spindle cell histology in nominally Trop2 wild-type tumors, Trop2 expression was absent and a gradient of decreasing expression was evident mirroring the decline in E-cadherin and increase in vimentin staining in transition zones bordering cells that had passaged through an EMT (Fig. 2C). These data indicate that Trop2 deficiency facilitates EMT during oncogenic transformation of keratinocytes in vivo. Furthermore, Trop2 loss occurs during transformation associated with mesenchymal transdifferentiation in Trop2 positive cells.
Figure 2.
Role of Trop2 loss in primary epithelial cell transformation. A, Squamous histology in ras- transformed Trop2+/+:Arf−/− keratinocytes and spindle cell histology in ras-transformed Trop2−/−:Arf−/− keratinocytes as demonstrated by demonstrated by hematoxylin and eosin staining. B, Results of measurements of the percentages of spindle relative to squamous cell histology in tumors from cells with the indicated genotypes using image-analysis software to calculate the areas. *, P=0.008, student’s t-test. C, Hematoxylin and eosin stained section from a tumor derived from Trop2+/+:Arf−/− keratinocytes showing a region of spontaneous progression from squamous to spindle cell histology (left panel). Indirect immunofluorescence staining reveals loss of E-cadherin (red) and gain of vimentin (green) as the tumor transitions from squamous to spindle cell histology (middle panel). Nuclei are stained with DAPI (blue). Indirect immunofluorescence staining of Trop2 (right panel) shows loss of Trop2 coincident with the EMT observed in the nominally Trop2+/+ tumor cells. Middle and right panel are from sections cut adjacent to the hematoxylin and eosin stained section. Abbreviations are: sq, squamous, tran, area of low E-cadherin and Trop2 representing a transition to spindle cells, and sp, spindle cell. Bar, 100 μm.
Trop2 loss promotes tumorigenesis in mice
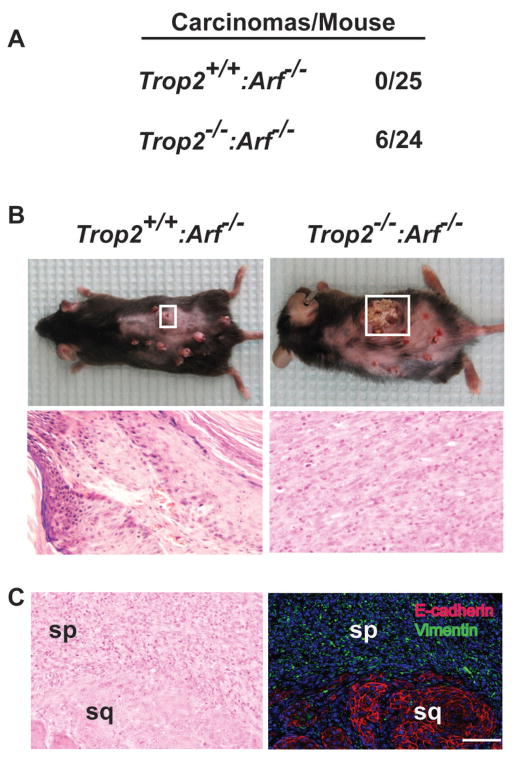
The ability of Trop2 loss to modify the histologic outcome of an identical set of transforming events at the cellular level raised the question whether Trop2 loss contributes to tumorigenesis. To investigate this possibility, we used a well-established model of skin carcinogenesis (DMBA-TPA) that in C57Bl/6 mice generates H-Ras mutations and papillomas but rarely invasive cancer (30). The absence of Trop2 did not alter the incidence or natural history of papilloma formation using this protocol (Supplementary Table 1A). However, given the cooperativity between Trop2 and Arf-ras pathway defects observed in keratinocytes, the effect of Trop2 loss on skin cancer development was assessed in Arf null animals exposed to DMBA-TPA. Over a standard thirty-six week treatment period, both strains developed papillomas at equivalent rates and sizes (Supplementary Table 1B) and equivalent percentages of mice survived the treatment protocol, some succumbing to the expected lymphoid and non-skin sarcomatoid appearing malignancies. However and most importantly, Trop2 loss in this context was sufficient to promote the formation of skin carcinomas (n=6/24 in Trop2−/−:Arf−/− mice) whereas none of the Trop2+/+:Arf−/− mice (n=0/25, P=0.009, Fisher’s exact test) developed cancer (Figs. 3A and B). All six tumors were invasive, five were classified as sarcomatoid carcinomas, and one was found to consist of poorly-differentiated high-grade cancer. Interestingly, some squamous cell histology was identified in three of the sarcomatoid carcinomas, consistent with their epithelial origin. Accordingly, these squamous cell components expressed E-cadherin whereas the spindle cells lacked this staining but were positive for vimentin (Fig. 3C). Thus, Trop2 loss, in collaboration with Arf inactivation, increases the susceptibility to skin carcinogenesis and promotes the development of tumors with mesenchymal features.
Figure 3.
Trop2 loss promotes the development of sarcomatoid carcinomas in mice. A, Enumeration of the incidence of carcinoma formation in Trop2+/+:Arf−/− and Trop2−/−:Arf−/− mice after thirty-six weeks of DMBA-TPA exposure (P=0.009 based on Fisher’s exact test). B, A representative Trop2+/+:Arf−/− mouse harbors multiple papillomas (left upper panel). The box circumscribes a typical lesion. The associated hematoxylin and eosin stained section is shown in the panel below. A representative large tumor (right upper panel, boxed in white) from a Trop2−/−:Arf−/− animal. The spindle cell histology of the tumor is shown by hematoxylin and eosin staining in the lower panel. C, A section from a tumor arising in Trop2−/−:Arf−/− mice showing biphasic histology by hematoxylin and eosin staining (left panel). Immunofluorescence staining of the corresponding section (right panel) reveals that the spindle cell component (sp) is positive for vimentin (green) while the squamous component (sq) is E-cadherin positive. Bar, 100 μm.
Immortalized Trop2 null keratinocytes exhibit increased proliferative and migratory capacity
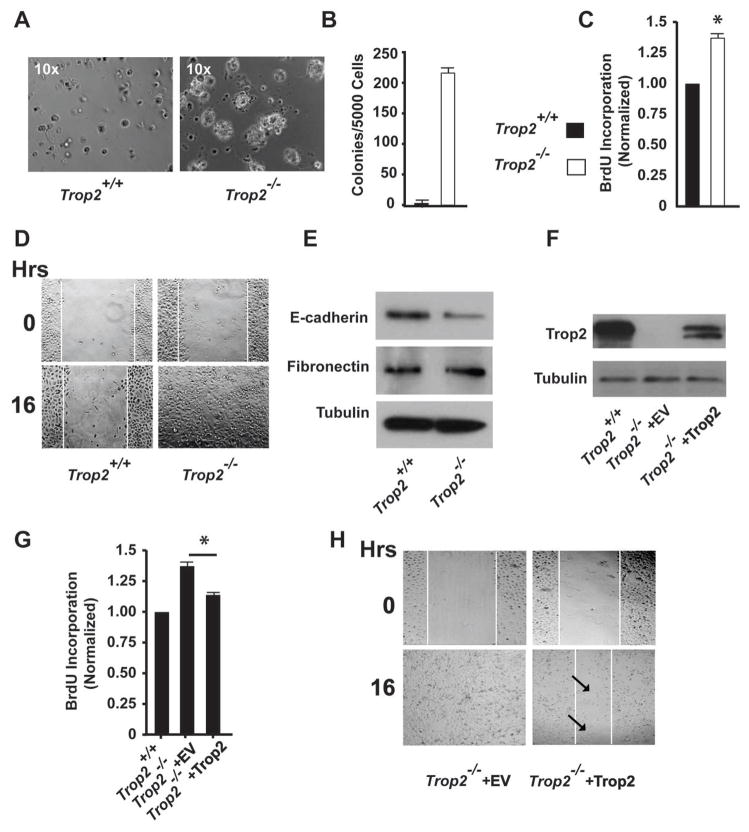
Trop2 null mice display no overt phenotypic abnormalities, yet Trop2 loss can contribute to tumor initiation and drive progression towards spindle cell histology. These observations suggest that non-transformed Trop2 null cells are more likely to acquire characteristics that can facilitate cancerous growth. To investigate whether such characteristics could be identified, we isolated Trop2+/+:Arf−/− and Trop2−/−:Arf−/− keratinocytes; the Arf deficient background imparts features of cellular immortalization in vitro (31). Loss of Trop2 did not alter cellular proliferation in standard tissue culture conditions (data not shown), but Trop2−/−:Arf−/− keratinocytes displayed a dramatic proliferative advantage in three-dimensional culture compared to Trop2+/+:Arf−/− cells. Measurement of bromodeoxyuridine (BrdU) incorporation revealed increased rates of DNA synthesis in Trop2 null cells when grown in 3-D culture, suggesting a proliferative advantage under these more physiologic conditions (Figs. 4A–C). We also compared the migratory potential of Trop2+/+:Arf−/− and Trop2−/−:Arf−/− keratinocytes and found the latter to possess a significantly increased mobility in a two-dimensional would healing assay (Fig. 4D). Next, we examined whether Trop2 loss was sufficient to promote features of EMT in immortalized keratinocytes. Trop2−/−:Arf−/− did not acquire a mesenchymal morphology and immunoblot analysis of Twist, Snail, Slug, and fibronectin revealed no detectable alterations in the levels of these proteins compared to wile-type cells. However, E-cadherin protein levels were found to be significantly decreased in the absences of Trop2 (Fig. 4E and data not shown). Lastly, we sought to reconstitute Trop2 expression in Trop2−/−:Arf−/− cells with a full-length mouse cDNA in an effort to reverse the observed phenotypic changes. After lentiviral transduction of Trop2 and antibiotic selection, Trop2 protein expression was partially restored relative to levels measured in Trop2+/+:Arf−/− cells (Fig. 4F), and the protein migrated as a doublet, suggesting differential post-translational modification(s) in this cellular environment. Importantly, expression of Trop2 in Trop2−/−:Arf−/− cells was found to be sufficient to reduce (but not normalize) both rates of proliferation in 3-D culture as well as migration in a wound healing assay relative to those measured in empty-vector transduced cells (Figs. 4G and H). Total E-cadherin levels were not measurably increased (data not shown), possibly owing to the need for higher levels of Trop2 protein expression to rescue this specific defect in knockout cells. Nevertheless, partial restoration of Trop2 protein levels is able to measurably reduce the proliferative and migratory advantages identified in Trop2 null keratinocytes, observations that point to a causal role for Trop2 loss in the development of these phenotypes.
Figure 4.
Trop2 loss confers a proliferative and migratory advantage in Arf−/− immortalized keratinocytes. A, Photomicrographs of Trop2+/+:Arf−/− and Trop2−/−:Arf−/− keratinocytes grown on Matrigel for seven days. 10× magnification. B, Graph of colony counts seven days after keratinocyte plating. C, Graph of BrdU incorporation of keratinocytes grown on Matrigel measured three days after plating. *, P=0.025, student’s t-test. D, Photomicrographs of a scratch assay using keratinocytes from Trop2+/+:Arf−/− and Trop2−/−:Arf−/− mice showing a completely closed wound in Trop2 null cells by sixteen hours. E, Immunoblot analysis of protein lysates from Trop2+/+:Arf−/− and Trop2−/−:Arf−/− keratinocytes showing decreased E-cadherin levels in cells lacking Trop2. F, Immunoblot analysis of Trop2−/−:Arf−/− keratinocytes infected with control (empty vector) or Trop2-expressing lentivirus. Protein levels of Trop2 in Trop2+/+:Arf−/− are shown for comparison. G, Graph of BrdU incorporation of Trop2−/−:Arf−/− keratinocytes infected with control or Trop2-expressing virus grown on Matrigel measured three days after plating. *, P=0.049, student’s t-test. BrdU incorporation of Trop2+/+:Arf−/− keratinocytes is shown for comparison. For BrdU experiments, results were normalized to Trop2+/+:Arf−/− cells. H, Photomicrographs of a scratch assay using keratinocytes from Trop2−/−:Arf−/− mice infected with control (empty vector) or Trop2-expressing virus showing retarded migration in Trop2 expressing cells. Arrows point to scattered cells in the unfilled region of the scratch. Results from each experiment are representative of three independent tests.
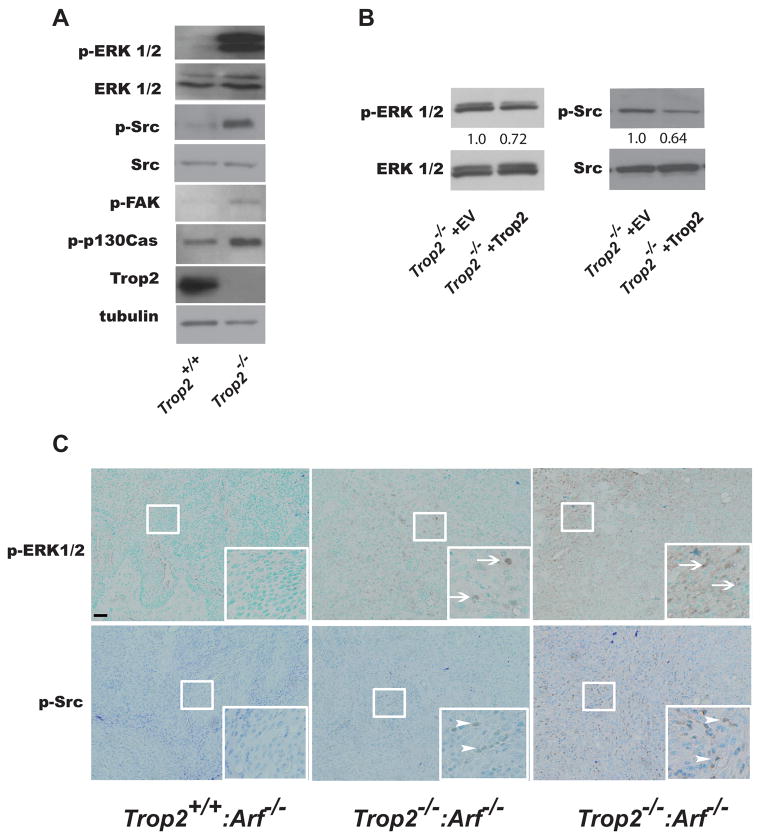
To identify Trop2-dependent mechanisms underlying the increased susceptibility to tumorigenesis, we examined whether Trop2 loss could increase the activity of common oncogenic pathways by measuring the phosphorylation status of EGFR, AKT, ERK, and Src. This analysis revealed that keratinocytes derived from Trop2−/−:Arf−/− mice exhibit an increase in MAPK and Src activity, the former showing a dramatic increase in the absence of Trop2, compared to Trop2+/+:Arf−/− cells (Fig. 5A). An increase in the activity of EGFR and AKT was not observed (data not shown). In Trop2 knockout cells, we also detected an increase in the level of phosphorylated forms of two Src substrates, FAK and p130Cas (Fig. 5A). Importantly and in consonance with the partial phenotypic rescue achieved by Trop2 expression, we observed reduced levels of active MAPK and Src in Trop2−/−:Arf−/− cells after infection with Trop2-expressing lentivirus (Fig. 5B). To determine whether increased MAPK and Src activity could be identified in the context of Trop2 deficiency in vivo, we performed an immunohistochemical analysis of papillomas and tumors. Neither MAPK nor Src hyperactivation was found in papillomas arising in Trop2+/+:Arf−/− mice. In contrast, increased staining of p-ERK and p-Src was readily detected in papillomas arising in Trop2−/−:Arf−/− mice (n=5 per group). In addition, extensive staining was observed in five carcinomas examined that developed in the double knockout animals (Fig. 5C). Interestingly, Src, staining was detected primarily in the cytoplasm, a pattern of localization that has previously been associated with poorly differentiated cancer (32). Collectively, these data reveal that Trop2 loss causes an increase in MAPK and Src activation prior to oncogenic transformation.
Figure 5.
Activation of MAPK and Src in Trop2−/−:Arf−/− keratinocytes and tissues. A, Immunoblot analysis of immortalized Trop2+/+:Arf−/− and Trop2−/−:Arf−/− keratinocytes reveals activation of ERK 1/2 (Thr 202/Tyr 204), Src (Tyr416), FAK (Tyr397)and p130Cas (Tyr165), in Trop2 null cells. B, Immunoblot analysis shows reduction in ERK and Src activation in Trop2-expressing Trop2−/−:Arf−/− cells compared to control cells harboring an empty vector. The numbers indicate densitometrically quantified protein levels (normalized to total ERK or Src). C, Immunohistochemical staining of p-ERK (Thr 202/Tyr 204) and p-Src (Tyr416) in papillomas and carcinomas from tissues arising in the denoted genotypes. Arrows and arrowheads point to cells staining positive. Sections were stained with methyl green as a counterstain. Bar is 50 μM.
Decreased Trop2 levels in squamous cell carcinoma with an EMT gene expression signature and sarcomatoid carcinomas of the head and neck
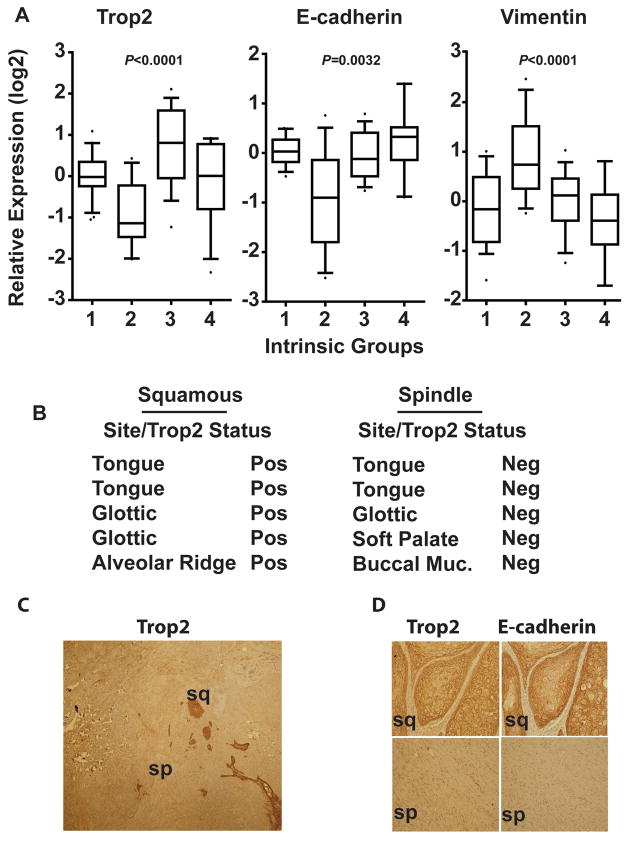
Our data showing that loss of Trop2 facilitates EMT in squamous cell tumors in mice prompted us to investigate Trop2 expression in human head and neck cancer. A subset of head and neck cancers exhibit morphologic and/or molecular features of EMT. In addition to sarcomatoid carcinomas of the head and neck, which exhibit mesenchymal markers as well as distinct histopathology suggestive of an EMT, a gene expression signature has previously been reported by us that identifies a subtype of tumors with molecular features of EMT (designated as Group 2). Pathologically, these tumors appear as poorly differentiated carcinomas (22). To determine whether decreased Trop2 expression associates with this EMT-like subtype, we queried its expression in our dataset that was used to develop this signature (22). Notably, Trop2 expression was found to be decreased in the EMT-like group relative to the other subtypes and normal tonsillar mucosa. Accordingly, reduced Trop2 expression segregates with low E-cadherin and high vimentin expression in this dataset (Fig. 6A). Given the relative decrease in these poorly differentiated, EMT-like yet non-sarcomatoid tumors and based on the results from the knockout animal experiments, we suspected that Trop2 expression might be completely undetectable in the spindle cell component of sarcomatoid carcinoma of the head and neck. Immunohistochemical staining of sarcomatoid carcinoma confirmed this possibility. Trop2 expression was detected in areas of squamous cell histology in cancers containing pure squamous cell elements and biphasic sarcomatoid carcinomas. However, in striking contrast, its expression was absent in the spindle cell component of sarcomatoid carcinomas (Figs. 6B–D) from various head and neck sites (n=5 of each histologic group, P=0.007, Fisher’s exact test). Consistent with their mesenchymal state, Trop2-negative sarcomatoid carcinomas also lacked E-cadherin expression, while tumors containing Trop2-positive cells with squamous histology expressed high levels (Fig. 6D). Therefore, decreased Trop2 expression is a feature of squamous cell carcinomas that possess molecular and histological characteristics of EMT.
Figure 6.
Trop2 expression in head and neck cancers. A, Association of Trop2 expression levels with intrinsic subtypes of head and neck cancers by in silico analysis. Group 2 is the EMT-like subtype consisting of poorly differentiated tumors. B, Association of negative Trop2 status with the spindle cell component of sarcomatoid carcinomas. Cancers denoted in the left column contain pure squamous cell histology. C, Example of loss of Trop2 staining in a biphasic head and neck cancer. Trop2 is absent in the spindle cell component (sp) while nests of squamous cancer cells (sq) stain positive. 10× magnification. D, Immunohistochemical staining of Trop2 and E-cadherin showing concomitant loss in spindle cell components. 20× magnification.
Discussion
The results presented here were not predicted based on previous epidemiologic and functional studies of Trop2, all of which pointed to a gain-of-function role for this protein in the development of aggressive cancer and tumor susceptibility (2–7, 11, 24, 33–35). As such, we expected that Trop2 ablation would hinder cancer development. Instead, through the use of a novel Trop2 knockout mouse model generated in our laboratory, we uncovered an unpredicted relationship between Trop2 loss and cancer development. In addition, we were able to confirm a relationship between Trop2 loss and an increased progression towards EMT during epithelial transformation. The epidemiologic analysis presented in this report is the first description, to our knowledge, of the association between Trop2 loss and EMT in any primary human tumor, and highlights the clinical relevance of our findings. Collectively, these observations reveal a far more complex functional role for this protein in the multistep development of cancer than has been previously suspected.
It has been reported that germline deletion of EpCAM, the closest homolog to Trop2, causes early embryonic lethality in mice (27). Additionally, Trop2 expression is elevated in stem cell compartments of the prostate (24) and liver (25), and the pattern of expression is dynamic during development (26). All of these observations suggested a high likelihood that Trop2 deletion would result in abnormalities in development or adulthood, but these animals are viable and lack overt developmental defects. However, a more detailed analysis revealed that Trop2 loss promotes skin cancer in mice lacking Arf, a tumor suppressor gene commonly inactivated in many cancers, including those arising in the head and neck. It is notable that mice lacking this gene are tumor prone and predominantly develop sarcomas and lymphomas rather than skin tumors (31), which do form when exposed to DMBA as neonates (36). C57Bl/6 mice, the strain employed in our experiments, are notoriously resistant to the standard TPA-DMBA-mediated carcinogenesis protocol that we used herein. Given the cancers that develop in Trop2−/−:Arf−/− animals in the C57Bl/6 background after TPA-DMBA exposure and the absence of a detectable cancer phenotype in Trop2−/− mice, our results indicate that Trop2 participates in multistep tumorigenesis as a modifier in collaboration with other pro-tumorigenic events. In this manner, Trop2 loss resembles the effects of Src, whose catalytic activity is frequently elevated in cancers. Owing to its weak transforming ability, Src is thought to facilitate other oncogenic signals rather than function as a dominant oncogene (37). The identification of Src hyperactivation in keratinocytes, papillomas and carcinomas derived from Trop2−/−:Arf−/− mice, and the suppression of this activity by re-expression of Trop2 supports the idea that Trop2-dependent modulation of Src likely contributes to the enhanced tumor susceptibility observed in Trop2−/−:Arf−/− animals. We also found that Trop2 loss causes elevation of MAPK activity, which lies downstream of several oncogenic pathways. We did not assess the relative contributions of these two pathways to the observed cellular phenotypes but it is notable that cross-talk between the Src and MAPK pathways is well established. These pathways are commonly linked in adhesion signaling mediated by integrin and hyluronic acid receptors, suggesting that Trop2 may be a component of one of such module (38).
Strikingly, the squamous cell tumors that arise in Trop2−/−:Arf−/− mice are primarily sarcomatoid or poorly differentiated carcinomas, an observation that is consistent with the primary human tumor data. The ability of Trop2 loss to promote spindle cell histology in primary keratinocyte transformation assays provides additional evidence for a role for Trop2 in modulating the epithelial state. Similar to what has been observed with established inducers of EMT, Trop2-loss induced mesenchymal transdifferentiation must occur in collaboration with other factors and may be cell-type specific (39). This is clear because Trop2 knockout mice are developmentally normal and the pattern of E-cadherin expression in two tissues that we have examined in situ (skin and breast, data not shown) is similar in wild-type and knockout animals. Additionally, EMT was not observed in immortalized Trop2−/−:Arf−/− null keratinocytes, although E-cadherin expression is reduced in these cells. The sarcomatoid histology and E-cadherin loss observed in tumors arising in Trop2−/−:Arf−/− animals are likely to be mediated at least in part through Src activity, a well-established negative regulator of E-cadherin. Activation of the MAPK pathway has also been implicated in the development of aggressive cancer and EMT (15, 40), and may also contribute to Trop2-loss induced EMT. Intriguingly, gene expression analyses of lung and ovarian cancer cell lines that exhibit features of EMT (as well as those from the head and neck) show strikingly low Trop2 expression compared to more epithelial lines originating from the same tumors. These results point to the possibility that Trop2 mediates EMT in a broad range of tissues when considering the data presented herein (41–43). Further dissection of the relationship between Trop2, Src and MAPK activation, is the goal of ongoing studies.
The ability of proteins to exert dual (oncogenic or tumor suppressor) functions is well established (44, 45). The observations that both gain and loss of Trop2 function provoke strong phenotypes suggest that this protein possesses potent signaling activities. Given the similarity to EpCAM, which regulates both adhesion (46) and intracellular signaling via cleavage products (47), Trop2 may also possess a pleiotropic mechanism of action. This possibility may reconcile the apparent contradiction that high levels of Trop2 in oral cancers have been observed and associate with a poor outcome in one study (5). As such, studies in the oral mucosa of Trop2 knockout animals are planned to more accurately define the functional role of Trop2 in HNSCC, where EMT is thought to be an important prognostic factor (48).
In summary, while previous studies have highlighted the potential importance of Trop2 gain-of-function in the development of aggressive epithelial cancers, the data presented herein identify for the first time a role for Trop2 loss-of-function in tumorigenesis and mesenchymal transdifferentiation. Ongoing efforts to target Trop2 immunologically (10, 49) will need to take into account the effects of its loss uncovered in this study. Further investigation into Trop2 function through the loss-of-function genetics described in this report should provide insights into EMT and elucidate novel mechanisms of cancer susceptibility.
Supplementary Material
Acknowledgments
LSM is supported by a Damon Runyon Clinical Investigator Award and Susan G. Komen for the Cure. JD Weber is supported by Department of Defense Era of Hope Scholar Award (W81XWH-08-1-0178). We thank Ms. Andrea C. Santeford for assistance with image analysis. We would like to thank Peter Siegel, Ph.D, for helpful comments and suggestions.
References
- 1.Tsujikawa M, Kurahashi H, Tanaka T, Nishida K, Shimomura Y, Tano Y, et al. Identification of the gene responsible for gelatinous drop-like corneal dystrophy. Nat Genet. 1999;21:420–3. doi: 10.1038/7759. [DOI] [PubMed] [Google Scholar]
- 2.Iacobuzio-Donahue CA, Maitra A, Shen-Ong GL, van Heek T, Ashfaq R, Meyer R, et al. Discovery of novel tumor markers of pancreatic cancer using global gene expression technology. Am J Pathol. 2002;160:1239–49. doi: 10.1016/S0002-9440(10)62551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santin AD, Zhan F, Bellone S, Palmieri M, Cane S, Bignotti E, et al. Gene expression profiles in primary ovarian serous papillary tumors and normal ovarian epithelium: identification of candidate molecular markers for ovarian cancer diagnosis and therapy. Int J Cancer. 2004;112:14–25. doi: 10.1002/ijc.20408. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima K, Shimada H, Ochiai T, Kuboshima M, Kuroiwa N, Okazumi S, et al. Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int J Cancer. 2004;112:1029–35. doi: 10.1002/ijc.20517. [DOI] [PubMed] [Google Scholar]
- 5.Fong D, Spizzo G, Gostner JM, Gastl G, Moser P, Krammel C, et al. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21:186–91. doi: 10.1038/modpathol.3801001. [DOI] [PubMed] [Google Scholar]
- 6.Muhlmann G, Spizzo G, Gostner J, Zitt M, Maier H, Moser P, et al. TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol. 2009;62:152–8. doi: 10.1136/jcp.2008.060590. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Day R, Dong Y, Weintraub SJ, Michel L. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther. 2008;7:280–5. doi: 10.1158/1535-7163.MCT-07-2003. [DOI] [PubMed] [Google Scholar]
- 8.Cubas R, Zhang S, Li M, Chen C, Yao Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer. 2010;9:253. doi: 10.1186/1476-4598-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang CH, Gupta P, Michel R, Loo M, Wang Y, Cardillo TM, et al. Ranpirnase (frog RNase) targeted with a humanized, internalizing, anti-Trop-2 antibody has potent cytotoxicity against diverse epithelial cancer cells. Mol Cancer Ther. 2010;9:2276–86. doi: 10.1158/1535-7163.MCT-10-0338. [DOI] [PubMed] [Google Scholar]
- 10.Varughese J, Cocco E, Bellone S, de Leon M, Bellone M, Todeschini P, et al. Uterine serous papillary carcinomas overexpress human trophoblast-cell-surface marker (trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized anti-trop-2 monoclonal antibody. Cancer. 2011 doi: 10.1002/cncr.25891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–71. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansari-Lari MA, Hoque MO, Califano J, Westra WH. Immunohistochemical p53 expression patterns in sarcomatoid carcinomas of the upper respiratory tract. Am J Surg Pathol. 2002;26:1024–31. doi: 10.1097/00000478-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Takaoka M, Nakamura T, Ban Y, Kinoshita S. Phenotypic investigation of cell junction-related proteins in gelatinous drop-like corneal dystrophy. Invest Ophthalmol Vis Sci. 2007;48:1095–101. doi: 10.1167/iovs.06-0740. [DOI] [PubMed] [Google Scholar]
- 14.Nakatsukasa M, Kawasaki S, Yamasaki K, Fukuoka H, Matsuda A, Tsujikawa M, et al. Tumor-Associated Calcium Signal Transducer 2 Is Required for the Proper Subcellular Localization of Claudin 1 and 7. Implications in the Pathogenesis of Gelatinous Drop-Like Corneal Dystrophy. Am J Pathol. 2010 doi: 10.2353/ajpath.2010.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–54. doi: 10.1158/0008-5472.CAN-07-2938. [DOI] [PubMed] [Google Scholar]
- 18.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis JS., Jr Spindle cell lesions--neoplastic or non-neoplastic?: spindle cell carcinoma and other atypical spindle cell lesions of the head and neck. Head Neck Pathol. 2008;2:103–10. doi: 10.1007/s12105-008-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung CH, Parker JS, Karaca G, Wu J, Funkhouser WK, Moore D, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 23.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte ON. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci U S A. 2008;105:20882–7. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, et al. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–60. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 26.Lu J, Izvolsky KI, Qian J, Cardoso WV. Identification of FGF10 targets in the embryonic lung epithelium during bud morphogenesis. J Biol Chem. 2005;280:4834–41. doi: 10.1074/jbc.M410714200. [DOI] [PubMed] [Google Scholar]
- 27.Nagao K, Zhu J, Heneghan MB, Hanson JC, Morasso MI, Tessarollo L, et al. Abnormal placental development and early embryonic lethality in EpCAM-null mice. PLoS One. 2009;4:e8543. doi: 10.1371/journal.pone.0008543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberg WC, Azzoli CG, Kadiwar N, Yuspa SH. p53 gene dosage modifies growth and malignant progression of keratinocytes expressing the v-rasHa oncogene. Cancer research. 1994;54:5584–92. [PubMed] [Google Scholar]
- 29.Lin AW, Lowe SW. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5025–30. doi: 10.1073/pnas.091100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–62. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–59. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 32.Serrels B, Serrels A, Mason SM, Baldeschi C, Ashton GH, Canel M, et al. A novel Src kinase inhibitor reduces tumour formation in a skin carcinogenesis model. Carcinogenesis. 2009;30:249–57. doi: 10.1093/carcin/bgn278. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–72. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 34.Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–63. doi: 10.1158/1078-0432.CCR-05-1961. [DOI] [PubMed] [Google Scholar]
- 35.Cubas R, Li M, Chen C, Yao Q. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796:309–14. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Kamijo T, Bodner S, van de Kamp E, Randle DH, Sherr CJ. Tumor spectrum in ARF-deficient mice. Cancer research. 1999;59:2217–22. [PubMed] [Google Scholar]
- 37.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–14. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol. 2002;156:299–313. doi: 10.1083/jcb.200109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17:542–7. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Coldren CD, Helfrich BA, Witta SE, Sugita M, Lapadat R, Zeng C, et al. Baseline gene expression predicts sensitivity to gefitinib in non-small cell lung cancer cell lines. Mol Cancer Res. 2006;4:521–8. doi: 10.1158/1541-7786.MCR-06-0095. [DOI] [PubMed] [Google Scholar]
- 42.Frederick BA, Helfrich BA, Coldren CD, Zheng D, Chan D, Bunn PA, Jr, et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Ther. 2007;6:1683–91. doi: 10.1158/1535-7163.MCT-07-0138. [DOI] [PubMed] [Google Scholar]
- 43.Tomassetti A, De Santis G, Castellano G, Miotti S, Mazzi M, Tomasoni D, et al. Variant HNF1 modulates epithelial plasticity of normal and transformed ovary cells. Neoplasia. 2008;10:1481–92. doi: 10.1593/neo.81004. 3p following 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–42. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 45.Winslow MM, Dayton TL, Verhaak RG, Kim-Kiselak C, Snyder EL, Feldser DM, et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature. 2011 doi: 10.1038/nature09881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J Cell Biol. 1994;125:437–46. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maetzel D, Denzel S, Mack B, Canis M, Went P, Benk M, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162–71. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 48.Chung CH, Parker JS, Ely K, Carter J, Yi Y, Murphy BA, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer research. 2006;66:8210–8. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- 49.Chang CH, Gupta P, Michel R, Loo M, Wang Y, Cardillo TM, et al. Ranpirnase (frog RNase) targeted with a humanized, internalizing, anti-Trop-2 antibody has potent cytotoxicity against diverse epithelial cancer cells. Mol Cancer Ther. 2010;9:2276–86. doi: 10.1158/1535-7163.MCT-10-0338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.