Abstract
Waking neurobehavioral performance is temporally regulated by a sleep/wake homeostatic process and a circadian process in interaction with a time-on-task effect. Neurobehavioral impairment resulting from these factors is task-specific, and characterized by performance variability. Several aspects of these phenomena are not well understood, and cannot be explained solely by a top-down (subcortically driven) view of sleep/wake and performance regulation. We present a bottom-up theory, where we postulate that task performance is degraded by local, use-dependent sleep in neuronal groups subserving cognitive processes associated with the task at hand. The theory offers explanations for the temporal dependence of neurobehavioral performance on time awake, time on task, and their interaction; for the effectiveness of task switching and rest breaks to overcome the time-on-task effect (but not the effects of sleep deprivation); for the task-specific nature of neurobehavioral impairment; and for the stochastic property of performance variability.
Keywords: Use-dependence, cognition, time on task, state instability, sleep homeostasis, near-infrared optical imaging, theory development
TEMPORAL REGULATION OF NEUROBEHAVIORAL PERFORMANCE
It has long been recognized that human waking functions change dynamically over time across a wide range of cognitive, motor, and behavioral domains – including task performance accuracy, reaction time, decision making, emotional reactivity, subjective sleepiness, etc. Describing these various domains collectively with the term neurobehavioral performance, their combined temporal dynamics over hours and days are regulated by two key biological processes: a homeostatic process that keeps track of time awake and time asleep, and a circadian process that keeps track of time of day [1–3].
The homeostatic process builds up a pressure for sleep over time awake that results in neurobehavioral performance degradation during periods of sleep deprivation or sleep restriction [4–6]. Conversely, the pressure for sleep is dissipated across time spent asleep, so that “recovery” sleep or even a brief nap results in improvement of previously degraded neurobehavioral performance [5, 7–9]. Concurrently, the circadian process modulates neurobehavioral performance over the 24 hours of the day, leading to relative improvement during the biological day and relative impairment during the biological night [10, 11].
In interaction, the homeostatic and circadian processes affect neurobehavioral performance as a function of the timing and duration of the waking periods in ways that are easily explained for conditions of total sleep deprivation – see Fig. (1) - but get more complicated for schedules in which the waking periods are temporally displaced (e.g., as tends to be the case for shift workers). To deal with this issue, mathematical models have been developed to predict neurobehavioral performance changes over time based on the dynamics of the homeostatic and circadian processes [12–14].
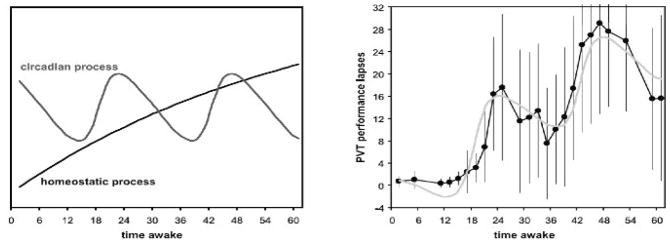
Fig. 1.
Homeostatic and circadian regulation of neurobehavioral performance during total sleep deprivation. The left panel shows the gradual increase of pressure for sleep from the homeostatic process across 62 hours of total sleep deprivation, and the modulatory influence of the circadian process with an oscillatory period of 24 hours. The right panel shows the sum of these two biological processes as a model of neurobehavioral performance impairment (gray curve), superimposed on actual measurements of performance in the laboratory (black curve). The dots in the right panel represent averages of performance lapses (reaction times longer than 500 ms) on a psychomotor vigilance test (PVT) [61] over 12 healthy adults. The whiskers represent the standard deviations over subjects, which reflect trait inter-individual differences [62] not further discussed in the present paper. Note how well the model predictions based on the dynamics of the homeostatic and circadian processes match the neurobehavioral performance averages. Figure taken from [63] with permission.
Many other factors besides the homeostatic and circadian processes affect neurobehavioral functioning. One noteworthy biological process additionally involved is sleep inertia, which produces the transient grogginess, neurobehavioral performance impairment and tendency to return to sleep experienced immediately after awakening [15]. As the sleep inertia effect dissipates rapidly within the first hour after awakening and is barely (if at all) still measurable after two hours [16, 17], it is not further discussed here.
Internal and external states can have a transient, albeit potentially powerful, effect on neurobehavioral performance. Performance may temporarily improve due to internal (e.g., motivation [18]) or external (e.g., bright light exposure [19]) factors. Conversely, it may temporarily deteriorate due to internal (e.g., anxiety [20]) or external (e.g., noise [21]) states as well. See Fig. (2) for a schematic of this interplay of multiple driving forces, in which the modifiers of neurobehavioral performance other than the homeostatic, circadian and sleep inertia processes are subsumed under the broad categories of endogenous and exogenous stimuli.
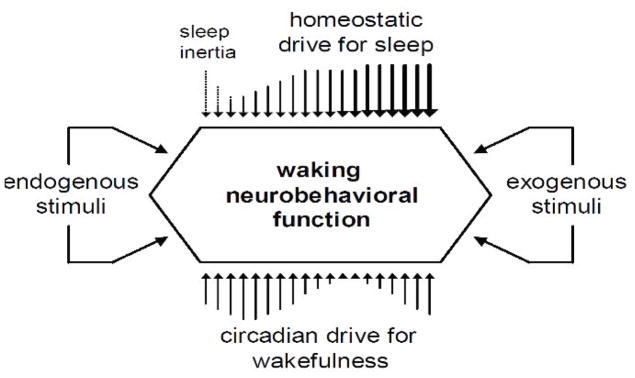
Fig. 2.
Schematic representation of the biological (homeostatic, circadian and sleep inertia) processes systematically driving temporal changes in waking neurobehavioral performance, and the transient modifying effects of endogenous (internal) and exogenous (external) stimuli. The circadian process is shown here as providing pressure for wakefulness that opposes the homeostatic pressure for sleep [57], which is one way to conceptualize the interplay between the two processes as they modulate neurobehavioral performance [3, 64]. A wide variety of internal and external influences may temporarily modify this interplay or otherwise affect neurobehavioral performance. Figure taken from [11] with permission.
In the present paper, the focus is on the main, continually operating neurobiological mechanisms of the homeostatic and circadian processes as they affect neurobehavioral performance. The circadian process is well characterized: the circadian rhythm is principally generated by the suprachiasmatic nuclei (SCN) in the hypothalamus [22], and its effect on neurobehavioral function involves the locus ceruleus [23] and other subcortical structures [24]. The biobehavioral function and evolutionary advantage of the circadian process is also clearly recognized [25, 26]. However, the neurobiology of the homeostatic process and its effects on performance are poorly understood.
As discussed in other papers in this special issue, there is little evidence that the homeostatic process is fundamentally regulated centrally (top-down) and/or generated by specific brain structures; nor is it known precisely what its function or evolutionary significance is. Yet, the effects of the homeostatic process on waking neurobehavioral function can be described in detail, and this may shed some light on the underlying mechanisms. Before pursuing this topic, it is useful to discuss another facet of the temporal regulation of neurobehavioral function: the time-on-task effect.
TIME-ON-TASK EFFECT
The time-on-task effect constitutes a performance decrement building over the duration of a cognitive performance task, which seems to reflect a cumulative increase in the effort required to deploy cognitive resources. The phenomenon has been operationalized as a progressive decline in performance (longer reaction times and/or greater number of errors) the longer one is required to sustain attention to perform the task. It is particularly noticeable in vigilance performance tasks [27] and related tasks such as the psychomotor vigilance test (PVT; see [3]). Rest breaks (with or without sleep) and task switching provide recuperation from the time-on-task effect [28–30].
Of relevance to our understanding of the mechanisms underlying neurobehavioral performance, the homeostatic and circadian processes interact with the time-on-task effect, such that the time-on-task effect is amplified during extended wakefulness and during the biological night [31–33]; see Fig. (3). An unresolved question is whether this apparent interaction is additive or synergistic. In case of the latter, it could mean that extending time on task – which could be seen as increasing task-specific workload – results in a more rapid build-up of homeostatic pressure for sleep. This intriguing possibility would imply that the time-on-task effect and the homeostatic process have some common underlying neurobiology [34].
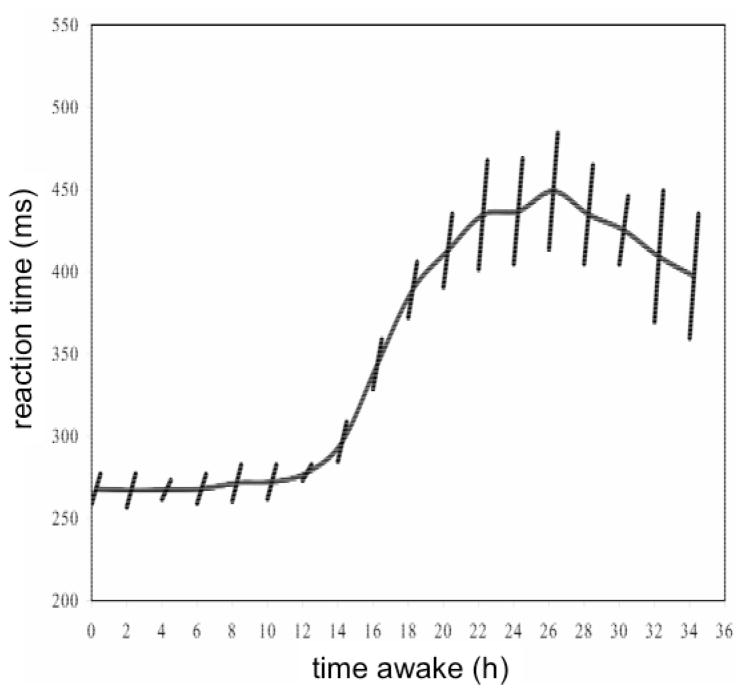
Fig. 3.
Pattern of neurobehavioral performance across 36 hours of total sleep deprivation. In this laboratory study, the sleep deprivation period began at 10:00 hours, and performance was measured by mean reaction time on a 10-minute PVT administered every 2 hours (averages over 10 healthy young adults are shown). The curve displays the reaction time averages from test bout to test bout, tracking the combined effect of the homeostatic and circadian processes. The slanted lines show deterioration of performance – approximated with linear regression – during each 10-minute test bout, representing the time-on-task effect. The rate of deterioration was greater when overall neurobehavioral performance was more impaired, revealing an interaction between the time-on-task effect and the homeostatic and circadian processes. Note also that the rest breaks between the test bouts provided recuperation from the time-on-task effect: performance at the beginning of a test bout was typically better than that at the end of the previous test bout. Figure taken from [65] with permission.
The cause of the time-on-task effect in terms of brain function is not known. Using near-infrared optical imaging [35, 36], we measured hemodynamic signals during performance of a 10-minute PVT at multiple time points across 62 hours of total sleep deprivation [37]. We used this brain imaging technique, suitable for the recording of relative changes in brain activation across time on task, to gauge changes in regional brain metabolism as measured from the scalp over the prefrontal cortex in 12 healthy young adults (7 males, 5 females; ages 22–37). Bilateral prefrontal hemodynamic signals were measured with the Hamamatsu NIRO-200 near-infrared optical topography device every 2 hours during performance of the PVT, while head position was fixed with a chin rest. Prefrontal oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb) were sampled at 0.17 Hz across the task duration.
The 10-minute hemodynamic profiles thus acquired were averaged across the multiple PVT test bouts in an attempt to isolate brain metabolic correlates of the time-on-task effect independent of homeostatic and circadian influences – see Fig. (4). The results suggested an increasing but saturating profile for O2Hb over the 10 minutes of time on task, with an inverse profile for HHb, leaving total hemoglobin more or less constant. This finding might be interpreted as an increasing need for metabolic supply over time on task, which cannot be fully met and therefore leads to increased performance impairment as task duration progresses. However, a closer look at moment-to-moment task performance suggested that additional or altogether different mechanisms are at play, as described in the next section.
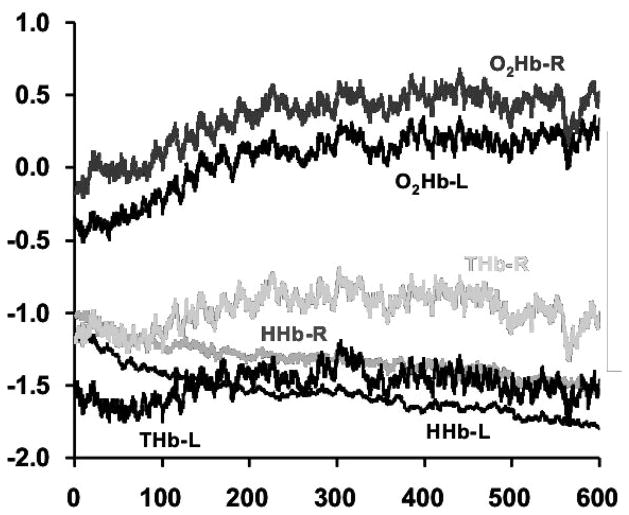
Fig. 4.
Left and right oxygenated (O2Hb-L, O2Hb-R), deoxygenated (HHb-L, HHb-R) and total (oxygenated + deoxygenated; THb-L, THb-R) hemoglobin measured over the scalp of the prefrontal cortex. Grand averages are shown (in relative, arbitrary units) as observed across repeated 10-minute PVT test bouts during 62 hours of total sleep deprivation in 12 healthy young adults.
WAKE STATE INSTABILITY
While sleep loss amplifies the time-on-task effect (see Fig. (3)), it also induces increased moment-to-moment performance variability [38, 39]. As such, the time-on-task effect is not strictly linear as the regression lines in Fig. (3) would suggest. Rather, it entails a progressive increase in response variability over the duration of the task [39, 40].
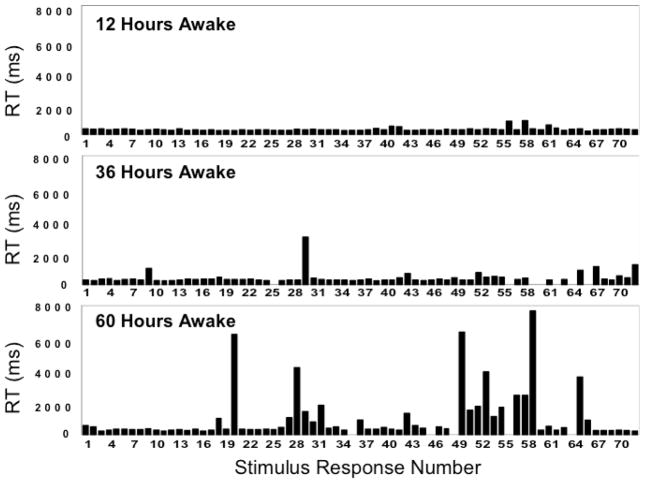
Fig. (5) shows the raw reaction time data of 10-minute PVT bouts performed by a single individual at 24-hour intervals across 60 hours of total sleep deprivation. The graphs show that performance deteriorated as time on task progressed, and that this occurred more rapidly the longer wakefulness was extended. However, the performance impairment did not take the form of a gradual lengthening of reaction times – it involved an increase in stochastic performance variability. In fact, on the PVT, performance impairment due to sleep loss results in what appears to be a mixture of normal task responses, false starts, and lapses of attention.
Fig. 5.
Raw reaction time (RT) data as observed during a 10-minute PVT for one individual undergoing total sleep deprivation. The panels show reaction times (on the ordinate) as a function of the successive stimuli (on the abscissa), after 12 hours, 36 hours and 60 hours of continuous wakefulness (each test bout took place at 20:00 hours). Gaps represent errors of commission (false starts). Figure taken from [41] as redrawn from [39] with permission.
Fig. (5) illustrates that both time on task and time awake induce stochastic performance variability. It has been posited that increased performance variability is a hallmark of the effect of the homeostatic process on neurobehavioral performance, and that this phenomenon is caused by wake state instability [39]. However, the similarity between the effect of time awake and that of time on task suggests that this theory needs to be refined, as it may need to account for the time-on-task effect and the homeostatic process having a shared underlying neurobiology.
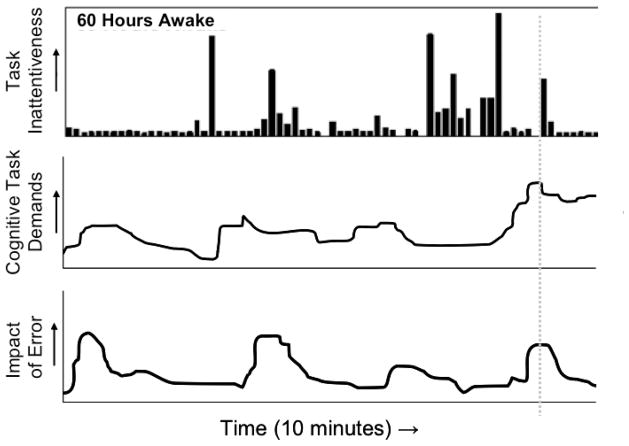
It has been postulated that stochastic performance variability under conditions of sleep deprivation provides a key explanation for why sleep loss-related accidents tend to be rare but catastrophic [41]. This can be understood in the context of the Swiss cheese model of accident causation [42], which implies that accidents occur predominantly under circumstances when multiple potential vulnerabilities are aligned in time. For example, a sleep-deprived automobile driver would be poised to have an accident when he or she simultaneously experiences a) a lapse of attention when b) approaching an intersection while c) another car is already on the intersection. However, if the driver were sleep-deprived but did not experience a lapse of attention upon approaching the intersection, the accident would not occur. In this sense, the timing of lapses of attention constitutes a key mediator of the occurrence of sleep loss-related accidents, as illustrated in Fig. (6).
Fig. 6.
Schematic of a mechanism postulated to explain how sleep loss contributes to accidents. The top panel, copied from Fig. (5), depicts performance variability across a 10-minute interval at 60 hours of total sleep deprivation; extended bars represent lapses of attention. The middle panel shows a hypothetical pattern of changing cognitive demands over the 10-minute interval. The bottom panel displays the hypothetical impact of human error over the course of the 10-minute interval. Accidents are most likely to occur when lapses of attention line up temporally with high performance demands as well as substantial consequences of error, as where illustrated by the dotted gray line. Thus, accidents related to sleep loss are unusual (rare), but typically profound (catastrophic). The schematic can be adapted for specific operational settings by replacing the middle panel with one or more particular, temporally varying safety risk (e.g., for road transportation: traffic density, weather conditions, etc.). Figure adapted from [41] with permission.
The timing of lapses of attention is stochastic and thus unpredictable, and for moderate amounts of sleep loss, lapses are relatively infrequent (see Fig. (5), middle panel). This may help to explain why, say, a night shift worker may have the same drive home in the morning – day after day, year after year – without ever having an incident, until one day a lapse of attention temporally aligns with a dangerous traffic situation, and a collision ensues. In hindsight, it would be hard to reconstruct why, on that specific day, the person suddenly failed to drive safely. Had the pattern of his/her performance variability on the day of the accident and on earlier days been known, however, it might have been revealed that an accident was waiting to happen all along, whenever the timing of a lapse of attention were to coincide with a safety-critical event. In other words, performance variability from sleep loss does not necessarily cause an accident – but it does result in an increase in risk which may lead to an accident sooner or later [41].
In technologically advanced, safety-sensitive settings, ranging from automobiles, trains, planes and other modes of transportation to factories, oil platforms and nuclear reactors, many detection and warning systems help to promote and maintain safe operations. The role of human beings in these settings is often focused on monitoring the warning systems and taking action as soon as any operational safeguards break down. Optimal human responsiveness is critical then, so that back-up systems and safety procedures are employed in a timely manner to avert overall systems failure. It is under such circumstances that stochastic performance variability can be particularly devastating, as exemplified by a number of well-known nocturnal catastrophes (e.g., the Chernobyl nuclear disaster). In those instances, a better understanding of neurobehavioral performance impairment and the underlying mechanisms could make a crucial difference.
TASK DEPENDENCE OF SLEEP DEPRIVATION EFFECTS
The effects of sleep deprivation on neurobehavioral performance vary depending on the performance task considered [43, 44]. To some extent, this variation is due to confounds in performance measurements intrinsic to many tasks, such as practice effects and speed/accuracy trade-offs [11]. However, there is also variation in the effects of sleep deprivation depending on the specific cognitive processes involved in the tasks at hand [45], as sleep deprivation appears to affect distinct cognitive processes differentially [37].
The differential effects of sleep deprivation on distinct cognitive processes have been demonstrated recently with a modified Sternberg task [46] designed to distinguish working memory scanning efficiency and resistance to proactive interference from other aspects of cognition [47]. In this task, subjects were shown a set of either two or four consonant letters, which they were required to hold in working memory. They were then shown a probe letter, which was present in the memory set at 50% chance; and they were asked to determine, as quickly and accurately as possible, whether or not the probe was indeed in the memory set. The slope of the linear relationship between reaction times and memory set size (two or four), measured for correct responses across 128 test trials, was used to measure deficits in working memory scanning efficiency [48]. Further, half the probes in the task for which the correct response was “no” (i.e., negative probes) had been encountered in the preceding trial, which caused proactive interference. The difference between reaction times to the previously encountered (i.e., recent) negative probes and reaction times to the other (non-recent) negative probes was used to measure deficits in the ability to resist proactive interference [49].
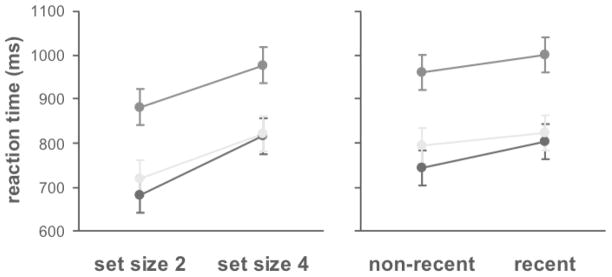
In a laboratory, the modified Sternberg task was administered at baseline, 48 hours later after 51 hours of total sleep deprivation, and 48 hours later after two nights of recovery sleep [37]. As illustrated in Fig. (7), overall reaction times were significantly degraded during sleep deprivation as compared to baseline and following recovery. However, the slope of the linear relationship between reaction times and memory set size was not significantly altered by sleep deprivation Fig. (7), left panel), nor was the difference in reaction times between recent and non-recent negative probes Fig. (7), right panel). Thus, sleep deprivation did not significantly affect working memory scanning efficiency [37, 50] and resistance to proactive interference [37], respectively. The effects of sleep deprivation on overall reaction times were due to one or more of the other cognitive processes involved in performing the modified Sternberg task, such as probe encoding and/or response selection [37, 51].
Fig. 7.
Effects of sleep deprivation on reaction times in a modified Sternberg task. Eleven healthy young adults performed the task at baseline (black dots), after 51 hours of total sleep deprivation (dark gray dots), and following two nights of recovery sleep (light gray dots). See the main text for details on the task. The left panel shows reaction times (mean ± standard error) for memory sets containing two items versus four items; the slopes of the lines represent working memory scanning efficiency. The right panel shows reaction times (mean ± standard error) for negative probes not seen recently versus negative probes seen recently (i.e., in the previous trial); the differences in reaction times represent the ability to resist proactive interference. Sleep deprivation caused an overall increase in reaction times regardless of set size or probe recency. However, working memory scanning efficiency and resistance to proactive interference were not significantly affected by sleep deprivation. Figure taken from [66]; data from [37].
BOTTOM-UP REGULATION OF TEMPORAL CHANGES IN NEUROBEHAVIORAL PERFORMANCE
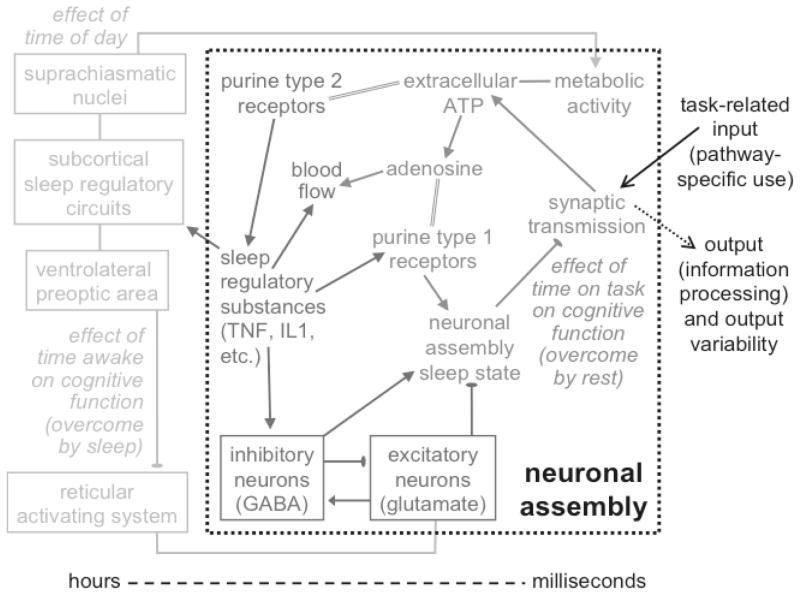
How and why sleep deprivation affects distinct cognitive processes differentially is not well understood. The phenomenology suggests that sleep deprivation may affect brain function locally in those neuronal pathways that subserve the cognitive processes invoked during task performance. The existence of the time-on-task effect and its interaction with sleep loss further suggest a dependence on (cumulative) activation of the neuronal groups involved – i.e., use-dependence. Similar mechanisms are observed in sleep regulation, as recognized in the bottom-up theory of local, use-dependent sleep [52, 53] discussed in this special issue. Here we extend the theory to waking neurobehavioral performance – see Fig. (8).
Fig. 8.
Simplified conceptual model of the effects of time on task, sleep deprivation and circadian rhythm on neurobehavioral performance. 1) On a time scale in the order of milliseconds, information processing in a neuronal assembly (group) associated with a given performance task triggers a biochemical cascade that induces the local sleep state. When the neuronal assembly is in the wake state and stimulated by input, it responds with synaptic transmission to process the input signal and generate corresponding output. This involves release of adenosine triphosphate (ATP) into the extracellular space and increased local metabolic activity. Breakdown of extracellular ATP results in accumulation of adenosine, at a rate approximately proportional to the amount of synaptic transmission in response to stimulation (i.e., use-dependent). Binding of adenosine at purine type 1 receptors promotes the neuronal assembly sleep state, during which the assembly’s synaptic transmission patterns are fundamentally altered. This functionally alters the contribution of the neuronal assembly to the coordinated response of the larger neuronal pathway involved in the performance task, causing degraded information processing. As such, the local sleep state causes output variability, which leads to (stochastic) neurobehavioral performance variability (see Fig. (5)). 2) On a time scale of minutes to hours, binding of ATP to purine type 2 receptors results in release of sleep regulatory substances (SRSs) such as tumor necrosis factor (TNF) and interleukin-1 (IL1). Continued use of the neuronal assembly causes these SRSs to accumulate and, via their receptors and nuclear factor κB (NF-κB, not shown), increase the density of post-synaptic adenosine (A1) receptors (purine type 1 receptors) [52]. As a consequence, the probability of entering the sleep state increases in a use-dependent manner, at the neurobehavioral level giving rise to the time-on-task effect. The SRSs also promote the neuronal assembly sleep state through activation of GABAergic inhibitory neurons. The GABAergic neurons inhibit the glutamatergic excitatory neurons, which prevents these glutamatergic neurons from promoting the local wake state. The SRSs together with metabolic products such as adenosine also influence regional blood supply (see Fig. (4)) and thereby oxygen and metabolic nutrient supply. A rest break allows SRS, ATP and adenosine levels to decay, resetting the time-on-task effect. 3) On a time scale of hours to days, basal metabolic activity present in all neuronal assemblies, and associated conversion of ATP to adenosine, leads to a build-up of SRSs over time awake. This is modulated by the SCN, which drive circadian rhythms in the cellular machinery and neuronal activation across the whole brain. Accordingly, the magnitude of the time-on-task effect is affected by both time awake and time of day (see Fig. (3)). Subcortical circuits involved in the coordination and consolidation of whole-brain sleep (depicted schematically in light gray) are influenced by the collective neuronal assembly states, integrated across the brain through neuronal mechanisms involving the SRSs. The subcortical circuits include the ventrolateral preoptic area (VLPO), which can shut down the wake-promoting (e.g., glutamatergic) neurons of the reticular activating system and other systems such as the cholinergic networks of the basal forebrain (not shown). These subcortical systems orchestrate sleep/wake states across the whole brain, and seek to induce global (i.e., whole-brain) sleep to prevent interaction with the environment when too many neuronal assemblies are in the local sleep state. The global sleep state allows SRS concentrations and receptor densities to be restored in a coordinated manner across all neuronal assemblies, resetting both the time-on-task effect and the time-awake effect in the process. Figure and caption adapted from [34] with permission.
We theorize that the regulation of temporal changes in neurobehavioral performance during sleep deprivation and across time-on-task, like the regulation of sleep, is fundamentally local and use-dependent in nature [34]. In particular, we hypothesize that neuronal groups involved in performing a given task will fall asleep locally as a homeostatic consequence of sustained use [54], thereby interrupting information processing and leading to performance impairment. This notion would explain a number of phenomena for which no satisfactory other explanation is available, as follows.
Performance variability arises from neuronal groups involved in the task at hand expressing the sleep state locally in homeostatic response to sustained use. When the sleep state occurs during a time-critical cognitive process (such as stimulus detection), performance impairment results. At the neurobehavioral level, this impairment is observed as stochastic (see Figs. (5) and (6), and conceptually the same for the time-on-task effect as for sleep loss.
The time-on-task effect is a result of the use-dependent property of the regulation of temporal changes in neurobehavioral performance. The longer the cumulative use, the greater the chance of neuronal groups involved in the task expressing the local sleep state, and thus the higher the degree of performance variability.
Rest breaks reset the time-on-task effect (see Fig. (3)), because they allow the homeostatic pressure for local sleep to be dissipated. This reduces the expression of local sleep in neuronal groups and thus diminishes the chance of performance interruption when task performance is resumed.
The homeostatic process (see Fig. (1)) is posited to be temporally driven by the build-up of use-dependent pressure for local sleep resulting from task-specific as well as background brain activation, as integrated across the collective of neuronal groups in the brain [53]. As such, wake extension causes a need for whole-brain sleep, which is fundamentally driven by integrated local sleep pressure [55] although globally orchestrated by subcortical nuclei [24]. Resisting this need for sleep (i.e., sleep deprivation) forces sleep to be expressed locally rather than globally, leading to stochastic interruption of cognitive processing and thus performance variability.
The time-on-task effect can be overcome by a rest break with or without sleep, whereas the effects of sleep deprivation on performance can only be overcome by a period of whole-brain sleep. This is paradoxical considering that the two phenomena are both theorized to results from local, use-dependent sleep. However, in the case of the time-on-task effect, the local sleep is expressed in response to use of specific neuronal groups involved in the task, and thus the homeostatic pressure for local sleep can be dissipated (and task performance capability restored) while engaging in a different activity not relying on the same neuronal groups. Sleep deprivation, on the other hand, extends the overall duration of activation – i.e., the cumulative use – in neuronal groups across the whole brain, and therefore requires whole-brain sleep to restore optimal neurobehavioral performance (see Fig. (8) caption).
The interaction of the time-on-task effect with sleep deprivation (see Fig. (3)) is based on the association of both with increased use of neuronal groups involved in task performance, in a manner hypothesized to be additive.
Neurobehavioral deficits due to sleep deprivation are task-dependent because engaging in a task particularly increases use of neuronal groups involved in cognitive processing specific to that task. Performance tasks requiring repeated, intensive use of a limited number of neuronal groups would be expected to show considerable impact of sleep loss (big effect size) as well as steep performance degradation across time on task. The PVT is a good example of this [39, 56].
It follows that the time-on-task effect must also be task-dependent. This would explain why task switching can reset the time-on-task effect [30]: it shifts neuronal use for cognitive processing to different neuronal groups, thus allowing local sleep to be expressed in the previously used neuronal groups (to dissipate the built-up homeostatic pressure for local sleep) without performance consequences.
It should be emphasized that these explanations are based on theory derived from a mixture of scientific evidence regarding sleep regulation (expounded in this special issue) and regarding waking neurobehavioral performance (discussed in the present paper) as well as some reasoned speculation. Also, it is recognized that not all aspects of neurobehavioral performance impairment in the context of sleep loss can be explained in terms of local, use-dependent sleep. Notably, the circadian process is known to be centrally driven (see Fig. (8)), originating in the SCN [57] as discussed earlier. Certain correlates of sleep loss such as oculomotor changes [58] and slow eyelid closures [59] are also likely to be centrally mediated [60].
Nonetheless, these top-down, central (predominantly subcortical) mechanisms do not explain either the reason or the time course of the homeostatic process and its effects and interactions on waking neurobehavioral function. Our proposed explanations are effectively the same as those put forth for sleep regulation and sleep function in this special issue, deriving from the concept of local, use-dependent sleep. We posit that to understand the temporal regulation of neurobehavioral performance in sufficient detail, such a bottom-up perspective is essential.
Acknowledgments
We thank David Rector, Devon Grant, Adrienne Tucker, Scott Doran and David Dinges for their contributions to experimental results discussed here. Original findings presented in this paper were obtained in studies supported by the W.M. Keck Foundation, CDMRP award W81XWH-05-1-0099, and DURIP grant FA9550-06-1-0281.
References
- 1.Daan S, Beersma DGM, Borbély AA. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R178. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 2.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 3.Van Dongen HPA, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clin Sports Med. 2005;24:237–249. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Patrick GTW, Gilbert JA. On the effects of loss of sleep. Psychol Rev. 1896;III(5):469–483. [Google Scholar]
- 5.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, Russo MB, Balkin TJ. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 7.Herscovitch J, Broughton R. Performance deficits following short-term partial sleep deprivation and subsequent recovery oversleeping. Can J Psychol. 1981;35:309–322. doi: 10.1037/h0081197. [DOI] [PubMed] [Google Scholar]
- 8.Rosa RR, Bonnet MH, Warm JS. Recovery of performance during sleep following sleep deprivation. Psychophysiology. 1983;20:152–159. doi: 10.1111/j.1469-8986.1983.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 9.Dinges DF, Orne MT, Whitehouse WG, Orne EC. Temporal placement of a nap for alertness: Contributions of circadian phase and prior wakefulness. Sleep. 1987;10:313–329. [PubMed] [Google Scholar]
- 10.Monk TH, Buysse DJ, Reynolds CF, III, Berga SL, Jarrett DB, Begley AE, Kupfer DJ. Circadian rhythms in human performance and mood under constant conditions. J Sleep Res. 1997;6:9–18. doi: 10.1046/j.1365-2869.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Dongen HPA, Dinges DF. Circadian rhythms in sleepiness, alertness, and performance. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4. Elsevier Saunders; Philadelphia: 2005. pp. 435–443. [Google Scholar]
- 12.Mallis MM, Mejdal S, Nguyen TT, Dinges DF. Summary of the key features of seven biomathematical models of human fatigue and performance. Aviat Space Environ Med. 2004;75:A4–A14. [PubMed] [Google Scholar]
- 13.Van Dongen HPA. Comparison of mathematical model predictions to experimental data of fatigue and performance. Aviat Space Environ Med. 2004;75:A15–A36. [PubMed] [Google Scholar]
- 14.McCauley P, Kalachev LV, Smith AD, Belenky G, Dinges DF, Van Dongen HPA. A new mathematical model for the homeostatic effects of sleep loss on neurobehavioral performance. J Theor Biol. 2009;256:227–239. doi: 10.1016/j.jtbi.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinges DF, Orne EC, Evans FJ, Orne MT. Performance after naps in sleep-conducive and alerting environments. In: Johnson LC, Tepas DI, Colquhoun WP, Colligan MJ, editors. Biological Rhythms, Sleep and Shift Work. Vol. 7. SP Medical & Scientific Books; New York: 1981. pp. 539–552. [Google Scholar]
- 16.Achermann P, Werth E, Dijk DJ, Borbély AA. Time course of sleep inertia after nighttime and daytime sleep episodes. Arch Ital Biol. 1995;134:109–119. [PubMed] [Google Scholar]
- 17.Jewett ME, Wyatt JK, Ritz-De Cecco A, Khalsa SB, Dijk DJ, Czeisler CA. Time course of sleep inertia dissipation in human performance and alertness. J Sleep Res. 1999;8:1–8. doi: 10.1111/j.1365-2869.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- 18.Horne JA, Pettitt AN. High incentive effects on vigilance performance during 72 hours of total sleep deprivation. Acta Psychol. 1985;58:123–139. doi: 10.1016/0001-6918(85)90003-4. [DOI] [PubMed] [Google Scholar]
- 19.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11:453–464. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Mueller JH. Anxiety and performance. In: Smith AP, Jones DM, editors. Handbook of Human Performance. State and Trait. Vol. 3. Academic Press; London: 1992. pp. 127–160. [Google Scholar]
- 21.Smith AP, Jones DM. Noise and performance. In: Jones DM, Smith AP, editors. Handbook of Human Performance. The Physical Environment. Vol. 1. Academic Press; London: 1992. pp. 1–28. [Google Scholar]
- 22.Moore RY. Organization of the mammalian circadian system. In: Chadwick DJ, Ackrill K, editors. Circadian Clocks and Their Adjustments; Ciba Foundation Symposium; Chichester: John Wiley & Sons; 1995. pp. 89–106. [PubMed] [Google Scholar]
- 23.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 24.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 25.Roenneberg T, Merrow M. Life before the clock: modeling circadian evolution. J Biol Rhythms. 2002;17:495–505. doi: 10.1177/0748730402238231. [DOI] [PubMed] [Google Scholar]
- 26.Waterhouse JM, DeCoursey PJ. The relevance of circadian rhythms for human welfare. In: Dunlap JC, Loros JJ, DeCoursey PJ, editors. Chronobiology Biological Timekeeping. Sinauer; Sunderland: 2003. pp. 325–356. [Google Scholar]
- 27.Davies DR, Parasuraman R. The Psychology of Vigilance. Academic Press; New York: 1982. [Google Scholar]
- 28.McCormack PD. Performance in a vigilance task as a function of inter-stimulus interval and interpolated rest. Can J Psychol. 1958;12:242–246. doi: 10.1037/h0083749. [DOI] [PubMed] [Google Scholar]
- 29.Bergum BO, Lehr DJ. Vigilance performance as a function of interpolated rest. J Appl Psychol. 1962;46:425–427. [Google Scholar]
- 30.Komaki J. Signal-source switching in human vigilance. Jpn Psychol Res. 1967;9:49–57. [Google Scholar]
- 31.Wilkinson RT. Effects of up to 60 hours’ sleep deprivation on different types of work. Ergonomics. 1964;7:175–186. [Google Scholar]
- 32.Angus RG, Heslegrave RJ. Effects of sleep loss on sustained cognitive performance during a command and control simulation. Behav Res Meth Instr Comp. 1985;17:55–67. [Google Scholar]
- 33.Wesensten NJ, Belenky G, Thorne DR, Kautz MA, Balkin TJ. Modafinil vs. caffeine: effects on fatigue during sleep deprivation. Aviat Space Environ Med. 2004;75:520–525. [PubMed] [Google Scholar]
- 34.Van Dongen HPA, Belenky G, Krueger JM. Investigating the Temporal Dynamics and Underlying Mechanisms of Cognitive Fatigue. In: Ackermann PL, editor. Cognitive Fatigue; American Psychological Association; Washington, D.C: 2010. pp. 127–147. [Google Scholar]
- 35.Al-Rawi PG, Smielewski P, Kirkpatrick PJ. Evaluation of a near-infrared spectrometer (NIRO 300) for the detection of intracranial oxygenation changes in the adult head. Stroke. 2001;32:2492–2500. doi: 10.1161/hs1101.098356. [DOI] [PubMed] [Google Scholar]
- 36.Rector DM, Schei JL, Van Dongen HPA, Belenky G, Krueger JM. Physiological markers of local sleep. Eur J Neurosci. 2009;29:1771–1778. doi: 10.1111/j.1460-9568.2009.06717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HPA. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33:47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams HL, Lubin A, Goodnow JJ. Impaired performance with acute sleep loss. Psychol Monogr Gen Appl. 1959;73:1–26. [Google Scholar]
- 39.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–267. [PubMed] [Google Scholar]
- 40.Bills AG. Blocking: a new principle of mental fatigue. Am J Psychol. 1931;43:230–245. [Google Scholar]
- 41.Van Dongen HPA, Hursh SR. Fatigue, performance, errors, and accidents. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5. Elsevier Saunders; St. Louis: 2010. pp. 753–759. [Google Scholar]
- 42.Reason J. Human Error. Cambridge University Press; Cambridge: 1990. [Google Scholar]
- 43.Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: A meta-analysis. Sleep. 1996;19:318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 44.Alhola P, Polo-Kantola P. Sleep deprivation: impact on cognitive performance. Neuropsychiat Dis Treat. 2007;3:553–567. [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson ML, Van Dongen HPA. Cognitive effects of sleepiness. In: Thorpy M, Billiard M, editors. Sleepiness. Cambridge University Press; Cambridge: 2011. pp. 72–81. [Google Scholar]
- 46.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 47.Whitney P, Jameson T, Hinson JM. Impulsiveness and executive control of working memory. Personality Indiv Diff. 2004;37:417–428. [Google Scholar]
- 48.Sternberg S. Memory-scanning: mental processes revealed by reaction time experiments. Am Sci. 1969;57:421–457. [PubMed] [Google Scholar]
- 49.Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci USA. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habeck C, Rakitin BC, Moeller J, Scarmeas N, Zarahn E, Brown T, Stern Y. An event-related fMRI study of the neurobehavioral impact of sleep deprivation on performance of a delayed-match-to-sample task. Cogn Brain Res. 2004;18:306–321. doi: 10.1016/j.cogbrainres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 51.Mu Q, Mishory A, Johnson KA, Nahas Z, Kozel FA, Yamanaka K, Bohning DE, George MS. Decreased brain activation during a working memory task at rested baseline is associated with vulnerability to sleep deprivation. Sleep. 2005;28:433–446. doi: 10.1093/sleep/28.4.433. [DOI] [PubMed] [Google Scholar]
- 52.Krueger JM, Obál F., Jr A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 53.Krueger JM, Rector DM, Roy S, Van Dongen HPA, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008;9:910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Roy S, Krueger JM, Rector DM, Wan Y. A network model for activity-dependent sleep regulation. J Theor Biol. 2008;253:462–468. doi: 10.1016/j.jtbi.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balkin TJ, Bliese PD, Belenky G, Sing H, Thorne DR, Thomas M, Redmond DP, Russo M, Wesensten NJ. Comparative utility of instruments for monitoring sleepiness-related performance decrements in the operational environment. J Sleep Res. 2004;13:219–227. doi: 10.1111/j.1365-2869.2004.00407.x. [DOI] [PubMed] [Google Scholar]
- 57.Edgar DM, Dement WC, Fuller CA. Effect of SCN lesions on sleep in squirrel monkeys: evidence for opponent processes in sleep-wake regulation. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Gennaro L, Devoto A, Lucidi F, Violani C. Oculomotor changes are associated to daytime sleepiness in the multiple sleep latency test. J Sleep Res. 2005;14:107–112. doi: 10.1111/j.1365-2869.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 59.Dinges DF, Grace R. Report no FHWA-MCRT-98-006. Federal Highway Administration; Washington, D.C: 1998. PERCLOS: A Valid Psychophysiological Measure of Alertness as Assessed by Psychomotor Vigilance. [Google Scholar]
- 60.Schmidtke K, Büttner-Ennever JA. Nervous control of eyelid function – a review of clinical, experimental and pathological data. Brain. 1992;115:227–247. doi: 10.1093/brain/115.1.227. [DOI] [PubMed] [Google Scholar]
- 61.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instr Comp. 1985;17:652–655. [Google Scholar]
- 62.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- 63.Van Dongen HPA, Belenky G. Individual differences in vulnerability to sleep loss in the work environment. Ind Health. 2009;47:518–526. doi: 10.2486/indhealth.47.518. [DOI] [PubMed] [Google Scholar]
- 64.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 65.Van Dongen HPA, Belenky G. Alertness level. In: Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of Neuroscience; Springer; Berlin: 2008. pp. 75–77. [Google Scholar]
- 66.Whitney P, Hinson JM. Measurement of cognition in studies of sleep deprivation. In: Kerkhof GA, Van Dongen HPA, editors. Human Sleep and Cognition. Part 1: Basic Research; Progress in Brain Research. Vol. 185. Elsevier; Amsterdam: 2010. pp. 37–48. [DOI] [PubMed] [Google Scholar]