Abstract
Objective
Matrix metalloproteinases (MMPs), especially MMP-2 and MMP-9 have been known to play an important role in secondary inflammatory reaction after spinal cord injury (SCI). The aim of this study was to investigate the expression and activity of MMP-2 and MMP-9 and to determine their relationship with disruption of endothelial blood-barrier after photochemically induced SCI in rats.
Methods
Female Sprague-Dawley rats, weighing between 250 and 300 g (aged 8 weeks) received focal spinal cord ischemia by photothrombosis using Rose Bengal. Expressions and activities of MMP-2 and MMP-9 were assessed by Western blot and gelatin zymography at various times from 6 h to 7 days. Endothelial blood-barrier integrity was assessed indirectly using spinal cord water content.
Results
Zymography and Western blot analysis demonstrated rapid up-regulation of MMP-9 protein levels in spinal cord after ischemic onset. Expressions and activities of MMP-9 showed a significant increased at 6 h after the photothrombotic ischemic event, and reached a maximum level at 24 h after the insult. By contrast, activated MMP-2 was not detected at any time point in either the experimental or the control groups. When compared with the control group, a significant increase in spinal cord water content was detected in rats at 24 h after photothrombotic SCI.
Conclusion
Early up-regulation of MMP-9 might be correlated with increased water content in the spinal cord at 24 h after SCI in rats. Results of this study suggest that MMP-9 is the key factor involved in disruption of the endothelial blood-barrier of the spinal cord and subsequent secondary damage after photothrombotic SCI in rats.
Keywords: Endothelial blood-barrier, Matrix metalloproteinases-9, Photothrombosis, Rat, Spinal cord injury
INTRODUCTION
Spinal cord injury (SCI) is a devastating clinical condition that may result in permanent functional deficits. The pathophysiology of spinal cord injury is very complex and two main mechanisms can be distinguished as primary and secondary damages. The primary injury is the initial mechanical damage to the spinal cord, which includes the kinetic impact and persistent compression of the cord by bone or disc fragments7). It typically leads to necrosis of a number of neuronal cells that generally cannot be improved or regenerated20). By contrast, secondary injury is considered to result from a number of proposed auto-destructive processes, such as lipid hydrolysis, free-radical induced lipid peroxidation, injury caused by hydroxyl radicals, tumor necrosis factor production, activation of non-N-methyl-D-aspartate ionotropic glutamate receptors, and inflammation with polymorphonuclear leukocytes1,4,9,11,14). Inflammatory and immune reactions have also been considered as the main component of secondary neuronal injury3). Most pathological changes after a SCI are the results of secondary injury after the initial impact, including edema, neutrophil infiltration, altered blood flow, and alteration of microvascular permeability19).
Proteinases, particularly matrix metalloproteinases (MMPs), have recently been known as mediators of early secondary neuronal damage after SCI10,18,25). MMPs are a family of zinc-binding proteolytic enzymes that degrade extracellular matrix components of the basement membrane12). Excessive proteolytic activity of MMPs after tissue injury can be detrimental, and lead to disruption of the endothelium and excessive inflammation16,24,26). MMPs have been implicated in numerous pathological conditions including atherosclerosis, inflammation, tumor growth, metastasis, and cerebral ischemia. In particular, MMP-9 has been implicated in abnormal vascular permeability associated with either hemorrhagic injury or inflammation16,24). Abnormal increases of MMP-9 in both inflammatory cells and endothelial cells may result in collective impairment of endothelial barrier function by degrading the vascular basement membrane.
In this study, in order to clarify the role of MMPs in the pathophysiologic cascade of neuronal tissue damage and endothelial disruption after SCI, we investigated the expression and activities of MMP-2 and MMP-9, and spinal cord water content after photochemicaelly induced SCI.
MATERIALS AND METHODS
Photothrombotic SCI model
Female Sprague-Dawley rats, weighing between 250 and 300 g were used in the present study. Female rats were used in order to facilitate management of posttraumatic urinary dysfunction. Rats were kept in 12 h light/dark cycles, and allowed free access to food and water. SCI was induced by photothrombosis of the cord microvessels according to a modified method described by Watson et al.28). Each rat was anesthetized with 5% isoflurane and maintained with 2% isoflurane in an oxygen/air mixture using a gas anesthesia mask in a stereotaxic frame (Stoelting, Wood Dale, IL, USA). After obtaining a deep level of anesthesia, the rat was placed in a prone position with a warmed surgical pad in order to preserve body temperature throughout the procedure. Rectal temperature was controlled during surgery at 37±0.5℃ with a homeothermic blanket (Harvard Apparatus). After midline skin incision and paravertebral muscles dissection, laminae and spinous processes of the T8-T9 vertebrae were removed in order to expose the spinal cord. The photochemical dye, Rose Bengal (80 mg/kg, Sigma Chemical Co., St. Louis, MO, USA) in normal saline was then administered into the right femoral vein. Immediately afterward, illumination of a cold light source (Zeiss KL1500 LCD, Jena, Germany) with a 4.5 mm aperture, was focused over the dorsal surface of the spinal cord for three minutes. After illumination, back muscles and skin were sutured. Animals were then returned to their individual cages, and kept heated under an infrared lamp until full recovery from anesthesia. Antibiotic coverage was provided for five days and the bladder was emptied twice a day by manual pressure until total recovery of function. Control animals, underwent only a laminectomy without a photochemical procedure.
Preparation of tissue extracts
For analysis of protein expression patterns in spinal cord tissue after injury, protein extracts of tissues were prepared for gelatin zymography and western blot analysis. Animals were deeply anesthetized with isoflurane and the injured spinal cord was then rapidly removed at 6 and 24 hours and 2, 3, and 7 days after injury. The spinal cord was homogenized in a protein extraction buffer consisting of 10 mM Tris-HCl (pH 7.6), 250 mM sucrose, 1 mM ethylenediaminetetraacetate and 0.1 mM phenylmethylsulfonyl fluoride, and then minced into small pieces. After the extraction was complete, samples were centrifuged at 3,000 rpm for 20 minutes at 4℃, and the supernatants were collected, and stored at -75℃. Bicinchoninic acid assays kit (Pierce, USA) were used routinely for determination of protein concentrations.
Western blot analysis
To further investigate the protein expression of MMP-2 and MMP-9 in spinal cord tissue extracts, samples containing 50 ug of protein were boiled for 5 minutes at 100℃ with an appropriate volume of 4X sample buffer [NuPAGE® LDS Sample Buffer (4X) NP0007; invitrogen]. Samples were then placed on ice to cool before being loaded onto an 8% sodium dodecyl sulfate (SDS)-polyacrylamide gel and separated at 120 V for 3 h. Gels were then transferred onto a polyvinylidene difluoride membrane at 300 mA for 90 min at 4℃. Membranes were reversibly stained with Ponceau S to confirm the transfer of proteins, and destained in water. Membranes were then incubated in blocking buffer for 1 h at room temperature (5% non-fat dry milk in Tris-buffered saline with 0.2% Tween 20), probed overnight at 4℃ with primary anti-MMP-9 (rabbit anti-Rat MMP-9 polyclonal, dilution 1 : 2,000; Chemocon International, Temecula, CA, USA) and anti MMP-2 (mouse monoclonal, dilution 1 : 2,000; Santa Cruz Biotechnology, Inc., CA, USA) antibodies, and finally incubated for 1 hr at room temperature with horseradish peroxidase-conjugated immunoglobulin (polyclonal Goat anti-rabbit Ig, dilution 1 : 5,000; Dako Cytomation, Glostrup, Denmark and Goat anti-mouse IgG, dilution 1 : 5,000; Zymed, California, USA respectively). β-actin (anti-β-actin, dilution 1 : 40,000; Sigma Aldrich Co., St Louis, Mo, USA) and GAPDH (rabbit monoclonal, dilution 1 : 20,000; Cell Signaling Technology, USA) were used as a control for loading. The reaction was developed with a chemiluminiscence reagent containing luminal reagent and peroxide solution (Millipore). Optical densities were measured by a Luminescent Image Analyzer (Fujifilm LAS-3000; Fuji Photo Film Co., Tokyo, Japan).
Gelatin zymography
Gelatin zymography was used for measurement of the activities of MMP-2 and MMP-9 in spinal cord homogenates following previously described techniques2,21). Equal amounts of protein samples (50 ug/lane) were loaded and separated by an 8% SDS-polyacrylamide gel containing 1 mg/mL gelatin as a substrate at 4℃. After separation by electrophoresis, the gel was rinsed briefly with distilled water and washed three times in 2.5% Triton X-100 solution for 30 minutes. The gel was then incubated with developing buffer at 37℃ overnight. After developing, the gel was stained with 0.5% Coomassie Brilliant Blue R-250 (Sigma Chemical) in a mixture of methanol : acetic acid : water (2 : 1 : 7) for 2 h and then destained. To quantify the degrees of MMP-9 and MMP-2 activity as detected by gelatin zymography, the gels were digitized, and the area of lysis for each band detected was quantified using the J-Image program of the lytic zone area in square millimeters.
Spinal cord water content
Spinal cords, 10 mm rostral and 10 mm caudal to the lesion site (20 mm long), were removed at 24 h after SCI (n=5 for each group). The spinal cord was weighed immediately after removal (wet weight) and again while drying in an oven at 105℃ for 24 h (dry weight). The percent water content was calculated as [(wet weight-dry weight)/wet weight]×100%.
Statistical analysis
The SPSS software program (version 17.0 for windows; SPSS INC., Chicago, IL, USA) was used for statistical analysis. Values are presented as the mean±standard error of the mean. Statistical analysis was performed using one-way ANOVA or the Student's t-test. Differences were considered significantly at a p<0.05.
RESULTS
Expressions of MMP-2 and MMP-9
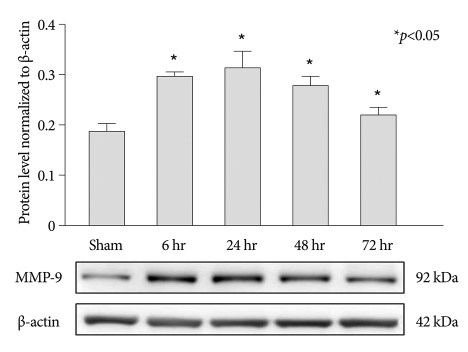
MMP-2 and MMP-9 protein expression were evaluated by Western blot analysis. Results demonstrated that expression of MMP-9 was rapidly increased at 6 h after the photothrombotic ischemic event, and reached a peak level at 24 h after the event (Fig. 1). However, MMP-9 expression was very low in the control group at each time point analyzed.
Fig. 1.
Western blots of MMP-9 demonstrated up-regulation of protein expression at 6, 24, 48, and 72 h after photothrombotic SCI (mean±SEM, n=5 per time point). *p<0.05 compared with the control group; the Mann-Whitney U test. MMP : matrix metalloproteinases, SCI : spinal cord injury, SEM : standard error of the mean.
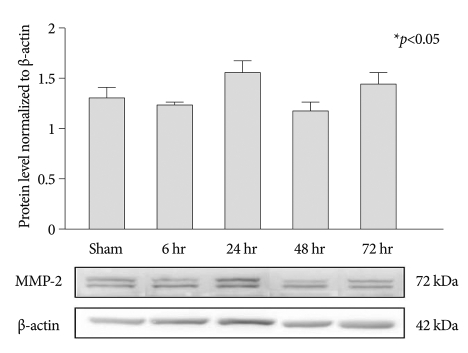
In MMP-2 expression, MMP-2 was observed in both the experimental and the control groups. However, there were no significant differences at each time point after SCI (Fig. 2).
Fig. 2.
Western blots of MMP-2. The statistically significant differences aree not observed at each time point after photothrombotic SCI (mean±SEM, n=5 per time point). *p<0.05 compared with the control group; the Mann-Whitney U test. MMP : matrix metalloproteinases.
Activities of MMP-2 and MMP-9
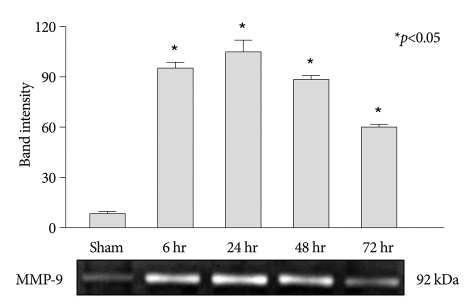
MMP-2 and MMP-9 protein activities were evaluated by gelatin zymography. Rapid increase of MMP-9 activity was observed at 6 h after the photothrombotic ischemic event, and reached a maximum level at 24 h after the insult. And then elevated MMP-9 activity showed a slow decrease (Fig. 3). By contrast, when compared with the control groups, elevation of MMP-2 activity was not observed at any time points in the experimental groups (Fig. 4).
Fig. 3.
Zymographic analysis of MMP-9 activity in injured spinal cord extracts. Up-regulation of MMP-9 is observed at 6, 24, 48, and 72 h after injury, and reached a maximum level at 24 h after the insult (mean±SEM, n=5 per time point). *p<0.05 compared with the control group; the Mann-Whitney U test. MMP : matrix metalloproteinases, SEM : standard error of the mean.
Fig. 4.
Zymographic finding of MMP-2 activity in injured spinal cord extracts. Elevation of MMP-2 activity is not observed at any time points in the experimental group (mean±SEM, n=5 per time point). *p<0.05 compared with the control group; the Mann-Whitney U test. MMP : matrix metalloproteinases, SEM : standard error of the mean.
Spinal cord water content
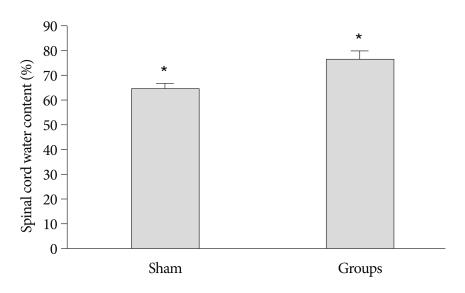
We determined spinal cord edema at 24 hours after the insult. When compared with the control group, a significant increase in spinal cord water content was detected at 24 h after photothrombotic SCI (p<0.05) (Fig. 5).
Fig. 5.
Spinal cord water content. When compared with the control group, a significant increase in water content is detected in rats at 24 h after photothrombotic SCI (mean±SEM, n=5 per time point). *p<0.05 compared with the control group; the Mann-Whitney U test. SCI : spinal cord injury.
DISCUSSION
MMPs are important for extracellular matrix remodeling and are integral to inflammation, morphogenesis, and wound healing29). However, MMPs have the ability to degrade gelatin, collagens, elastin, vitronectin, and the major components of the basal lamina comprising the vascular endothelial blood-barrier. MMP-2 and MMP-9 have been implicated in degradation of constituents of basal lamina, and are the key enzymes associated with secondary damage after spinal cord or brain injuries, including ischemia and traumatic accidents2,15). MMP-2 is constitutively expressed in certain populations of astrocytes and is up-regulated in these cells after central nervous system (CNS) injury23). MMP-9 is expressed at low levels in neural cells, including anterior horn motor neurons, and is markedly up-regulated in blood vessels, macrophages, and glia after CNS injury18,22,23). Therefore, up-regulation of MMPs can be a critical event for disruption of endothelial blood-barrier with changes of vascular permeability after SCI2,5,10,18). Esposito et al.8) established strong induction of pro-MMP-2 and pro-MMP-9 activity in cord tissue extracts from an SCI mice model using an aneurysm clip. When compared with sham mice, significant up-regulation of MMP-2 and MMP-9 expression was also observed in the cord tissues from SCI operated mice. However, several studies have reported that central nervous tissue after a SCI or a stroke revealed delayed expression of MMP-2 compared with MMP-95,30). MMP-9 is elevated within several hours, whereas MMP-2 is elevated at several days or weeks after infarction or SCI. Therefore, some researchers have advocated that the later increase of MMP-2 expression and activity would correlate with better outcome with the post-traumatic time course of capillary remodeling, and might contribute to wound healing and functional recovery after SCI10).
Expressions and activities of MMP-2 and MMP-9 only were investigated in this study due to their established association with early neuronal damage, including inflammation, angiogenesis, endothelial blood-barrier disruption, and scar formation in the spinal cord17,23). Expressions and activities of MMP-9 showed a significant increase within 24 h after photochemical induction of SCI, and the level of MMP-9 expression and activity reached a peak at 24 h after the event. By contrast, Western blot did not show a significant increase in elevation of MMP-2 expression until 72 h. Neither change nor elevation of MMP-2 activity was observed until 72 h after photothrombotic insult in gelatin zymography. Spinal cord water content was measured for evaluation of endothelial blood-barrier disruption and edema formation of the spinal cord after photothrombotic insults. When compared with the control groups, a significant increase in spinal cord water content was detected in rats at 24 h after photothrombotic SCI. These findings indicate that activation and increased expression of MMP-9 may be associated with disruption of the endothelial blood-barrier of the spinal cord after photothrombotic insults. Consequently, MMP-9 might be involved in early tissue damage and contributes to initial development of spinal cord edema after photothrombotic SCI. Therefore, MMP-9 may play a significant role in disruption of the blood-barrier of the spinal cord and aggravation of SCI with edema formation caused by change of vascular permeability after photothrombotic insult. Elevation of MMP-2 expression and activity was not observed until 72 h after photothrombotic insult, therefore, MMP-2 might not be involved in early damage to the blood-barrier of the spinal cord after photothrombotic insults in our study, and additional study with a prolonged time interval would be required the demonstration of the role of MMP-2.
Among a variety of SCI models, the weight drop technique has been the most widely used method for experimental induction of SCI. The rat model of contusive SCI has been shown to provide a reliable, reproducible, graded injury, and offers an easier method for establishment of large-scale screening of potential therapeutic agents. However, in order to produce a consistent and reliable injury, sufficient experience with strict adherence to control of variability of the injury is necessary. Nonetheless, lesions tend to be variable in size and have the potential for difficulty in reproducing a consistent injury. Photothrombosis has mainly been used for development a stroke model6,13,27). We adopted the photothrombotic ischemia model in order to establish an SCI model in rats19). Photochemical damage to the spinal cord has histopathological similarities with experimental contusion and compression lesions. A prolonged secondary injury phase associated with photochemical lesions has been demonstrated in photochemical models in rats. Photothrombosis can induce a relatively constant lesion in the target spinal cord. Therefore, the photothrombotic SCI model could be a suitable model for study of MMPs in association with a breakdown of blood-cord barrier disruption.
The limitation of this study is that the observation period for expression and activation of MMP-2 and MMP-9 was 3 days and spinal cord water content was only measured at 24 hr after photothrombotic insults, even although initiation of spinal cord edema and secondary injury have been known to occur several hours after SCI. In the future, studies with a longer experimental period and pharmacological trials with neuroprotective effects will be required for investigationg of the relationship between MMPs and secondary damage to the spinal cord with blood-barrier disruption.
CONCLUSION
Early up-regulation of MMP-9 might be correlated with increased water content in the spinal cord at 24 h after SCI in rats. Results of this study suggest that MMP-9 may play an important role in disruption of the blood-barrier of the spinal cord and aggravation of SCI with edema formation caused by alteration of vascular permeability after photothrombotic insult. Therefore, MMP-9 inhibition could result in reduced alteration and destruction of the blood-barrier of the spinal cord and secondary injury after an ischemic insult.
Acknowledgements
This study was financially supported by Chonnam National University, 2009.
References
- 1.Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci. 1997;17:1055–1063. doi: 10.1523/JNEUROSCI.17-03-01055.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia : effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 2002;15:355–360. doi: 10.1097/00019052-200206000-00021. [DOI] [PubMed] [Google Scholar]
- 4.Carlson GD, Gorden C. Current developments in spinal cord injury research. Spine J. 2002;2:116–128. doi: 10.1016/s1529-9430(01)00029-8. [DOI] [PubMed] [Google Scholar]
- 5.Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/s0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich WD, Busto R, Watson BD, Scheinberg P, Ginsberg MD. Photochemically induced cerebral infarction. II. Edema and blood-brain barrier disruption. Acta Neuropathol. 1987;72:326–334. doi: 10.1007/BF00687263. [DOI] [PubMed] [Google Scholar]
- 7.Ducker TB, Salcman M, Perot PL, Jr, Ballantine D. Experimental spinal cord trauma, I : correlation of blood flow, tissue oxygen and neurologic status in the dog. Surg Neurol. 1978;10:60–63. [PubMed] [Google Scholar]
- 8.Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. Melatonin regulates matrix metalloproteinases after traumatic experimental spinal cord injury. J Pineal Res. 2008;45:149–156. doi: 10.1111/j.1600-079X.2008.00569.x. [DOI] [PubMed] [Google Scholar]
- 9.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 10.Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26:9841–9850. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasa K, Ikata T, Fukuzawa K. Protective effect of vitamin E on spinal cord injury by compression and concurrent lipid peroxidation. Free Radic Biol Med. 1989;6:599–606. doi: 10.1016/0891-5849(89)90067-1. [DOI] [PubMed] [Google Scholar]
- 12.Lee JE, Yoon YJ, Moseley ME, Yenari MA. Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. J Neurosurg. 2005;103:289–297. doi: 10.3171/jns.2005.103.2.0289. [DOI] [PubMed] [Google Scholar]
- 13.Lee JK, Park MS, Kim YS, Moon KS, Joo SP, Kim TS, et al. Photochemically induced cerebral ischemia in a mouse model. Surg Neurol. 2007;67:620–625. doi: 10.1016/j.surneu.2006.08.077. discussion 625. [DOI] [PubMed] [Google Scholar]
- 14.Liu D, Yang R, Yan X, McAdoo DJ. Hydroxyl radicals generated in vivo kill neurons in the rat spinal cord : electrophysiological, histological, and neurochemical results. J Neurochem. 1994;62:37–44. doi: 10.1046/j.1471-4159.1994.62010037.x. [DOI] [PubMed] [Google Scholar]
- 15.Maegele M, Riess P, Sauerland S, Bouillon B, Hess S, McIntosh TK, et al. Characterization of a new rat model of experimental combined neurotrauma. Shock. 2005;23:476–481. doi: 10.1097/01.shk.0000159929.87737.5c. [DOI] [PubMed] [Google Scholar]
- 16.Mun-Bryce S, Rosenberg GA. Gelatinase B modulates selective opening of the blood-brain barrier during inflammation. Am J Physiol. 1998;274:R1203–R1211. doi: 10.1152/ajpregu.1998.274.5.R1203. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen M, Arkell J, Jackson CJ. Human endothelial gelatinases and angiogenesis. Int J Biochem Cell Biol. 2001;33:960–970. doi: 10.1016/s1357-2725(01)00007-3. [DOI] [PubMed] [Google Scholar]
- 18.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piao MS, Lee JK, Jang JW, Kim SH, Kim HS. A mouse model of photochemically induced spinal cord injury. J Korean Neurosurg Soc. 2009;46:479–483. doi: 10.3340/jkns.2009.46.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15:415–436. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Romanic AM, White RF, Arleth AJ, Ohlstein EH, Barone FC. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats : inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29:1020–1030. doi: 10.1161/01.str.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg GA. Matrix metalloproteinases in brain injury. J Neurotrauma. 1995;12:833–842. doi: 10.1089/neu.1995.12.833. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg GA, Dencoff JE, McGuire PG, Liotta LA, Stetler-Stevenson WG. Injury-induced 92-kilodalton gelatinase and urokinase expression in rat brain. Lab Invest. 1994;71:417–422. [PubMed] [Google Scholar]
- 25.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier : an expanded therapeutic window. Brain Res. 1995;703:151–155. doi: 10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- 27.Schroeter M, Jander S, Stoll G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels : characterization of inflammatory responses. J Neurosci Methods. 2002;117:43–49. doi: 10.1016/s0165-0270(02)00072-9. [DOI] [PubMed] [Google Scholar]
- 28.Watson BD, Holets VR, Prado R, Bunge MB. Laser-driven photochemical induction of spinal cord injury in the rat : Methodology, histopathology, and applications. Neurorotocols. 1993;3:3–15. [Google Scholar]
- 29.Werb Z. ECM and cell surface proteolysis : regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 30.Yu F, Kamada H, Niizuma K, Endo H, Chan PH. Induction of mmp-9 expression and endothelial injury by oxidative stress after spinal cord injury. J Neurotrauma. 2008;25:184–195. doi: 10.1089/neu.2007.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]