Abstract
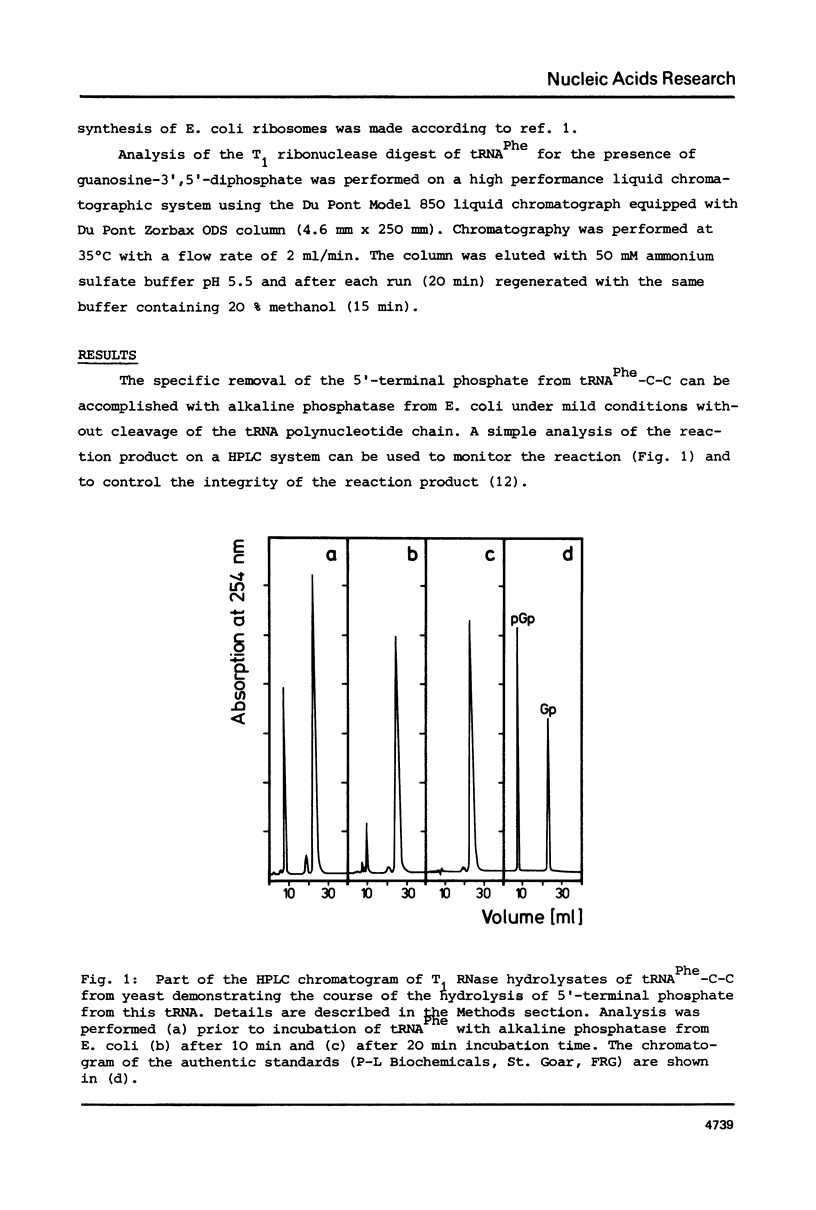
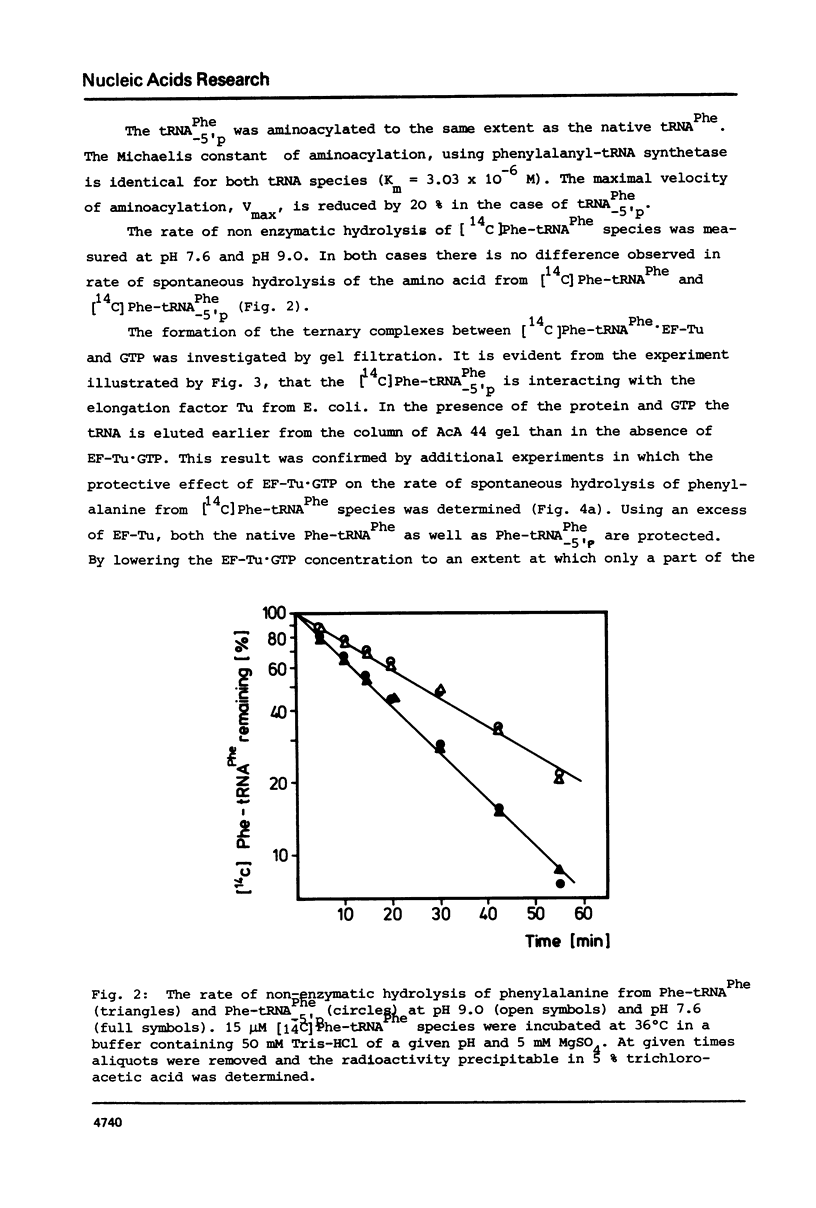
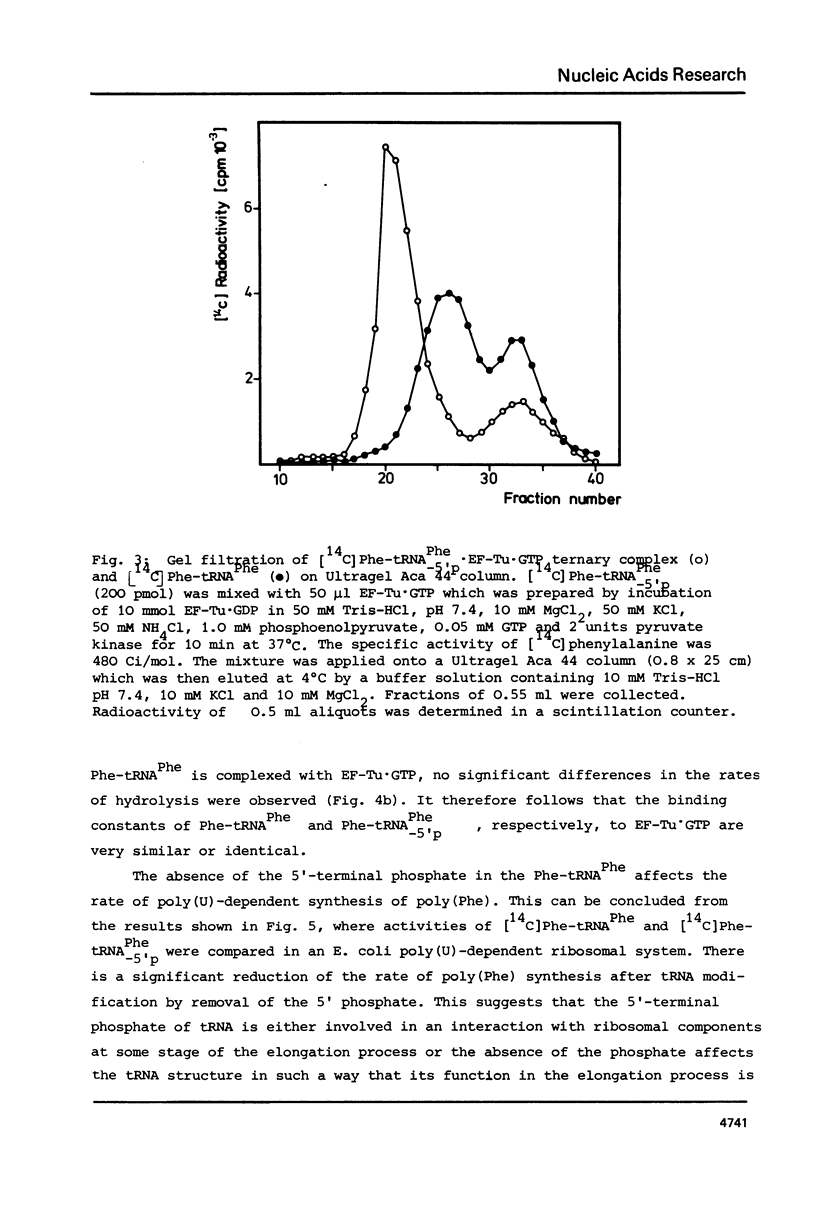
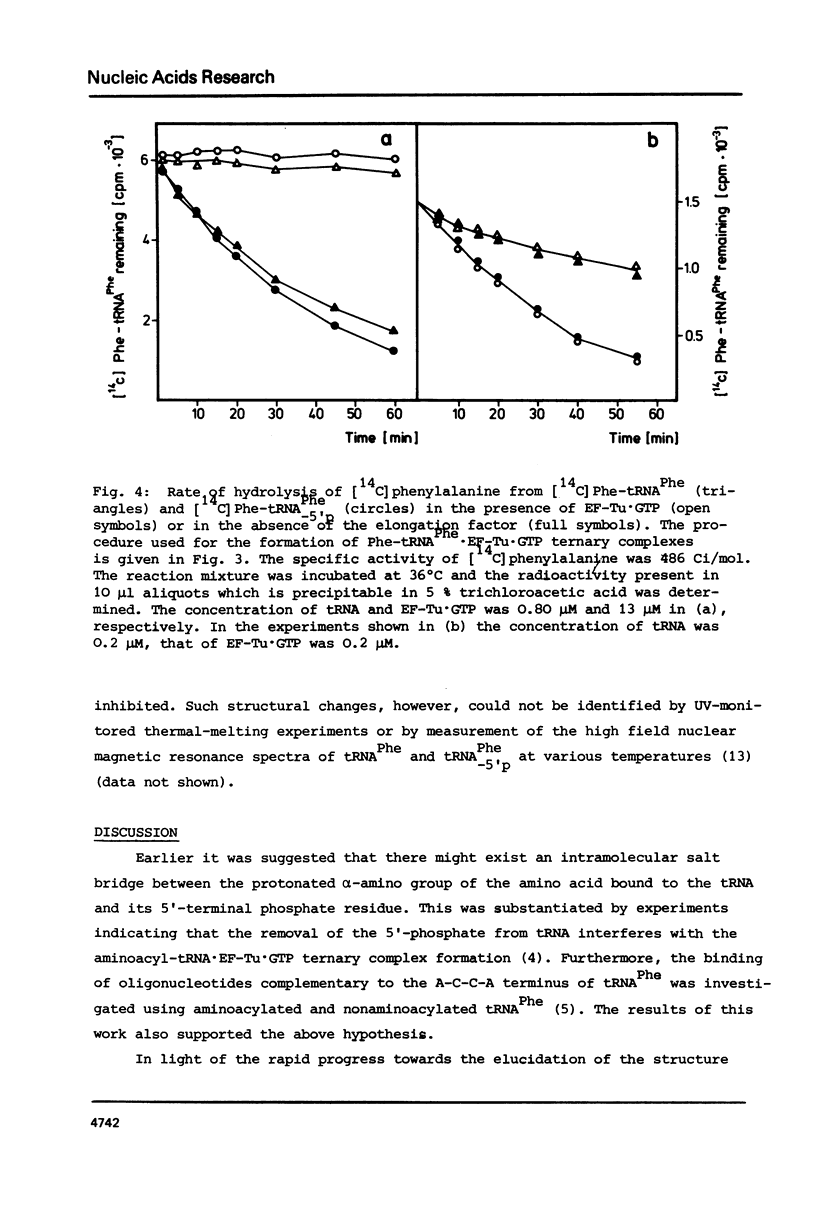
The 5'-terminal phosphate of tRNAPhe from yeast was removed using tRNAPhe lacking its 3'-terminal adenosine. After regeneration of the C-C-A terminus this tRNA was investigated in following reactions: aminoacylation, spontaneous hydrolysis of the amino acid from aminoacyl-tRNA, aminoacyl-tRNA.EF-Tu.GTP ternary complex formation and poly(U)-dependent synthesis of poly(Phe). The absence of the 5'-terminal phosphate of Phe-tRNAPhe does not influence the rate of hydrolysis of the amino acid or the ability of this rRNA to participate in complex formation with EF-Tu.GTP. The translation of the polyuridylic acid is slightly inhibited whereas the rate and extent of the enzymatic aminoacylation is not affected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alford B. L., Pezzuto J. M., Tan K. H., Hecht S. M. Both positional isomers of aminoacyl-tRNA's are bound by elongation factor Tu. J Biol Chem. 1979 Aug 10;254(15):6894–6903. [PubMed] [Google Scholar]
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Arai K., Clark B. F., Duffy L., Jones M. D., Kaziro Y., Laursen R. A., L'Italien J., Miller D. L., Nagarkatti S., Nakamura S. Primary structure of elongation factor Tu from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1326–1330. doi: 10.1073/pnas.77.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanloo P., Sprinzl M., Cramer F. Proton nuclear magnetic resonance of minor nucleosides in yeast phenylalanine transfer ribonucleic acid. Conformational changes as a consequence of aminoacylation, removal of the Y base, and codon--anticodon interaction. Biochemistry. 1979 Jul 24;18(15):3189–3199. doi: 10.1021/bi00582a001. [DOI] [PubMed] [Google Scholar]
- Morikawa K., la Cour T. F., Nyborg J., Rasmussen K. M., Miller D. L., Clark B. F. High resolution x-ray crystallographic analysis of a modified form of the elongation factor Tu: guanosine diphosphate complex. J Mol Biol. 1978 Nov 5;125(3):325–338. doi: 10.1016/0022-2836(78)90406-0. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Urbanke C., Krauss G., Peters F., Maass G. Ternary complex formation between elongation factor Tu, GTP and aminoacyl-tRNA: an equilibrium study. Eur J Biochem. 1977 Sep;78(2):403–409. doi: 10.1111/j.1432-1033.1977.tb11752.x. [DOI] [PubMed] [Google Scholar]
- Pongs O., Wrede P., Erdmann V. A. Binding of complementary oligonucleotides to amino-acylated tRNAPhe from yeast. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1025–1033. doi: 10.1016/0006-291x(76)90757-9. [DOI] [PubMed] [Google Scholar]
- Schneider D., Solfert R., von der Haar F. Large scale purification of tRNA ser , tRNA tyr and tRNA phe from Baker's yeast. Hoppe Seylers Z Physiol Chem. 1972 Aug;353(8):1330–1336. doi: 10.1515/bchm2.1972.353.2.1330. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H., Sundari R. M. Structural requirements for recognition of Escherichia coli initiator and non-initiator transfer ribonucleic acids by bacterial T factor. J Biol Chem. 1974 Nov 25;249(22):7102–7110. [PubMed] [Google Scholar]
- Sprinzl M., Kucharzewski M., Hobbs J. B., Cramer F. Specificity of elongation factor Tu from Escherichia coli with respect to attachment to the amino acid to the 2' or 3'-hydroxyl group of the terminal adenosine of tRNA. Eur J Biochem. 1977 Aug 15;78(1):55–61. doi: 10.1111/j.1432-1033.1977.tb11713.x. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Sternbach H., von der Haar F., Cramer F. Enzymatic incorporation of ATP and CTP analogues into the 3' end of tRNA. Eur J Biochem. 1977 Dec;81(3):579–589. doi: 10.1111/j.1432-1033.1977.tb11985.x. [DOI] [PubMed] [Google Scholar]
- Sternbach H., von der Haar F., Schlimme E., Gaertner E., Cramer F. Isolation and properties of tRNA nucleotidyl transferase from yeast. Eur J Biochem. 1971 Sep 24;22(2):166–172. doi: 10.1111/j.1432-1033.1971.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Wagner T., Sprinzl M. The complex formation between Escherichia coli aminoacyl-tRNA, elongation factor Tu and GTP. The effect of the side-chain of the amino acid linked to tRNA. Eur J Biochem. 1980;108(1):213–221. doi: 10.1111/j.1432-1033.1980.tb04714.x. [DOI] [PubMed] [Google Scholar]
- von der Haar F. Affinity elution as a purification method for aminoacyl-tRNA synthetases. Eur J Biochem. 1973 Apr 2;34(1):84–90. doi: 10.1111/j.1432-1033.1973.tb02731.x. [DOI] [PubMed] [Google Scholar]


