Abstract
Objective
Brainstem metastases are rarely operable and generally unresponsive to conventional radiation therapy or chemotherapy. Recently, Gamma Knife Radiosurgery (GKRS) was used as feasible treatment option for brainstem metastasis. The present study evaluated our experience of brainstem metastasis which was treated with GKRS.
Methods
Between November 1992 and June 2010, 32 patients (23 men and 9 women, mean age 56.1 years, range 39-73) were treated with GKRS for brainstem metastases. There were metastatic lesions in pons in 23, the midbrain in 6, and the medulla oblongata in 3 patients, respectively. The primary tumor site was lung in 21, breast in 3, kidney in 2 and other locations in 6 patients. The mean tumor volume was 1,517 mm3 (range, 9-6,000), and the mean marginal dose was 15.9 Gy (range, 6-23). Magnetic Resonance Imaging (MRI) was obtained every 2-3 months following GKRS. Follow-up MRI was possible in 24 patients at a mean follow-up duration of 12.0 months (range, 1-45). Kaplan-Meier survival analysis was used to evaluate the prognostic factors.
Results
Follow-up MRI showed tumor disappearance in 6, tumor shrinkage in 14, no change in tumor size in 1, and tumor growth in 3 patients, which translated into a local tumor control rate of 87.5% (21 of 24 tumors). The mean progression free survival was 12.2 months (range, 2-45) after GKRS. Nine patients were alive at the completion of the study, and the overall mean survival time after GKRS was 7.7 months (range, 1-22). One patient with metastatic melanoma experienced intratumoral hemorrhage during the follow-up period. Survival was found to be associated with score of more than 70 on Karnofsky performance status and low recursive partitioning analysis class (class 1 or 2), in terms of favorable prognostic factors.
Conclusion
GKRS was found to be safe and effective for management of brainstem metastasis. The integral clinical status of patient seems to be important in determining the overall survival time.
Keywords: Brainstem tumor, Gamma knife radiosurgery, Metastasis, Stereotactic radiosurgery
INTRODUCTION
Metastasis to the brain is a frequent complication of malignant tumors of the lung, breast, kidney, and of malignant melanoma2). Metastatic brain lesions are estimated to eventually develop in 15 to 40% of patients with cancer31), and autopsy studies show brain metastases in 24% of cancer patients. However, brainstem metastases are uncommon, and account for only 3 to 5% of all brain metastases8,17,30). Brainstem metastases are generally not treated surgically due to the risk of causing neurological damage. Whole brain radiation therapy (WBRT) and stereotactic radiosurgery have been reported to provide benefits in brain metastases patients3,4,9,10,14,19,29). However, the benefits of such treatment in brainstem metastasis patients remain unclear.
Since the first report of Huang et al. series15), there were some small case series for the management of brainstem metastases using Gamma Knife Radiosurgery (GKRS)12,16,18,22,26,35). The present study assessed outcomes following our use of GKRS for the management of brainstem metastasis, and we investigated the effect of treatment on tumor size and survival time.
MATERIALS AND METHODS
Patient characteristics
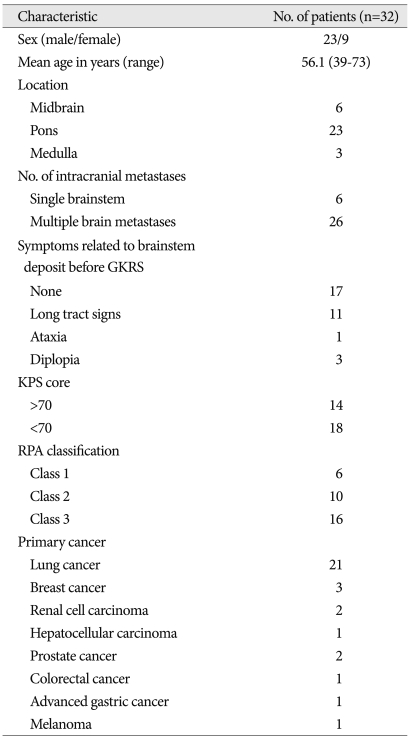
From November 1992 to June 2010, a total of 32 patients underwent GKRS for local control of brainstem metastasis at our institute. The clinical characteristics of the 32 patients are summarized in Table 1. The study population consisted of 23 men and 9 women, with a mean age of 56.1 years (range, 39-73). The primary malignancy was lung cancer in 21 patients, breast cancer in 3, renal cell cancer in 2, prostate cancer in 2, and hepatocellular carcinoma, colorectal cancer in 1, gastric cancer in 1, and melanoma in 1. Fourteen patients had score of more than 70 on the Karnofsky performance status (KPS), and 18 had scores of less than 70. Lesions were located in the pons in 23 patients, the midbrain in 6 and the medulla oblongata in 3 patients. Fifteen patients presented with brainstem signs which comprised long tract signs in 11, ataxia in 1, and diplopia in 3 patients. Six patients had a single brainstem metastasis, and the remainder had multiple intracranial deposits (range 2-15). Eighteen patients presented with active extracranial systemic disease.
Table 1.
Summary of patients and tumor characteristics
GKRS : Gamma Knife Radiosurgery, KPS : Karnofsky performance status, RPA : recursive partitioning analysis
Radiosurgery technique
All radiosurgical procedures in this study were carried using a Leksell Gamma Knife (B. and C. Elekta, Stockholm, Sweden). A Leksell stereotactic coordinate frame was applied to the patient's head under local anesthesia and stereotactic gadolinium-enhanced MR imaging was then performed for target coordinate determination and dose planning. The mean tumor volume at the time of GKRS was 1,517 mm3 (range, 9-6,000). The mean marginal radiation dose was 15.9 Gy (range, 6-23). In most patients, the 50% isodose line was selected. The marginal dose was selected on the basis of histology, lesion size, lesion location and previous radiotherapy.
Clinical and radiological follow-up
Follow-up MRI was performed at every 2 or 3 months after GKRS. Radiological follow-up was available for twenty-four of 32 patients and mean follow-up duration was 12.0 months (range, 1-45). Follow-up MRI was not available for 8 patients due to survival of less than 3 months after GKRS (7 patients), and loss to follow-up (1 patient).
For statistical analysis, we constructed Kaplan-Meier plots for survival and progression free survival using the dates of diagnosis, first GKRS, follow-up MRIs, and death or last follow-up. Progression free survival and overall survival times were calculated from the day of the first GKRS, using the Kaplan-Meier method with a p value of <0.05 set as significant. Primary site, age (<65 years vs. >65 years), KPS (<70 vs. >70), single vs. multiple brain metastases, active vs. absent or controlled extracranial disease, RPA classificiation13) were assessed for influence on survival. Standard statistical processing soft ware (SPSS, version 12.0) was used.
RESULTS
Tumor control
MRI was available for 24 patients. Follow-up MRI showed tumor disappearance in 6 patients (25.0%), tumor shrinkage in 14 patients (58.3%), no change in tumor size in 1 patient (4.2%), and an increase in tumor size in 3 patients (12.5%). This translated into a local tumor control rate of 87.5% (21 of 24 tumors). In three cases, tumor progression was observed at 6, 8, and 16 months, respectively. The mean progression free survival (PFS) was 12.2 months after GKRS.
Patient survival
At the time of the assessment, 9 (28.1%) patients were alive at a mean of 20.5 months (ranged 2 to 45 months) and 22 (68.8%) patients had died after GKRS. The overall mean survival time was 7.7 months (ranged 1 to 22 months). For one patient, follow-up data was not available due to loss to follow-up.
The cause of death was documented in 15 patients. Death was related to brainstem metastasis in one patient (increase in hemorrhagic tumor with clinical worsening), development of other brain metastases in five, and aggravation of primary cancer in nine patients.
Prognostic factors for survival
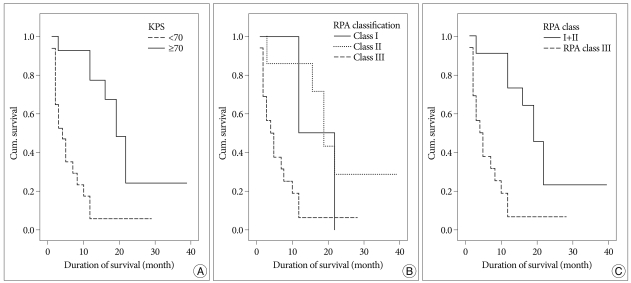
KPS score and recursive partitioning analysis (RPA) classification were shown to have affected survival. Patients with scores more than 70 on KPS group had better overall survival than those with less than 70 (Fig. 1A). RPA class 1 comprised 3 patients who had a mean survival of 17 months, RPA class 2 comprised 5 patients who had a mean survival of 22 months, and RPA class 3 comprised 15 patients who had a mean survival of 7 months (p=0.0057) (Fig. 1B). Due to the small patient population in RPA class 1 (3 patients), we compared RPA class 3 with RPA class 1 and 2 together. RPA class 1 and 2 showed better overall survival (p=0.0014) (Fig. 1C).
Fig. 1.
A : There is a significant association between survival time and KPS score at the time of GKRS for brainstem metastasis (mean survival time 22 months among 15 patients in score more than 70 on KPS vs. 6 months for 17 in score less than 70 on KPS, p=0.0001). B : RPA Kaplan-Meier survival curve shows significant a difference between the three classes (p=0.0059), with the expected mean survival for class 1 patients to be 17 months, 22 months for class 2, and 7 months for class 3. C : Survival of 15 patients in RPA 3 (mean survival=7 months) compared with 8 patients in RPA 1 and 2 together (mean survival=21 months), p=0.0014.
However, survival curves for sex, primary tumor, lesion location, and extracranial metastases did not demonstrate significant difference among subsets.
Complication
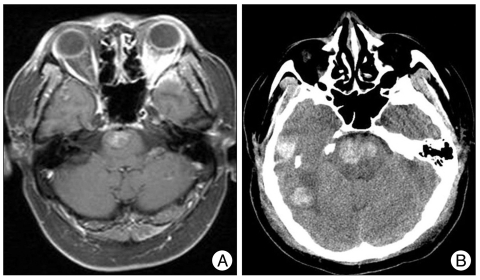
Complication was observed in only 1 case (Fig. 2). This patient was a 51-year-old man who had a metastatic tumor with hemorrhage from a malignant melanoma in the right temporal lobe. After tumor resection, 4 new supratentorial metastatic lesions appeared, and they were treated using GKRS. MRI at 1 month after GKRS showed that all 4 tumors had slightly decreased in size. However, 10 months after GKRS, the patient presented with headache and seizures. Brain MRI revealed a pontine and multiple supratentorial tumors with hemorrhage. GKRS was performed at 14.8 Gy on the pontine deposit. The next day, the patient presented with swallowing difficulty and progressive impairment of consciousness. Brain CT demonstrated an increase in the extent of multifocal hemorrhagic mass lesions, and that the lesions were compressing the brainstem. The patient died of hemorrhagic tumor increase.
Fig. 2.
Axial enhanced T1-weighted MR image demonstrating metastatic deposits with hemorrhage involving the pons (A). CT image revealing aggravation of hemorrhagic mass lesions in the right temporal lobe and pons with brain stem compression (B).
DISCUSSION
Brainstem metastases are relatively rare and have a poor prognosis. The median survival in patients with untreated metastasis to the brain is 1 to 2 months21,23). Survival time is reported to be increased to 3 to 6 months following fractionated radiotherapy, and is even longer in patients with a single or limited numbers of metastatic tumors who undergo surgical resection7,20,25,33). Smalley et al. reported a median survival of 11.7 months after resection of a solitary brain metastasis28). Stereotactic radiosurgery has been reported to be as effective as open surgery in local tumor control24,27). GKRS is reported to result in local control rates of 83 to 94%, and median survival times ranging from 7 to 12 months1,5,6,10,11,15).
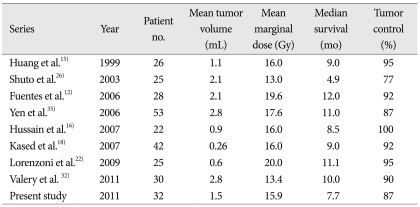
The advantages of GKRS are that it is minimally invasive and suitable for lesions inaccessible via surgery. To date, 8 studies have reported on outcomes following stereotactic radiosurgery for brainstem metastases, and their findings are summarized in Table 2. Even though they are small, retrospective, non-controlled studies due to rarity of this disease, they showed the usefulness of GKRS for brainstem metastasis. Those studies reported local control rates of 77 to 100%, which are similar to rates reported for brain metastases in other locations3,9,22,29,34). The median survival times ranged from 4.9 to 12 months. Most series used a low radiation dose, ranging from 13 to 20 Gy, with the most common prescribed marginal dose being 16 Gy15,16,18). In 1999, Huang et al. reported a local control rate of 95% and a median survival time of 9 months in 26 patients with brainstem metastasis using a median prescribed dose of 16 Gy15). The current study used a mean prescribed dose of 15.9 Gy, had a local control rate of 87.5%, a mean survival time of 7.7 months, all of which are comparable to previous series. No direct relationship between peripheral dose delivered and local control were observed. Hussain et al.16) reported a local control rate of 100% using a median prescribed dose of 16 Gy, whereas Lorenzoni et al. 22) used a mean prescribed dose of 20 Gy, reported a local control rate of 95%.
Table 2.
Summary of published series on brainstem metastasis treated with stereotactic radiosurgery
The advantage of dose reduction is to limit severe adverse effects involving normal tissue included in the prescription isodose. Valery et al.32) tried to limit peripheral isodose to 13 Gy and to improve conformal dosimetry, reported a local control rate of 90.0%, a mean survival time of 10.0 months. There are possible explanations for the good local control rate compared with hemispherical locations usually treated with higher doses. Targeting or defining an intraparenchymal target is more accurate in this area; high-density brainstem white fibers surrounding the lesion may constitute a barrier against cell spread; and a combination of both may come into play32).
Lorenzoni et al.22) reported that the only patient with tumor re-currence was treated initially with a marginal dose of 18 Gy; the lesion was retreated 8 months later with a marginal dose of 20 Gy. This patient did not develop radiation-induced morbidity and lived 21.5 months after the first treatment. In the present study, two of three patients with tumor recurrence underwent a second GKRS for brainstem metastasis. At 6 and 16 months after the first treatment, they were retreated with a marginal dose of 12.5 and 10 Gy respectively, who had been treated initially with a marginal dose of 12 Gy. These patients lived 12 and 19 months after the first treatment and did not develop radiation-induced morbidity. The prescribed dose was lower compared with the study by Lorenzoni et al. This suggests that the brainstem may tolerate such doses at least for this relatively short period.
Yen et al.35) found that the absence of extracranial disease was the only favorable prognostic factor. Kased et al. reported that factors associated with longer survival were a single metastasis, non-melanoma histology, and extracranial disease control. As mentioned above, the present study found that Karnofsky Performance Status (KPS) and Recursive partitioning analysis (RPA) classification were factors associated with survival rate.
Common GKRS complications include headache, nausea, vomiting, brain edema, and seizure due to radiation-induced injury or intratumoral hemorrhage. GKRS complications related to brainstem include ataxia, dysequilibrium, paresthesia and hemiparesis18). The reported morbidity rate after GKRS for brainstem metastasis is 5.5 to 10%24,27,30). Shuto et al. reported 2 of 25 patients experienced radiation-induced damage. In the study of Fuentes et al, a higher marginal dose (19.6 Gy), compared with 13 Gy in the study of Shuto et al., was administered, and no radiation-induced complication was documented. Huang et al. reported one case of vomiting and three cases of seizure among 26 patients. In the present study, a dose of 15.9 Gy was administrated. During the follow-up period, one patient with metastatic melanoma experienced intratumoral hemorrhage after GKRS.
CONCLUSION
Brainstem metastasis accounts for only a small proportion of brain metastases (3-5%), but the prognosis is highly unfavorable. GKRS resulted in an excellent local control rate with relatively low morbidity and complication rates in patients with brainstem metastasis, indicating it is effective and safe. Patient survival was linked to systemic disease severity.
References
- 1.Adler JR, Cox RS, Kaplan I, Martin DP. Stereotactic radiosurgical treatment of brain metastases. J Neurosurg. 1992;76:444–449. doi: 10.3171/jns.1992.76.3.0444. [DOI] [PubMed] [Google Scholar]
- 2.Alexander E, 3rd, Moriarty TM, Davis RB, Wen PY, Fine HA, Black PM, et al. Stereotactic radiosurgery for the definitive, noninvasive treatment of brain metastases. J Natl Cancer Inst. 1995;87:34–40. doi: 10.1093/jnci/87.1.34. [DOI] [PubMed] [Google Scholar]
- 3.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases : phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 4.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases : a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 5.Auchter RM, Lamond JP, Alexander E, Buatti JM, Chappell R, Friedman WA, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys. 1996;35:27–35. doi: 10.1016/s0360-3016(96)85008-5. [DOI] [PubMed] [Google Scholar]
- 6.Bindal AK, Bindal RK, Hess KR, Shiu A, Hassenbusch SJ, Shi WM, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84:748–754. doi: 10.3171/jns.1996.84.5.0748. [DOI] [PubMed] [Google Scholar]
- 7.Cairncross JG, Kim JH, Posner JB. Radiation therapy for brain metastases. Ann Neurol. 1980;7:529–541. doi: 10.1002/ana.410070606. [DOI] [PubMed] [Google Scholar]
- 8.Chason JL, Walker FB, Landers JW. Metastatic carcinoma in the central nervous system and dorsal root ganglia. A prospective autopsy study. Cancer. 1963;16:781–787. doi: 10.1002/1097-0142(196306)16:6<781::aid-cncr2820160614>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Chidel MA, Suh JH, Reddy CA, Chao ST, Lundbeck MF, Barnett GH. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:993–999. doi: 10.1016/s0360-3016(00)00527-7. [DOI] [PubMed] [Google Scholar]
- 10.Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. 1994;28:797–802. doi: 10.1016/0360-3016(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 11.Flickinger JC, Lunsford LD, Somaza S, Kondziolka D. Radiosurgery: its role in brain metastasis management. Neurosurg Clin N Am. 1996;7:497–504. [PubMed] [Google Scholar]
- 12.Fuentes S, Delsanti C, Metellus P, Peragut JC, Grisoli F, Regis J. Brainstem metastases : management using gamma knife radiosurgery. Neurosurgery. 2006;58:37–42. doi: 10.1227/01.neu.0000190655.95669.5c. discussion 37-42. [DOI] [PubMed] [Google Scholar]
- 13.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 14.Goodman KA, Sneed PK, McDermott MW, Shiau CY, Lamborn KR, Chang S, et al. Relationship between pattern of enhancement and local control of brain metastases after radiosurgery. Int J Radiat Oncol Biol Phys. 2001;50:139–146. doi: 10.1016/s0360-3016(00)01584-4. [DOI] [PubMed] [Google Scholar]
- 15.Huang CF, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery for brainstem metastases. J Neurosurg. 1999;91:563–568. doi: 10.3171/jns.1999.91.4.0563. [DOI] [PubMed] [Google Scholar]
- 16.Hussain A, Brown PD, Stafford SL, Pollock BE. Stereotactic radiosurgery for brainstem metastases : Survival, tumor control, and patient outcomes. Int J Radiat Oncol Biol Phys. 2007;67:521–524. doi: 10.1016/j.ijrobp.2006.08.081. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JD, Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7:337–344. [PubMed] [Google Scholar]
- 18.Kased N, Huang K, Nakamura JL, Sahgal A, Larson DA, McDermott MW, et al. Gamma knife radiosurgery for brainstem metastases : the UCSF experience. J Neurooncol. 2008;86:195–205. doi: 10.1007/s11060-007-9458-4. [DOI] [PubMed] [Google Scholar]
- 19.Kondziolka D, Patel A, Lunsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 20.Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schmitz PI. Identification of prognostic factors in patients with brain metastases : a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 21.Lang EF, Slater J. Metastatic brain tumours: results of surgical and nonsurgical treatment. Surg Clin North Am. 1964;44:865–872. doi: 10.1016/s0039-6109(16)37308-x. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzoni JG, Devriendt D, Massager N, Desmedt F, Simon S, Van Houtte P, et al. Brain stem metastases treated with radiosurgery : prognostic factors of survival and life expectancy estimation. Surg Neurol. 2009;71:188–195. doi: 10.1016/j.surneu.2008.01.029. discussion 195, 195-196. [DOI] [PubMed] [Google Scholar]
- 23.Markesbery WR, Brooks WH, Gupta GD, Young AB. Treatment for patients with cerebral metastases. Arch Neurol. 1978;35:754–756. doi: 10.1001/archneur.1978.00500350058012. [DOI] [PubMed] [Google Scholar]
- 24.Muacevic A, Kreth FW, Horstmann GA, Schmid-Elsaesser R, Wowra B, Steiger HJ, et al. Surgery and radiotherapy compared with gamma knife radiosurgery in the treatment of solitary cerebral metastases of small diameter. J Neurosurg. 1999;91:35–43. doi: 10.3171/jns.1999.91.1.0035. [DOI] [PubMed] [Google Scholar]
- 25.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 26.Shuto T, Fujino H, Asada H, Inomori S, Nagano H. Gamma knife radiosurgery for metastatic tumours in the brain stem. Acta Neurochir (Wien) 2003;145:755–760. doi: 10.1007/s00701-003-0034-1. [DOI] [PubMed] [Google Scholar]
- 27.Simonová G, Liscák R, Novotný J, Jr, Novotný J. Solitary brain metastases treated with the Leksell gamma knife : prognostic factors for patients. Radiother Oncol. 2000;57:207–213. doi: 10.1016/s0167-8140(00)00267-x. [DOI] [PubMed] [Google Scholar]
- 28.Smalley SR, Laws ER, Jr, O'Fallon JR, Shaw EG, Schray MF. Resection for solitary brain metastasis. Role of adjuvant radiation and prognostic variables in 229 patients. J Neurosurg. 1992;77:531–540. doi: 10.3171/jns.1992.77.4.0531. [DOI] [PubMed] [Google Scholar]
- 29.Sneed PK, Lamborn KR, Forstner JM, McDermott MW, Chang S, Park E, et al. Radiosurgery for brain metastases : is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43:549–558. doi: 10.1016/s0360-3016(98)00447-7. [DOI] [PubMed] [Google Scholar]
- 30.Soffietti R, Rudā R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357–1369. doi: 10.1007/s00415-002-0870-6. [DOI] [PubMed] [Google Scholar]
- 31.Tosoni A, Ermani M, Brandes AA. The pathogenesis and treatment of brain metastases : a comprehensive review. Crit Rev Oncol Hematol. 2004;52:199–215. doi: 10.1016/j.critrevonc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Valery CA, Boskos C, Boisserie G, Lamproglou I, Cornu P, Mazeron JJ, et al. Minimized doses for linear accelerator radiosurgery of brainstem metastasis. Int J Radiat Oncol Biol Phys. 2011;80:362–368. doi: 10.1016/j.ijrobp.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 33.Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis : radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 34.Weltman E, Salvajoli JV, Brandt RA, de Morais Hanriot R, Prisco FE, Cruz JC, et al. Radiosurgery for brain metastases : a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46:1155–1161. doi: 10.1016/s0360-3016(99)00549-0. [DOI] [PubMed] [Google Scholar]
- 35.Yen CP, Sheehan J, Patterson G, Steiner L. Gamma knife surgery for metastatic brainstem tumors. J Neurosurg. 2006;105:213–219. doi: 10.3171/jns.2006.105.2.213. [DOI] [PubMed] [Google Scholar]