Abstract
Objective
Stenting of symptomatic intracranial stenosis has recently become an alternative treatment modality. However, urgent intracranial stenting in patients with intracranial stenosis following a transient ischemic attack (TIA) or minor stroke is open to dispute. We sought to assess the feasibility, safety, and effectiveness of urgent intracranial stenting for severe stenosis (>70%) in TIA or minor stroke patients.
Methods
Between June 2009 and October 2010, stent-assisted angioplasty by using a balloon-expandable coronary stent for intracranial severe stenosis (>70%) was performed in 7 patients after TIA and 5 patients after minor stroke (14 stenotic lesions). Technical success rates, complications, angiographic findings, and clinical outcomes were retrospectively analyzed.
Results
Stenting was successful in all 12 patients. The mean time from symptom onset to stenting was 2.1 days (1-8 days). Post-procedural angiography showed restoration to a normal luminal diameter in all patients. In-stent thrombosis occurred in one patient (n=1, 8.3%), and was lysed with abciximab. No device-related complications, such as perforations or dissections at the target arteries or intracranial hemorrhaging, occurred in any patient. The mortality rate was 0%. No patient had an ischemic event over the mean follow-up period of 12.5 months (range, 7-21 months), and follow-up angiography (n=7) revealed no significant in-stent restenosis (>50%).
Conclusion
Urgent recanalization with stenting is feasible, safe, and effective in patients with TIA or acute minor stroke with intracranial stenosis of ≥70%.
Keywords: Acute ischemic stroke, Transient ischemic attack, Intracranial atherosclerosis, Intracranial stenting
INTRODUCTION
Symptomatic intracranial stenosis carries a high risk of recurrent stroke within the first year, particularly in Asian and black patients9). Despite risk factor modification and the use of warfarin or aspirin, the rate of ipsilateral stroke to the intracranial stenosis (50-99%) is 11% in the first year and 14% in the second. Furthermore, in patients with severe stenosis of >70%, the risk of subsequent stroke in the vicinity of a stenotic artery is greater, with 23% risk at 1 year and 25% risk at 2 years1).
Several studies have shown that the recurrent stroke rate in patients with intracranial stenosis decreases after angioplasty with a stent13). However, no clinical randomized trial to date has provided standard therapeutic guidelines for intracranial stenosis management by using an endovascular approach, and the necessity of early management of symptomatic intracranial stenosis remains the subject of debate. Our inability to predict which patient is at an early high risk of a recurrent stroke after a cerebrovascular event [transient ischemic attack (TIA) or stroke] may explain current variations in the management of acute stroke with intracranial stenosis.
Recently, the ABCD2 scoring system was devised and validated for assessing the short-term stroke risk of patients after TIA. This system is based on 5 clinical factors : age, blood pressure, symptom duration, unilateral weakness or speech impairment and a history of diabetes. According to this system, a score of >3 is associated with an increased general risk of vascular events in the medium-to-long-term after TIA3).
We aimed to assess the effectiveness and safety of urgent intracranial stenting for severe symptomatic stenosis (>70%) in TIA patients with an ABCD2 score of >3 and in acute minor stroke patients with intracranial stenosis of >70%.
MATERIALS AND METHODS
We retrospectively reviewed 12 patients with symptomatic intracranial stenosis treated by stent-assisted angioplasty between June 2009 and October 2010. The inclusion criteria were TIA with an ABCD2 score of >3 or acute minor stroke with intracranial stenosis of >70%.
Patients presented to the clinic with the sudden onset of ≥1 of the following symptoms : hemiparesis, speech disorder, hemianopsia, gait disturbance, vertigo, dysphasia, or disturbance of consciousness. Diagnosis was made by a vascular neurologist before patients were included in the study. On admission, computerized tomography (CT) was performed to exclude hemorrhage, and cerebral magnetic resonance imaging, including diffusion-weighted imaging (DWI) and magnetic resonance angiography, were conducted.
For stroke patients, the National Institutes of Health Stroke scales (NIHSS) were checked on admission and at 7 days after the procedure, and an independent neurologist checked the modified Rankin Scale when the patient was to be discharged. Minor stroke was defined as any acute neurological deficit with a new lesion by DWI and a NIHSS score of ≤4.
TIA was defined as an acute transient focal neurological deficit caused by vascular disease, which completely reversed within 24 h12) and caused no acute lesion by DWI. In TIA patients, ABCD2 scoring was performed by assigning points as follows : age (≥60 years, 1 point); blood pressure (BP) at first assessment after symptom onset (systolic BP of ≥140 mm Hg or diastolic BP of ≥90 mm Hg, 1 point); clinical features of TIA (unilateral weakness, 2 points; speech impairment without weakness, 1 point); duration of symptoms (≥60 min, 2 points; 10-59 min, 1 point); diabetes (1 point)3).
Stenosis percentages were calculated by measuring the narrowest vessel diameter visualized within stenosis and expressing this as a ratio of its proximal diameter1). Intracranial stents were placed 1-2 days after the onset of symptoms in TIA patients and as soon as possible in minor stroke patients. A loading dose of 300 mg clopidogrel plus 300 mg aspirin was administered before endovascular treatment. All angiographic procedures were performed using a transfemoral approach under local anesthesia using an electrocardiogram, arterial oxygen saturation, and blood pressure monitoring. After diagnostic angiography had been performed, baseline activated clotting times (ACT) were obtained to establish an intracranial stenosis.
Prior to the therapeutic procedure, patients were administered with a systemic heparinization and a bolus injection of heparin 3,000 IU. An additional 1,000 IU bolus of heparin was administered every hour to maintain an ACT of >200 s throughout the procedure. Stent placement was conducted as follows. A 6F guiding catheter Envoy (Cordis Endovascular Corporation, Miami, FL, USA) was positioned in the distal cervical ICA, and preprocedural angiograms were then obtained in single planes. The stenotic segment was crossed with a 205-cm-long, 0.014-inch-thick microwire (Transcend 14; Target/Boston Scientific, Natick, MA, USA) placed in the insular portion of the MCA to ensure maximal support. A Flexmaster coronary stent (JoMed GmbH, Rangendirgen, Germany) was used in all patients. The coronary stent was then advanced over the microwire and positioned across the stenosis using roadmap imaging and external stent markings. Correct stent positioning was confirmed by angiography and stent deployment was initiated using the roadmap image. The balloon was slowly inflated by using a multistage technique to prevent vessel dissection or rupture. When no gap was present between the stent and the parent artery, deployment was terminated, and was followed by a 30-min wait to identify possible complications, such as acute in-stent thrombosis or previously undetected vascular rupture. When post-stenting angiography showed a filling defect, we considered the defect to be due to acute in-stent thrombosis and instantly attempted thrombolysis by administering Abciximab (4 mg) (Reopro; Centocor, Netherlands) intra-arterially through a guiding catheter. Angiography was performed 10 min later to detect for undissolved thrombus. Repeat Abciximab boluses (2 mg) were administered until complete thrombolysis was achieved. Complete dissolution of the acute in-stent thrombus was deemed to have occurred when no filling defect was identified on subsequent angiography, and if there were no complications, the procedure was completed. After the procedure, hemostasis of the femoral artery was achieved using an occlusion device (Proglide, Abbott Vascular Incorporated, USA).
Technical success was defined as revascularization of >50% of the stenotic segment after stent deployment. Immediately after stent placement, we obtained the detailed neurologic history and performed a complete examination of all patients. The patients underwent non-enhanced brain CT for the evaluation of possible hemorrhagic complications. Following endovascular stent placement, patients were transferred to a stroke intensive care unit for strict blood pressure control to prevent hyperperfusion syndrome.
After the procedure, patients were administered aspirin 100 mg and clopidogrel 75 mg daily. Low-molecular-weight nadroparin calcium (Fraxiparine; GlaxoSmithKline, France) 2,850 IU was also subcutaneously administered 3 times a day for at least 3 days. Patients were discharged 7 days after the procedure if their neurologic conditions stabilized.
RESULTS
Between June 2009 and October 2010, 12 patients (7 men and 5 women) with a mean age of 65.9 years (age range of 48-75 years) were treated by stent-assisted angioplasty for symptomatic intracranial stenosis. In this study, we retrospectively reviewed the anamnesis of these 12 patients to determine whether urgent stenting in TIA or minor stroke patients is warranted. Seven TIA patients had an ABCD2 score of >3 (Table 1) and 5 minor stroke patients had an NIHSS score of ≤4 (Table 2).
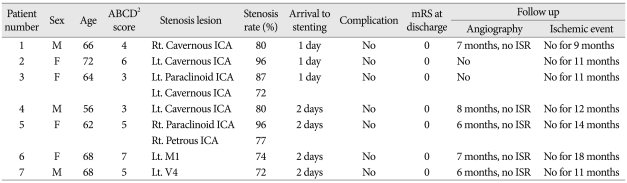
Table 1.
Characteristics and clinical outcomes of patients with a transient ischemic attack and an ABCD2 score of >3
ICA : Internal carotid artery, MCA : middle cerebral artery, M1 : first segment of MCA, V4 : fourth segment of vertebral artery, Rt. : right, Lt. : left, mRS : modified Rankin Scale, ISR : in-stent restenosis
Table 2.
Characteristics and clinical outcomes of patients with minor stroke and an NIHSS score of ≤4
*Complete thrombolysis with Abciximab. ICA : Internal carotid artery, M1 : first segment of MCA, Rt. : right, Lt. : left, NIHSS : National Institutes of Health Stroke Scale, mRS : modified Rankin Scale, ISR : in-stent restenosis
In the 7 TIA patients, stent-assisted angioplasty was performed within 24-48 h of symptom onset (mean, 1.6 days). In the 5 minor stroke patients, the mean time from symptom onset to intracranial stent placement (Fig. 1) was 2.8 days (range, 1-8 days).
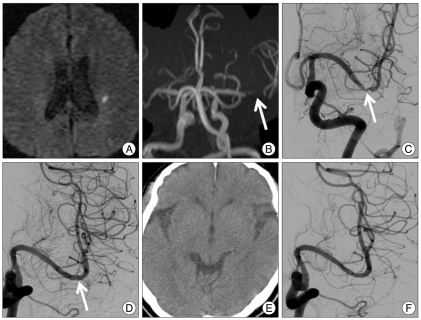
Fig. 1.
A 70-year-old man presented with minor stroke with national Institutes of Health Stroke scales 2. A : Diffusion-weighted image shows a focal acute infarction in the left corona radiata. B and C : Magnetic resonance angiography (B) and left internal carotid artery angiography (C) images show a focal severe stenosis of the distal first segment of the left middle cerebral artery. D : Immediate post-stenting angiogram shows complete recanalization of the stenotic segment with normalized luminal diameter. E : Post-procedural computed tomography shows no intracranial hemorrhage. F : Angiogram obtained 19 months after the stenting reveals no significant in-stent restenosis.
Intracranial stent placement was technically successful in all 12 patients. The mean preprocedural stenosis was 80.4% (range, 72-96%).
Acute in-stent thrombosis occurred in 1 patient (8.3%), but this was completely resolved by intra-arterial Abciximab (6 mg). There were no device-related complications, such as perforation or dissection at the target artery, and none of the patients showed evidence of an intracranial hemorrhage by control CT. The mortality rate was 0%.
The mean NIHSS score of the 5 stroke patients on admission was 2.4 (range, 2-4), with a standard deviation of 0.4 (0-2) points. At 7 days after recanalization, the mean NIHSS score of all patients was 0.4 (range, 0-2). No ischemic event occurred over 12.5 months (range, 7-21 months).
No significant in-stent restenosis (>50%) was observed in the 7 TIA patients during angiographic follow-up (mean, 8.6 months; range, 6-19 months).
DISCUSSION
The paucity of intracranial stent studies with supportive scientific evidence makes it difficult to derive differential indications for endovascular or medical treatment. Although a large number of studies have addressed rates of recurrent ischemic events after intracranial stent placement in symptomatic intracranial arterial stenosis, little data exist regarding the rate of ischemic events during the deferral period in patients being considered for an intracranial procedure.
According to the WASID trial, symptomatic patients with high-grade (≥70%) stenosis have a poor prognosis after medical treatment1). In patients with acute cerebral symptoms due to severe intracranial stenosis, the goal of early intervention is to stop plaque embolization from a vulnerable lesion11). This is chiefly because the risk for stroke is the highest during the first 48-h following a TIA3,6,7), and urgent TIA treatment is associated with an 80-90% reduction in early stroke incidence2).
Nevertheless, the early management of symptomatic intracranial stenosis remains the subject of debate, and our inability to identify patients at early high risk of a recurrent stroke after a cerebrovascular event (TIA or stroke) may explain why numerous protocols exist for the treatment of intracranial stenosis in patients who have experienced acute stroke.
Currently, intracranial angioplasty with a stent placement is deferred in favor of optimizing medical treatment and initiating anti-platelet agent therapy. Intracranial angioplasty with a stent is also delayed to allow plaque stabilization and the resolution of ischemic blood-brain barrier disruption, both of which reduce stent thrombosis and intracranial hemorrhagic complications. However, it remains controversial as to whether deferred intervention is associated with a lower periprocedural rate of stroke or death. Furthermore, any benefit associated with delayed intervention may be offset by high rates of recurrent ischemic events during the interim period between the initial ischemic event and intracranial angioplasty with a stent.
Kasner SE et al.4) reported that patients with 70-99% stenosis and recent symptoms (≤17 days) have a high risk of stroke. This is particularly the case for patients with symptomatic intracranial stenosis, predominantly in the region of the stenotic artery. Potential intervention should be considered soon after clinical presentation, unless early intervention increases the short-term risk. The early risk of recurrent stroke with intracranial stenosis has been recognized, with reports showing that three-fourths of recurrent events occur during the first months, and predominantly the first 7 days, after starting a medical treatment or performing a planned endovascular procedure5). In the GESICA study, despite optimal medical treatment, 38% of patients at the 2-year follow-up had experienced a recurrent cerebrovascular event in the territory of the stenotic artery at a median of 2 months8). Furthermore, a consensus conference on intracranial atherosclerotic disease concluded that the risk of recurrent ischemia is highest within the first 2 weeks after the initial event in patients presenting with symptomatic intracranial atherosclerotic disease10).
In this trial, the prognostic value of anti-thrombotic medication failure could not be ascertained. Furthermore, this study is limited by its retrospective nature, the small sample size, and the single center study.
CONCLUSION
This study indicates that urgent recanalization with stenting is feasible, safe and effective in patients with TIA and an ABCD2 score of >3, or in patients with acute minor stroke with intracranial stenosis ≤70%. Although this study was performed on a small number of patients, it reveals that endovascular treatment provide a satisfactory outcome.
References
- 1.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stem BJ, Hertzberg VS, Frankel MR, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 2.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack : a systematic review and meta-analysis. Lancet Neurol. 2007;6:1063–1072. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischemic attack. Lancet. 2007;369:283–292. doi: 10.1016/S0140-6736(07)60150-0. [DOI] [PubMed] [Google Scholar]
- 4.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–563. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 5.Kozak O, Tariq N, Suri MF, Taylor RA, Qureshi AI. High risk of recurrent ischemic events among patients with deferred intracranial angioplasty and stent placement for symptomatic intracranial atherosclerosis. Neurosurgery. 2011;69:334–342. doi: 10.1227/NEU.0b013e31821789ad. discussion 342-343. [DOI] [PubMed] [Google Scholar]
- 6.Lisabeth LD, Ireland JK, Risser JM, Brown DL, Smith MA, Garcia NM, et al. Stroke risk after transient ischemic attack in a population-based setting. Stroke. 2004;35:1842–1846. doi: 10.1161/01.STR.0000134416.89389.9d. [DOI] [PubMed] [Google Scholar]
- 7.Lovett JK, Dennis MS, Sandercock PA, Bamford J, Warlow CP, Rothwell PM. Very early risk of stroke after a first transient ischemic attack. Stroke. 2003;34:e138–e140. doi: 10.1161/01.STR.0000080935.01264.91. [DOI] [PubMed] [Google Scholar]
- 8.Mazighi M, Tanasescu R, Ducrocq X, Vicaut E, Bracard S, Houdart E, et al. Prospective study of symptomatic atherothrombotic intracranial stenosis : the GESICA study. Neurology. 2006;66:1187–1191. doi: 10.1212/01.wnl.0000208404.94585.b2. [DOI] [PubMed] [Google Scholar]
- 9.Miao ZR, Feng L, Li S, Zhu F, Ji X, Jiao L, et al. Treatment of symptomatic middle cerebral artery stenosis with balloon-mounted stents : long-term follow-up at a single center. Neurosurgery. 2009;64:79–84. doi: 10.1227/01.NEU.0000335648.31874.37. discussion 84-85. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi AI, Feldmann E, Gomex CR, Johnston SC, Kasner SE, Quick DC, et al. Consensus conference on intracranial atherosclerotic disease : rationale, methodology, and results. J Neuroimaging. 2009;19(Suppl 1):1S–10S. doi: 10.1111/j.1552-6569.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- 11.Setacci C, de Donato G, Chisci E, Setacci F. Carotid artery stenting in recently symptomatic patients : a single center experience. Ann Vasc Surg. 2010;24:474–479. doi: 10.1016/j.avsg.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990;21:637–676. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 13.Zhao ZW, Deng JP, He SM, Qin HZ, Gao L, Gao GD. Intracranial angioplasty with Gateway-Wingspan system for symptomatic atherosclerotic stenosis : preliminary results of 27 Chinese patients. Surg Neurol. 2009;72:607–611. doi: 10.1016/j.surneu.2009.06.017. discussion 611. [DOI] [PubMed] [Google Scholar]