Abstract
Purpose
Pancreatic leakage is a serious complication of gastrectomy due to stomach cancer. Therefore, we analyzed amylase and lipase concentrations in blood and drainage fluid, and evaluated the volume of drainage fluid to discern their usefulness as markers for the early detection of serious pancreatic leakage requiring reoperation after gastrectomy.
Methods
From January 2001 to December 2007, we retrospectively analyzed data from 24,072 patient samples. We divided patients into two groups; 1) complications with pancreatic leakage (CG), and 2) no complications associated with pancreatic leakage (NCG). Values of amylase and lipase in the blood and drainage fluid, volume of the drainage fluid, and relationships among the volumes, amylase values, and lipase values in the drainage fluid were evaluated, respectively in the two groups.
Results
The mean amylase values of CG were significantly higher than those of NCG in blood and drainage fluid (P < 0.05). For lipase, statistically significant differences were observed in drainage fluid (P < 0.05). The mean volume (standard deviation) of the drained fluid through the tube between CG (n = 22) and NCG (n = 236) on postoperative day 1 were 368.41 (266.25) and 299.26 (300.28), respectively. There were no statistically significant differences between the groups (P = 0.298). There was a correlation between the amylase and lipase values in the drainage fluid (r = 0.812, P = 0.000).
Conclusion
Among postoperative amylase and lipase values in blood and drainage fluid, and the volume of drainage fluid, the amylase in drainage fluid was better differentiated between CG and NCG than other markers. The volume of the drainage fluid did not differ significantly between groups.
Keywords: Drainage fluid, Pancreatic leakage, Stomach neoplasms, Gastrectomy
INTRODUCTION
Radical gastrectomy with extensive lymph node dissection for the treatment of gastric cancer is associated with high morbidity and mortality rates [1-4]. Partial resection or mobilization of the pancreas is often required when extended total gastrectomy (TG) with splenectomy and D2 lymph node dissection is performed. Even minimal damage to pancreatic tissue could result in pancreatic fistula in patients with high visceral fat after total gastrectomy, which was thought to be due to surgical difficulties associated with deeper and poorer view of the surgical field as well as the fragile, easily bleeding tissue in the high visceral fat group [5]. Gastrointestinal anastomosis has been also associated with leakage-related mortality rate of which ranged from 4.8 to 75% [6]. After all, pancreatic leakage after gastrectomy seemed to be mainly related to the technique and difficulty in surgery, which is sometimes unavoidable.
Pancreatic fistula is a serious potential complication after subtotal or total gastrectomy, and clinical efforts in preventing postoperative pancreatic fistula after gastrectomy have been performed [7-9]. High levels of amylase in drainage fluid have been reported to be a predictive factor for the development of postoperative pancreatic fistulas in pancreatic resection patients [10,11]. The concentration of amylase in drainage fluid after TG was suggested to be a simple and useful method for the prediction of pancreatic fistula formation [1].
In experimental models of pancreatitis, ascites accumulates rapidly, and in human diseases its early appearance is especially common in fatal cases [12,13]. Ascites may follow abdominal trauma involving the pancreas, leading to the production of small amounts of peritoneal fluid [14]; it is frequently the first sign of pancreatic disease, whether acute or chronic [15]. Ascitic fluid may reflect the peripancreatic necrotizing process [16]. The presence of drainage fluid after gastrectomy is thought to be related to the status of the pancreas in the abdominal cavity. The authors of this study wanted to ascertain the probable possibility of relationship between the volume of drainage fluid and complications requiring reoperation after gastrectomy.
Here, we analyzed amylase and lipase concentrations both in blood and drainage fluids, as well as the volume of drainage fluid to evaluate their usefulness as markers for the early detection of serious pancreatic leakage requiring reoperation after gastrectomy.
METHODS
Analysis of patient data
The records of 3,183 patients who had undergone gastric operation for pathologically confirmed gastric adenocarcinoma from January 2001 to December 2007 at the Department of Surgery, Kosin University College of Medicine were reviewed retrospectively. Among them, 100, 114, and 163 patients who underwent bypass gastrojejunostomy, open and closure, and partial resection due to their comorbidity were excluded, respectively. 2,215,539, and 52 patients who underwent subtotal gastrectomy, total gastrectomy, and TG with splenectomy or distal pancreatectomy were included, respectively in this study. We retrospectively analyzed amylase and lipase values from the blood and drainage fluids of 24,072 samples of 3,183 patients, as well as the volume of the drainage fluid at the operation site after total and subtotal gastrectomy. The Institutional Review Board approved the review of the clinical data for this study. We excluded data that were measured qualitatively. We divided patients into two groups; 1) complications with pancreatic leakage (CG); this group consisted of patients who underwent reoperation due to pancreatic leakage, and 2) no complications associated with pancreatic leakage (NCG); this group consisted of patients who had pancreatic leakage without serious complications requiring reoperation. The highest postoperative amylase and lipase values in the drainage fluid and blood were used. The volume in the drainage fluid bag was measured from postoperative day 1.
We compared mean values of amylase and lipase in blood and drainage fluid and the volumes of drainage fluid, between the two groups. To assess the validity of amylase and lipase values in the blood and drainage fluid, we performed receiver operating characteristic (ROC) analyses. To evaluate the relationships among the volume, amylase values, and lipase values in drainage fluid, values were analyzed from postoperative day 1.
Surgical procedures
TG or subtotal gastrectomy was performed depending on the location of the cancer in the stomach. Gastroduodenostomy (Billroth I) or gastrojejunostomy (Billroth II or Roux-en-Y) in the subtotal gastrectomy and Roux-en-Y esophagojejunostomy in the TG were the standard surgical procedures used according to our institute's policy. Lymph node dissection was performed according to the Japanese gastric cancer treatment guideline. After completion of intestinal reconstruction duple-type drains were placed near the subhepatic and esophagojejunostomy sites. Drains were connected to a bag for drainage fluid collection through an expansion tube.
Pancreatic leakage check-up
After surgery, all patients had drainage tubes installed at their operation sites, through which drainage fluids were collected in a bag. From postoperative day 1, discharge was collected for examination of amylase, lipase and the amount of drainage fluid every other day until the extraction of the drainage tube.
Statistical methods
We performed all statistical analyses using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). To compare means of amylase and lipase levels in the blood and drainage fluid and volume of the drainage fluid between the CG and NCG groups, independent t-tests were performed. To test the validity of amylase and lipase in blood and drainage fluid, the area under the ROC curve (AUC) was measured in this setting. To identify any correlations among the volume, amylase value, and lipase value in the drainage fluid, Pearson's correlation coefficients were calculated. Differences were considered statistically significant at P < 0.05.
RESULTS
Blood and drainage fluid samples from 1,534 patients were analyzed for both amylase and lipase.
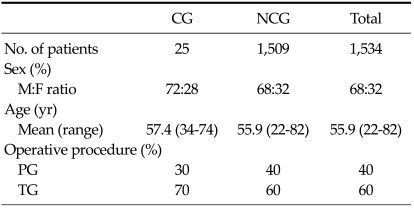
The male:female sex ratio/mean age in the amylase and lipase cases were 68:32/55.9 in blood and drainage fluid groups. TG was performed more frequently than partial gastrectomy in all analyses (Table 1).
Table 1.
Clinical information and operative procedures for amylase and lipase test groups
CG, complications with pancreatic leakage; NCG, no complications associated with pancreatic leakage; PG, partial gastrectomy; TG, total gastrectomy.
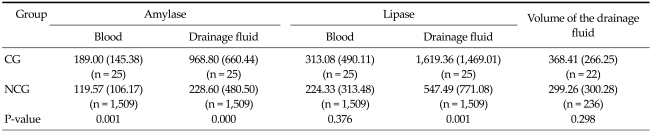
The mean amylase values of CG were significantly higher than those of NCG in blood and drainage fluid (P < 0.05, Table 2). Statistically significant differences were also observed in lipase levels in the drainage fluid (P < 0.05, Table 2). The mean volumes (standard deviation) of the drained fluid through the tube between CG (n = 22) and NCG (n = 236) on postoperative day one were 368.41 (266.25) and 299.26 (300.28), respectively. The difference between the groups was not statistically significant (P = 0.298, Table 2).
Table 2.
Mean values (SD) of amylase and lipase in blood and drainage fluid and the volume of the drainage fluid in groups
Values are presented as mean (standard deviation [SD]).
CG, complication with pancreatic leakage group; NCG, no complication associated with pancreatic leakage group.
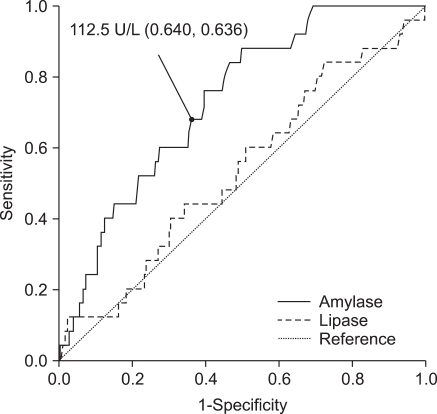
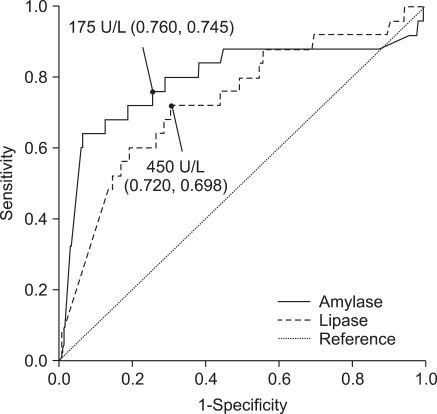
AUC analyses of lipase levels in blood and drainage fluid were 0.535, and 0.735, respectively while AUC of amylase levels in blood and drainage fluid were 0.731, and 0.797, respectively (Figs. 1, 2). When the cut-off values of amylase and lipase concentrations in drainage fluid were set at 175 and 450 U/L, respectively; sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for predicting pancreas leakage-related complications were 76.0, 74.5, 4.7, 99.5, and 74.6% for amylase, and 72.0, 69.8, 3.8, 99.3, and 69.9% for lipase.
Fig. 1.
Receiver operating characteristic (ROC) of amylase and lipase in blood. ROC curve created to identify the cut-off value of amylase concentration. Figures in parentheses are sensitivity and specificity. The area under the ROC curve (AUC) is 0.731 and 0.535 for amylase and lipase, respectively.
Fig. 2.
Receiver operating characteristic (ROC) of amylase and lipase in drainage fluid. ROC curve created to identify the cut-off value of amylase concentration. Figures in parentheses are sensitivity and specificity. The area under the ROC curve (AUC) is 0.797 and 0.735 for amylase and lipase, respectively. The AUC value was highest for amylase from the drainage fluid.
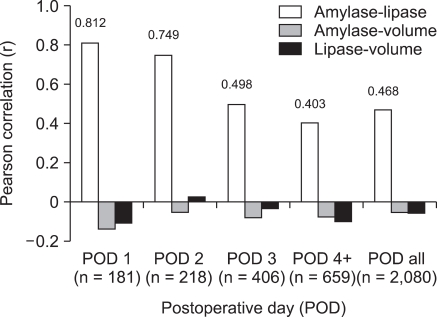
There was a correlation between the amylase and lipase values in drainage fluid (r = 0.812, P = 0.000), however, no correlations were observed between the volume and amylase value or between the volume and lipase value on postoperative day one (volume and amylase value; volume and lipase value: r = -0.140, P = 0.060; r = -0.108, P = 0.149, respectively) (Fig. 3).
Fig. 3.
Correlations among amylase, lipase, and volume in the drainage fluid by postoperative day. No significant correlation between the volume of drainage fluid and amylase or lipase concentrations was observed. POD, Postoperative day; POD 4+, From POD 4 to the rest POD; POD all, All postoperative days' data included.
DISCUSSION
Gastrectomy with Japanese-style D2 lymph node dissection is the standard surgical procedure for the treatment of advanced gastric cancer in Korea. When D2 lymph node dissection procedure is performed during gastrectomy, pancreatitis may follow due to the manipulation of the tissues around the pancreas; this may subsequently elevate amylase values both in blood and drainage fluid at the operation site and is believed to be closely related to the development of a pancreatic fistula (PF).
The International Study Group in Pancreatic Fistula has defined postoperative PF as follows: output via an operatively placed drain (or a subsequently placed, percutaneous drain) of any measurable volume of drain fluid on or after postoperative day 3, with an amylase content greater than three times the upper normal serum value [17].
The relationship between the amylase concentrations in drainage fluids and PF has been studied previously. Iwata et al. [18] reported that amylase concentration higher than 1,000 IU/L in drainage fluid on postoperative day one, along with body mass index, was an independent risk factor of pancreatic fistula and related abdominal abscess following radical gastrectomy for gastric carcinoma. Sano et al. [1] suggested that when a drain is properly placed near the pancreas and the drainage fluid amylase levels are less than 2,000 units on postoperative day one, the risk of pancreatic fistula formation is low, and the drain can be removed within a few days. They also stated that when the drainage fluid amylase levels exceed 4,000 units, a pancreatic fistula may develop in one third of cases, necessitating meticulous drain management.
Increases in serum amylase during the first few days after surgery in the upper abdomen have been reported [19-24]. Once amylase values increase after gastrectomy, serious complications requiring reoperation may occur. Reoperation itself may be a last resort for complicated patients with pancreatic leakage; however, it is never an easy decision to re-operate, thus making it necessary to identify a critical determinant for reoperation in the urgent case of complicated patients.
Here, we attempted to determine an adequate cut-off value to differentiate between the CG and NCG groups in this study. When the cut-off value of amylase was set at 175 U/L, the positive predictive values and negative predictive values (NPV) of amylase in drainage fluid were 4.7% and 99.5%, respectively. Although the prevalence of disease in a population also affects screening test performance; in low-prevalence settings, even very good tests have poor positive predictive values [25], and NPV should be more favored by surgeons. When the value of amylase in drainage fluid is less than the value set for the cut-off, reoperation is not a consideration.
When performing D2 lymphadenectomies, irrespective of the extent of gastrectomy, we routinely placed suction drains near the pancreas. Although a drainage tube placed near the area of operation can cause complications such as ascending infection [26-29], we placed drains during gastrectomy because such safety devices improve the probability of favorable prognosis for the following reasons; first, by analyzing the contents of the drainage fluid we could determine the status inside the abdominal cavity, and, second, the exudate, which is basically the aggravating material of infection, could be removed through the tube. In Japan, placement and management of drainage tubes is thought to play an important role in the postoperative care of radical gastrectomy patients, since adequate management through well-placed drainage tubes may save a patient from reoperation and reduce mortality [23].
We evaluated whether the volume of drainage fluid was related to prognosis. We were unable to find a significant correlation between the volume of drainage fluid and amylase or lipase concentrations (Fig. 3). Although the mean value of the volume was higher in CG (368.41) than in NCG (299.26) (Table 2), the differences were not statistically significant, indicating that the volume of drainage fluid itself is not a significant factor influencing complications relating to pancreatic leakage. The correlation between amylase and lipase was highest on postoperative day 1 (r = 0.812, P = 0.000) and the correlation after postoperative day 3 was thought to be similar regardless of days after operation.
In conclusion, among postoperative amylase and lipase values in blood and drainage fluid, and the volume of the drainage fluid, amylase levels in drainage fluid seemed to be better than other markers for differentiating between the CG and NCG groups. Lipase was not superior to amylase in differentiating between the two groups. The volume of drainage fluid was not significantly different between the two groups. Cautious observation for reoperation might be needed when amylase levels are higher than 175 U/L in drainage fluid after radical gastrectomy.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Sano T, Sasako M, Katai H, Maruyama K. Amylase concentration of drainage fluid after total gastrectomy. Br J Surg. 1997;84:1310–1312. [PubMed] [Google Scholar]
- 2.Robertson CS, Chung SC, Woods SD, Griffin SM, Raimes SA, Lau JT, et al. A prospective randomized trial comparing R1 subtotal gastrectomy with R3 total gastrectomy for antral cancer. Ann Surg. 1994;220:176–182. doi: 10.1097/00000658-199408000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 4.Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346–351. doi: 10.1002/bjs.1800820321. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K, Miyashiro I, Yano M, Kishi K, Motoori M, Seki Y, et al. Accumulation of excess visceral fat is a risk factor for pancreatic fistula formation after total gastrectomy. Ann Surg Oncol. 2009;16:1520–1525. doi: 10.1245/s10434-009-0391-y. [DOI] [PubMed] [Google Scholar]
- 6.Hur H, Lim YS, Jeon HM, Kim W. Management of anastomotic leakage after gastrointestinal surgery using fluoroscopy-guided foley catheter. J Korean Surg Soc. 2010;78:165–170. [Google Scholar]
- 7.Nobuoka D, Gotohda N, Konishi M, Nakagohri T, Takahashi S, Kinoshita T. Prevention of postoperative pancreatic fistula after total gastrectomy. World J Surg. 2008;32:2261–2266. doi: 10.1007/s00268-008-9683-9. [DOI] [PubMed] [Google Scholar]
- 8.Okabayashi T, Kobayashi M, Sugimoto T, Okamoto K, Matsuura K, Araki K. Postoperative pancreatic fistula following surgery for gastric and pancreatic neoplasm; is distal pancreaticosplenectomy truly safe? Hepatogastroenterology. 2005;52:233–236. [PubMed] [Google Scholar]
- 9.Oh ST, Kim WS, Kim BS. Changing patterns of pancreatic enzyme after distal gastrectomy and the effect of protease inhibitor treatment. J Korean Surg Soc. 1997;52:846–851. [Google Scholar]
- 10.Molinari E, Bassi C, Salvia R, Butturini G, Crippa S, Talamini G, et al. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg. 2007;246:281–287. doi: 10.1097/SLA.0b013e3180caa42f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi M, Nakano H, Midorikawa T, Yoshizawa Y, Sanada Y, Kumada K. Prediction of pancreatic fistula by amylase levels of drainage fluid on the first day after pancreatectomy. Hepatogastroenterology. 2003;50:1155–1158. [PubMed] [Google Scholar]
- 12.Geokas MC, Rinderknecht H, Brodrick JW, Largman C. Studies on the ascites fluid of acute pancreatitis in man. Am J Dig Dis. 1978;23:182–188. doi: 10.1007/BF01073198. [DOI] [PubMed] [Google Scholar]
- 13.Smith RB, 3rd, Warren WD, Rivard AA, Jr, Amerson JR. Pancreatic ascites: diagnosis and management with particular reference to surgical technics. Ann Surg. 1973;177:538–546. doi: 10.1097/00000658-197305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barua RL, Villa F, Steigmann F. Massive ascites due to pancreatitis. Am J Dig Dis. 1962;7:900–906. doi: 10.1007/BF02231868. [DOI] [PubMed] [Google Scholar]
- 15.Geffroy Y, Colin R, Testart J, Bourreille J, Ledouarec P, Paillot B, et al. Massive ascites in pancreatitis. Review apropos of 10 personal cases. Sem Hop. 1975;51:927–934. [PubMed] [Google Scholar]
- 16.Dugernier T, Laterre PF, Reynaert MS. Ascites fluid in severe acute pancreatitis: from pathophysiology to therapy. Acta Gastroenterol Belg. 2000;63:264–268. [PubMed] [Google Scholar]
- 17.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Iwata N, Kodera Y, Eguchi T, Ohashi N, Nakayama G, Koike M, et al. Amylase concentration of the drainage fluid as a risk factor for intra-abdominal abscess following gastrectomy for gastric cancer. World J Surg. 2010;34:1534–1539. doi: 10.1007/s00268-010-0516-2. [DOI] [PubMed] [Google Scholar]
- 19.Perryman RG, Hoerr SO. Observations on postoperative pancreatitis and postoperative elevation of the serum amylase. Am J Surg. 1954;88:417–420. doi: 10.1016/0002-9610(54)90359-1. [DOI] [PubMed] [Google Scholar]
- 20.Singh LM, Okukubo F, James PM, Jr, Salmon J, Howard JM. Further studies on postoperative pancreatitis. Arch Surg. 1965;90:43–49. doi: 10.1001/archsurg.1965.01320070045009. [DOI] [PubMed] [Google Scholar]
- 21.Harada K, Kitamura M, Ikenaga T. Isoenzyme study on postoperative transient hyperamylasemia. Am J Gastroenterol. 1974;61:121–126. [PubMed] [Google Scholar]
- 22.Keighley MR, Johnson AG, Stevens AE. Raised serum amylase after upper abdominal operation. Br J Surg. 1969;56:424–427. doi: 10.1002/bjs.1800560606. [DOI] [PubMed] [Google Scholar]
- 23.Bardenheier JA, Kaminski DL, Willman VL. Pancreatitis after biliary tract surgery. Am J Surg. 1968;116:773–776. doi: 10.1016/0002-9610(68)90366-8. [DOI] [PubMed] [Google Scholar]
- 24.Miller SF, Whitaker JR, Jr, Snyder RD. Incidence of elevated serum amylase levels and pancreatitis after upper abdominal surgery. Am J Surg. 1973;125:535–537. doi: 10.1016/0002-9610(73)90132-3. [DOI] [PubMed] [Google Scholar]
- 25.Grimes DA, Schulz KF. Uses and abuses of screening tests. Lancet. 2002;359:881–884. doi: 10.1016/S0140-6736(02)07948-5. [DOI] [PubMed] [Google Scholar]
- 26.Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006;244:1–7. doi: 10.1097/01.sla.0000218077.14035.a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tani M, Kawai M, Terasawa H, Ueno M, Hama T, Hirono S, et al. Complications with reconstruction procedures in pylorus-preserving pancreaticoduodenectomy. World J Surg. 2005;29:881–884. doi: 10.1007/s00268-005-7697-0. [DOI] [PubMed] [Google Scholar]
- 28.Tani M, Onishi H, Kinoshita H, Kawai M, Ueno M, Hama T, et al. The evaluation of duct-to-mucosal pancreaticojejunostomy in pancreaticoduodenectomy. World J Surg. 2005;29:76–79. doi: 10.1007/s00268-004-7507-0. [DOI] [PubMed] [Google Scholar]
- 29.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–366. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]