Abstract
AIM: To investigate how many discrepancies occur in patients before and after endoscopic treatment of referred adenoma and the reason for these results.
METHODS: We retrospectively reviewed data from 554 cases of 534 patients who were referred from primary care centres for adenoma treatment and treated for endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) at Chungnam National University Hospital, from July 2006 to June 2009. Re-endoscopy was examined in 142 cases and biopsy was performed in 108 cases prior to treatment. Three endoscopists (1, 2 and 3) performed all EMRs or ESDs and three pathologists (1, 2 and 3) diagnosed most of the cases. Transfer notes, medical records and endoscopic pictures of these cases were retrospectively reviewed and analyzed.
RESULTS: Adenocarcinoma was 72 (13.0%) cases in total 554 cases after endoscopic treatment of referred adenoma. When the grade of dysplasia was high (55.0%), biopsy number was more than three (22.7%), size was no smaller than 2.0 cm (23.2%), morphologic type was depressed (35.8%) or yamada type IV (100%), and color was red (30.9%) or mixed-or-undetermined (25.0%), it had much more malignancy rate than the others (P < 0.05). All 18 cases diagnosed as adenocarcinoma in the re-endoscopic forceps biopsy were performed by endoscopist 1. There were different malignancy rates according to the pathologist (P = 0.027).
CONCLUSION: High grade dysplasia is the most im-portant factor for predicting malignancy as a final pathologic diagnosis before treating the referred gastric adenoma. This discrepancy can occur mainly through inappropriately selecting a biopsy site where cancer cells do not exist, but it also depends on the pathologist to some extent.
Keywords: Discrepancy, Adenoma, High grade dysplasia, Endoscopic mucosal resection, Endoscopic submucosal dissection
INTRODUCTION
Since gastric adenoma can progress to higher grade dysplasia or cancer, as shown in long-term follow up studies, it should be treated by endoscopic resection or surgical resection[1-3]. Endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) have been approved as standard treatments for gastric adenoma[4]. Pathologic results from the mucosectomy specimens taken from endoscopic resection of gastric adenoma can be different from those of an endoscopic forceps biopsy[5,6]. As endoscopy has been examined more commonly and extensively, prevalence of adenoma referred from a primary care center for endoscopic resection has increased. However, there have been no reports on the histologic discrepancy between the endoscopy-based diagnosis of the referred gastric adenoma and the final pathologic diagnosis, and previous studies did not include analysis of other possible factors for discrepancy[5-7]. This study aimed to elucidate and analyze possible factors affecting discrepancy for referred gastric adenoma.
MATERIALS AND METHODS
A total of 1049 patients with gastric adenoma were endo-scopically treated by EMR or ESD between July 2006 and June 2009 at Chungnam National University Hospital. Among these, 534 patients were referred from primary care centres, most of them from the Tae-jeon Chungchoeng province in South Korea. Because it was intended for all the referred patients to undergo endoscopic treatment, most patients were treated with EMR or ESD, except for an extreme few who had a tendency toward bleeding, or were untreatable due to size, location, or comorbidity. Endoscopists decided resection methods (EMR or ESD) from clinical information such as age, size, morphology, color, location and pathologic grade, but there were no strict criteria. Transfer notes, medical records, and endoscopic pictures of these cases were retrospectively reviewed and analyzed. One hundred and forty-two cases were examined by re-endoscopy and 108 cases underwent re-biopsy prior to endoscopic resection according to the judgment of the endoscopist. The main reason for pre-evaluations of endoscopic examination and biopsy before the resection was incomplete or confusing referred medical records for determining treatment methods. This is schematically described in Figure 1. Transfer reports were reviewed for information of histologic grade, biopsy number, date of the biopsy, and the name of the referring center. Pathologic reports on 54 patients were written as mild, moderate, or severe grade dysplasia of the adenoma, instead of low or high grade dysplasia. Grading terms of adenoma required unification for statistical analysis. “Mild grade”or “moderate grade” was classified as low grade and “severe grade” or “moderate to severe grade” was classified as high grade. Three endoscopists performed all EMRs or ESDs and three pathologists diagnosed most of the cases. Endoscopic reports and saved pictures of procedures were reviewed for morphologic type, color, size, and location.
Figure 1.
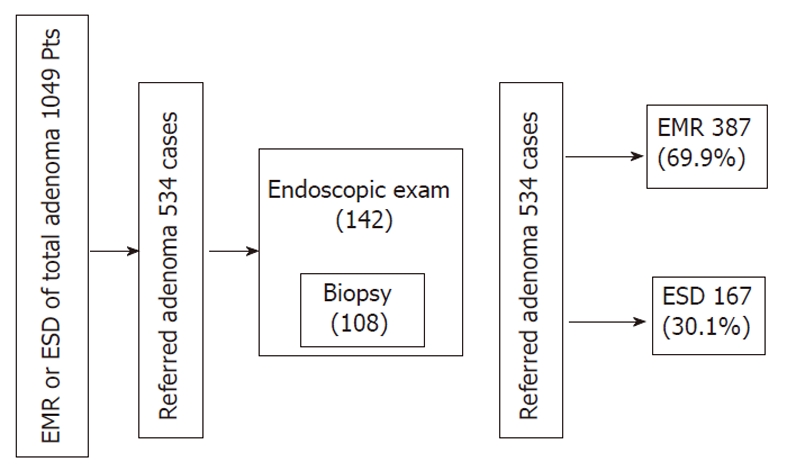
Schematic description of the study design. EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection; Pts: Patients.
SPSS version 13.0 was used for statistic analysis. The one-way analysis of variance test was used for comparison of continuous variables; for example, age, size, day duration and biopsy number. The χ2 test was used for other parameters of nominal variables.
RESULTS
Baseline characteristics, endoscopic features and treatment results of referred adenoma
Baseline characteristics and endoscopic features of referred adenomas from primary care centres are shown in Table 1. The mean age of the 554 cases was 66.1 years old. More than 86.4% of cases were located within and under the lower body. Results showed adenomas with no grading record in the transfer note in 92 cases (16.6%), low grade adenomas in 382 cases (69.0%), and high grade adenomas in 80 cases (14.4%). Treatment results of referred adenoma are shown in Table 2. Early gastric cancers were found in 72 cases (13.0%), no adenomatous lesions were found in 56 cases (10.1%), low grade adenomas were found in 356 cases (64.3%), and high grade adenomas were found in 68 cases (12.3%). One case involved mucosa associated lymphatic tissue lymphoma (MALToma) and one complicated case of bleeding were referred. Histologic results of pre-procedure re-endoscopic biopsy were various, from gastritis to adenocarcinoma. In the re-endoscopic biopsy, there were 18 cases (16.7%) of adenocarcinoma and one case of MALToma. The most common complication of EMR and ESD was bleeding (14 cases, 2.5%) which is defined as a case requiring an endoscopic procedure for bleeding control. Perforation (2 cases, 0.4%) and stricture (2 cases, 0.4%) were rare complications of EMR or ESD. There was one case of positive resection margin, in which surgery was performed for completion of treatment. Sixteen patients had multiple adenomas, 12 patients had 2 adenomas and 4 patients had 3 adenomas (Table 2).
Table 1.
The baseline characteristics and endoscopic features of referred adenomas n (%)
| No. of cases | 554 |
| No. of patients | 534 |
| Age (yr), mean ± SD | 62.1 ± 9.6 |
| Male:female | 372:182 (2.04:1) |
| Histologic grade | |
| Adenoma (no grading) | 92 (16.6) |
| Low grade | 382 (69.0) |
| High grade | 80 (14.4) |
| No. of referring hospitals | 116 |
| No. of Bx, mean ± SD | 2.24 ± 1.75 |
| Mean duration between biopsy and procedure | 40.7 d |
| Information of endoscopic photo | 449 (81.0) |
| Size (cm), mean ± SD | 1.2 ± 0.8 |
| Morphologic type | |
| Elevated | 275 (49.6) |
| Flat | 206 (37.2) |
| Depressed | 67 (12.1) |
| Y-IV | 6 (1.1) |
| Color | |
| Whitish | 332 (59.9) |
| Reddish | 94 (17.0) |
| Mixed or undetermined | 128 (23.1) |
| Longitudinal location | |
| Antrum | 298 (53.8) |
| Angle | 57 (10.3) |
| Body | 191 (34.4) |
| High | 12 (2.2) |
| Middle | 55 (9.9) |
| Lower | 124 (22.4) |
| Cardia or fundus | 8 (1.5) |
| Circular location | |
| Anterior | 123 (22.2) |
| Posterior | 119 (21.5) |
| Lesser curvature | 193 (34.8) |
| Greater curvature | 113 (20.4) |
Bx: Biopsy; Y-IV: Yamada type IV.
Table 2.
Treatment results of referred adenomas n (%)
| Repeat endoscopy | 142 (25.6) |
| Repeat biopsy | 108 (19.5) |
| No. of biopsy, mean ± SD | 2.4 ± 1.0 |
| Histologic results of repeat biopsy | |
| Low grade adenoma | 73 (67.6) |
| High grade adenoma | 9 (8.3) |
| Adenocarcinoma | 18 (16.7) |
| Gastritis | 7 (6.5) |
| Others | 1 (0.9) (MALToma) |
| Endoscopist | |
| 1 | 462 (83.4) |
| 2 | 64 (11.6) |
| 3 | 28 (5.1) |
| Pathologist | |
| 1 | 340 (61.4) |
| 2 | 124 (22.4) |
| 3 | 83 (15.0) |
| Others | 8 (1.1) |
| Histologic type; tubulovillous adenoma | 10 (1.8) |
| Type of procedure | |
| EMR | 387 (69.9) |
| ESD | 167 (30.1) |
| Histologic results of post-procedure | |
| Low grade adenoma | 356 (64.3) |
| High grade adenoma | 68 (12.3) |
| EGC | 72 (13.0) |
| No adenomatous lesion | 56 (10.1) |
| Others | 2 (0.4) (MALToma: 1, transfer by Cx: 1) |
| Complication | |
| Bleeding | 14 (2.5) |
| Perforation | 2 (0.4) |
| Stricture | 2 (0.4) |
| Cases with multiple adenoma | |
| 2 adenoma in a patient | 12 patients/534 (2.2) |
| 3 adenoma in a patient | 4 patients/534 (0.7) |
MALToma: Mucosa associated lymphoid tissue lymphoma; EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection; EGC: Early gastric cancer; Cx: Complication.
Agreement and discrepancy of histologic diagnosis
Comparison of histologic diagnoses between local clinic endoscopic biopsy and repeat endoscopic biopsy and post-procedure specimens are described in Table 3. The rate of discrepancy between primary care center and repeat biopsy was 42.4% (39 cases/92), 38.1% (176 cases/462) between primary care center and post procedure specimens, and 29.6% (32 cases/108) between repeat biopsy and post procedure specimens. The rate of complete agreement was 57.6% (53 cases/108), 61.9% (286 cases/554), and 70.4% (76 cases/108), respectively. In all comparisons, the discrepancy rate of high grade dysplasia was higher than that of other forms of adenomas.
Table 3.
Comparisons of histologic diagnoses n (%)
| LG | HG | EGC | NAL | Others | Total | Agreement | Discrepancy | |
| Between local clinic biopsy and repeat biopsy | ||||||||
| Adenoma | 11 (68.8) | 3 (18.8) | 0 (0) | 1 (6.3) | 1 (6.3) | 16 (100) | ||
| LG | 51 (83.6) | 4 (6.6) | 2 (3.3) | 4 (6.6) | 0 (0) | 61 (100) | 51 (83.6) | 10 (16.4) |
| HG | 11 (35.5) | 2 (6.5) | 16 (51.6) | 2 (6.5) | 0 (0) | 31 (100) | 2 (6.5) | 29 (93.5) |
| Total | 73 (67.6) | 9 (8.3) | 18 (16.7) | 7 (6.5) | 1 (0.9) | 108 (100) | 53 (57.6) | 39 (42.4) |
| Between local clinic and post procedure | ||||||||
| Adenoma | 60 (65.2) | 14 (15.2) | 6 (6.5) | 11 (12.0) | 1 (1.1) | 92 (100) | ||
| LG | 274 (71.7) | 42 (11.0) | 22 (5.8) | 43 (11.3) | 1 (0.3) | 382 (100) | 274 (71.7) | 108 (28.3) |
| HG | 22 (27.5) | 12 (15.0) | 44 (55.0) | 2 (2.5) | 0 (0) | 80 (100) | 12 (15.0) | 68 (85.0) |
| Total | 356 (64.3) | 68 (12.3) | 72 (13.0) | 56 (10.1) | 2 (0.4) | 554 (100) | 286 (61.9) | 176 (38.1) |
| Between repeat forcep biopsy and post procedure | ||||||||
| LG | 52 (71.2) | 1 (15.1) | 6 (8.2) | 3 (4.1) | 1 (1.4) | 73 (100) | 52 (71.2) | 21 (28.8) |
| HG | 3 (33.3) | 4 (44.4) | 2 (22.2) | 0 (0) | 0 (0) | 9 (100) | 4 (44.4) | 5 (45.6) |
| Adenocarcinoma | 0 (0) | 0 (0) | 17 (94.4) | 1 (5.6) | 0 (0) | 18 (100) | 17 (94.4) | 1 (5.6) |
| Benign lesion | 3 (42.9) | 0 (0) | 2 (28.6) | 2 (28.6) | 0 (0) | 7 (100) | 2 (28.6) | 5 (71.4) |
| Others | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 1 (100) | 1 (100) | 0 (0) |
| Total | 58 (53.7) | 15 (13.9) | 27 (25.0) | 6 (5.6) | 2 (1.9) | 108 (100) | 76 (70.4) | 32 (29.6) |
LG: Low grade; HG: High grade; NAL: No adenomatous lesion; EGC: Early gastric cancer.
Although the histologic diagnosis of referred adenoma was as low grade dysplasia, it could be high grade (11.0%) or adenocarcinoma (5.8%) in the post procedure. High grade adenoma of the primary care center could also be low grade adenoma (27.5%) or early gastric cancer (55.0%) as a final pathologic diagnosis. Consistent high grade adenoma was only 15.0% between local clinics and post procedure biopsy. All adenocarcinomas of repeat endoscopic biopsies were early gastric cancer in the post procedure, except for one case, which had no adenomatous lesion. When this one case was reviewed with pathologists, the specimen from the repeat endoscopic biopsy was not enough for adenocarcinoma. There were only a few atypical glands, but this could still be suggestive of malignancy (Table 3).
Detection of adenocarcinoma in re-endoscopic repeat biopsy prior to the procedure
Histologic results of re-endoscopic biopsy (108 cases) are shown in (Table 4), according to the endoscopists and pathologists. All of the adenocarcinoma biopsies were performed by endoscopist 1 (P < 0.001). Pathologist 1 diagnosed a much larger number of adenocarcinomas than pathologist 2 (P = 0.048).
Table 4.
Results of re-endoscopic forceps biopsy according to endoscopists and pathologists n (%)
| LG | HG | Ade | NAL | Others | Total | |
| Endoscopist | ||||||
| 1 | 34 (54.0) | 6 (9.5) | 18 (28.6) | 4 (6.3) | 1 (1.6) | 63 |
| 2 | 36 (92.3) | 3 (7.7) | 0 | 0 | 0 | 39 |
| 3 | 3 (50.0) | 0 | 0 | 3 (50.0) | 0 | 6 |
| Pathologist | ||||||
| 1 | 40 (64.5) | 4 (6.5) | 15 (24.2) | 2 (3.2) | 1 (1.6) | 62 |
| 2 | 20 (66.7) | 4 (13.3) | 2 (6.7) | 4 (13.3) | 0 | 30 |
| 3 | 8 (80.0) | 1 (10.0) | 1 (10.0) | 0 | 0 | 10 |
| Others | 5 (83.3) | 0 | 0 | 1 (16.7) | 0 | 6 |
| Deletion of minority | ||||||
| Adenocarcinoma | Non-adenocarcinoma | P value | ||||
| Endoscopist | ||||||
| 1 | 18 (28.6) | 45 (71.4) | < 0.001 | |||
| 2 | 0 (0) | 39 (100) | ||||
| Pathologist | ||||||
| 1 | 15 (24.2) | 47 (77.8) | 0.048 | |||
| 2 | 2 (6.7) | 15 (93.3) | ||||
Ade: Adenocarcinoma; LG: Low grade; HG: High grade; NAL: No adenomatous lesion.
Risk factors for predicting malignancy of referred adenoma
There was no difference between the malignancy group and the non-malignancy group as a final pathologic diagnosis with regard to age, sex, histologic type, duration between local clinic biopsy and procedure, longitudinal and circular location, endoscopist, local clinics, and multiplicity. There was a difference with regard to histologic grade, number of biopsies, size, morphologic type, color, type of procedure, examination of repeat endoscopy, pathologist, and complications (Table 5).
Table 5.
Comparison of the non-malignancy group and the malignancy group n (%)
| Non-malignancy group | Malignancy group | P value | |
| No. of cases | 482 (87.0) | 72 (13.0) | |
| No. of patients | 462 (86.5) | 72 (13.5) | |
| Age (yr), mean ± SD | 61.8 ± 9.8 | 63.7 ± 9.0 | 0.132 |
| Sex | |||
| Male | 320 (86.0) | 52 (14.0) | 0.350 |
| Female | 162 (89.0) | 20 (11.0) | |
| Histologic grade | |||
| Adenoma | 86 (93.5) | 6 (6.5) | < 0.001 |
| Low grade | 360 (94.2) | 22 (5.8) | |
| High grade | 36 (45.0) | 44 (55.0) | |
| Histologic type | |||
| Tubulovillous | 7 (70) | 3 (30) | 0.129 |
| Tubular | 475 (83.3) | 69 (12.7) | |
| No. of Bx, mean ± SD | 2.1 ± 1.6 | 2.9 ± 2.2 | < 0.001 |
| No. of biopsy | |||
| Undetermined | 111 (86.0) | 18 (14.0) | |
| 1 | 47 (92.2) | 4 (7.8) | |
| 2 | 117 (94.4) | 7 (5.6) | |
| 3 | 105 (92.1) | 9 (7.9) | |
| 4 | 47 (72.3) | 18 (27.7) | |
| 5 | 25 (80.6) | 6 (19.4) | |
| 6 | 12 (70.6) | 5 (29.4) | |
| 7 | 1 (33.3) | 2 (66.7) | |
| 8 | 1 (33.3) | 2 (66.7) | |
| 3 | 269 (93.1) | 20 (6.9 | < 0.001 |
| 4 | 86 (72.3) | 33 (27.7) | |
| Mean duration between Bx and procedure (d) | 40.9 | 39.3 | 0.869 |
| Duration between biopsy and procedure | |||
| 14 d | 24 (92.3) | 2 (7.7) | 0.658 |
| 90 d | 29 (87.9) | 4 (12.1) | |
| Size (cm), mean ± SD | 1.2 ± 0.8 | 1.5 ± 1.1 | 0.003 |
| < 1.0 | 201 (89.7) | 23 (10.3) | 0.010 |
| > 1.0, < 2.0 | 218 (87.9) | 30 (12.1) | |
| > 2.0 | 63 (76.8) | 19 (23.2) | |
| Morphologic type | |||
| Elevated | 252 (91.6) | 23 (8.4) | < 0.001 |
| Flat | 187 (90.8) | 19 (9.2) | |
| Depressed | 43 (64.2) | 24 (35.8) | |
| Y-IV | 0 (0) | 6 (100) | |
| Color | |||
| Whitish | 321 (96.7) | 11 (3.3) | < 0.001 |
| Reddish | 65 (69.1) | 29 (30.9) | |
| Mixed or undetermined | 96 (75.0) | 32 (25.0) | |
| Longitudinal location | |||
| Antrum | 255 (85.6) | 43 (14.4) | 0.291 |
| Angle | 47 (82.5) | 10 (17.5) | |
| Body | 173 (90.6) | 18 (9.4) | |
| Cardia or fundus | 7 (87.5) | 1 (12.5) | |
| Circular location | |||
| Anterior | 104 (84.6) | 19 (15.4) | 0.573 |
| Posterior | 107 (89.9) | 12 (10.1) | |
| Lesser curvature | 171 (88.6) | 22 (11.4) | |
| Greater curvature | 95 (84.1) | 18 (15.9) | |
| Type of procedure | |||
| EMR | 371 (95.9) | 16 (4.1) | < 0.001 |
| ESD | 111 (66.5) | 56 (33.5) | |
| Repeat endoscopy | |||
| Yes | 110 (77.5) | 32 (22.5) | < 0.001 |
| No | 372 (90.3) | 40 (9.7) | |
| Endoscopist | |||
| 1 | 397 (85.6) | 65 (14.1) | 0.204 |
| 2 | 60 (93.8) | 4 (6.3) | |
| 3 | 25 (89.3) | 3 (10.7) | |
| Pathologist | |||
| 1 | 284 (83.5) | 56 (16.5) | 0.027 |
| 2 | 117 (94.4) | 7 (5.6) | |
| 3 | 74 (89.2) | 9 (10.8) | |
| Others | 6 (100) | 0 (0) | |
| Local clinics (> 30 cases) | |||
| 1 | 39 (95.1) | 2 (4.9) | 0.224 |
| 2 | 37 (90.2) | 4 (9.8) | |
| 3 | 35 (97.2) | 1 (2.8) | |
| 4 | 28 (84.8) | 5 (15.2) | |
| Complication | |||
| Bleeding | 9 (64.3) | 5 (35.7) | |
| Perforation | 1 (50) | 1 (50) | |
| Stricture | 1 (50) | 1 (50) | |
| Total complications | 11 (61.1) | 7 (38.9) | 0.005 |
| No complications | 471 (87.9) | 65 (12.1) | |
| Multiplicity | |||
| Patient of single case | 449 (86.7) | 69 (13.3) | 0.464 |
| Patient of multiple cases | 13 (81.3) | 3 (18.8) | |
Bx: Biopsy; Y: Yamada type; EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection.
Before the resection, predictive factors for a malignant result were high grade dysplasia (55.0%), a biopsy number of more than three (22.7%), a size of no less than 2.0 cm (23.2%), a morphologic type of depressed (35.8%) or yamada type IV (100%), and a red (30.9%) or mixed-or-undetermined (25.0%) coloration. There was no statistical significance between less than 1.0 cm and no less than 1.0 cm (P = 0.124).
Cases of ESD, repeat endoscopy, or complicated cases had many more malignant results than cases of EMR or direct procedures without re-endoscopy or non-complicated cases. The rate of malignancy was different according to the pathologist (P = 0.027). Mean duration from local clinic biopsy to endoscopic treatment did not differ between the malignancy group and the non-malignancy group. There was also no difference between cases (26 cases) with duration of no more than 14 d and cases (33 cases) of duration of more than 90 d. High grade dysplasia showed the highest odds ratio (19.5) with regard to risk factors for malignancy (Table 6).
Table 6.
Odds ratio of risk factors for malignancy as a final diagnosis
| Odds ratio | |
| High grade dysplasia | 19.5 |
| Biopsy number ≥ 4 | 5.1 |
| Size ≥ 2.0 cm | 2.4 |
| Depressed or Y-III, Y-IV | 7.3 |
| Reddish or undetermined | 11.1 |
| ESD | 11.7 |
| Repeat endoscopy | 2.7 |
| Pathologist 1 | 2.4 |
| Complications | 4.6 |
ESD: Endoscopic submucosal dissection; Y: Yamada type.
DISCUSSION
Histologically, gastric adenomas are composed of cells with hyperchromatic, elongated nuclei arranged in a picket-fence pattern with cystic glands and nuclear atypia being occasionally present[8,9]. The malignant potential of adenomas has been demonstrated in long term follow up studies, even in low grade dysplasia, therefore, resection is recommended[3,10]. Since the introduction of EMR in Japan, techniques for endoscopic resection have been continuously advancing; therefore, EMR and ESD are now approved for use in standard treatment of gastric adenoma[4,11,12].
Predictive factors for malignancy
In univariate analysis, risk factors for malignant transformation included location, histologic type (tubulovillous), redness, and high grade dysplasia in the study by Park et al[5], and depressed type, high grade dysplasia, redness, ulceration in the study by Jung et al[6] in the univariate analysis. In multivariate analysis, only high grade dysplasia had a significant relationship with malignant transformation in the two studies. In our study, predictive factors for malignancy as a final diagnosis included histologic grade, biopsy number, size, morphologic type and color. High grade dysplasia was the most important risk factor for malignancy, as in previous studies[5,6], with the highest odds ratio (Table 6).
Three out of ten cases (30%) of tubulovillous adenoma were malignancies, compared to only 69/475 cases (12.7%) of tubular adenoma, although this was not statistically significant. Cases with more than three biopsies were more often malignant than cases with fewer biopsies. This might be explained by the assumption that the endoscopist has taken more biopsies when he suspected a malignancy. ESD, re-endoscopy, and complicated groups had more many malignancies than EMR, direct procedure without re-endoscopy, and non-complicated groups, but those are not the cause of malignancy, but the result of strict treatment. Other possible factors affecting malignancy will be discussed below.
Possible causes affecting malignant discrepancy: (1) geographic variety of histology; forceps biopsy can be done only on the adenoma site, when cancer cells are mixed in the same lesion; (2) chronological difference between the time of forceps biopsy and the time of resection; adenoma can be transformed to malignancy; (3) different criteria of pathologist with regard to malignancy; and (4) different location between forceps biopsy and resection.
Geographic variety of histology
Because relatively small forceps biopsy foci of the polyp cannot represent the entire lesion, there can be a discrepancy between the forceps biopsy and resection specimen of the polyp[13]. Discrepancies before and after endoscopic resection in adenoma are mainly due to the geographic distribution of malignant cells within the adenoma[5,6,14], which means that the discrepancy depends on the location of the initial endoscopic forceps biopsy. It is noteworthy that all of the adenocarcinomas (18 cases) in the re-endoscopic forceps biopsy before the procedure were performed by endoscopist 1, although there was no difference in the discrepancy rate between primary care center and post-treatment according to the endoscopist (Table 4). This may be due to the experience of the endoscopist. An expert endoscopist who can reduce the rate of discrepancy has the ability to determine the location of the cancer cells grossly, approximately to the real histology. A similar two studies on malignant transformation of adenoma presented different discrepancy rates in spite of similar study designs[5,6]. The rate of malignant transformation of adenoma was 6.8% (8/118) in the study by Park et al[5] and 55.3% (63/114) in the study by Jung et al[6]. These large differences can be understood in the same context.
Chronological difference between the time of forceps biopsy and the time of resection
Gastric adenoma can progress to early gastric cancer, as shown in long term follow-up studies[3,10,15]; even low grade dysplasia has malignant potential. This change can occur over a long period of time. Yamada et al[3] reported on one case of 37 low grade dysplasia and one case of 10 high grade dysplasia that progressed to invasive carcinoma over a period of 212 mo and 55 mo, respectively. In our study, duration from the time of initial biopsy to the time of resection was not different between the malignant group and the nonmalignant group. Statistically, the rate of malignancy was also not different between fewer than two weeks (7.7%, 2 cases/33) and fewer than 90 d (12.1%, 4 cases/26) in duration. This means that a treatment delay of roughly three months is not a problem.
Different criteria of pathologist with regard to malignancy
Because criteria between Japanese and Western pathologists are different, international workshops have been steadily and persistently organized in an effort to establish a consensus[16-18]. In 1996, eight pathologists from Japan and Western countries met in Tokyo and individually reviewed a set of 35 gastric biopsy and resection specimens of lesions with potential early neoplasias[16]. There was agreement between Japanese and Western viewpoints in only 11 of the 35 specimens. A different diagnosis can be made for the same specimen, even by an intraobserver in the time interval of three years[19]. Table 5 shows that the malignant discrepancy rate of pathologist 1 is approximately three times greater than that of pathologist 2. The rate of adenocarcinoma diagnosis for re-endoscopic forceps biopsy is also higher for pathologist 1 than pathologist 2 (Table 4). Although the forceps biopsy specimen was not reviewed, it can be assumed that forceps biopsy by the primary care center can be underestimated by the pathologist. However, no difference in the malignant discrepancy rate was observed between primary care centers (Table 5). It is a limitation of our study that the same specimens were not reviewed by pathologists.
Different location between forceps biopsy and resection
Logically, it is possible that either the patient or the sample changed, or that a different mucosectomy site was selected from the diagnostic biopsy site; however, this was not included in the discussion.
COMMENTS
Background
Endoscopic examination is performed more commonly in the primary care center, and gastric adenoma is more frequently referred to tertiary care units.
Research frontiers
There have been so many embarrassing events when previous and post-procedure diagnoses have been different. This research is performed to predict the treatment result and to discover the reasons for these events.
Innovations and breakthroughs
There have been no reports about the discrepancy of referred gastric adenoma and diverse predictive factors, and possible causes are included in this study.
Applications
The results of this study will help endoscopists to predict the results of treatment and to decide the proper treatment option.
Peer review
The authors studied various predictive factors for discrepancy of gastric adenoma and deeply analyzed possible causes of discrepancy.
Footnotes
Supported by Chung-Nam National University Hospital Fund
Peer reviewer: Barbara Braden, Professor, Department of Gastroenterology, John Radcliffe Hospital, Headley Way, OX3 9DU Oxford, United Kingdom
S- Editor Sun H L- Editor Rutherford A E- Editor Li JY
References
- 1.Kamiya T, Morishita T, Asakura H, Miura S, Munakata Y, Tsuchiya M. Long-term follow-up study on gastric adenoma and its relation to gastric protruded carcinoma. Cancer. 1982;50:2496–2503. doi: 10.1002/1097-0142(19821201)50:11<2496::aid-cncr2820501140>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Farinati F, Rugge M, Di Mario F, Valiante F, Baffa R. Early and advanced gastric cancer in the follow-up of moderate and severe gastric dysplasia patients. A prospective study. I.G.G.E.D.--Interdisciplinary group on gastric epithelial dysplasia. Endoscopy. 1993;25:261–264. doi: 10.1055/s-2007-1010310. [DOI] [PubMed] [Google Scholar]
- 3.Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390–396. doi: 10.1055/s-2004-814330. [DOI] [PubMed] [Google Scholar]
- 4.Lambert R. Treatment of esophagogastric tumors. Endoscopy. 2003;35:118–126. doi: 10.1055/s-2003-37016. [DOI] [PubMed] [Google Scholar]
- 5.Park DI, Rhee PL, Kim JE, Hyun JG, Kim YH, Son HJ, Kim JJ, Paik SW, Rhee JC, Choi KW, et al. Risk factors suggesting malignant transformation of gastric adenoma: univariate and multivariate analysis. Endoscopy. 2001;33:501–506. doi: 10.1055/s-2001-15089. [DOI] [PubMed] [Google Scholar]
- 6.Jung MK, Jeon SW, Park SY, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Bae HI. Endoscopic characteristics of gastric adenomas suggesting carcinomatous transformation. Surg Endosc. 2008;22:2705–2711. doi: 10.1007/s00464-008-9875-2. [DOI] [PubMed] [Google Scholar]
- 7.Muehldorfer SM, Stolte M, Martus P, Hahn EG, Ell C. Diagnostic accuracy of forceps biopsy versus polypectomy for gastric polyps: a prospective multicentre study. Gut. 2002;50:465–470. doi: 10.1136/gut.50.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ming SC, Goldman H. Gastric polyps; a histogenetic classification and its relation to carcinoma. Cancer. 1965;18:721–726. doi: 10.1002/1097-0142(196506)18:6<721::aid-cncr2820180609>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Tomasulo J. Gastric polyps. Histologic types and their relationship to gastric carcinoma. Cancer. 1971;27:1346–1355. doi: 10.1002/1097-0142(197106)27:6<1346::aid-cncr2820270612>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Fertitta AM, Comin U, Terruzzi V, Minoli G, Zambelli A, Cannatelli G, Bodini P, Bertoli G, Negri R, Brunati S. Clinical significance of gastric dysplasia: a multicenter follow-up study. Gastrointestinal endoscopic pathology study group. Endoscopy. 1993;25:265–268. doi: 10.1055/s-2007-1010311. [DOI] [PubMed] [Google Scholar]
- 11.Tada M, Murakami M, Karita H, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Enoscopy. 1993;25:445–450. doi: 10.1055/s-2007-1010365. [DOI] [PubMed] [Google Scholar]
- 12.Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58–62. doi: 10.1016/s0016-5107(93)70012-7. [DOI] [PubMed] [Google Scholar]
- 13.Yoon WJ, Lee DH, Jung YJ, Jeong JB, Kim JW, Kim BG, Lee KL, Lee KH, Park YS, Hwang JH, et al. Histologic characteristics of gastric polyps in Korea: emphasis on discrepancy between endoscopic forceps biopsy and endoscopic mucosal resection specimen. World J Gastroenterol. 2006;12:4029–4032. doi: 10.3748/wjg.v12.i25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park EH, Kang KT, Kim BH, Kim KT, Lee SW, Lee JH, Roh MH, Han SY, Choi SR, Jeong JS, et al. The histologic discrepancy before and after endoscopic submucosal dissection of gastric adenoma and early gastric cancer. Korean J Gastrointest Endoc. 2007;34:125–131. [Google Scholar]
- 15.Coma del Corral MJ, Pardo-Mindan FJ, Razquin S, Ojeda C. Risk of cancer in patients with gastric dysplasia. Follow-up study of 67 patients. Cancer. 1990;65:2078–2085. doi: 10.1002/1097-0142(19900501)65:9<2078::aid-cncr2820650932>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Schlemper RJ, Itabashi M, Kato Y, Lewin KJ, Riddell RH, Shimoda T, Sipponen P, Stolte M, Watanabe H, Takahashi H, et al. Differences in diagnostic criteria for gastric carcinoma between Japanese and Western pathologists. Lancet. 1997;349:1725–1729. doi: 10.1016/S0140-6736(96)12249-2. [DOI] [PubMed] [Google Scholar]
- 17.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlemper RJ, Kato Y, Stolte M. Review of histological classifications of gastrointestinal epithelial neoplasia: differences in diagnosis of early carcinomas between Japanese and Western pathologists. J Gastroenterol. 2001;36:445–456. doi: 10.1007/s005350170067. [DOI] [PubMed] [Google Scholar]
- 19.Palli D, Bianchi S, Cipriani F, Duca P, Amorosi A, Avellini C, Russo A, Saragoni A, Todde P, Valdes E. Reproducibility of histologic classification of gastric cancer. Br J Cancer. 1991;63:765–768. doi: 10.1038/bjc.1991.171. [DOI] [PMC free article] [PubMed] [Google Scholar]


