Abstract
AIM: To determine whether adding vitamin D, a potent immunomodulator, improves the hepatitis C virus (HCV) response to antiviral therapy.
METHODS: Seventy-two consecutive patients with chronic HCV genotype 1 were randomized into two groups: the treatment group (n = 36, 50% male, mean age 47 ± 11 years) received Peg-α-2b interferon (1.5 μg/kg per week) plus ribavirin (1000-1200 mg/d) together with vitamin D3 (2000 IU/d, target serum level > 32 ng/mL), and the control group (n = 36, 60% male, mean age 49 ± 7 years) received identical therapy without vitamin D. HCV-RNA was assessed by real-time polymerase chain reaction (sensitivity, 10 IU/mL). The sustained virologic response (SVR) was defined as undetectable HCV-RNA at 24 wk post-treatment.
RESULTS: Clinical characteristics were similar in both groups. The treatment group had a higher mean body mass index (27 ± 4 kg/m2 vs 24 ± 3 kg/m2; P < 0.01), viral load (50% vs 42%, P < 0.01), and fibrosis score (> F2: 42% vs 19%, P < 0.001) than the controls. At week 4, 16 (44%) treated patients and 6 (17%) controls were HCV-RNA negative (P < 0.001). At week 12, 34 (94%) treated patients and 17 (48%) controls were HCV-RNA negative (P < 0.001). At 24 wk post-treatment (SVR), 31 (86%) treated patients and 15 (42%) controls were HCV-RNA negative (P < 0.001). Viral load, advanced fibrosis and vitamin D supplementation were strongly and independently associated with SVR (multivariate analysis). Adverse events were mild and typical of Peg-α-2b/ribavirin.
CONCLUSION: Adding vitamin D to conventional Peg-α-2b/ribavirin therapy for treatment-naïve patients with chronic HCV genotype 1 infection significantly improves the viral response.
Keywords: Hepatitis C, Vitamin D, Sustained viral response, Genotype 1, Fibrosis
INTRODUCTION
The current treatment for hepatitis C virus (HCV) infection is pegylated interferon α combined with ribavirin (Peg/RBV) administered for 24 wk for HCV genotypes 2 or 3, or 48 wk for HCV genotype 1, the most prevalent genotype in Israel, Europe, and North America[1]. The aim of HCV therapy is a sustained virologic response (SVR), defined as an undetectable serum HCV-RNA level at 24 wk after the cessation of therapy. For patients with HCV genotype 1, the rate of SVR ranges between 38% and 46%[2,3]. In subgroups of this population (e.g., Hispanics and African-Americans), the rate of SVR is even lower, reaching only 19%[4]. These differences are not explained by baseline viral load or compliance to treatment. Recent efforts to improve patient outcomes have focused on adding new antiviral therapies specifically targeted to HCV, including inhibitors of either HCV polymerase or protease[5]. However, few studies have addressed the issue of improving the host factors.
Vitamin D is a potent immunomodulator[6,7]. Increased production of 1, 25-dihydroxy vitamin D3 results in the synthesis of cathelicidin, a peptide capable of destroying many viral infectious agents as well as M. tuberculosis. Low serum levels of 25-hydroxyvitamin D (< 20 ng/mL) prevent macrophages from initiating this innate immune response, which may explain why African-Americans, who are often vitamin D deficient, are more prone to contracting tuberculosis and viral infections than Caucasians[8]. Moreover, vitamin D improves insulin sensitivity[9], su-ppresses proinflammatory cytokines, increases anti-inflammatory cytokines, and improves CD4 T cell hyper-responsiveness[10]. Vitamin D deficiency is very common (92%) among patients with chronic liver disease, and at least one-third suffer from severe vitamin D deficiency (< 12 ng/mL)[11]. Israeli subjects from various ethnic backgrounds are at higher risk of vitamin D deficiency[12]. Pettas and co-workers recently showed a low serum vitamin D level to be related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C (CHC)[13]. Its role and relationship to SVR and therapy in CHC are unknown. We reasoned that adding vitamin D to conventional therapy could improve treatment efficacy at weeks 4 [rapid viral response (RVR)] and 12 [early viral response (EVR)] during therapy, and 24 wk after cessation of therapy (SVR).
MATERIALS AND METHODS
Subjects
Study inclusion criteria were age 18-65 years, a chronic HCV genotype 1 infection, no previous treatment for hepatitis C, seronegative for HBV, HDV, and human immunodeficiency virus infections, an absolute neutrophil count of > 1500 per mm3, a platelet count of > 90 000 per mm3, and a normal hemoglobin level. Liver biopsies were required within 2 years prior to study entry, and the samples were examined by two pathologists who were unaware of patient identity and treatment regimen. The severity of hepatic inflammation and fibrosis was evaluated by the Ishak score in separate reports for grading and staging[14]. Exclusion criteria were decompensated liver disease (cirrhosis with a Child-Pugh score > 9), another cause of clinically significant liver disease, or the presence of hepatocellular carcinoma.
Study design
This was an intention-to-treat prospective randomized study. The experimental procedures were approved by the institutional review boards of the two participating medical centers. Informed consent was obtained from all participants (Clinical Trial Gov: NCT00804752)
The study included 72 consecutive CHC genotype 1 treatment-naïve patients who were stratified according to ethnic group (i.e., Russian/Jewish/Arab) due to possible differences in vitamin D levels. They were randomly assigned to one of two study groups. The treatment group comprising 36 patients (mean age 47 ± 11 years, 50% male) who received pegylated (peg)-interferon-α-2b (1.5 μg per kg body weight) plus oral ribavirin 1000 mg/d (for body weight < 75 kg) or 1200 mg/d (for body weight > 75 kg) and vitamin D3 (Vitamidyne D, Fischer Pharmaceuticals, Israel) 2000 IU/d, target serum level > 32 ng/mL) for 48 wk. Vitamin D3 was given by oral drops for 4 wk before the initiation of antiviral treatment and after serum levels had reached > 32 ng/mL in all patients in the treatment group. The supplemented vitamin D levels were maintained during the course of therapy with the same dosage as in the lead-in phase. The control group of 36 patients (mean age 49 ± 7 years, 60% male) received peg-interferon-α-2b (1.5 μg/kg body weight) plus ribavirin (1000-1200 mg/d) without vitamin D3 for 48 wk.
Efficacy assessments
Plasma HCV-RNA levels were measured using the COBAS Taq Man HCV assay, version 1.0 (Roche Molecular Systems), with a lower limit of quantification of 35-45 IU/mL and a lower limit of detection of 10 IU/mL. HCV-RNA levels were measured at the time of screening and during the treatment period at weeks 4, 12 and 48. All subjects had at least one follow-up visit at 24 wk after the completion of treatment. Those who had undetectable HCV-RNA levels had another follow-up visit 24 wk later, at which time HCV-RNA levels were measured again. Treatment efficacy was defined as SVR, i.e., undetectable HCV-RNA at 24 wk post-treatment. Clearance of HCV-RNA by real-time polymerase chain reaction (RT-PCR) was assessed at week 4 (RVR), week 12 (complete EVR), and at week 48 of treatment response (early treatment response, ETR). Patients with ETR who tested HCV-RNA positive during follow-up were classified as relapsers. Breakthrough was defined as an increase in the HCV-RNA level of one log10 unit compared with the lowest value. Therapy was discontinued if quantitative HCV-RNA levels at week 12 dropped by < 2 log compared with baseline values (non-responders), and at week 24 if HCV-RNA was still detectable in those patients in whom HCV-RNA dropped > 2 log at week 12[3,15].
Safety assessments
Biochemical assessments were performed at each visit during the treatment period and at the post-treatment follow-up visit. Data on adverse events were collected and physical examinations were also performed each time. The safety assessment included complete blood count, antinuclear antibody, and thyroid-stimulating hormone levels. Peg-interferon α 2b was reduced to 1.0 μg/kg body weight in patients with a < 750 neutrophil count and withdrawn temporarily in patients with a < 500 neutrophil count. The same dose reduction was applied if platelet levels fell under 50 000 cells/mm3, with peg-interferon being discontinued when the 25 000 cell/mm3 threshold was reached. In both treatment arms, the ribavirin dose was tapered by 200 mg/d in patients with a hemoglobin level < 10 g/dL, and discontinued altogether in patients with a level < 8.0 g/dL.
Clinical and laboratory measurements
Vitamin D levels: 25 (OH)-vitamin D3 levels were determined by 125 I-radioimmunoassay (Dia-Sorin, Stillwater, MN, United States)[16]. 25-OH vitamin D is the major circulating form of vitamin D and is used as an indicator of vitamin D status. Vitamin D deficiency was defined as a 25 (OH)-vitamin D serum level < 12 ng/mL, vitamin D insufficiency as 25 (OH)-vitamin D levels of 12-32 ng/mL, and vitamin D sufficiency as levels > 32 ng/mL[12]. Insulin resistance was estimated using the homeostasis model assessment (HOMA-IR)[17]. HOMA-IR was measured at baseline and at 4 wk in both study groups. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Obesity was defined as a BMI exceeding 28 kg/m². C-reactive protein was determined by the nephelometric method[18]. Paraoxonase activity was measured according to a method using phenylacetate as a substrate[19]. α tocopherol (vitamin E) was estimated spectrophotometrically[20]. Malondialdehyde concentration was estimated spectrophotometrically using the thiobarbituric acid assay[21]. Calcium, phosphor, vitamin B12, thyroid-stimulating hormone, glucose, insulin, liver enzymes, albumin, bilirubin, prothrombin time, and creatinine were measured by standard biochemical tests.
Statistical analysis
Results were expressed as mean ± SD. The difference between the two groups was assessed by the chi-squared test for categorical variables and by the Mann-Whitney rank test for continuous variables. The Spearman correlation was used to express correlations between variables. The primary study endpoint was evidence of the influence of vitamin D on the viral response at weeks 4 and 12 during therapy and at week 24 post-treatment. Logistic regression analysis was performed to detect independent predictors for SVR. The significance level was set at P < 0.05. The statistical analyses were carried out with the WINSTAT (Kalmia, CA, United States) software program.
RESULTS
At baseline, 21% of the patients in the treatment group had severe vitamin D deficiency (< 12 ng/mL), 59% had insufficiency, and 20% had sufficient vitamin D levels. The control group baseline tests showed that 27% had vitamin D deficiency, 60% had insufficiency, and 13% had sufficient vitamin D levels. Table 1 shows the clinical and biochemical parameters of the patient populations.
Table 1.
Baseline demographic, clinical and virologic characteristics of all patients
| Baseline demographics | Peg/RBV (n = 36) | Peg/RBV + Vit D (n = 36) | P value |
| Age (yr) | 49 ± 7 | 47 ± 11 | 0.123 |
| Males (%) | 60 | 50 | 0.015 |
| Body mass index (kg/m2) | 24 ± 3 | 27 ± 4 | 0.014 |
| HCV genotype: 1a/1b (n) | 3/33 | 3/33 | 0.138 |
| Baseline HCV-RNA (log IU/mL) | 6.2 ± 0.8 | 6.1 ± 0.7 | 0.126 |
| High viral load | 15 (42%) | 18 (50%) | 0.033 |
| HCV-RNA > 800 000 IU/mL | |||
| Baseline ALT (U/L) | 56 ± 31 | 55 ± 28 | 0.587 |
| Advanced fibrosis (> F2) | 7 (19%) | 15 (42%) | 0.001 |
| Ethnicity (Russian /Jewish/Arab) | 28/6/2 | 29/3/4 | 0.194 |
Peg/RBV: Pegylated interferon α and ribavirin; Vit D: Vitamin D; ALT: Alanine aminotransferase; HCV: Hepatitis C virus.
The treatment group had higher BMI levels, viral loads, and fibrosis scores > F2 than the controls (27 ± 4 kg/m2vs 24 ± 3 kg/m2, P = 0.014; > 800 000 IU/mL, 50% vs 42%, P = 0.033; 42% vs 19%, P = 0.001, respectively). There were no differences between the two groups in terms of age, HCV genotype, baseline HCV-RNA, ethnic background, or aminotransferases levels.
Figure 1 depicts the baseline and week 4 vitamin D levels at the beginning of antiviral therapy. Serum vitamin D levels were significantly lower at baseline (20.5 ± 9.0 ng/mL) and increased after 4 wk of vitamin D treatment to a mean level of 37 ± 10 ng/mL. Baseline vitamin D levels were also lower in the control group (19 ± 6 ng/mL, Table 2).
Figure 1.
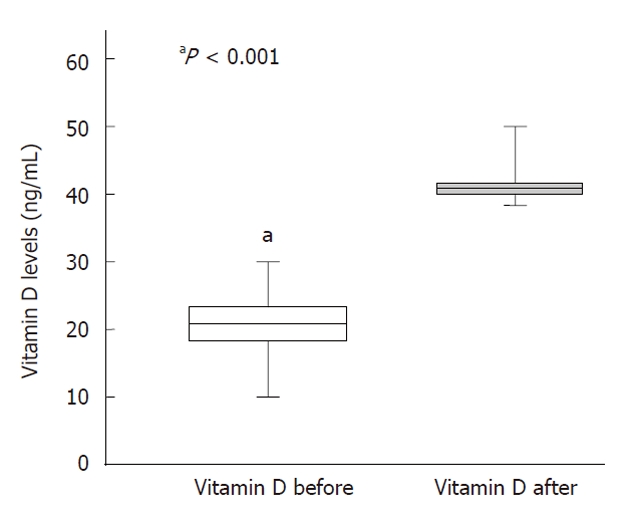
Vitamin D serum levels before and at 4 wk after the beginning of antiviral treatment + vitamin D supplementation (n = 36). Bars represent standard error.
Table 2.
Viral response, vitamin D levels, biomarkers of inflammation, insulin resistance, and oxidative stress in all patients
| Parameter | Peg/RBV (n = 36) | Peg/RBV + Vit D (n = 36) | P value |
| Viral response | |||
| Relapser | 13 (36%) | 3 (8%) | 0.001 |
| Non-responder | 8 (22%) | 2 (6%) | 0.010 |
| HOMA-IR | |||
| Baseline | 4.6 ± 5.7 | 4.5 ± 1.4 | 0.123 |
| After 4 wk | 5.0 ± 4.0 | 2.3 ± 1.0a | 0.001 |
| Basal vitamin D-25-OH levels (ng/mL) | 19 ± 6 | 20.5 ± 9.0 | 0.177 |
| Malondialdehyde (mmol/L) | 0.11 ± 0.05 | 0.13 ± 0.04 | 0.810 |
| Paraoxonase (mmol/L/min) | 0.57 ± 0.1 | 0.64 ± 0.1 | 0.120 |
| Vitamin E (μg/mL) | 19.7 ± 8.8 | 21 ± 8.0 | 0.510 |
| Vitamin B12 pmol/L | 316 ± 190 | 331 ± 170 | 0.103 |
| CRP mg/dL | 0.39 ± 0.3 | 0.45 ± 0.4 | 0.100 |
| Triglycerides (mg/dL) | 200 ± 80 | 220 ± 60 | 0.110 |
P < 0.001 between HOMA-IR at baseline and HOMA-IR after 4 wk of treatment with vitamin D. HOMA-IR: Homeostasis model assessment of insulin resistance; Peg/RBV: Pegylated interferon α and ribavirin; Vit D: Vitamin D; CRP: C-reactive protein.
Figures 2 and 3 show the rates of RVR, EVR, and SVR in the treatment and control groups. At week 4, 16 (44%) patients in the treatment group and 6 (17%) controls were HCV-RNA negative, and at week 12, 34 (94%) and 17 (48%), respectively, were HCV-RNA negative (P < 0.001 for each week). Twenty-four weeks after the cessation of therapy (SVR), 31 (86%) patients in the treatment group and 15 (42%) controls were HCV-RNA negative (P < 0.001). The percentage of relapses and non-responders and the biomarkers of insulin resistance, inflammation, pro-oxidant levels, antioxidant levels, and baseline vitamin D, vitamin E, and vitamin B12 serum levels are shown in Table 2 for both groups.
Figure 2.
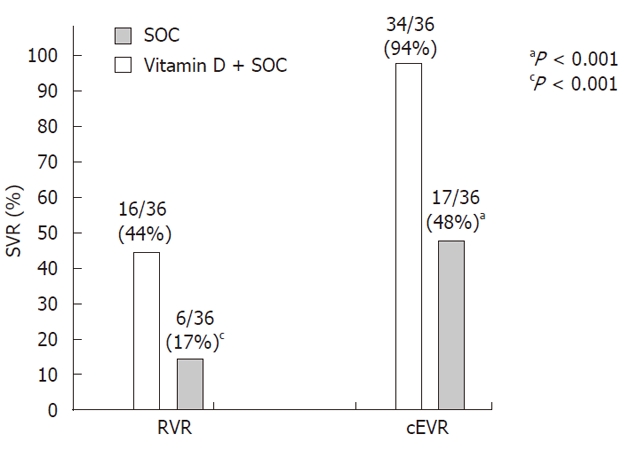
Rate (%) of the rapid viral response and rate of early viral response in the treatment (n = 36) and control (n = 36) groups. RVR was defined as undetectable HCV RNA at 4 wk during treatment. Complete EVR (cEVR) was defined as undetectable HCV RNA at 12 wk during treatment. SOC: Standard of care; RVR: Rapid viral response; EVR: Early viral response; HCV: Hepatitis C virus; SVR: Sustained viral response.
Figure 3.
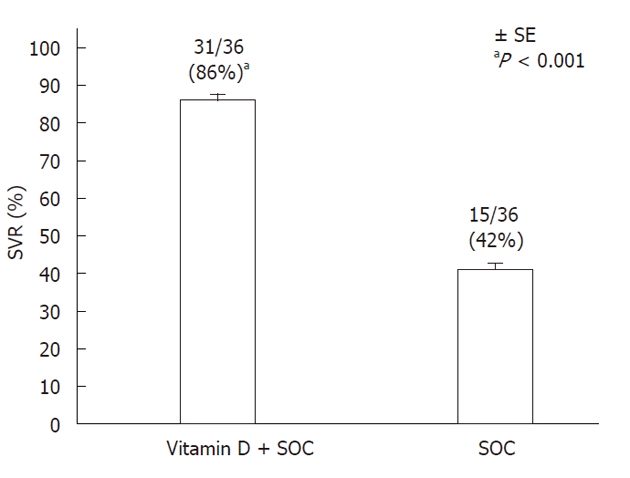
Rate of sustained viral response in the treatment group (Vitamin D + SOC, n = 31/36) and the control group (n = 15/36, SOC) 6 mo after cessation of treatment. SVR was defined as undetectable HCV-RNA at 24 wk post-treatment. Bars represent standard error. SOC: Standard of care; SVR: Sustained viral response; HCV: Hepatitis C virus.
The rate of viral breakthrough was null. The rates of relapse and non-response were significantly lower in the treatment group compared with the control group [n = 3 (8%) vs n = 13 (36%), P < 0.001, and 2 (6%) vs 8 (22%), P < 0.01, respectively]. The HOMA-IR index decreased significantly after 4 wk of treatment with vitamin D compared with the control group (from 4.5 ± 1.4 to 2.3 ± 1.0, P < 0.01 vs 4.6 ± 5.7 to 5.0 ± 4.0, respectively, P < 0.1). There was no difference between groups for malondialdehyde, paraoxonase, vitamin E, vitamin B12, C-reactive protein, and triglyceride levels.
The adherence to vitamin D treatment was excellent during the entire course, and all patients in the treatment group achieved the target level. Vitamin D levels were maintained during the course of therapy with the same dosage (2000 IU/d) as in the lead-in phase. Predictive factors for SVR in patients treated with Peg/RBV combination therapy are shown in Table 3.
Table 3.
Predictors for sustained virologic response in treatment-naïve hepatitis C virus genotype 1 patients with pegylated interferon α and ribavirin combination therapy
| Odds ratio | 95% CI | P value | |
| Vitamin D treatment (Yes vs No) | 2.5 | 2.0-4.9 | < 0.001 |
| Baseline vitamin D (< 20 or > 20 IU/mL) | 1.5 | 1.2-3.8 | 0.080 |
| Advanced fibrosis (< F2 or > F2) | 2.0 | 1.0-3.6 | 0.001 |
| High viral load (< 800 000 or > 800 000 IU/mL) | 2.8 | 1.2-4.0 | 0.001 |
| Baseline CRP (< 0.05 or > 0.5 mg/dL) | 1.0 | 0.5-1.9 | 0.510 |
| Changes in homeostasis model assessment (%) | 1.8 | 0.5-3.0 | 0.030 |
CRP: C-reactive protein; CI: Confidence interval.
Viral load, advanced fibrosis, baseline vitamin D levels, changes in HOMA-IR, and vitamin D supplementation were significant univariate predictors of SVR. Viral load, vitamin D supplementation, advanced fibrosis and changes in HOMA-IR remained as independent predictors in the multivariate analysis. Thus, vitamin D supplementation emerged as being more responsible for higher SVR than the baseline vitamin D level.
The most common adverse events were mild in nature, similar in both groups, and consistent with typical Peg/RBV-induced systemic symptoms. They included nausea (n = 4), headache (n = 4), insomnia (n = 5), chills (n = 4), myalgia (n = 3), pyrexia (n = 3), pruritus (n = 2), mild neutropenia (n = 3), mild thrombocytopenia (n = 5), and mild anemia (n = 3). There were no serious adverse events. Adherence to Peg/RBV combination therapy was excellent, and there was no difference in dose reduction Peg/RBV combination therapy due to adverse events in either group. No patient discontinued treatment. Changes in laboratory values during the study were consistent with those reported in association with the combined use of Peg/RBV[3].
DISCUSSION
The results of this study suggest that the addition of a vitamin D supplement to current standard therapy can significantly improve the rate of RVR, EVR and SVR in treatment-naïve patients with HCV genotype 1 compared the rates with standard therapy alone. The observed SVR in the control group (42%) was consistent with previous reports[2,3]. Overall there was a marked increase in the virologic response at week 4 (44% vs 17%), week 12 (94% vs 48%), and week 24 after the cessation of therapy (86% vs 42%), and a low rate of relapse (8% vs 36%) with vitamin D supplementation compared with no supplementation. The rate of relapse in the control group was within the reported 18%-40% range for current standard HCV antiviral therapy[2,22].
There are only two reports examining the association between vitamin D status and outcome of antiviral therapy in patients with chronic HCV viral infection. Petta and co-workers retrospectively analyzed a cohort of 167 patients treated with Peg/RBV for hepatitis C, and detected an association between lower vitamin D serum levels and failure to achieve SVR[23]. Our results provide further support for that data. The second study by Bitetto and co-workers showed that vitamin D supplementation improved the response to antiviral treatment for recurrent HCV in liver transplant recipients[24]. Several differences between those two studies should be noted. Bitetto and co-workers’ HCV patients were immunocompromised, and they were supplemented with low-dose vitamin D (800 IU/d) after liver transplantation. In addition, most of their HCV patients (75%) had low vitamin D levels despite treatment. Finally, that study was retrospective and focused on the prevention of osteoporosis, not on the treatment of hepatitis C.
The exact mechanism of action leading to improved RVR, EVR, and SVR in patients receiving vitamin D is unknown. Vitamin D is metabolized by the liver and converted to 1,25-dihydroxy-vitamin D3, which is the active form of the vitamin[6,7]. Individuals with chronic liver disease may have poor conversion from vitamin D3 or any of its other biologically active metabolites[11]. 1,25 vitamin D3 appears to modulate immunity principally via regulation of T-cell function[25]. The vitamin D receptor (VDR) is expressed on virtually every type of cell involved in immunity[26]. The immunomodulatory actions of vitamin D are elicited through its direct action on T-cell antigen-presenting cell function[27]. T helper cell type 1 (TH1) actions are intensified when vitamin D is insufficient, as in the majority of our patient population, or when signals through VDR are weak. Regulatory T cell and TH2 cells are diminished, thus favoring an autoimmune TH1 response[28]. This is a pro-inflammatory response which may impair IFN and insulin signaling, thus decreasing the viral response[29,30]. A recent study on 120 patients with chronic HCV genotype 1 infections reported that a TH1 to TH2 ratio of < 15.5 was significantly associated with SVR (odds ratio 9.6)[31]. TH1 and TH2 measurements were not performed in the present study. Persistent HCV infection modulates the balance between immune stimulatory and inhibitory cytokines which can prolong inflammation and lead to fibrosis and chronic liver diseases[32]. More recently, Gutierrez and co-workers showed that vitamin D3 increased VDR protein expression and inhibited viral replication in cell culture[33].
It is well known that people of African and Hispanic descent are less likely to respond to standard therapy[34]. This may be due to a polymorphism of the interleukin (IL)-28B gene, polymorphism of VDR or vitamin D deficiency[13,35]. The vast majority of the Russian/Jewish/Arab patients in the present study had vitamin D insufficiency, possibly related to paradoxically low exposure to the sun in this predominantly sunny country and/or to a low supply of vitamin D from their diet.
The impact of diet on liver fibrosis and on response to IFN therapy in patients with HCV chronic hepatitis has been reported before[36]. HCV patients also lack vitamins E and B12[37,38]. A recent study showed that higher levels of vitamin B12 were associated with SVR, but there was no difference in serum levels of those vitamins between the group treated with vitamin D and the controls[39].
Insulin resistance emerged as one of the most important host factors in the prediction of the response in non-diabetic HCV patients treated with Peg/RBV, and is a common factor in the features associated with difficult-to-treat patients[40]. Vitamin D is also known to help prevent type 2 diabetes, and it is possible that low levels of vitamin D lead to insulin resistance[9]. The direct effect of vitamin D may be mediated by binding of its circulating active form to the pancreatic B cell vitamin D receptor[41]. Vitamin D deficiency or insufficiency may alter the balance between the extracellular and intracellular cell calcium pools, which may interfere with normal insulin release[42]. Thus, a lack of either calcium or vitamin D can result in peripheral insulin resistance[41]. Moreover, oxidative stress leeches calcium, and vitamin D helps absorb calcium[43]. Our current results confirm these findings: the HOMA-IR was higher at baseline in the vitamin D treatment group and improved after 4 wk of therapy compared to the control group. Moreover, the changes in HOMA-IR were strongly associated with SVR (multivariate analysis).
The definition of normal vitamin D serum levels is a subject of debate. In the current study, increasing the vitamin level D to > 32 ng/mL increased the response to antiviral therapy to the same extent in patients with vitamin D deficiency as well as those with vitamin D insufficiency. Multivariate analysis revealed that viral load, advanced fibrosis and vitamin D supplementation remained as independent predictors. Thus, it can be concluded that vitamin D supplementation is responsible for a higher SVR, rather than the baseline vitamin D level. It remains to be determined whether the addition of vitamin D acts by a mechanism other than improvement of insulin resistance or immune function such as the upregulation of toll-like receptors involved in the immune response in HCV-infected patients
Limitations of the present study include the small number of patients, lack of vitamin D level assessment during therapy for the treatment and control groups, and that this prospective and randomized study was not placebo-controlled, thus the patients knew whether or not they received a vitamin D supplement. Another limitation is the lack of data on the TH1 and TH2 immune response. The identification of determinants of the response, such as polymorphisms of the IL28B gene, polymorphism of the VDR and immune function[13,35], may help explain the difference in response rates between patients with different ethnic backgrounds. This was not done in our study since data on IL-28B and on VDR polymorphism were not available at the time the study was designed.
In conclusion, the addition of vitamin D to Peg/RBV combination therapy in treatment-naïve patients who were infected with HCV genotype 1 significantly increased the rates of rapid, early, and sustained viral responses.
COMMENTS
Background
Treating chronic hepatitis C virus (HCV) (genotype1) patients with pegylated interferon and ribavirin, which is considered to be the standard of care, has achieved viral clearance in less than 50% of the patients. Vitamin D is a potent immunomodulator with a beneficial effect against viral and bacterial infections. The vast majority of patients with chronic hepatitis C have low levels of vitamin D. Different new drugs such as protease or polymerase inhibitors are still under investigation and are expensive and have many side effects like rash.
Research frontiers
Vitamin D deficiency is well documented in patients with chronic liver disease. However, treating patients with chronic HCV infection by adding a vitamin D supplement to the standard of care has not been addressed. There are only two reports dealing with the association between vitamin D status and outcome of antiviral therapy for chronic HCV infection.
Innovations and breakthroughs
The current study shows that adding a vitamin D supplement to pegylated interferon and ribavirin significantly increases the rapid, early and late clearance of the virus, in chronic hepatitis C genotype 1 treatment-naïve patients.
Applications
This study emphasizes the importance of vitamin D supplementation when added to standard treatment in all patients with chronic hepatitis C. Further studies are needed to explain the mechanism of vitamin D supplementation for these patients.
Terminology
Hepatitis C is a chronic liver infection that can be complicated by liver failure and liver cancer. Clearance of the virus from the blood is achievable by a combination of pegylated interferon and ribavirin in less than 50% of the patients. Vitamin D has an important role in the treatment of different bacterial and viral infections; this vitamin is synthesized in the skin by absorption of ultraviolet from the sun light. The mechanism of action of this vitamin is unknown, but it may improve the activities of immune cells that are important in the eradication of HCV.
Peer review
This is a well conducted study with a relevant finding, and it is well written.
Footnotes
Peer reviewer: Sabine Mihm, Professor, Department of Gastroenterology, Georg-August-University, Robert-Koch-Str 40, Göttingen D-37099, Germany
S- Editor Tian L L- Editor Cant MR E- Editor Zhang DN
References
- 1.Global surveillance and control of hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat. 1999;6:35–47. [PubMed] [Google Scholar]
- 2.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 3.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 4.Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;350:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- 5.Pawlotsky JM, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132:1979–1998. doi: 10.1053/j.gastro.2007.03.116. [DOI] [PubMed] [Google Scholar]
- 6.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 7.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 8.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez JA, Ashraf A. Role of vitamin d in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol. 2010;2010:351385. doi: 10.1155/2010/351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 11.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–2628. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 12.Hochwald O, Harman-Boehm I, Castel H. Hypovitaminosis D among inpatients in a sunny country. Isr Med Assoc J. 2004;6:82–87. [PubMed] [Google Scholar]
- 13.Petta S, Cammà C, Scazzone C, Tripodo C, Di Marco V, Bono A, Cabibi D, Licata G, Porcasi R, Marchesini G, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158–1167. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- 14.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 15.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–2451. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- 16.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 17.Haffner SM, Kennedy E, Gonzalez C, Stern MP, Miettinen H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care. 1996;19:1138–1141. doi: 10.2337/diacare.19.10.1138. [DOI] [PubMed] [Google Scholar]
- 18.Montagne P, Laroche P, Cuillière ML, Varcin P, Pau B, Duheille J. Microparticle-enhanced nephelometric immunoassay for human C-reactive protein. J Clin Lab Anal. 1992;6:24–29. doi: 10.1002/jcla.1860060106. [DOI] [PubMed] [Google Scholar]
- 19.Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991;19:100–106. [PubMed] [Google Scholar]
- 20.Baker H, Handelman GJ, Short S, Machlin LJ, Bhagavan HN, Dratz EA, Frank O. Comparison of plasma alpha and gamma tocopherol levels following chronic oral administration of either all-rac-alpha-tocopheryl acetate or RRR-alpha-tocopheryl acetate in normal adult male subjects. Am J Clin Nutr. 1986;43:382–387. doi: 10.1093/ajcn/43.3.382. [DOI] [PubMed] [Google Scholar]
- 21.Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987;45:337–351. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson IM, Brown RS, Freilich B, Afdhal N, Kwo PY, Santoro J, Becker S, Wakil AE, Pound D, Godofsky E, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46:971–981. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 23.Petta S, Cammà C, Scazzone C, Tripodo C, Di Marco V, Bono A, Cabibi D, Licata G, Porcasi R, Marchesini G, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158–1167. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- 24.Bitetto D, Fabris C, Fornasiere E, Pipan C, Fumolo E, Cussigh A, Bignulin S, Cmet S, Fontanini E, Falleti E, et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl Int. 2011;24:43–50. doi: 10.1111/j.1432-2277.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 25.Müller K, Bendtzen K. 1,25-Dihydroxyvitamin D3 as a natural regulator of human immune functions. J Investig Dermatol Symp Proc. 1996;1:68–71. [PubMed] [Google Scholar]
- 26.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sochorová K, Budinský V, Rozková D, Tobiasová Z, Dusilová-Sulková S, Spísek R, Bartůnková J. Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) and calcitriol (1,25-dihydroxyvitamin D3) exert potent immunomodulatory effects on dendritic cells and inhibit induction of antigen-specific T cells. Clin Immunol. 2009;133:69–77. doi: 10.1016/j.clim.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Jirapongsananuruk O, Melamed I, Leung DY. Additive immunosuppressive effects of 1,25-dihydroxyvitamin D3 and corticosteroids on TH1, but not TH2, responses. J Allergy Clin Immunol. 2000;106:981–985. doi: 10.1067/mai.2000.110101. [DOI] [PubMed] [Google Scholar]
- 29.Lecube A, Hernández C, Genescà J, Simó R. Proinflammatory cytokines, insulin resistance, and insulin secretion in chronic hepatitis C patients: A case-control study. Diabetes Care. 2006;29:1096–1101. doi: 10.2337/diacare.2951096. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Feld JJ, Sapp RK, Nanda S, Lin JH, Blatt LM, Fried MW, Murthy K, Liang TJ. Defective hepatic response to interferon and activation of suppressor of cytokine signaling 3 in chronic hepatitis C. Gastroenterology. 2007;132:733–744. doi: 10.1053/j.gastro.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirakawa H, Matsumoto A, Joshita S, Komatsu M, Tanaka N, Umemura T, Ichijo T, Yoshizawa K, Kiyosawa K, Tanaka E. Pretreatment prediction of virological response to peginterferon plus ribavirin therapy in chronic hepatitis C patients using viral and host factors. Hepatology. 2008;48:1753–1760. doi: 10.1002/hep.22543. [DOI] [PubMed] [Google Scholar]
- 32.Larrubia JR, Benito-Martínez S, Calvino M, Sanz-de-Villalobos E, Parra-Cid T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J Gastroenterol. 2008;14:7149–7159. doi: 10.3748/wjg.14.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gal-Tanamy M, Bachmetov L, Ravid A, Koren R, Erman A, Tur-Kaspa R, Zemel R. Vitamin D: an innate antiviral agent suppressing hepatitis C virus in human hepatocytes. Hepatology. 2011;54:1570–1579. doi: 10.1002/hep.24575. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Torres M, Jeffers LJ, Sheikh MY, Rossaro L, Ankoma-Sey V, Hamzeh FM, Martin P. Peginterferon alfa-2a and ribavirin in Latino and non-Latino whites with hepatitis C. N Engl J Med. 2009;360:257–267. doi: 10.1056/NEJMoa0805062. [DOI] [PubMed] [Google Scholar]
- 35.Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 36.Loguercio C, Federico A, Masarone M, Torella R, Blanco Cdel V, Persico M. The impact of diet on liver fibrosis and on response to interferon therapy in patients with HCV-related chronic hepatitis. Am J Gastroenterol. 2008;103:3159–3166. doi: 10.1111/j.1572-0241.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 37.Yadav D, Hertan HI, Schweitzer P, Norkus EP, Pitchumoni CS. Serum and liver micronutrient antioxidants and serum oxidative stress in patients with chronic hepatitis C. Am J Gastroenterol. 2002;97:2634–2639. doi: 10.1111/j.1572-0241.2002.06041.x. [DOI] [PubMed] [Google Scholar]
- 38.Lott WB, Takyar SS, Tuppen J, Crawford DH, Harrison M, Sloots TP, Gowans EJ. Vitamin B12 and hepatitis C: molecular biology and human pathology. Proc Natl Acad Sci USA. 2001;98:4916–4921. doi: 10.1073/pnas.081072798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg P, Hagen K. Serum B12 levels predict response to treatment with interferon and ribavirin in patients with chronic HCV infection. J Viral Hepat. 2011;18:129–134. doi: 10.1111/j.1365-2893.2010.01288.x. [DOI] [PubMed] [Google Scholar]
- 40.Grasso A, Malfatti F, De Leo P, Martines H, Fabris P, Toscanini F, Anselmo M, Menardo G. Insulin resistance predicts rapid virological response in non-diabetic, non-cirrhotic genotype 1 HCV patients treated with peginterferon alpha-2b plus ribavirin. J Hepatol. 2009;51:984–990. doi: 10.1016/j.jhep.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 42.Resnick LM. Calcium metabolism in hypertension and allied metabolic disorders. Diabetes Care. 1991;14:505–520. doi: 10.2337/diacare.14.6.505. [DOI] [PubMed] [Google Scholar]
- 43.Sun X, Zemel MB. Calcitriol and calcium regulate cytokine production and adipocyte-macrophage cross-talk. J Nutr Biochem. 2008;19:392–399. doi: 10.1016/j.jnutbio.2007.05.013. [DOI] [PubMed] [Google Scholar]


