Abstract
Psychophysical studies of interactions between retronasal olfaction and taste have focused most often on the enhancement of tastes by odors, which has been attributed primarily to a response bias (i.e., halo dumping). Based upon preliminary evidence that retronasal odors could also be enhanced by taste, the present study measured both forms of enhancement using appropriate response categories. In the first experiment, subjects rated taste (“sweet,” “sour,” “salty,” and “bitter”) and odor (“other”) intensity for aqueous samples of 3 tastants (sucrose, NaCl, and citric acid) and 3 odorants (vanillin, citral, and furaneol), both alone and in taste–odor mixtures. The results showed that sucrose, but not the other taste stimuli, significantly increased the perceived intensity of all 3 odors. Enhancement of tastes by odors was inconsistent and generally weaker than enhancement of odors by sucrose. A second experiment used a flavored beverage and a custard dessert to test whether the findings from the first experiment would hold for the perception of actual foods. Adding sucrose significantly enhanced the intensity of “cherry” and “vanilla” flavors, whereas adding vanillin did not significantly enhance the intensity of sweetness. It is proposed that enhancement of retronasal odors by a sweet stimulus results from an adaptive sensory mechanism that serves to increase the salience of the flavor of nutritive foods.
Keywords: enhancement, flavor, psychophysics, retronasal odor, suppression, taste
Introduction
Modern research on sensory interactions between retronasal odor and taste can be traced to the benchmark studies of Murphy and colleagues (Murphy et al. 1977; Murphy and Cain 1980), which showed that stimulation in the 2 modalities did not interact in the perception of overall intensity but that retronasal odors could be confused with tastes and thereby increase their perceived intensity. Subsequent studies of intensity interactions in which odors were delivered orthonasally (Gillan 1983; Hornung and Enns 1984, 1986; Enns and Hornung 1985) supported the conclusion that taste and olfaction contribute independently to overall intensity. The apparent enhancement of taste by retronasal odors was not addressed again until Frank and Byram (1988) reported that sweetness was enhanced by a strawberry odor but not by a peanut butter odor, indicating that the effect was odorant dependent. However, Frank and his colleagues later discovered that enhancement of sweetness occurred in significant amounts only when the psychophysical rating task lacked a suitable response category for the odor (Frank et al. 1990, 1993; Frank and Van der Klaauw 1992; Van der Klaauw and Frank 1996). Specifically, when a strawberry odor was presented with sucrose and subjects were instructed to rate fruitiness as well as sweetness, enhancement of sweetness was nil. This pivotal finding was confirmed by Clark and Lawless (1992, 1994) who dubbed the effect of too few response categories on ratings of perceived intensity “halo dumping.” Enhancement of taste by retronasal odors has since been reported by other investigators (Schifferstein and Verlegh 1996; Stevenson et al. 1999; Prescott et al. 2004), but in none of the studies were subjects asked to rate both odor intensity and taste intensity on every trial, a procedure that would have ruled out halo dumping and provided data on any possible effects of taste on the perception of retronasal odors.
Measuring possible effects of taste on retronasal olfaction is important because in the field of food science, the study of interactions between tastes and odors has focused on how tastes can increase the perception of the volatile components of foods (“flavor enhancement”) (e.g., Valdes, Hinreiner, and Simone 1956; Valdes, Simone, and Hinreiner 1956; Wiseman and McDaniel 1991; Bonnans and Noble 1993; Kuo et al. 1993; Noble 1996; Davidson et al. 1999; Hollowood et al. 2002). Once again, however, in nearly all these experiments, subjects rated only the flavor (i.e., the retronasal odor) or rated the taste and flavor in separate sessions or trials, thereby inviting halo dumping. In a few studies in which panelists rated taste and flavor on every trial (Valdes, Hinreiner, and Simone 1956; Philipsen et al. 1995), taste-induced flavor enhancement was still significant. In addition, numerous other studies employing descriptive analysis techniques have shown that the addition of tastants changes the flavor profile of complex foods and beverages, often by enhancing specific flavors (e.g., Lindley et al. 1993; Baldwin et al. 2008; Cardoso and Bolini 2008). On balance, therefore, the evidence that taste can enhance the perception of retronasal odor seems to be stronger than the evidence that retronasal odors can enhance the perception of taste.
The purpose of the present study was therefore to measure the potential for enhancement, both of taste by odors and of odors by tastes, using a psychophysical procedure that gave subjects appropriate response categories with which to rate the intensities of both kinds of sensations. After an initial experiment using aqueous solutions showed that enhancement of odors by sucrose was more frequently encountered and greater in magnitude than was enhancement of taste by odors, we ran a second experiment to verify that odor enhancement by taste also predominates when subjects rate the tastes and retronasal odors of actual foods and beverages.
Experiment 1
The first experiment was designed to investigate the phenomena of taste enhancement by odor and odor enhancement by taste using aqueous model solutions of odors, tastes, and their mixtures. This approach enabled measurement of odor–taste interactions under conditions of retronasal stimulation that broadly mimicked those encountered during normal tasting. Consistent with this approach, odor and taste intensity were measured over multiple consecutive trials to assess the possible effects of sensory adaptation in odor–taste interactions.
Materials and methods
Subjects
A total of 31 subjects (18 females and 13 males) between 18 and 45 years of age were recruited from public postings on the Yale University Medical School and Yale College campuses. Each person gave informed consent and was paid for their participation. All subjects were self-reported healthy nonsmokers who had no known taste or smell disorders or deficiencies and had no lingual piercings. Subjects were asked to refrain from eating or drinking foods or beverages for at least 1 h prior to their scheduled session.
Stimuli
The experiment included a total of 19 test stimuli: 4 taste stimuli (0.56 M sucrose, 0.32 M NaCl, 10 mM citric acid, and the mixture of 0.56 M sucrose and 10 mM citric acid), 3 odor stimuli (0.56 mM furaneol, 0.00025% citral, and 1.8 mM vanillin), and 12 odor–taste mixtures. The concentrations of NaCl and citric acid were chosen to produce tastes approximately equal in intensity to the sweetness of 0.56 M sucrose. The concentrations of odor stimuli were selected to produce clearly perceptible retronasal odors without evoking tastes or oral irritation. All stimuli were prepared weekly with deionized water in 100-mL volumes and stored in airtight 100-mL flasks. A container of deionized water for rinsing between stimuli was heated in circulated water baths to 37 °C to avoid cooling the mouth and tongue during repeated rinsing.
Procedure
Practice session.
Prior to the first data collection session, all subjects attended a short practice session in which they were instructed in how to use the general version of the labeled magnitude scale (gLMS; Green et al. 1993, 1996; Bartoshuk et al. 2002). After the instructions were given, the subjects were asked to rate a list of 20 remembered or imagined oral sensations (e.g., the sweetness of cotton candy and the burn of cinnamon gum) to give them experience using the gLMS in the broad context of normal oral perception. Subjects were then instructed to rate the intensity of taste and retronasal odor of 7 practice stimuli (0.28 M sucrose, 0.16 M NaCl, 5 mM citric acid, 1.8 mM vanillin, a binary taste mixture with sucrose and citric acid, 2 binary odor–taste mixtures with 0.00025% citral and NaCl, and 0.56 mM furaneol and sucrose). The stimuli were pipetted onto the tongue in 1-mL volumes, tasted in the mouth for 2 s, and expectorated. Preliminary tests indicated that this procedure induced reliable tastes and retronasal odors and encouraged natural tasting movements both before and after expectoration. Subjects then rated sweetness, saltiness, sourness, bitterness, and “other” on the gLMS. The instructions were to use the category “other” to rate any sensations other than sweetness, sourness, saltiness, or bitterness. This general response category was used instead of specific odor qualities such as lemon or vanilla because furaneol evokes an unfamiliar odor that is variously described fruit-like (e.g., strawberry) or a burnt sugar-like quality. The 5 categories of sensation were rated sequentially on a separate computer screen as the subjects continued tasting and breathed normally with the mouth closed. Each stimulus was presented 5 times in a row at 60-s intervals for a total of 35 practice trials, with rinsing between each trial. The subjects were not aware of the names of the specific odorants delivered or that each stimulus would be presented in blocks of 5.
Experimental sessions.
Subjects were randomly assigned to 1 of 3 groups, with each group receiving the stimuli in a different pseudorandom order. On each trial, 1 mL of a stimulus was pipetted onto the subject’s tongue and actively tasted in the closed mouth for 2 s before being expectorated. Subjects continued to taste the stimulus by moving the tongue and breathing normally through the nose for another 5 s before making the intensity ratings. The additional time ensured that the taste and retronasal odors would be perceived together (during multiple exhalations) when taste–odor mixtures were presented. Subjects were instructed to make their ratings based on the maximum sensation intensities of each quality they perceived. Subjects rinsed vigorously at least 3 times with 37 °C deionized water after each trial during a 60-s intertrial interval. The experiment consisted of 3 sessions on separate days, with 6 stimuli presented in each of the first 2 sessions and 7 presented in the last session. The order of stimulus delivery was pseudorandom, with 3 different orders to which subjects were randomly assigned. As in the practice session, each stimulus was presented on 5 consecutive trials to assess the possible effects of adaption. Thus, there were a total of 30 trials in the first 2 experimental sessions and 35 trials in the third session.
Data analysis
Before statistical analysis, the perceived intensity ratings were log transformed. After the transformation, the data were analyzed using repeated-measures analysis of variance (ANOVA) followed by Tukey honestly significant difference (HSD) post hoc tests. All statistical analyses were performed using Statistica 8 (StatSoft, Inc.).
Results and discussion
Effects of tastes on retronasal odors
Figures 1–3 contain graphs of the perceived intensity of “other” sensations produced by citral, vanillin, and furaneol experienced either alone or in mixture with sucrose, citric acid, NaCl, or the mixture of sucrose and citric acid. Because subjects also rated sweetness, saltiness, sourness, and bitterness, ratings of “other” can be inferred to reflect the perceived intensity of retronasal odors. Consistent with prior evidence that taste can enhance flavor intensity (Valdes, Hinreiner, and Simone 1956; Valdes, Simone, and Hinreiner 1956; McBride and Johnson 1987; Bonnans and Noble 1993; Kuo et al. 1993; Philipsen et al. 1995; Davidson et al. 1999; Hollowood et al. 2002; Hort and Hollowood 2004), ratings of ‘other’ sensations were often higher in the presence of taste stimulation, specifically sucrose.
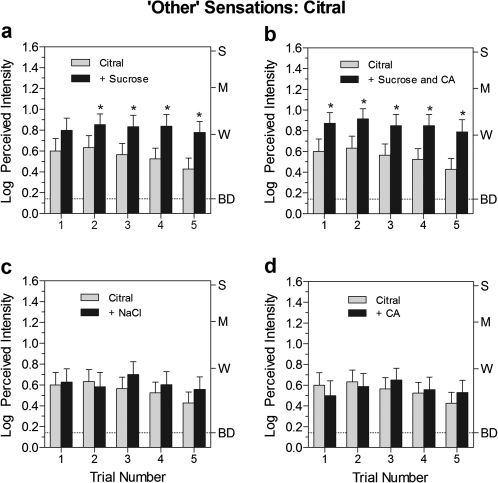
Figure 1.
Log mean ratings of the perceived intensity of “other” sensations over 5 trials for the retronasal odorant citral presented alone (light bars) or with 1 of 4 taste stimuli (black bars). Asterisks indicate significant differences between conditions (Tukey HSD, P < 0.05). Letters on the right y axis represent labels on the gLMS: BD, barely detectable; W, weak; M, moderate; S, strong. Vertical bars represent the standard errors of the mean.
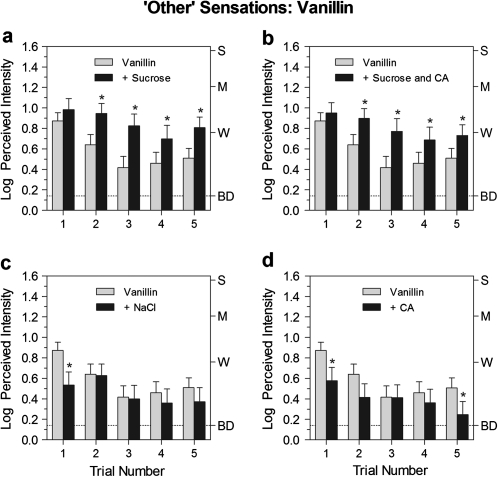
Figure 2.
Same as Figure 1, except the retronasal odorant was vanillin.
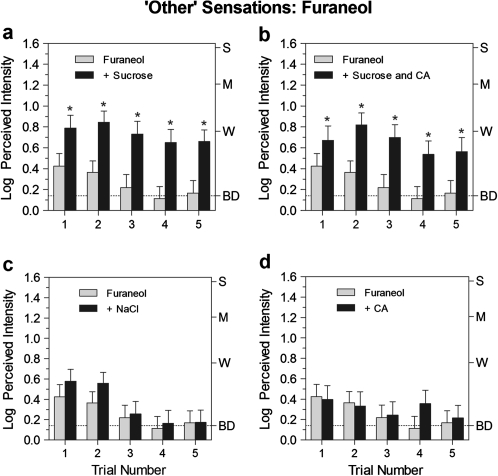
Figure 3.
Same as Figure 1, except the retronasal odorant was furaneol.
Looking first at “other” ratings for citral (Figure 1a–d), its lemon odor was consistently rated as more intense when it was presented together with sucrose (Figure 1a). A repeated-measures ANOVA with stimulus and trial as factors confirmed that there was a significant main effect of stimulus, that is, “other” ratings were higher when sucrose was present [F(1,30) = 14.4, P < 0.001]. Post hoc tests showed that the difference between stimulus conditions was significant on trials 2–5 (Tukey HSD, P < 0.05), with ratings on the final 2 trials being more than twice as high when sucrose was present. Similarly, “other” ratings were significantly higher [F(1,30) = 10.3, P < 0.005] when citral was presented with the mixture of sucrose and citric acid (Figure 1b). Although odor enhancement by the mixture of sucrose and citric acid appeared more uniform across trials than when citral was paired with sucrose alone, a repeated-measures ANOVA of the difference scores (i.e., the amount of enhancement) for citral plus sucrose and for citral plus both sucrose and citric acid found no significant effect of stimulus [F(1,30) = 0.026, P = 0.89]. In contrast, no enhancement was found when citral was mixed with either NaCl or citric acid (Figure 1c,d).
The results for vanillin and sucrose were similar to those for citral and sucrose (Figure 2a). Although there was a main effect of stimulus [F(1,30) = 12.2, P < 0.005], as with citral, enhancement by sucrose did not attain statistical significance on the first trial. However, “other” sensations from vanillin were rated as much as 2.5 times stronger on the 4 subsequent trials (Tukey HSD, P < 0.05). The combination of sucrose and citric acid (Figure 2b) was again the only other stimulus that led to significantly higher “other” ratings [F(1,30) = 6.7, P < 0.05]. Without sucrose, adding NaCl or citric acid to vanillin tended to suppress odor sensations, causing significant reductions on the first trial for both taste stimuli and as well as on the fifth trial for citric acid (Tukey HSD, P < 0.05). This result further demonstrates that retronasal odor enhancement by taste and retronasal odor suppression by taste are both stimulus dependent.
The magnitude of odor enhancement was greatest for the least familiar odor stimulus, furaneol. Figure 3a,b shows that in mixture with sucrose, subjects rated “other” sensations from furaneol significantly higher on every trial [main effect of stimulus; F(1,30) = 21.3, P < 0.0001], with ratings on trials averaging more than 3 times higher than for furaneol alone. Once again, enhancement occurred only (and always) when sucrose was present. There was a main effect of stimulus for the sucrose–citric acid mixture [F(1,30) = 19.0, P < 0.001], and “other” ratings were significantly higher on all trials (Tukey HSD, P < 0.05), whereas there were no significant effects of NaCl or citric acid alone.
Effects of retronasal odor on taste
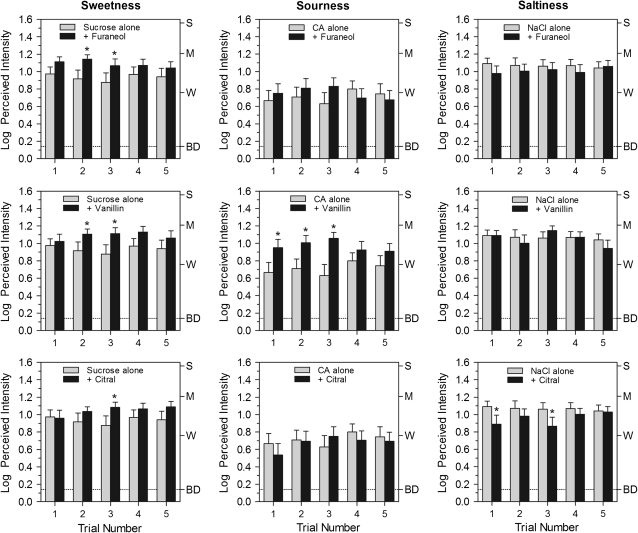
In general, the effects of retronasal odor on perceived taste intensity were smaller and less consistent than were the effects of tastes (particularly sucrose) on perceived odor intensity. This finding is consistent with the evidence that enhancement of taste by odors is relatively weak, if present at all, when subjects have an appropriate response category with which to rate the odors (Frank et al. 1993; Clark and Lawless 1994). Figure 4 displays the log mean perceived intensity ratings for the primary taste qualities—sweetness, sourness, and saltiness—evoked by sucrose, citric acid, and NaCl alone and in mixture with the 3 odor stimuli. There were only 2 significant main effects of stimulus: enhancement of the sweetness of sucrose by furaneol [F(1,30) = 7.4, P < 0.05] and enhancement of the sourness of citric acid by vanillin [F(1,30) = 11.0, P < 0.005]. A main effect of sucrose fell just short of statistical significance for vanillin [F(1,30) = 3.4, P = 0.077]. Post hoc tests showed that for both furaneol and vanillin, sweetness ratings were significantly higher on the second and third trials (Tukey HSD, P < 0.05). However, the addition of vanillin caused a significant increase in rated intensity of sourness of citric acid on each of the first 3 trials, and on the third trial, sourness was rated more than twice as strong (i.e., a difference of >0.3 log units) than when citric acid was tasted by itself. This was a surprising result, particularly given that the lemony odor of citral showed no tendency to enhance sourness. In fact, the opposite effect was found, with citric acid tending to suppress vanilla odor (Figure 2d). Perhaps both of these effects resulted from the discordance of the sour–vanilla combination.
Figure 4.
Log mean ratings of the perceived intensity of sweetness, sourness, and saltiness for the sucrose, citric acid (CA), and NaCl stimuli presented alone (light bars) or with the retronasal odors furaneol, vanillin, or citral over 5 trials. Asterisks indicate significant differences between conditions (Tukey HSD, P < 0.05). Letters on the right y axis represent labels on the gLMS: BD, barely detectable; W, weak; M, moderate; S, strong. Vertical bars represent the standard errors of the mean.
None of the odors had a significant effect on the perceived intensity of the saltiness of NaCl, although a tendency for citral to suppress saltiness fell just short of statistical significance [F(1,30) = 4.09, P = 0.052]. Post hoc tests showed that saltiness was, however, rated significantly less intense on trials 1 and 3 when citral was present (Tukey HSD, P < 0.05).
Effects of repeated exposures
Data were collected over 5 trials to study the effect on odor–taste interactions of intermittent, repeated exposures similar to what occurs during normal eating and drinking. Informal preliminary observations had suggested to us that retronasal odors adapted faster than tastes and thus that interactions between odors and tastes might change over repeated exposures. Consistent with those observations, intensity ratings (Figures 1–3) trended downward across trials for all 3 odors when they were presented alone. Repeated-measures ANOVAs indicated that this trend was significant for both furaneol [F(4,120) = 3.02, P < 0.02] and vanillin [F(4,120) = 14.375, P < 0.0001] but fell short of significance for citral [F(4,120) = 1.7629, P = 0.14]. When the 3 odorants were paired with sucrose (Figures 1a and 3a), only vanillin still exhibited a significant effect of trial [F(4,120) = 3.50673, P < 0.01]. In contrast to the odors, none of the tastes (see Figure 4), including sucrose sweetness, declined significantly in intensity over trials (all P values > 0.05). These results indicate that the more stable sensation of sucrose sweetness not only enhanced odor intensity but also tended to counteract adaptation to the odors over time. A similar effect of sucrose has been reported for the minty flavor or chewing gum (Hollowood et al. 2002).
Experiment 2
After finding that sucrose had significantly enhanced “other” sensations produced by all 3 odor stimuli in aqueous model solutions, we went on to investigate whether sucrose would also enhance retronasal odors in actual food and beverage systems. Based upon the results of previous studies of flavor enhancement by taste, we expected that enhancement would occur at least as much as with the model solutions. However, because most such studies had designs that were conducive to halo dumping, we wished to confirm that flavor enhancement can occur when appropriate response categories are made available to subjects. In addition, to test our assumption in Experiment 1 that the increased ratings of “other” sensations by sucrose reflected enhancement of the retronasal odor, subjects in Experiment 2 were asked to rate the intensity of specific odor qualities (i.e., “vanilla” and “cherry”) along with the 4 basic taste qualities.
Materials and methods
Subjects
A total of 19 subjects (6 males and 13 females) between 19 and 32 years of age (mean = 23 years old) were recruited from the Oregon State University campus and were paid to participate. All were nonsmoking and nonpregnant individuals who were free from deficits in taste or smell by self-report. Subjects were asked to refrain from eating/drinking or using menthol products for a minimum of 1 h prior to their scheduled session. The experimental protocol was approved by the Oregon State University Institutional Review Board, and subjects gave written informed consent.
Stimuli
Two product systems were used in the experiment: a cherry-flavored beverage and vanilla-flavored custard. For the flavored beverage, stock-flavored juice was made following package directions with 1.9 g/L concentrated powder (Kool-Aid, Kraft Foods Inc.) dissolved in deionized water, to which 3 different levels of sucrose were added (0.14 M, 0.28 M, and 0.56 M; J.T. Baker). The vanilla custard samples consisted of 4 different formulations of custard (Bird’s Custard Powder, Premier Ambient Products Ltd; Ingredients: corn flour, salt, vanilla, and artificial color) varying concentrations of sucrose (none or 0.4 M added) and vanillin (baseline or 0.2% added; Sigma-Aldrich). Twenty grams of the custard powder and sucrose (0 or 71.2 g) were combined with 500 mL of whole milk and whisked until blended. The mixture was then microwaved on high for a total of 6 min. Once the custard reached room temperature, vanillin (0 or 1.05 g) was added into the custard. The cherry-flavored drink and vanilla custard samples were prepared weekly and were stored at 4–6 °C. All stimuli were presented in 10-mL aliquots at room temperature (20–22 °C).
Procedure
Each subject attended a single experimental session. At the beginning of the session, verbal instructions were given about how to use the gLMS. Following the instructions, the subjects were given practice rating remembered and/or imagined oral sensations on the gLMS (as in Experiment 1). Subjects were then instructed to rate the perceived intensities of sweetness, sourness, saltiness, bitterness, and the specific flavor (i.e., cherry or vanilla) for 3 practice stimuli (a 0.32 M sucrose solution, a cherry-flavored beverage with 0.18 M sucrose, and a mixture of 0.1% vanillin and 0.18 M sucrose solution). After taking 3-min break, subjects were presented with 2 blocks of 7 stimuli with a 5-min break between testing blocks. In each test block, there were 2 subblocks consisting of 3 cherry-flavored beverages and 4 vanilla custard samples. For the flavored beverage, the subjects sipped 10-mL samples, tasted them for 2 s, then expectorated, and rated the 4 taste qualities and cherry flavor. For the vanilla custard, the subjects placed a small plastic spoonful of the custard in the mouth, made normal tasting movements, then expectorated, and rated the 4 taste qualities and vanilla flavor. There was a 60-s intertrial interval during which subjects rinsed vigorously at least 3 times with 37 °C deionized water. After the first set of stimuli had been sampled, they were presented a second time using the same procedure. The presenting order of the food and beverage systems was counterbalanced across subjects, and the stimuli within each product system were randomly presented.
Data analysis
Before statistical analysis, the perceived intensity ratings were again log transformed. After the transformation, the data were analyzed using repeated-measures ANOVA followed by Tukey HSD post hoc tests. All statistical analyses were performed using Statistica 8 (StatSoft, Inc.).
Results and discussion
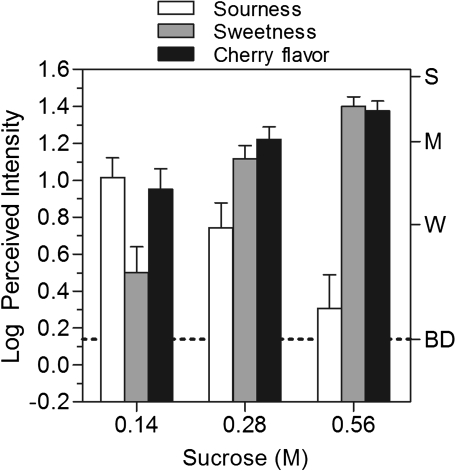
Figure 5 shows the log mean perceived intensity ratings of sourness, sweetness, and cherry flavor for the cherry-flavored beverage with 3 different amounts of sucrose added. As expected, the perceived intensity of sweetness increased significantly with the increase in sucrose concentration [F(2,36) = 16.64, P < 0.0001] and the perceived intensity of sourness decreased significantly [F(2,36) = 40.09, P < 0.0001]. Sucrose has previously been shown to be a powerful suppressor of sourness (Frank and Archambo 1986; McBride and Finlay 1990; Schifferstein and Frijters 1991; Green et al. 2010). More importantly, a repeated-measures ANOVA confirmed that there was a significant main effect of stimulus, with cherry flavor rated progressively higher as sucrose concentration increased [F(2,36) = 18.65, P < 0.0001]. Increasing sucrose concentration from 0.14 to 0.28 M enhanced the cherry flavor by more than two and a half times (a difference of 0.44 log units). The further increase in sucrose concentration from 0.28 to 0.56 M led to a smaller increase in rated cherry flavor (a difference of 0.27 log units), which post hoc tests showed were not significant.
Figure 5.
Log mean ratings of the perceived intensity of sourness (empty bars), sweetness (light bars), and cherry flavor (black bars) of a flavored beverage with 3 different concentrations of sucrose added. Sweetness and cherry flavor increased monotonically with sucrose concentration, whereas sourness declined. Letters on the right y axis represent labels on the gLMS: BD, barely detectable; W, weak; M, moderate; S, strong. Vertical bars represent the standard errors of the mean.
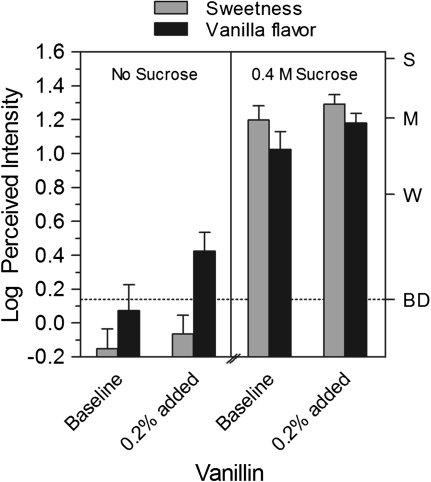
The results for vanilla custard were consistent with those for the cherry-flavored beverage, but even more dramatic. Although the vanilla flavor was rated below “barely detectable” when sucrose was missing from the formulation (Figure 6, the left bars) and when the recommended amount of sucrose was added (equivalent to 0.4 M), vanilla flavor was rated a little below “moderate” on the gLMS, which reflected a nearly10-fold increase in perceived intensity (a difference of 0.95 log units). Note that the custard mix itself contains an unknown amount of vanilla flavor. A repeated-measures ANOVA confirmed there was a significant main effect of stimulus for both vanilla flavor [F(3,54) = 36.88, P < 0.0001] and sweetness [F(3,54) = 119.45, P < 0.0001]. Post hoc tests showed that adding more vanillin (0.2%) to the custard mix significantly enhanced vanilla ratings when no sucrose was added (left panel). However, the added vanillin did not produce a significant further increase in vanilla flavor beyond the enhancement produced by sucrose (right panel). Thus, the introduction of sweetness drove the perception of vanilla much more effectively than did adding more vanilla flavor. In contrast, adding vanillin failed to increase the perceived sweetness of the custard significantly, whether or not sucrose was added.
Figure 6.
The effect of sucrose (0.4 M) on log mean ratings of the sweetness (light bars) and vanilla flavor (black bars) of a commercial vanilla-flavored custard mix with or without 0.2% more vanillin added. Note that the vanilla flavor of the custard mix without sucrose added was below “barely detectable.” Thus, as formulated, the vanilla flavor of the custard depends upon enhancement from sucrose. Letters on the right y axis represent labels on the gLMS: BD, barely detectable; W, weak; M, moderate; S, strong. Vertical bars represent the standard errors of the mean.
Taken together, the consistent finding of odor enhancement by taste for both the cherry-flavored beverage and the vanilla custard rule out the possibility that the higher ratings of “other” sensations in Experiment 1 were an artifact of using such a broad, nonspecific response category. Equal and greater amounts of enhancement were measured in the present experiment when subjects rated the intensity of the specific odor qualities of cherry and vanilla.
General discussion
The present finding that sucrose can enhance retronasal odors is surprising given that previous psychophysical studies of suprathreshold interactions between retronasal odors and tastes had reported no significant odor enhancement (Murphy et al. 1977; Murphy and Cain 1980; Frank et al. 1989, 1993; Clark and Lawless 1994; Stevenson et al. 1999; Stevenson 2001a; Auvray and Spence 2008). However, in nearly all previous studies, odor intensity was either not rated at all or was not rated together with taste. An important feature of the present study was that halo dumping was avoided by including response categories that were appropriate for both tastes and odors (Frank et al. 1993; Clark and Lawless 1994; Van der Klaauw and Frank 1996). Under those conditions, odor enhancement by taste was found in much greater amounts than taste enhancement by odor. This outcome indicates that adopting an analytical perceptual strategy does not necessarily interfere with the occurrence of perceptual interactions between odors and tastes (Stevenson et al. 1999).
Additional evidence that retronasal odors can be enhanced by taste comes from the previously mentioned studies of flavor enhancement in foods and beverages, which generally found that adding sucrose or increasing its concentration led to higher flavor ratings (e.g., Valdes, Hinreiner, and Simone 1956; Valdes, Simone, and Hinreiner 1956; Wiseman and McDaniel 1991; Bonnans and Noble 1993; Kuo et al. 1993; Philipsen et al. 1995; Noble 1996; Davidson et al. 1999; Hollowood et al. 2002). However, basic research on taste–smell interactions seems to have progressed independently of this body of work. One reason may be that studies of flavor enhancement within the field of food science are most often designed to learn how interactions between food ingredients affect flavor, not how the senses of olfaction and taste interact in flavor perception. This emphasis appears to have deflected attention away from the possibility that taste stimulation might increase flavor intensity in part by enhancing the perception of retronasal odors.
Another reason that attention has been directed away from the effects of taste on retronasal odors among chemosensory scientists was the evidence that retronasal odors can be referred to the mouth (Murphy and Cain 1980; Rozin 1982). Murphy and Cain (1980) reported results that implied referral caused confusion of odors with tastes and therefore led to higher intensity ratings for taste, an effect which Schifferstein and Verlegh (1996) later proposed depended on the similarity of the odors and tastes. The latter view is consistent with the hypothesis of Stevenson and colleagues that the taste-like qualities of odors (e.g., “sweet” or “sour”), which can be produced or enhanced by association with tastes (Stevenson et al. 2000; Stevenson 2001b; Yeomans et al. 2006; Stevenson and Tomiczek 2007), play an important role in the enhancement of tastes by retronasal odors (Stevenson et al. 1999). However, in Experiment 2 of the present study, when subjects were invited to rate all taste qualities and odor intensity, the sweetness of the vanilla custard without sucrose was rated below barely detectable, even when more vanillin was added.
Thus, the present results do not support a major role for perceptual confusions in normal flavor and food perception. They instead suggest that enhancement of retronasal odors, and perhaps also enhancement of tastes, result from a central neural mechanism that is triggered by the co-occurrence of perceptually congruent tastes and odors (Lim & Johnson, 2011) that is, tastes and odors that are commonly experienced together in foods and so have become associated. This hypothesis is consistent with the idea that experiencing retronasal odors, tastes, and somatosensations together in foods leads to the formation of multisensory percepts of foods and beverages, that is, “flavor objects” (Auvray and Spence 2008; Small and Green 2011). Support for this idea comes from the finding that all 3 test odors (citral, vanilla, and furaneol), which are commonly experienced in foods with sweet carbohydrates, were enhanced only by sucrose, and from the extraordinary amount of enhancement that occurred for the custard dessert. As an actual food system, the vanilla custard included congruent tactile as well as gustatory and olfactory stimulation, which produced a “complete” flavor object. Notably, citric acid failed to significantly enhance the lemon odor of citral, even though sour taste is highly congruent with lemon odor. On the other hand, noncongruent odor–taste pairings resulted in either no enhancement or even suppression (e.g., the odor of vanillin by NaCl and citric acid). These results suggest that taste–odor congruence is a necessary but not sufficient condition for retronasal odor enhancement. The fact that sucrose was the only taste stimulus that enhanced retronasal odors implies that only “nutritive” substances (in this case, a sweet carbohydrate) may be able to cause enhancement. A recent report suggests that the same 2 requirements may hold for the referral of retronasal odors to the mouth (Lim and Johnson 2011). In that study, subjects localized food odors (vanilla or soy sauce) to the oral cavity and/or to the tongue significantly more often when a congruent and nutritive taste (sucrose or NaCl, respectively) was delivered to the mouth. This finding implies that odor referral and enhancement are parallel sensory processes in flavor perception and that referral is important for binding odors and tastes into coherent flavor objects.
The function of retronasal odor enhancement in flavor perception is less obvious. However, one possibility is that by increasing the salience of a flavor, odor enhancement may help to strengthen the associative link between the flavor of a food and its metabolic consequences. Whereas ingestion of sweet carbohydrates can directly trigger brain reward mechanisms (e.g., dopamine and opioid release) related to caloric content (Lenoir et al. 2007; de Araujo et al. 2008; Hajnal et al. 2009), it is the odor of a food (in association with its sweetness) that provides a unique sensory signature that identifies it as a specific source of nutrition (Ackroff and Sclafani 2004; Bernal et al. 2008, 2010). Notable in this regard is the evidence from the present study that retronasal odor enhancement was greater for the weaker odors and as adaptation occurred over trials. The possibility that odor enhancement is inversely proportional to odor intensity and thus may be particularly important for increasing the odor profile of foods with relatively weak odors is now being tested systematically. Also consistent with a functional interpretation of odor enhancement is the possibility that flavor enhancement by sweetness may depend upon the amount of sugar a food contains and thus upon its degree of sweetness (Valdes, Hinreiner, and Simone 1956; Cliff and Noble 1990), which is the trend seen in Figure 5.
The hypothesis that retronasal odor enhancement increases the salience of the flavor of nutritious foods implies that enhancement should occur for other “nutritive tastes,” that is, NaCl and monosodium glutamate, when they appear with congruent odors. This hypothesis is currently being tested. A related question is whether enhancement also occurs when a nutritive taste is perceived with a novel odor. The data for furaneol in Experiment 1 suggest that it can: odor enhancement was greatest for furaneol, which is a common molecule in fruits (e.g., strawberries) but lacks a clear identity. Most subjects have difficulty describing its quality and assign it labels as diverse as “fruity” and “burnt sugar” (unpublished data). Experiments are also planned that will test this hypothesis, as well as its corollary, that nonnutritive tastes, specifically acids or bitter-tasting substances which can signal spoilage or poisons, do not enhance retronasal odors. Although a bitter taste was not tested in the present study, the failure of citric acid to enhance the congruent odor of citral provides preliminary support for this hypothesis.
Summary and implications
When tastes and retronasal odors were presented together and subjects were invited to rate the full range of sensations they perceived, enhancement of retronasal odors by sucrose was the most pronounced and reliable effect. We speculate that this phenomenon results from an adaptive sensory mechanism that increases the salience of retronasal odors and strengthens their associative link with the metabolic effects of the food. The fact that odor enhancement was limited to sucrose suggests that as in mixtures with other tastes (Green et al. 2010), sweetness may have a privileged role in flavor perception as the signal for sweet carbohydrates. However, additional studies are required to determine with confidence the stimulus conditions that are necessary or sufficient to produce enhancement, including whether it can occur for other tastes that also signal nutritive content, and the importance of cognitive factors such as congruence and familiarity.
Funding
This research was funded in part by a grant from the National Institutes of Health (RO1 DC05002) and by startup funds (to J.L.) from the Department of Food Science and Technology, Oregon State University.
References
- Ackroff K, Sclafani A. Fructose-conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite. 2004;42:287–297. doi: 10.1016/j.appet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Auvray M, Spence C. The multisensory perception of flavor. Conscious Cogn. 2008;17:1016–1031. doi: 10.1016/j.concog.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Baldwin EA, Goodner K, Plotto A. Interaction of volatiles, sugars, and acids on perception of tomato aroma and flavor descriptors. J Food Sci. 2008;73:S294–S307. doi: 10.1111/j.1750-3841.2008.00825.x. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. Labeled scales (e.g., category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Qual Prefer. 2002;14:125–138. [Google Scholar]
- Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ. Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats. Behav Brain Res. 2008;190:59–66. doi: 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal SY, Touzani K, Gerges M, Abayev Y, Sclafani A, Bodnar RJ. Opioid receptor antagonism in the nucleus accumbens fails to block the expression of sugar-conditioned flavor preferences in rats. Pharmacol Biochem Behav. 2010;95:56–62. doi: 10.1016/j.pbb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans S, Noble AC. Effect of sweetener type and of sweetener and acid levels on temporal perception of sweetness, sourness and fruitiness. Chem Senses. 1993;18:273–283. [Google Scholar]
- Cardoso JMP, Bolini HMA. Descriptive profile of peach nectar sweetened with sucrose and different sweeteners. J Sensory Stud. 2008;23:804–816. [Google Scholar]
- Clark CC, Lawless HT. Psychological basis in time-intensity scaling. Food Technol. 1992;46:81–90. [Google Scholar]
- Clark CC, Lawless HT. Limiting response alternatives in time-intensity scaling: an examination of the halo-dumping effect. Chem Senses. 1994;19:583–594. doi: 10.1093/chemse/19.6.583. [DOI] [PubMed] [Google Scholar]
- Cliff M, Noble AC. Time-intensity evaluation of sweetness and fruitiness and their interaction in a model solution. J Food Sci. 1990;55:450–454. [Google Scholar]
- Davidson JM, Linforth RS, Hollowood TA, Taylor AJ. Effect of sucrose on the perceived flavor intensity of chewing gum. J Agric Food Chem. 1999;47:4336–4340. doi: 10.1021/jf9901082. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Enns MP, Hornung DE. Contributions of smell and taste to overall intensity. Chem Senses. 1985;10:357–366. [Google Scholar]
- Frank RA, Archambo G. Intensity and hedonic judgments of taste mixtures: an information integration analysis. Chem Senses. 1986;11:427–438. [Google Scholar]
- Frank RA, Byram J. Taste-smell interactions are tastant and odorant dependent. Chem Senses. 1988;13:445–455. [Google Scholar]
- Frank RA, Ducheny K, Mize SJS. Strawberry odor, but not red color, enhances the sweetness of sucrose solutions. Chem Senses. 1989;14:371–377. [Google Scholar]
- Frank RA, Van der Klaauw NJ. The influence of stimulus context and instructional set on odor-induced enhancement of taste. Chem Senses. 1992;17:625. [Google Scholar]
- Frank RA, Van der Klaauw NJ, Schifferstein HN. Both perceptual and conceptual factors influence taste-odor and taste–taste interactions. Percept Psychophys. 1993;54:343–354. doi: 10.3758/bf03205269. [DOI] [PubMed] [Google Scholar]
- Frank RA, Wessel N, Shaffer GS. The enhancement of sweetness by strawberry odor is instruction-dependent. Chem Senses. 1990;15:576. [Google Scholar]
- Gillan DJ. Taste-taste, odor-odor, and taste-odor mixtures: greater suppression within than between modalities. Percept Psychophys. 1983;33:183–185. doi: 10.3758/bf03202837. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart BJ, Shaffer GS, Rankin KM, Higgins J. Evaluating the 'Labeled Magnitude Scale' for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Green BG, Lim J, Osterhoff F, Blacher K, Nachtigal D. Taste mixture interactions: suppression, additivity, and the predominance of sweetness. Physiol Behav. 2010;101:731–737. doi: 10.1016/j.physbeh.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18:683–702. [Google Scholar]
- Hajnal A, Norgren R, Kovacs P. Parabrachial coding of sapid sucrose: relevance to reward and obesity. Ann N Y Acad Sci. 2009;1170:347–364. doi: 10.1111/j.1749-6632.2009.03930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollowood TA, Davidson JM, DeGroot L, Linforth RST, Taylor AJ. Taste release and its effect on overall flavor perception. In: Given P, Paredes D, editors. ACS symposium series: Chemistry of taste: mechanisms, behaviors, and mimics. Vol. 825. Washington (DC): American Chemical Society; 2002. pp. 66–178. [Google Scholar]
- Hornung DE, Enns MP. The independence and integration of olfaction and taste. Chem Senses. 1984;9:97–106. [Google Scholar]
- Hornung DE, Enns MP. The contributions of smell and taste to overall intensity: a model. Percept Psychophys. 1986;39:385–391. doi: 10.3758/bf03207066. [DOI] [PubMed] [Google Scholar]
- Hort J, Hollowood TA. Controlled continuous flow delivery system for investigating taste-aroma interactions. J Agric Food Chem. 2004;52:4834–4843. doi: 10.1021/jf049681y. [DOI] [PubMed] [Google Scholar]
- Kuo YL, Pangborn RM, Noble AC. Temporal patterns of nasal, oral, and retronasal perception of citral and vanillin and interaction of these odorants with selected tastants. Int J Food Sci Technol. 1993;28:127–137. [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Johnson MB. Potential mechanisms of retronasal odor referral to the mouth. Chem Senses. 2011;36:283–289. doi: 10.1093/chemse/bjq125. [DOI] [PubMed] [Google Scholar]
- Lindley MG, Beyts PK, Canales I, Borrego F. Flavor modifying characteristics of the intense sweetener neohesperidin dihydrochalcone. J Food Sci. 1993;58:592–594. [Google Scholar]
- McBride RL, Finlay DC. Perceptual integration of tertiary taste mixtures. Percept Psychophys. 1990;48:326–330. doi: 10.3758/bf03206683. [DOI] [PubMed] [Google Scholar]
- McBride RL, Johnson RL. Perception of sugar acid mixtures in lemon juice drink. Int J Food Sci Technol. 1987;22:399–408. [Google Scholar]
- Murphy C, Cain WS. Taste and olfaction: independence vs interaction. Physiol Behav. 1980;24:601–605. doi: 10.1016/0031-9384(80)90257-7. [DOI] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Bartoshuk LM. Mutual action of taste and olfaction. Sens Processes. 1977;1:204–211. [PubMed] [Google Scholar]
- Noble AC. Taste-aroma interactions. Trends Food Sci Technol. 1996;7:439–444. [Google Scholar]
- Philipsen DH, Clydesdale FM, Griffin RW, Stern P. Consumer age affects response to sensory characteristics of a cherry flavored beverage. J Food Sci. 1995;60:364–368. [Google Scholar]
- Prescott J, Johnstone V, Francis J. Odor-taste interactions: effects of attentional strategies during exposure. Chem Senses. 2004;29:331–340. doi: 10.1093/chemse/bjh036. [DOI] [PubMed] [Google Scholar]
- Rozin P. “Taste-smell confusions” and the duality of the olfactory sense. Percept Psychophys. 1982;31:397–401. doi: 10.3758/bf03202667. [DOI] [PubMed] [Google Scholar]
- Schifferstein HN, Frijters JE. The effectiveness of different sweeteners in suppressing citric acid sourness. Percept Psychophys. 1991;49:1–9. doi: 10.3758/bf03211610. [DOI] [PubMed] [Google Scholar]
- Schifferstein HN, Verlegh PW. The role of congruency and pleasantness in odor-induced taste enhancement. Acta Psychol (Amst) 1996;94:87–105. doi: 10.1016/0001-6918(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Small DM, Green BG. A model of flavor perception. Frontiers in the neural bases of multisensory processes. London: Taylor Frances; 2011. [Google Scholar]
- Stevenson RJ. Is sweetness taste enhancement cognitively impenetrable? Effects of exposure, training and knowledge. Appetite. 2001a;36:241–242. doi: 10.1006/appe.2001.0401. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ. The acquisition of odour qualities. Q J Exp Psychol A. 2001b;54:561–577. doi: 10.1080/713755972. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Boakes RA, Wilson JP. Resistance to extinction of conditioned odor perceptions: evaluative conditioning is not unique. J Exp Psychol Learn Mem Cogn. 2000;26:423–440. doi: 10.1037//0278-7393.26.2.423. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Prescott J, Boakes RA. Confusing tastes and smells: how odours can influence the perception of sweet and sour tastes. Chem Senses. 1999;24:627–635. doi: 10.1093/chemse/24.6.627. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Tomiczek C. Olfactory-induced synesthesias: a review and model. Psychol Bull. 2007;133:294–309. doi: 10.1037/0033-2909.133.2.294. [DOI] [PubMed] [Google Scholar]
- Valdes RM, Hinreiner EH, Simone MJ. Effect of sucrose and organic acids on apparent flavor intensity. 1. Aqueous solutions. Food Technol. 1956;10:282–285. [Google Scholar]
- Valdes RM, Simone MJ, Hinreiner EH. Effect of sucrose and organic acids on apparent flavor intensity. 2. Fruit nectars. Food Technol. 1956;10:387–390. [Google Scholar]
- Van der Klaauw NJ, Frank RA. Scaling component intensities of complex stimuli: the influence of response alternatives. Environ Int. 1996;22:21–31. [Google Scholar]
- Wiseman JJ, McDaniel MR. Modification of fruit flavors by aspartame and sucrose. J Food Sci. 1991;56:1668–1670. [Google Scholar]
- Yeomans MR, Mobini S, Elliman TD, Walker HC, Stevenson RJ. Hedonic and sensory characteristics of odors conditioned by pairing with tastants in humans. J Exp Psychol Anim Behav Process. 2006;32:215–228. doi: 10.1037/0097-7403.32.3.215. [DOI] [PubMed] [Google Scholar]