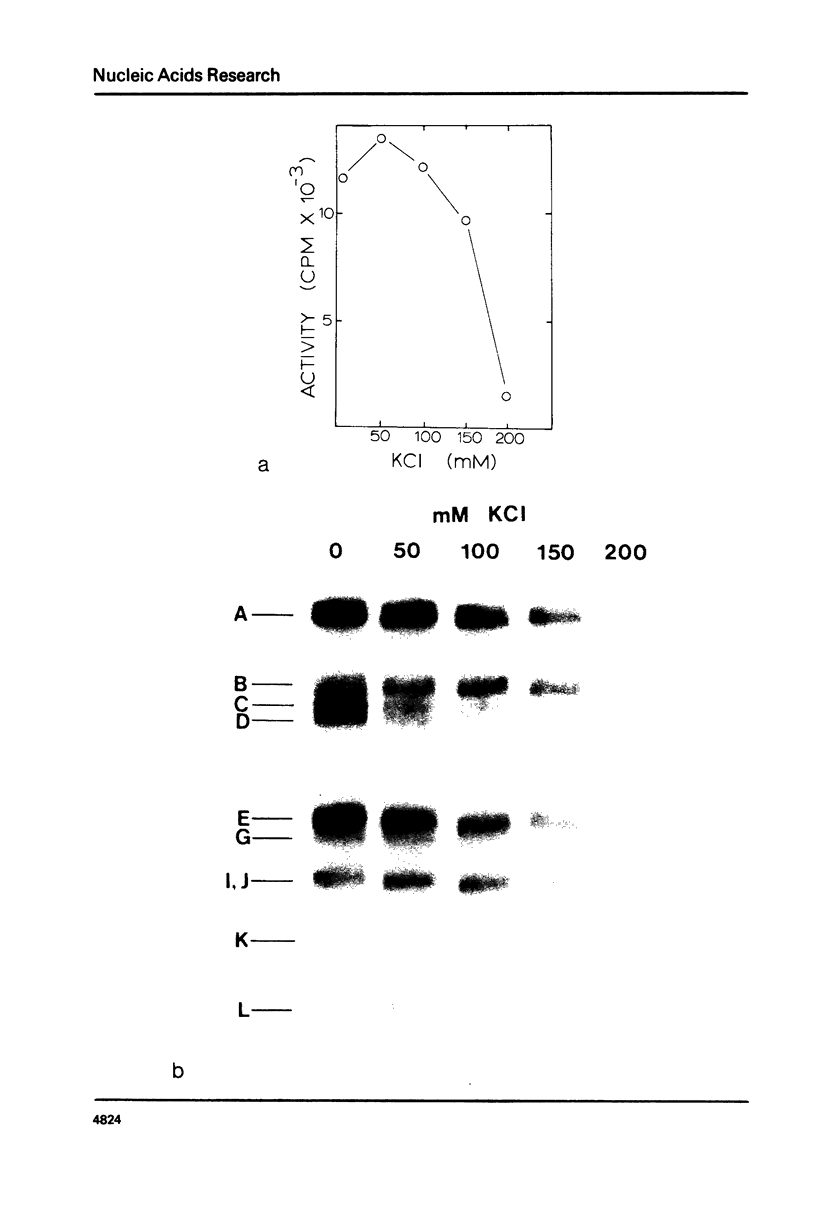
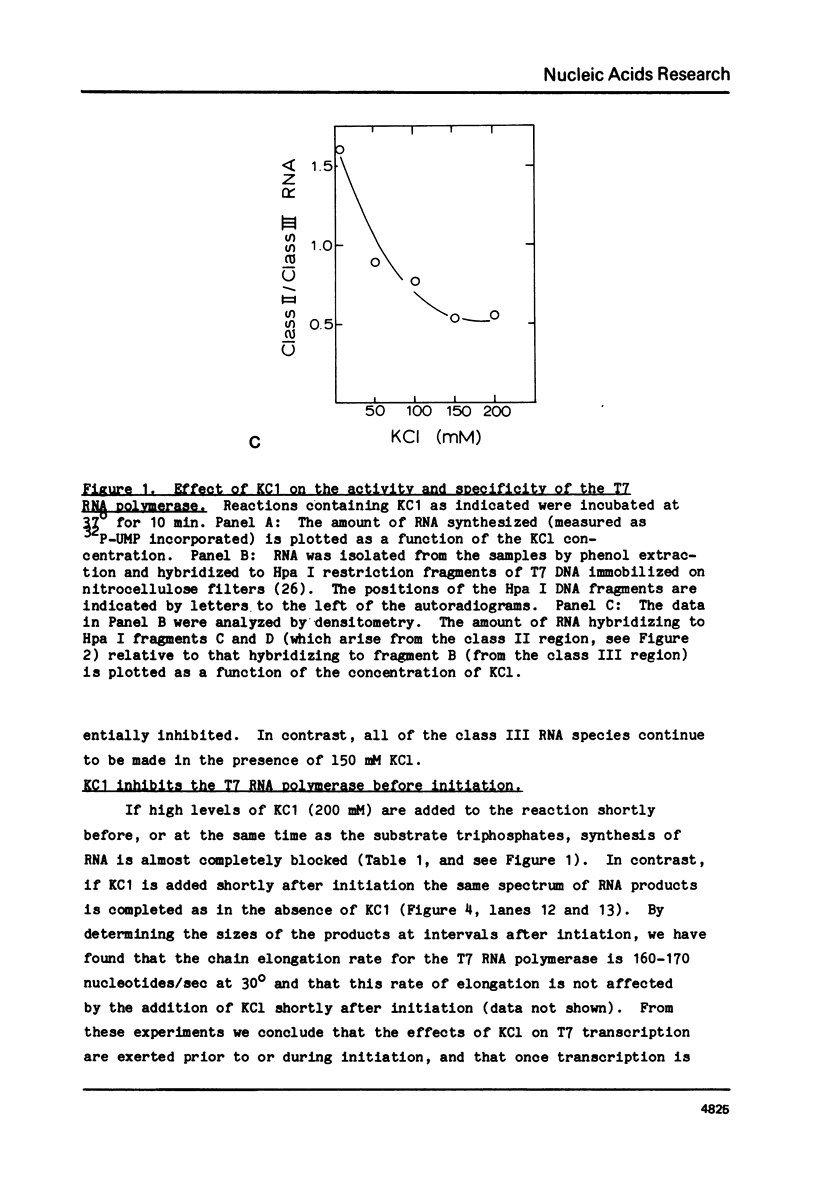
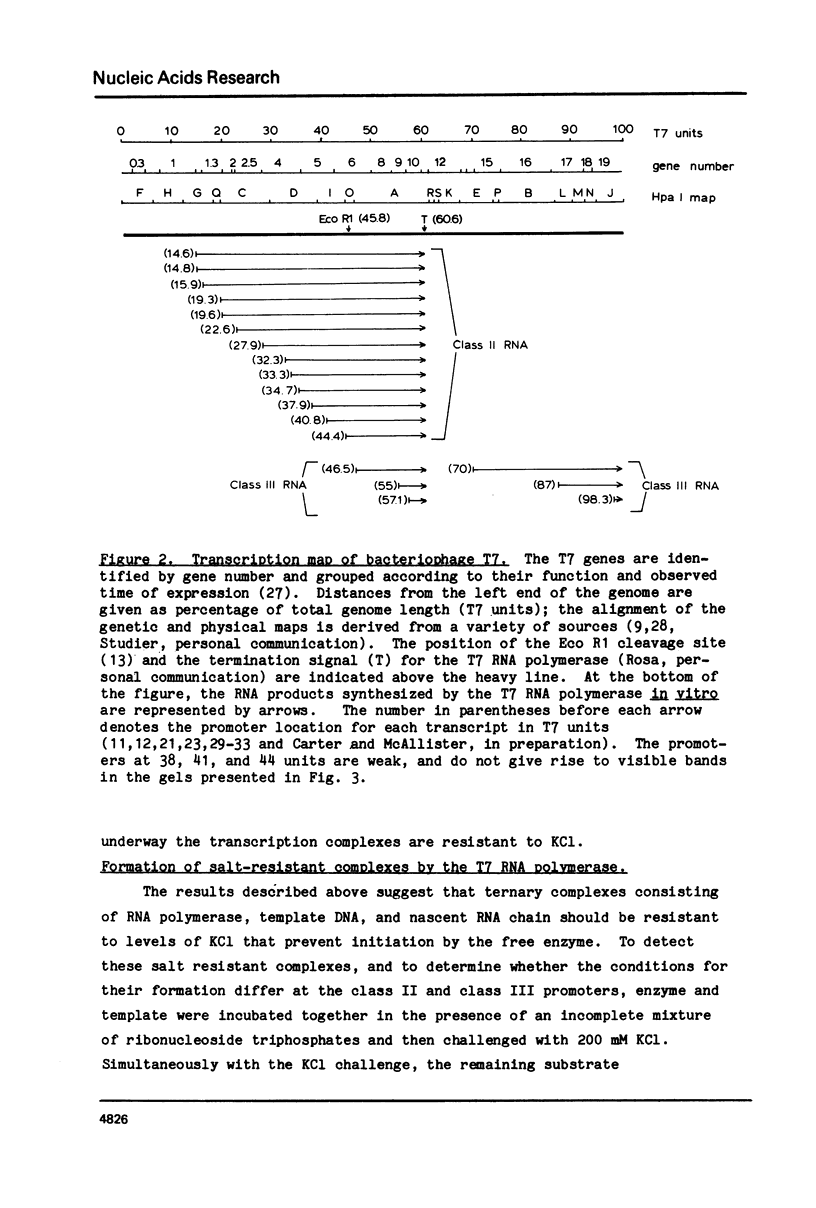
Abstract
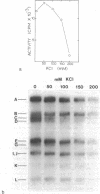
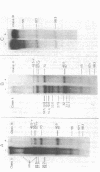
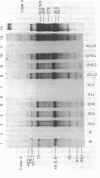
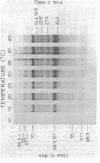
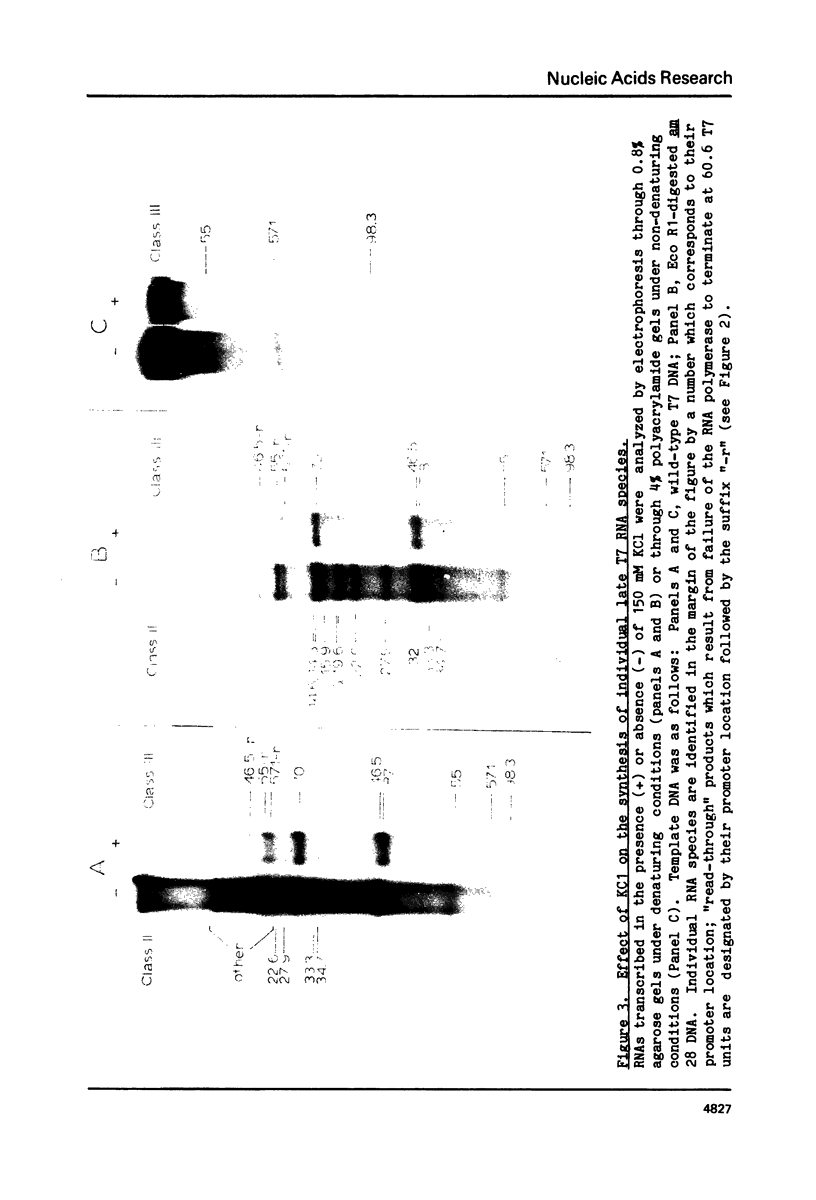
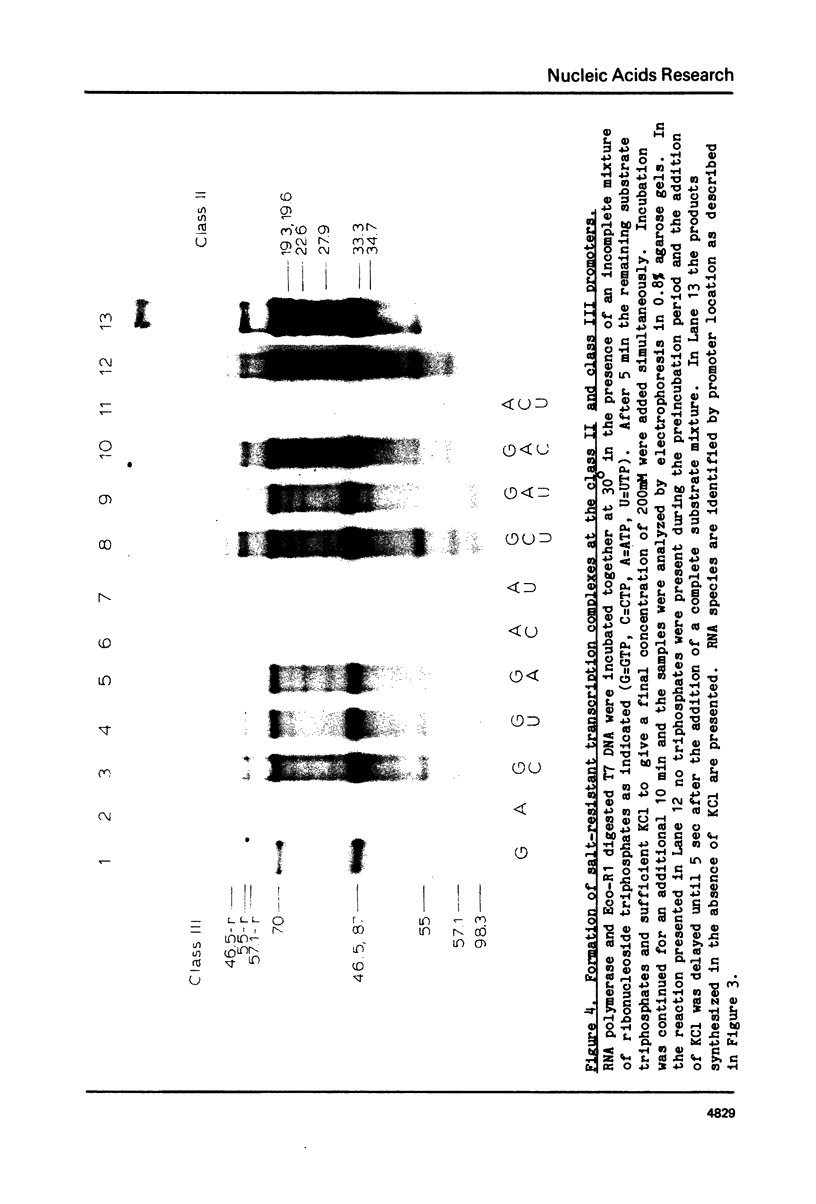
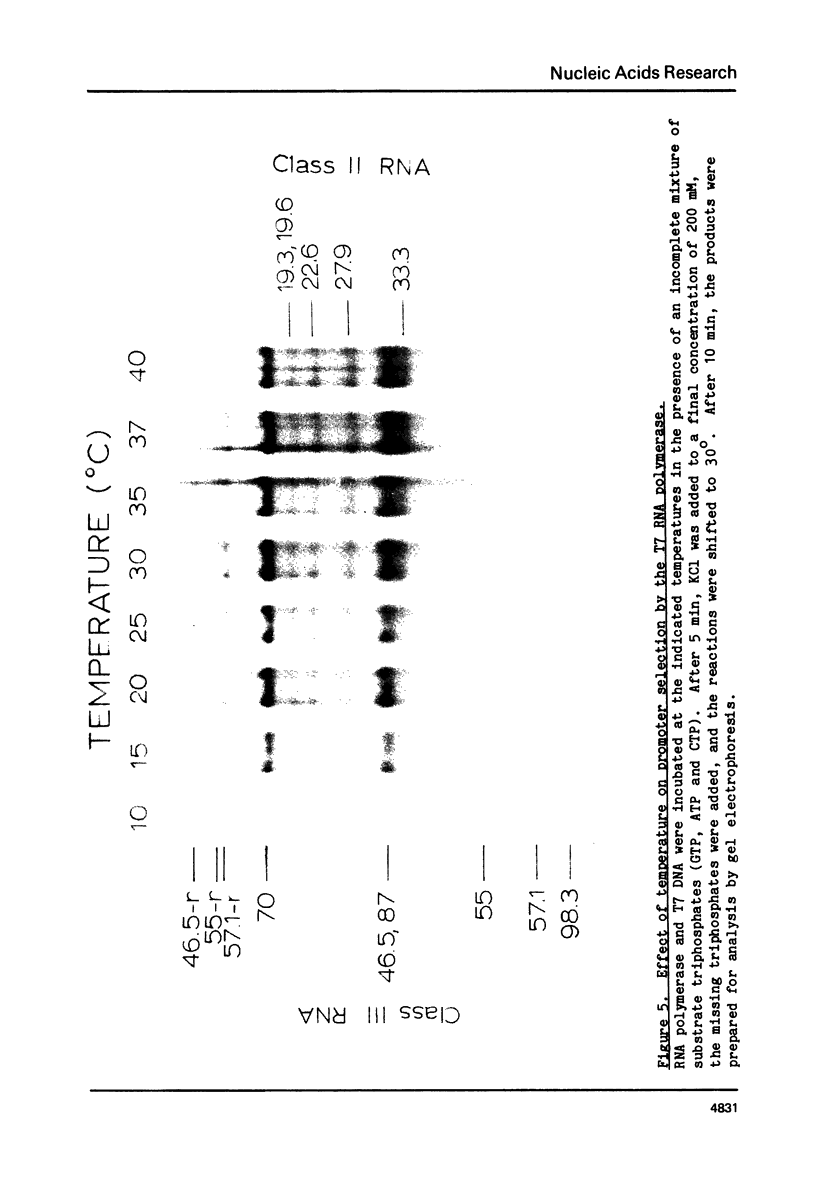
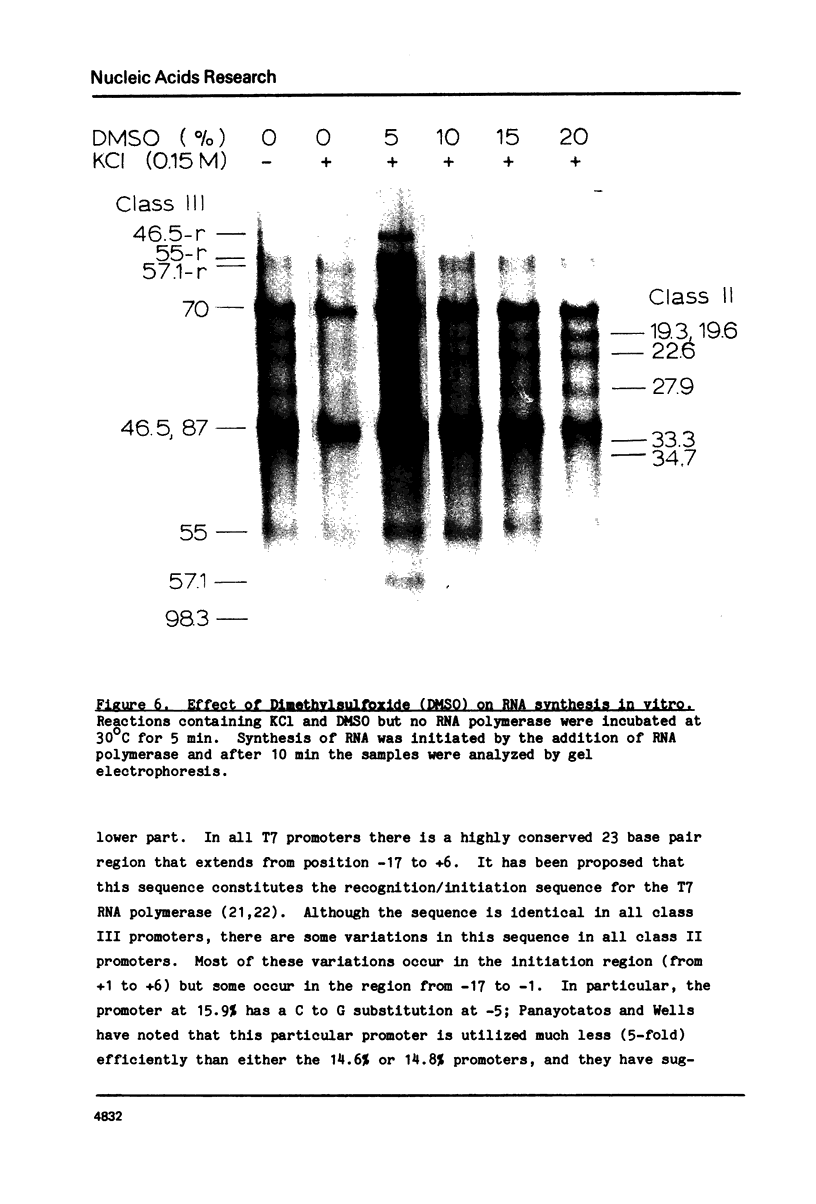
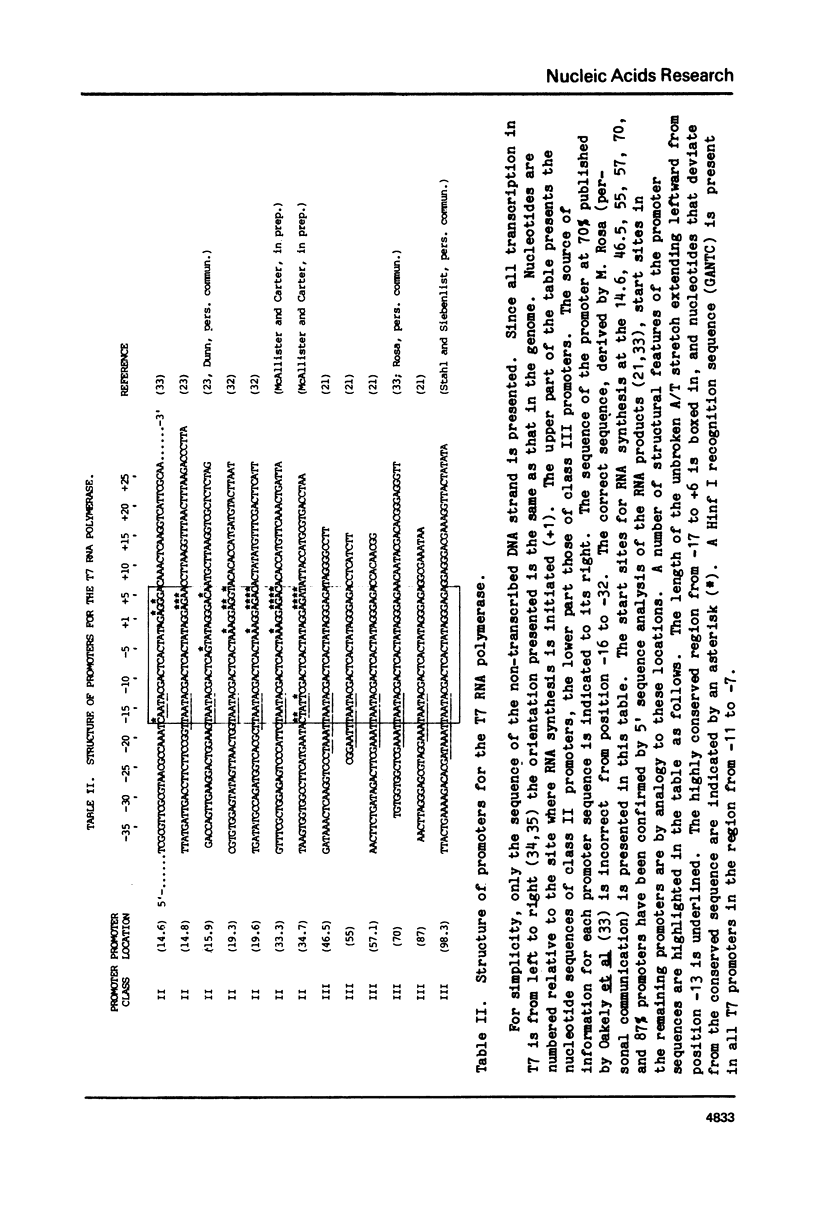
During bacteriophage T7 infection a phage-specified RNA polymerase transcribes the late phage genes in two temporal classes (class II and class III). In this report, we show that the purified phage polymerase discriminates between the class II and class III promoters in vitro as a function of variables that alter the stability of the DNA helix. These variables include ionic strength, temperature, and the presence of denaturing agents such as dimethyl sulfoxide. In general, initiation at the class II promoters is preferentially inhibited as helix stability is increased. Conditions required for the establishment of salt-resistant transcription complexes by the T7 RNA polymerase have been determined; the establishment of stable complexes at the class II promoters requires the synthesis of a longer nascent RNA transcript than does formation of such complexes at the class III promoters. A comparison of the nucleotide sequences of several class II and class III promoters suggests certain features that may be responsible for the different responses of these promoters to helix destabilization. The conservation of structural features that are peculiar to the class II or class III promoters indicates that these features are important in regulation of T7 transcription in vivo. Experiments which bear on the physiological significance of these features are discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier H., Golomb M., Chamberlin M. Isolation of recombinants between T7 and T3 bacteriophages and their use in vitro transcriptional mapping. J Virol. 1977 Feb;21(2):753–765. doi: 10.1128/jvi.21.2.753-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J. C., Hayward R. S. New genes and promoters suggested by the DNA sequence near the end of the coliphage T7 early operon. Nucleic Acids Res. 1979 Dec 11;7(7):1931–1943. doi: 10.1093/nar/7.7.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Richardson C. C., Studier F. W. Genetic recombination and complementation between bacteriophage T7 and cloned fragments of T7 DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2276–2280. doi: 10.1073/pnas.75.5.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., Ring J. Characterization of T7-specific ribonucleic acid polymerase. 1. General properties of the enzymatic reaction and the template specificity of the enzyme. J Biol Chem. 1973 Mar 25;248(6):2235–2244. [PubMed] [Google Scholar]
- Condit R. C. F factor-mediated inhibition of bacteriophage T7 growth: increased membrane permeability and decreased ATP levels following T7 infection of male Escherichia coli. J Mol Biol. 1975 Oct 15;98(1):45–59. doi: 10.1016/s0022-2836(75)80100-8. [DOI] [PubMed] [Google Scholar]
- De Wyngaert M., Hinkle D. C. Involvement of DNA gyrase in replication and transcription of bacteriophage T7 DNA. J Virol. 1979 Feb;29(2):529–535. doi: 10.1128/jvi.29.2.529-535.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb M., Chamberlin M. A preliminary map of the major transcription units read by T7 RNA polymerase on the T7 and T3 bacteriophage chromosomes. Proc Natl Acad Sci U S A. 1974 Mar;71(3):760–764. doi: 10.1073/pnas.71.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J. E., Ko G., Young E. T. Comparative analysis of the in vivo and in vitro expression of bacteriophage T7 messenger RNAs during infection of Escherichia coli. J Mol Biol. 1975 Jun 5;94(4):539–554. doi: 10.1016/0022-2836(75)90320-4. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Chamberlin M. J. Mapping of class II promoter sites utilized in vitro by T7-specific RNA polymerase on bacteriophage T7 DNA. J Virol. 1979 Jan;29(1):196–208. doi: 10.1128/jvi.29.1.196-208.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Barrett C. L. Hybridization mapping of restriction fragments from the early region of bacteriophage T7 DNA. Virology. 1977 Oct 15;82(2):275–287. doi: 10.1016/0042-6822(77)90003-4. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., McCarron R. J. Hybridization of the in vitro products of bacteriop&hage T7 RNA polymerase to restriction fragments of T7 DNA. Virology. 1977 Oct 15;82(2):288–298. doi: 10.1016/0042-6822(77)90004-6. [DOI] [PubMed] [Google Scholar]
- McAllister W. T., Wu H. L. Regulation of transcription of the late genes of bacteriophage T7. Proc Natl Acad Sci U S A. 1978 Feb;75(2):804–808. doi: 10.1073/pnas.75.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Activation of transcription at specific promoters by glycerol. J Biol Chem. 1974 Jul 10;249(13):4050–4056. [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Effects of dimethylsulfoxide on the E. coli gal operon and on bacteriophage lambda in vivo. Cell. 1974 Sep;3(1):39–46. doi: 10.1016/0092-8674(74)90035-x. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Selective effects of MgCl2 and temperature on the initiation of transcription at lac, gal, and lambda promoters. J Biol Chem. 1975 Oct 25;250(20):8202–8208. [PubMed] [Google Scholar]
- Niles E. G., Condit R. C. Translational Mapping of Bacteriophage T7 RNAs synthesized in vitro by purified T7 RNA polymerase. J Mol Biol. 1975 Oct 15;98(1):57–67. doi: 10.1016/s0022-2836(75)80101-x. [DOI] [PubMed] [Google Scholar]
- Oakley J. L., Strothkamp R. E., Sarris A. H., Coleman J. E. T7 RNA polymerase: promoter structure and polymerase binding. Biochemistry. 1979 Feb 6;18(3):528–537. doi: 10.1021/bi00570a023. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Identification, cloning and characterization of three late promoters at 14.6, 14.8 and 15.9% of T7 DNA. J Mol Biol. 1979 Nov 25;135(1):91–109. doi: 10.1016/0022-2836(79)90342-5. [DOI] [PubMed] [Google Scholar]
- Panayotatos N., Wells R. D. Recognition and initiation site for four late promoters of phage T7 is a 22-base pair DNA sequence. Nature. 1979 Jul 5;280(5717):35–39. doi: 10.1038/280035a0. [DOI] [PubMed] [Google Scholar]
- Rosa M. D. Four T7 RNA polymerase promoters contain an identical 23 bp sequence. Cell. 1979 Apr;16(4):815–825. doi: 10.1016/0092-8674(79)90097-7. [DOI] [PubMed] [Google Scholar]
- Siegel R. B., Summers W. C. The process of infection with coliphage T7. 3. Control of phage-specific RNA synthesis in vivo by an early phage gene. J Mol Biol. 1970 Apr 14;49(1):115–123. doi: 10.1016/0022-2836(70)90380-3. [DOI] [PubMed] [Google Scholar]
- Silberstein S., Inouye M. Studies on the role of bacteriophage T7 lysozyme during phage infection. J Mol Biol. 1975 Jul 25;96(1):1–11. doi: 10.1016/0022-2836(75)90178-3. [DOI] [PubMed] [Google Scholar]
- Strothkamp R. E., Oakley J. L., Coleman J. E. Promoter melting by T7 ribonucleic acid polymerase as detected by single-stranded endonuclease digestion. Biochemistry. 1980 Mar 18;19(6):1074–1080. doi: 10.1021/bi00547a005. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Travers A. RNA polymerase--promoter interactions: some general principles. Cell. 1974 Oct;3(2):97–104. doi: 10.1016/0092-8674(74)90112-3. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Ogawa H. EcoRI-sensitive mutation of T7 phage. Mol Gen Genet. 1977 Jan 18;150(2):221–223. doi: 10.1007/BF00695402. [DOI] [PubMed] [Google Scholar]
- Vollenweider H. J., Fiandt M., Szybalski W. A relationship between DNA helix stability and recognition sites for RNA polymerase. Science. 1979 Aug 3;205(4405):508–511. doi: 10.1126/science.377494. [DOI] [PubMed] [Google Scholar]