Abstract
A post-prandial increase in saturated fatty acids (SFAs) and glucose (Glc) activates an inflammatory response, which may be prolonged following restoration of physiological SFAs and Glc levels — a finding referred to as ‘metabolic memory'.
This study examined chronic and oscillating SFAs and Glc on the inflammatory signalling pathway in human adipose tissue (AT) and adipocytes (Ads) and determined whether Ads are subject to “metabolic memory.”
Abdominal (Abd) subcutaneous (Sc) explants and Ads were treated with chronic low glucose (L-Glc): 5.6 mM and high glucose (H-Glc): 17.5 mM, with low (0.2 mM) and high (2 mM) SFA for 48 h. Abd Sc explants and Ads were also exposed to the aforementioned treatment regimen for 12-h periods, with alternating rest periods of 12 h in L-Glc.
Chronic treatment with L-Glc and high SFAs, H-Glc and high SFAs up-regulated key factors of the nuclear factor-κB (NFκB) pathway in Abd Sc AT and Ads (TLR4, NFκB; P<.05), whilst down-regulating MyD88. Oscillating Glc and SFA concentrations increased TLR4, NFκB, IKKβ (P<.05) in explants and Ads and up-regulated MyD88 expression (P<.05). Both tumor necrosis factor α and interleukin 6 (P<.05) secretion were markedly increased in chronically treated Abd Sc explants and Ads whilst, with oscillating treatments, a sustained inflammatory effect was noted in absence of treatment.
Therefore, SFAs may act as key instigators of the inflammatory response in human AT via NFκB activation, which suggests that short-term exposure of cells to uncontrolled levels of SFAs and Glc leads to a longer-term inflammatory insult within the Ad, which may have important implications for patients with obesity and Type 2 diabetes.
Keywords: Saturated fatty acids, Glucose, Obesity, Toll-like receptors, Human adipose tissue, Inflammation
1. Introduction
Obesity and inflammation are highly integrated processes in the pathogenesis of insulin resistance, Type 2 diabetes (T2DM) and cardiovascular disease. Whilst it is clear that dietary habits are key determinants in the development of obesity associated T2DM, emerging evidence identifies distinct ‘cross-talk' between metabolic and inflammatory processes during such pathogenesis [1–3]. Previous findings have determined that postprandial hyperglycaemia is strongly associated with macro-and micro-vascular complications, with several studies identifying the role of glucotoxicity in oxidative stress and the subsequent induction of inflammatory cytokines, chemokines, reactive oxygen species and the nuclear factor-κB (NFκB) signalling pathway, which includes activation of NFκB's regulatory molecule, I kappa B kinase (IKKβ) [4–8]. Studies have also observed that free fatty acids have the potential to activate the innate immune signalling pathways, indicating a causative role for both glucose and saturated fatty acids (SFAs) in the pathogenesis of T2DM, via the initiation of these inflammatory processes [9–11].
In recent years the role of adipose tissue in the development of inflammatory linked disease states has been established, with numerous pro-inflammatory cytokines (adipocytokines) being secreted from adipose tissue. These factors often have a duality of function in their capacity to signal an organism's nutritional status in addition to having pro-inflammatory effects [1]. The identification of innate immune receptors- toll-like receptors (TLRs) — on human and murine adipocytes — and their activation by bacterial and fungal pathogens supports the theory that adipocytes have an innate immune capacity. In conditions such as obesity and T2DM, there are underlying subclinical inflammatory processes, which adipose tissue appears central to, exacerbated by macrophage infiltration, thus increasing the potential inflammatory response from this tissue [12].
With prospective studies highlighting the presence of low grade inflammation prior to the onset of T2DM or the metabolic syndrome [13–16], understanding the mechanisms that propagate this low level inflammatory state is key to unravelling its pathology. In recent years, studies have suggested that SFAs act as ligands for several members of the TLRs [10,11], leading to activation of putative inflammatory pathways. Furthermore, hyperglycaemia also mediates an inflammatory response, which may be perpetuated long after the original insult- a phenomenon referred to as “metabolic memory” [17–19]. As such, postprandial glucose and lipids appear to increase oxidative stress, inflammation and the production of free radicals [4–8,20], which may have a direct impact on adipose cells as well as the endothelium [17–19]. Studies indicate that inflammatory associated complications may arise many years later as a consequence of the initial insult and subsequent events [13–16,20,21].
As such, grazing dietary habits in pre-diabetic subjects may lead to an almost continual chronic elevation of glucose and SFAs, which may have a direct impact on the inflammatory response from adipose tissue, which is exacerbated in conditions of weight gain, insulin resistance and overt T2DM. Hence, adipose tissue would become a source of inflammatory response due to exposure to glucose and SFAs. Therefore, the aims of these studies were to (1) examine whether chronic exposure to glucose and/or SFAs may have a more substantial impact on human adipose to mediate inflammation, (2) examine the effect of intermittent exposure to such insults and if they alleviate or exacerbate such an inflammatory response and (3) determine whether the adipocyte retains a metabolic memory of the insult, in its absence. To examine this, specifically, we investigated whether chronic or oscillating exposure to glucose and SFA concentrations would promote the TLR4/NFκB signalling pathway in human adipose tissue and/or isolated adipocytes. Hence, in addition to observing TLR4 and NFκB protein expression, we investigated key components of the TLR4/NFκB intracellular signalling pathways, including MyD88, TRAF6 and IKKβ. Furthermore, we also determined whether the effects induced by glucose and SFAs and long-term or oscillating exposure are mediated through both the c-Jun N terminal kinase (JNK) and NFκB pathways.
2. Methods and materials
2.1. Subjects
For the purposes of tissue culture, abdominal subcutaneous (Abd Sc) adipose tissue was obtained from a cohort of female subjects (age: 45±3.3 years; body mass index: 21.9±2.4 kg/m2, n=6). All human adipose tissue was obtained through elective, liposuction surgery with informed consent, in accordance with guidelines of the Coventry and Warwickshire ethics committee. Subjects providing fat samples were not on endocrine therapy (e.g., steroids, HRT, thyroxine) or receiving any antihypertensive therapy.
2.2. Isolation and cell culture of adipocyte and Abd Sc AT
As previously published [22], Abd Sc adipose tissue was initially washed with lysis buffer [ammonium chloride (NH4Cl) 8.24g, potassium bicarbonate (KHCO3) 1.001g, EDTA 0.5 M] to remove erythrocytes and leukocytes. The Abd Sc adipose tissue was then separated from the blood fraction by centrifugation at 360×g for 30 s.
To isolate the Abd Sc adipocytes, the adipose tissue was washed with 1× Hank's balanced salt solution (HBSS) containing penicillin (100 U/ml) and streptomycin (100 μg/ml). All adipose tissue was digested with the same batch of collagenase class 1 (2 mg/ml, Worthington Biochemical) in 1× HBSS (Gibco, Paisley, UK) for between 30 min and 1 h at 37°C in a water bath and shaken at 100 cycles per minute [23–25]. The disrupted tissue was filtered through a double-layered cotton mesh and pre-adipocytes and adipocytes separated by centrifugation at 360×g for 5 min.
Following centrifugation, the upper layer of mature adipocytes was removed from the collagenase-dispersed preparation, washed in phenol red-free medium DMEM:F12 twice and centrifuged at 360×g for 2 min.
Abd Sc adipose tissue (500 μl) and Abd Sc adipocytes (500 μl, equivalent to 250,000 adipocytes) were placed in specialised permeable inserts within 12-well plates to allow easy transfer of samples to new 12-well plates with fresh media. For the chronic treatments, the samples were cultured in phenol red-free DMEM-F-12 medium containing penicillin and streptomycin, in addition to low-glucose (L-Glc): 5.6 mM or high glucose (H-Glc): 17.5 mM in combination with low (0.2 mM) and high (2 mM) doses of a palmitate:stearic mix (referred to hereafter as SFA), as well as high glucose alone 17.5 mM, for 48 h.
SFA was prepared as 40 mM stocks by dissolving Stearic:Palmitic acid Mixture (Sigma-Aldrich, Cambridge, UK) in absolute ethanol and then lyophilising it. The lyophilised SFA was re-constituted in 1 ml 3% bovine serum albumin (free fatty acid-free) in Geys Buffer by vortexing and sonication. Abd Sc adipose tissue and adipocytes maintained in L-Glc: 5.6-mM medium, as well as dissolving buffer without SFA, were used as controls.
For the intermittent treatments, Abd Sc adipose tissue and Abd Sc adipocytes were also cultured using the specialised permeable inserts within the wells of the 12-well plates. The samples were then intermittently exposed to 12-h alternate phases of control media incubation followed by the same previous glucose/SFA treatment, as outlined for chronic treatment. Cell viability was then assessed according to previously described methods [26]. Following incubation of adipocytes (37°C/5% CO2) with their respective treatments, the conditioned media, adipocytes and adipose tissue were separated by centrifugation (360×g for 2 min). The media were removed, aliquoted and stored at −80°C.
Protein was extracted from isolated Abd Sc adipose tissue and adipocytes with RIPA buffer. These samples were subsequently flash-frozen in liquid nitrogen, thawed and spun at 1800×g for 30 min at 4°C. The resulting infranatant was extracted and stored immediately at −80°C.
2.3. Protein determination and Western blot analysis
Extracted protein was quantified via the Bio-Rad DC (detergent compatible) protein assay kit (Bio-Rad, Hemel Hempstead, UK). Adipocyte and adipose tissue protein samples were assessed to determine there was no significant statistical variation between control and treatment regimens. Denatured protein samples (25–50 μg/lane) were loaded onto a 10% gel and blotted onto a Hybond-P membrane (GE Healthcare, Little Chalfont, UK). Following gel electrophoresis and electroblotting, filters were incubated overnight at 4°C with continual motion, with monoclonal NFκB Primary antibody (65 kDa, 1:250; Cambridge BioScience, Cambridge, UK), polyclonal anti-JNK1 and two stress-activated protein kinase phosphospecifics (49 kDa, 55 kDa, 1:1,750, BioSource International) and MyD88 (32 kDa, 1:250, TCS Cell works, Buckingham, UK). Monoclonal antibody to TLR4 (97 kDa, 1:250, ABCAM, Cambridge, UK) and a monoclonal antibody to IKKβ (1:250, TCS Cellworks, Buckingham, UK) followed by anti-mouse conjugated to horseradish peroxidase secondary antibody (The Binding Site, Birmingham, UK) were used. A chemiluminescent detection system ECL/ECL+ (Amersham, Little Chalfont, UK) enabled visualization after exposure to X-ray film. Equal protein loading was confirmed by actin Western blotting, as previously described [27]. Furthermore, absence of contaminating cells (i.e., macrophages) in the isolated adipocytes was confirmed via Western blot for CD45, a specific marker of mononuclear blood cells, as outlined in previous studies [28]. Autoradiographs were quantified by densitometry using Synoptics Group Gene tools Bio Imaging system software (Syngene, Cambridge, UK) according to the manufacturer's guidelines. The bands were first normalised as a function of the loading control (β-actin), then converted to fold change compared with controls (untreated samples).
2.4. Conditioned media cytokine assessment
Cytokine levels secreted into the media from Abd Sc adipose tissue and adipocytes treated with varying glucose and SFA concentrations were measured. For this, conditioned media was utilised in commercially available enzyme-linked immunosorbent assay (ELISA)-based colorimetric kits to determine the quantities of interleukin (IL)-6 and tumor necrosis factor α (TNFα) (QuantiGlo ELISA; R and D Systems, Abingdon, UK).
2.5. Statistical analysis
For assessment of protein expression and secretion, statistical analysis was undertaken using analysis of variance for comparison of control versus treatments. The threshold for significance was P<.05. Data in the text and figures are presented as mean±S.D. or mean±S.E.M. SPSS version 15 was used to perform the statistical analysis.
3. Results
3.1. Effects of chronic treatment with SFA and glucose on TLR4, MyD88 and TRAF-6 protein expression
TLR4 protein levels were significantly increased by exposure to H-Glc (P<.001) or by SFA treatment (explant: P<.001, Ad: P<.01), at either concentration, compared with respective controls (L-Glc 5.6 mM) in both Abd Sc explants and isolated Abd Sc adipocytes (Fig. 1A and 1B). Low SFA treatment was found to significantly reduce MyD88 expression in adipocytes in the presence of H-Glc (P<.01), whereas high SFA treatment suppressed MyD88 expression, independent of glucose concentration, in adipocytes (P<.01). Only H-Glc treatment combined with high SFA treatment produced a decrease in MyD88 expression in explants, compared with control (P<.01; Fig. 1C and 1D). In adipocytes, TRAF-6 protein expression was significantly increased by the presence of SFA treatment (P<.001), with H-Glc levels, alone, inducing a more modest but significant rise (P<.01). TRAF-6 expression in explants was not as strongly influenced by treatment, with only low SFA producing an increase in TRAF6 expression compared with the L-Glc control (P<.01; Fig. 1E and 1F).
Fig. 1.
(A–F) The mean relative protein expression (±S.E.M.) of TLR4, MYD88 and TRAF-6 in explants and isolated, mature adipocytes compared with their respective controls (explant tissue and adipocyte cells maintained in L-Glc (5.6 mM), with representative Western blots shown above. Statistical analysis compared expression of the proteins in explants and cells chronically treated with L-Glc: 5.6 mM or H-Glc: 17.5 mM in combination with low (0.2 mM) and high (2 mM) doses of a palmitate: SFA, as well as high glucose alone 17.5 mM, for 48 h (n=6; ⁎P<.05, ⁎⁎P<.01, ⁎⁎⁎P<.001).
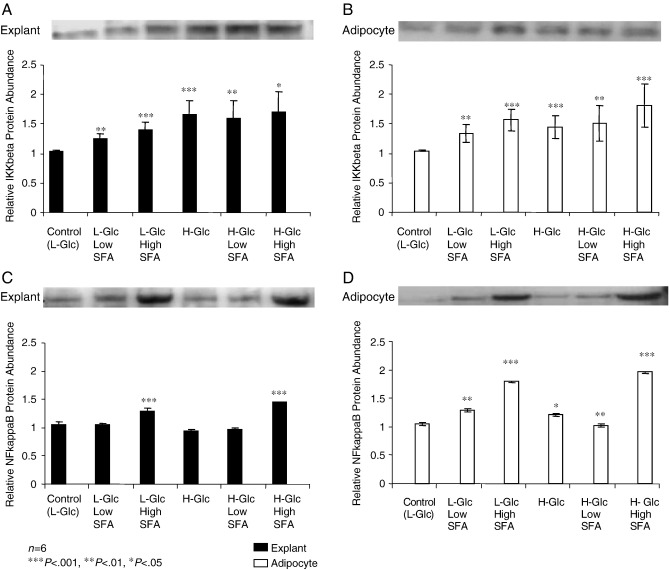
3.2. Effects of chronic treatment with SFA and glucose on IKKβ and NFκB protein expression
IKKβ protein levels were significantly increased when exposed to chronic H-Glc alone in both adipose tissue explants (P<.001) and isolated adipocytes (P<.001) compared with control. Both low and high SFA concentrations induced a significant increase in IKKβ levels, which remained consistent in the presence of H-Glc in adipose tissue explants (P<.01 and P<.05, respectively; Fig. 2A and 2B). A similar significant increase in IKKβ was further observed in isolated adipocytes. In contrast, NFκB protein levels in adipocytes did not mirror the pattern of expression in the explants. Protein expression in adipocytes was significantly increased in the presence of low SFA (P<.01) and high SFA (P<.001) treatment, independent of concentration, whilst H-Glc, alone, induced only a moderate increase (P<.05). However, high SFA concentration led to a highly significant increase in NFκB expression in the adipose tissue explants in the presence of L- and H-Glc (P<.001 and P<.001 respectively; Fig. 2C and 2D).
Fig. 2.
A–D The mean relative protein expression of IKKβ and NFκB (±S.E.M.) in explants and mature adipocytes compared with their respective controls, with representative Western blots shown above. The explants and cells were chronically treated with L-Glc: 5.6 mM or H-Glc: 17.5 mM in combination with low (0.2 mM) and high (2 mM) doses of SFAs, as well as H-Glc alone, for 48 h (n=6; ⁎P<.05, ⁎⁎P<.01, ⁎⁎⁎P<.001).
3.3. Effects of chronic treatment with SFA and glucose on JNK1 and JNK2 protein expression
No statistically significant differences in treatments were observed for either JNK1 or JNK2 protein levels in either adipose tissue explants or in isolated adipocytes compared with controls (data not shown).
3.4. Effects of chronic treatment with SFA and glucose on TNFα and IL-6 secretion
In explants high SFA concentration resulted in marked increases in both IL-6 (Fig. 3A) and TNFα (Fig. 3C) secretion, independent of glucose concentration, compared with control (SFA: plus either L-Glc: P<.05, or H-Glc: P<.01). Adipocytes cultured in high SFA concentration showed a similar pattern (SFA: plus either L-Glc: P<.01, or H-Glc: P<.01; Fig 3B and 3D).
Fig. 3.
(A–D) IL-6 and TNFα fold change in secretion levels (mean±S.E.M.) from control (L-Glc: 5.6 mM) and treated explants and adipocytes. Statistical analysis compared the effect of L-Glc: 5.6 mM or H-Glc: 17.5 mM in combination with low (0.2 mM) and high (2 mM) doses of SFAs, as well as H-Glc alone, on secretion of IL-6 and TNFα, respectively, for 48 h (n=6; ⁎P<.05, ⁎⁎P<.01, ⁎⁎⁎P<.001). (a and b) IL-6 control (mean±S.E.M.) Explants (Exp): 1212.0±299.22 pg/ml; Adipocytes (Ad): 12798.0±5954.16 pg/ml. (c and d) TNFα control (mean±S.E.M.) Exp: 0.30±0.14 pg/ml; Ad: 0.93±0.16 pg/ml.
3.5. Effects of intermittent treatment with SFA and glucose on TLR4, MyD88 and TRAF-6 protein expression
Protein levels of TLR4 were significantly increased in both explants and adipocytes, following 48 h of intermittent treatment with Glc and SFA (Fig. 4A and 4B), with the exception of L-Glc and low SFA on adipocytes. Similarly, MyD88 adaptor protein expression increased with all combinations of Glc and SFA in explants and adipocytes, excluding L-Glc combined with low and high SFA, respectively, in adipocytes — although the latter was close to significance at P=.055 (Fig. 4C and 4D). The intermediary signalling molecule, TRAF-6, also showed increased expression in explants and adipocytes with the varying treatments of Glc and SFA (Fig. 4E and 4F).
Fig. 4.
(A–F) The mean relative protein expression (±S.E.M.) of TLR-4, MyD88 and TRAF6 in explants and isolated adipocytes intermittently treated with L-Glc: 5.6 mM or H-Glc: 17.5 mM in combination with low (0.2 mM) and high (2 mM) doses of SFAs, as well as high glucose alone 17.5 mM, for 48 h, compared with controls (n=6; ⁎P<.05; ⁎⁎P<.01; ⁎⁎⁎P<.001).
3.6. Effects of intermittent treatment with SFA and glucose on IKKβ and NFκB protein expression
In the case of IKKβ (Fig. 5A and 5B) and NFκB (Fig. 5C and 5D), all combinations of Glc and SFA resulted in statistically significant increases in protein expression (range P<.05, P<.001), although for IKKβ expression, the increase was more prominent in the adipocytes than the explants.
Fig. 5.
(A–D) The mean relative protein expression (±S.E.M.) of IKKβ and NFκB in explants and isolated adipocytes intermittently treated with L-Glc: 5.6 mM or H-Glc: 17.5 mM in combination with low (0.2 mM) and high (2 mM) doses of SFAs, as well as high glucose alone 17.5 mM, for 48 h, compared with controls (n=6; ⁎P<.05; ⁎⁎P<.01; ⁎⁎⁎P<.001).
3.7. Effects of intermittent treatment with SFA and glucose on JNK1 and JNK2 protein expression
In contrast to the increased levels of TLR4, MyD88, IKKβ and NFκB proteins, following intermittent treatment with glucose and SFA, no effect was observed when JNK1 and JNK2 proteins were analysed by Western blot (data not shown).
3.8. Effects of intermittent treatment with SFA and glucose on TNFα and IL-6 secretion
3.8.1. 0–12 h
For the duration of 0–12 h, the tissues/cells remained untreated, resulting in no significant change in TNFα or IL-6 secretion (Figs. 6A, 6E, 7A, and 7E).
Fig. 6.
(A–H) The fold change in TNFα secretion levels (mean±S.E.M.) from control (L-Glc:5.6 mM) and explants and adipocytes that went through an alternating treatment regime that consisted of L-Glc for 12 h, then L-Glc: 5.6 mM or H-Glc: 17.5 mM in combination with low (0.2 mM) and high (2 mM) doses of SFAs, as well as H-Glc alone, for a period of 48 h in total (n=6; ⁎P<.05). (a) 0–12 h control (mean±S.E.M.) Exp: 0.39±0.18 pg/ml. (b) 12–24 h Control Exp: 0.32±0.13 pg/ml. (c) 24–36 h Control Exp: 0.29±0.15 pg/ml. (d) 36–48 h Control Exp: 0.28±0.13 pg/ml. (e) 0–12 h Control (mean±S.E.M.) Ad: 6.13±1.18 pg/ml. (f) 12–24 h Control Ad: 0.90±0.20 pg/ml. (g) 24–36 h Control Ad: 0.57±0.20 pg/ml. (h) 36–48 h Control Ad: 0.66±0.23 pg/ml.
Fig. 7.
A–H. The fold change in IL-6 secretion levels (mean±S.E.M.) from control (L-Glc:5.6 mM) and explants and adipocytes that went through alternating treatments of L-Glc for 12 h, then L-Glc: 5.6 mM or H-Glc: 17.5 mM in combination with low (0.2 mM) and high (2 mM) doses of SFAs, as well as H-Glc alone, for a period of 48 h in total (n=6, p-values: P<.05⁎). (a) 0–12 h Control (mean±S.E.M.) Exp: 447.9±160.90 pg/ml. (b) 12–24 h Control (mean±S.E.M.) Exp: 240.22±53.85 pg/ml. (c) 24–36 h Control (mean±S.E.M.) Exp: 193.04±45.16 pg/ml. (d) 36–48 h Control (mean±S.E.M.) Exp: 479.24±247.40 pg/ml. (e) 0–12 h Control (mean±S.E.M.) Ad: 1863.7±95.90 pg/ml. (f) 12–24 h Control Ad: 860.60±272.93 pg/ml. (g) 24–36 h Control Ad: 739.18±221.14 pg/ml. (h) 36–48 h Control Ad: 593.46±193.86 pg/ml.
3.8.2. 12–24 h
Following 12-h treatment with Glc and SFA, at 12–24 h, there was an obvious increase in TNFα and IL-6 secretion from explants and adipocytes with high SFA, independent of glucose concentration, but this was only significant for IL-6 (L-Glc/high SFA: explants P=.05, adipocytes P=.02; H-Glc/high SFA: explants P=.01, adipocytes P=.006 — although TNFα was often close to significance (Figs. 6B, 6F, 7B and 7F).
3.8.3. 24–36 h
During the “rest” phase of 24–36 h, in which the tissue and cells were incubated in low Glc media alone, the explants and cells, previously treated with high SFA, produced higher levels of IL-6, irrespective of glucose concentration (L-Glc/high SFA: explants P=.05; adipocytes P=.016; H-Glc/high SFA: adipocytes P=.004; Fig. 7C and 7G). However, only high glucose and high SFA influenced TNFα secretion in adipocytes (P=.04; Fig. 6G).
3.8.4. 36–48 h
Following a 12-h treatment period (36–48 h), both TNFα and IL-6 levels remained high, overall, with high SFA treatment (Figs. 6D, 6H, 7D, and 7H). However, only IL-6 demonstrated significantly higher levels in adipocytes treated with low glucose and high SFA compared with control (P=.03), although several samples were close to significance (Fig. 7H).
4. Discussion
The current study addressed the hypothesis that chronic over-nutrition of glucose and dietary lipid may induce long-term adverse effects via activation of the innate immune response within human adipose tissue. More specifically, that chronic or oscillating glucose and SFA levels may activate the adipose tissue TLR4/NFκB signalling pathway, leading to the production of downstream proinflammatory adipocytokines. As such, acute oscillating fluctuation in the cells' nutrient environment may induce a chronic, longer lasting proinflammatory response, which, in turn, could mediate further deleterious consequences for the progression of metabolic disease.
Our present studies demonstrated that both SFAs and high glucose exposure up-regulated TLR4 expression in Abd Sc adipose tissue and isolated Abd Sc adipocytes — a finding consistent with previous observations in a variety of other cell types [22,29–32]. Both chronic and oscillating treatment regimens significantly increased expression of TLR4 and the intracellular signalling molecule, TRAF6. However, chronic and oscillating treatments had subtle differential effects on the adaptor molecule, MyD88. As such, chronically treated cell cultures appeared to suppress MyD88 expression whilst oscillating treatment significantly up regulated the protein. This divergence between the two types of treatment conditions may occur due to activation of both the MyD88-dependent and the MyD88 independent pathways, which respond to the inflammatory insult via TLR4 stimulation [33,34]. These data suggest that the MyD88 independent pathway is of key importance in adipose tissue in maintaining sensitivity to the stimuli, as our findings highlight that chronically treated adipose cells have increased pro-inflammatory cytokine release. This indicates that, whilst intracellularly the cell may aim to desensitise itself to the insult, in the innate immune pathway there is still a heightened, continued inflammatory response. In an evolutionary context this may have been important so that adipose tissue, which resides underneath the dermal basal layer, may continue to mount a response to an infection induced by dermal abrasion, whilst the infection persists. However a systemic based insult may lead to a disproportional response, as adipose tissue mass expands beyond its' essential requirements. It should also be stressed that, although distinct pathways may be invoked, there is still a strong interplay between the MyD88 dependent and independent pathways, with TRAF6 being modulated by both signalling systems and therefore possessing an intermediary role. Stimulation of these MyD88 pathways ultimately results in the activation of IKKβ and NFκB, the latter of which is a master switch and central regulator of innate immunity and related functions [35]. The activation of IKKβ and NFκB is noted by our studies, as adipose cells treated with either chronic or oscillating culture conditions increased protein expression of these factors, although interestingly, chronic treatment with SFAs had a more pronounced effect on both the intracellular signal and resulting adipocytokines in both explants and isolated Abd Sc adipocytes.
In terms of adipocytokines, it was evident that high levels of SFA had a prominent effect in both the chronic and oscillating treated cultures. In both instances, secretion of IL-6 and TNFα followed a similar pattern — although significance was only observed for both IL-6 and TNFα in the chronically treated explants and adipocytes. Such data highlights the potential risk of a continued high fat diet on inducing an inflammatory response, as it appears to be more pronounced than the glucose induced response. This would also appear to align with clinical studies that suggest hyperlipidaemia may impact more significantly over time than hyperglycaemia in the pathogenesis of metabolic disease [21].
In addition to our observations on chronically treated cultures, analysis of adipokine release in the adipose cells undergoing oscillating treatment demonstrated a form of metabolic memory, as high SFA treated cultures continued to secrete high levels of IL-6 in absence of treatment when cultures were exposed to normal levels of glucose (24–36 h). This observation may, in part, be attributed to activation of the MyD88 independent pathway, as this mechanism leads to delayed kinetics in the instigation of NFκB and, hence, the subsequent later onset of IL-6 secretion [36]. Previous studies have also observed this late phase activation of NFκB in the macrophages of MyD88 knockout mice [37]. The findings from these and our present studies indicate a system of inducing a fast, early response to invading pathogens and a late phase inflammatory response — perhaps to prolong the inflammatory defence mechanisms and ensure neutralisation of the potential pathogen.
The present studies also noted a strong innate immune response from both the explants and the isolated adipocytes with chronic and oscillating stimuli. These findings corroborate our previous studies identifying the importance of the role of the adipocyte within adipose tissue [23–25,38]. Many studies have suggested that it is the macrophage within adipose tissue that exerts the defining inflammatory response, yet it is interesting to note the order of magnitude in response to SFA or glucose did not differ greatly between adipose tissue explants and isolated adipocytes. Therefore, our current findings continue to highlight the importance of the adipocyte and indicate that macrophage infiltration of adipose tissue, commonly observed in obese/T2DM subjects, may be initiated by the capacity of the isolated adipocyte to mount an autonomous immune response to environmental factors. However, it should be noted that the adipose tissue was not taken from subjects with either T2DM or a high degree of adiposity, which could clearly impact on the findings and would be important to consider in future studies.
These current findings provide evidence that adipose tissue plays a dynamic role in mechanisms of the innate immune response in human subjects and provides additional data to our growing understanding of the inflammatory pathways in adipose tissue and adipocytes, as well as the factors that activate them [12,39–41]. Irrespective of hyperglycaemic conditions, hyperlipidaemia has a profound effect on the increased production of the inflammatory cytokines, ranging from a 30–40-fold increase in TNF-α and IL-6 production, with consistent up-regulation of NFκB components in human Abd Sc adipose tissue explants and adipocytes. This study implicates elevated SFAs as a key instigator of the inflammatory response in both adipose tissue and adipocytes, via NFκB. The temporal differences that arise with activation of the TLR4 signalling pathways may once have been advantageous in maintaining a sustained inflammatory response to endotoxin/invading pathogens. However, in an environment of continuous dietary grazing, in which these pathways are stimulated by SFAs and glucose, there are clear implications for the pathogenesis of metabolic disease [34,42,43]. Based upon our present in vitro findings, it is apparent that maintained periods of fasting and potential change in dietary lipids are required to protect against sustained inflammation.
Acknowledgments
This is a contribution from the Warwickshire Institute for the study of Diabetes, Endocrinology and Metabolism (WISDEM). We would like to acknowledge the Egyptian Government for funding Elham Youssef-Elabd, a visiting PhD student within the team. We would also like to thank the British Heart Foundation for funding Alison Harte on an Intermediate fellowship and the Research Council UK for funding Gyanendra Tripathi on a RCUK fellowship. Lastly, we would like to thank Birmingham Science City for supporting this research.
Footnotes
The authors have nothing to disclose and state that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;14(444(7121)):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 2.Schaeffler A., Gross P., Buettner R., Bollheimer C., Buechler C., Neumeier M. Fatty acid-induction of Toll-like receptor-4/nuclear factor-B pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126(2):233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolowczuk I., Verwaerde C., Viltart O., Delanoye A., Delacre M., Pot B. Feeding our immune system: impact on metabolism. Clin Dev Immunol. 2008;639803:2008. doi: 10.1155/2008/639803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ceolotto G., Gallo A., Miola M., Sartori M., Trevisan R., Del Prato S. Protein kinase C activity is acutely regulated by plasma glucose concentration in human monocytes in vivo. Diabetes. 1999;48(6):1316–1322. doi: 10.2337/diabetes.48.6.1316. [DOI] [PubMed] [Google Scholar]
- 5.Dandona P., Chaudhuri A., Ghanim H., Mohanty P. Proinflammatory effects of glucose and anti-inflammatory effect of insulin: relevance to cardiovascular disease. J Cardiol. 2007;99(4A):15B–26B. doi: 10.1016/j.amjcard.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Jain S.K., Kannan K., Lim G., Matthews-Greer J., McVie R., Bocchini J.A., Jr Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003;26(7):2139–2143. doi: 10.2337/diacare.26.7.2139. [DOI] [PubMed] [Google Scholar]
- 7.Jialal I., Devaraj S., Venugopal S.K. Oxidative stress, inflammation, and diabetic vasculopathies: the role of alpha tocopherol therapy. Free Radic Res. 2002;36(12):1331–1336. doi: 10.1080/1071576021000038531. [DOI] [PubMed] [Google Scholar]
- 8.Shanmugam N., Reddy M.A., Guha M., Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52(5):1256–1264. doi: 10.2337/diabetes.52.5.1256. [DOI] [PubMed] [Google Scholar]
- 9.Kim F., Pham M., Luttrell I., Bannerman D.D., Tupper J., Thaler J. Toll-Like Receptor-4 mediates vascular inflammation and insulin resistance in diet induced obesity. Circ Res. 2007;100(11):1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.Y., Sohn K.H., Rhee S.H., Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276(20):16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.Y., Hwang D.H. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cells. 2006;21(2):174–185. [PubMed] [Google Scholar]
- 12.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15(8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 13.Festa A., D'Agostino R., Jr, Tracy R.P., Haffner S.M. Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of Type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;4:1131–1137. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 14.Hu F.B., Meigs J.B., Li T.Y., Rifai N., Manson J.E. Inflammatory markers and risk of developing Type 2 diabetes in women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M.I., Duncan B.B., Sharrett A.R., Lindberg G., Savage P.J., Offenbacher S. Markers of Inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;15(353(9165)):1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 16.Spranger J., Kroke A., Möhlig M., Hoffmann K., Bergmann M.M., Ristow M. Inflammatory cytokines and the risk to develop Type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52(3):812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 17.Frank R.N. Metabolic memory in diabetes is true long-term memory. Arch Ophthalmol. 2009;127(3):330–331. doi: 10.1001/archophthalmol.2008.607. [DOI] [PubMed] [Google Scholar]
- 18.Ihnat M.A., Thorpe J.E., Ceriello A. Hypothesis: the ‘metabolic memory’, the new challenge of diabetes. Diab Med. 2007;24(6):582–586. doi: 10.1111/j.1464-5491.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 19.LeRoith D., Fonseca V., Vinik A. Metabolic memory in diabetes — focus on insulin. Diabetes Metab Res Rev. 2005;21(2):85–90. doi: 10.1002/dmrr.530. [DOI] [PubMed] [Google Scholar]
- 20.O'Keefe J.H., Bell D.S. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100(5):899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 21.Haffner S.M., Stern M.P., Hazuda H.P., Mitchell B.D., Patterson J.K. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263:2893–2898. doi: 10.1001/jama.263.21.2893. [DOI] [PubMed] [Google Scholar]
- 22.Kim H.S., Han M.S., Chung K.W., Kim S., Kim E., Kim M.J. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27(2):321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Creely S.J., McTernan P.G., Kusminski C.M., Fisher M., Da Silva N.F., Khanolkar M. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and Type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292(3):E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 24.Kos K., Baker A.R., Jernas M., Harte A.L., Clapham J.C., O'Hare J.P. DPP-IV inhibition enhances the antilipolytic action of NPY in human adipose tissue. Diabetes Obes Metab. 2009;11(4):285–292. doi: 10.1111/j.1463-1326.2008.00909.x. [DOI] [PubMed] [Google Scholar]
- 25.Kos K., Harte A.L., O'Hare P.J., Kumar S., McTernan P.G. Ghrelin and the differential regulation of des-acyl (DSG) and oct-anoyl ghrelin (OTG) in human adipose tissue (AT) Clin Endocrinol (Oxf) 2009;70(3):383–389. doi: 10.1111/j.1365-2265.2008.03321.x. [DOI] [PubMed] [Google Scholar]
- 26.McTernan P.G., Anwar A., Eggo M.C., Barnett A.H., Stewart P.M., Kumar S. Gender differences in the regulation of P450 aromatase expression and activity in human adipose tissue. Int J Obes Relat Metab Disord. 2000;24(7):875–881. doi: 10.1038/sj.ijo.0801254. [DOI] [PubMed] [Google Scholar]
- 27.McTernan P.G., Harte A.L., Anderson L.A., Green A., Smith S.A., Holder J.C. Insulin and rosiglitazone regulation of lipolysis and lipogenesis in human adipose tissue in vitro. Diabetes. 2002;51(5):1493–1498. doi: 10.2337/diabetes.51.5.1493. [DOI] [PubMed] [Google Scholar]
- 28.McTernan P.G., McTernan C.L., Chetty R., Jenner K., Fisher F.M., Lauer M.N. Increased resistin gene and protein expression in human abdominal adipose tissue. J Clin Endocrinol Metab. 2002;87(5):2407. doi: 10.1210/jcem.87.5.8627. [DOI] [PubMed] [Google Scholar]
- 29.Dasu M.R., Devaraj S., Zhao L., Hwang D.H., Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57(11):3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan J., Frey R.S., Malik A.B. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J Clin Invest. 2003;112(8):1234–1243. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saillan-Barreau C., Cousin B., André M., Villena P., Casteilla L., Pénicaud L. Human adipose cells as candidates in defense and tissue remodeling phenomena. Biochem Biophys Res Commun. 2003;309(3):502–505. doi: 10.1016/j.bbrc.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 32.Yang X., Murthy V., Schultz K., Tatro J.B., Fitzgerald K.A., Beasley D. Toll-like receptor 3 signaling evokes a proinflammatory and proliferative phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291(5):H2334–H2343. doi: 10.1152/ajpheart.00252.2006. [DOI] [PubMed] [Google Scholar]
- 33.Björkbacka H., Fitzgerald K.A., Huet F., Li X., Gregory J.A., Lee M.A. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol Genomics. 2004;19(3):319–330. doi: 10.1152/physiolgenomics.00128.2004. [DOI] [PubMed] [Google Scholar]
- 34.Kelley D.S., Siegel D., Fedor D.M., Adkins Y., Mackey B.E. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr. 2009;139(3):495–501. doi: 10.3945/jn.108.100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayden R.E., Davies N.J., Khanim F.L., Birtwistle J., Delgado J., Pearce C. Treatment of primary CLL cells with bezafibrate and medroxyprogesterone acetate induces apoptosis and represses the pro-proliferative signal of CD40-ligand, in part through increased 15dDelta(12,14,)PGJ(2) Leukemia. 2009;23(2):292–304. doi: 10.1038/leu.2008.283. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T., Takeuchi O., Fujita T., Inoue J., Mühlradt P.F., Sato S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167(10):5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11(1):115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 38.Baker A.R., Harte A.L., Howell N., Pritlove D.C., Ranasinghe A.M., da Silva N.F., Youssef E.M., Khunti K., Davies M.J., Bonser R.S. Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab. 2008;94(1):261–267. doi: 10.1210/jc.2007-2579. [DOI] [PubMed] [Google Scholar]
- 39.Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shoelson S.E., Goldfine A.B. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nat Med. 2009;15(4):373–374. doi: 10.1038/nm0409-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;(8):921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall W.L. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev. 2009;22(1):18–38. doi: 10.1017/S095442240925846X. [DOI] [PubMed] [Google Scholar]
- 43.Sharma A.M., Chetty V.T. Obesity, hypertension and insulin resistance. Acta Diabetol. 2005;42(Suppl 1):S. doi: 10.1007/s00592-005-0175-1. [DOI] [PubMed] [Google Scholar]