Abstract
Provoked vestibulodynia, the most common form of vulvodynia (unexplained pain of the vulva), is a prevalent, idiopathic pain disorder associated with a history of recurrent candidiasis (yeast infections). It is characterized by vulvar allodynia (painful hypersensitivity to touch) and hyperinnervation. We tested whether repeated, localized exposure of the vulva to a common fungal pathogen can lead to the development of chronic pain. A subset of female mice subjected to recurrent Candida albicans infection developed mechanical allodynia localized to the vulva. The mice with allodynia also exhibited hyperinnervation with peptidergic nociceptor and sympathetic fibers (as indicated by increased protein gene product 9.5, calcitonin gene–related peptide, and vesicular monoamine transporter 2 immunoreactivity in the vaginal epithelium). Long-lasting behavioral allodynia in a subset of mice was also observed after a single, extended Candida infection, as well as after repeated vulvar (but not hind paw) inflammation induced with zymosan, a mixture of fungal antigens. The hypersensitivity and hyperinnervation were both present at least 3 weeks after the resolution of infection and inflammation. Our data show that infection can cause persistent pain long after its resolution and that recurrent yeast infection replicates important features of human provoked vulvodynia in the mouse.
INTRODUCTION
Pain is a cardinal feature of the inflammatory response to fungal, bacterial, and viral infections. In most cases, pain rapidly disappears with the resolution of the infection. Although acute pain is effectively managed with currently available analgesic strategies, chronic pain remains poorly treated. Pain secondary to previous and resolved infection is suspected to underlie numerous idiopathic chronic pain conditions, including urogenital pain (vulvodynia, endometriosis, and prostatitis), interstitial cystitis, and inflammatory bowel syndrome [for example, (1)], but a causal relationship between infection and persistent pain has not been demonstrated. To test whether such a causal relationship can exist, we developed a mouse model of provoked vestibulodynia (PVD), a chronic urogenital pain condition suspected to result from repeated infection by a common pathogen, the yeast Candida albicans.
Of the idiopathic pain conditions associated with a history of previous infection, vulvodynia (vulvar pain) is the most prevalent, affecting 9 to 12% of women of childbearing age (2). The predominant form of vulvodynia, PVD (previously known as vulvar vestibulitis), is characterized by burning and cutting pain localized to the vulvar vestibule in response to light touch (vulvar mechanical allodynia), with physical findings limited to occasional erythema (3). Chronic vulvar pain is associated with significant psychological distress because of its interference with sexual intercourse and nonsexual activities (bike riding, walking, and even standing); as a result, mood disturbances and reduced quality of life are often reported in this population (4). Reduced vulvar tactile and pain thresholds in PVD have been experimentally confirmed with standardized mechanical stimuli (5). Histological changes in vulvar vestibule tissue, including increased density of free nerve endings (6, 7), suggest that neural mechanisms may underlie these clinical symptoms. Brain imaging of patients with PVD reveals patterns of activity similar to those observed in experimental and clinical pain, as well as neuroanatomical abnormalities suggestive of compensatory central reorganization secondary to chronic pain (8).
No definitive causes of PVD have yet been identified. However, women with PVD have a high prevalence of recurrent vulvovaginal candidiasis (RVVC; or recurrent yeast infections), defined as three or more yeast infections annually, compared to healthy women: 42 to 60% (9, 10) versus 5 to 8%, respectively (11). The commensal yeast, C. albicans, is thought to cause 85 to 90% of all yeast infections in women (11). The comorbidity between RVVC and chronic vulvar pain has led to the hypothesis that vulvar hypersensitivity in PVD results from abnormal sensory processing secondary to past inflammation from prolonged and/or repeated vaginal yeast colonization. This correlational hypothesis remains untested. Here, we assess whether persistent vulvar mechanical hypersensitivity can develop in mice after multiple rounds of vulvovaginal infection or after a single, long-lasting infection with C. albicans.
RESULTS
RVVC can cause vulvar allodynia
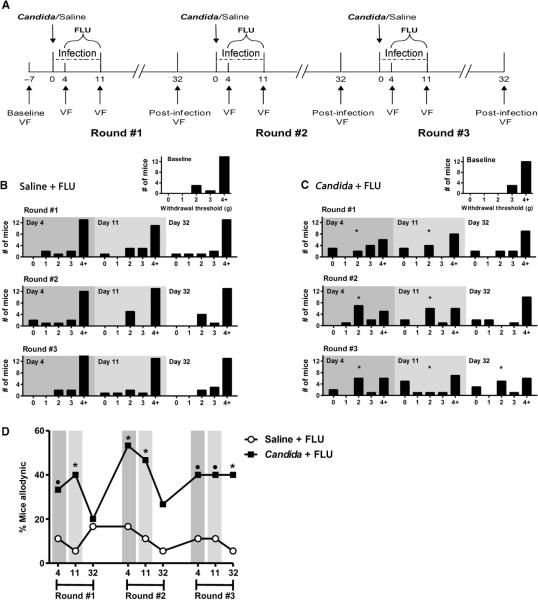
Mice were tested for baseline mechanical sensitivity of the vulva and hind paws with von Frey filaments. For each round of infection with C. albicans strain SC5314 cells, mice were tested again at 4 days (active infection), 11 days (treated infection), and 32 days (3 weeks after infection resolution) after inoculation (Fig. 1A). Quantification of vaginal fungal burden confirmed infection status at each time point (fig. S1A). At baseline, most mice (79%) exhibited vulvar mechanical withdrawal thresholds exceeding 4.0 g, with no between-group differences (χ2 = 5.0, P = 0.17). This was true at every testing session for mice in the fluconazole control group (Saline + FLU; Fig. 1B). By contrast, the group of mice with repeated infections (Candida + FLU; Fig. 1C) was allodynic during active SC5314 infection (day 4) and was still allodynic after treatment (day 11). By day 32, when the infection was long resolved, evidence of allodynia was absent after the first two rounds of infection. However, after the third round of infection, persistent allodynia was present (χ2 = 9.5, P = 0.02). We obtained similar results when we applied a within-subjects analysis, defining allodynic mice as those displaying a ≥33% decrease in vulvar threshold from their own baseline (Fig. 1D). On day 32 after the third round of infection, 40% (6 of 15) of the infected subjects were allodynic by this definition compared to 5.5% (1 of 18) of the fluconazole control subjects (P = 0.02, one-tailed Fisher's exact test). This allowed the separation of mice in the Candida + FLU group into allodynic and nonallodynic subgroups, from which tissues were obtained for immunohistochemical studies (see below). There were no statistically significant alterations in mechanical sensitivity of the hind paw produced by fluconazole or strain SC5314 over the course of the three rounds of infection (fig. S2).
Fig. 1.
Development of vulvar mechanical allodynia in a subset of mice after multiple rounds of vulvovaginal candidiasis with C. albicans strain SC5314. (A) The experimental timeline illustrates the experimental procedures across three rounds of vulvovaginal infections with SC5314. Inoculations of 5 × 104 cells were given on day 0 for each of three infections (for second and third inoculations, SC5314 was administered no more than 1 week after the previous vulvar and hind paw sensitivity testing). Behavioral measurements of vulvar mechanical sensitivity [von Frey (VF)] were taken at baseline and at three points during each infection: days 4, 11, and 32. Note that for each day 32 measurement, yeast was absent from the vaginal cavity for 3 weeks before testing. (B and C) Frequency histograms showing the number of subjects displaying 50% withdrawal thresholds (jumping up with all four paws; see Materials and Methods) in five arbitrarily defined bins (0: 0 to 0.99 g; 1: 1.0 to 1.99 g; 2: 2.0 to 2.99 g; 3: 3.0 to 3.99 g; 4+: >4.0 g) at each testing session. Infection status is indicated by shading. Mice were inoculated vaginally with saline (B) or 5 × 104 SC5314 cells (C) on day 0 of each infection round (n = 15 to 18 per group); all mice received fluconazole (FLU; 15 mg/kg, orally, once daily) from day 4 to day 11. *P < 0.05 by χ2 analysis compared to within-group baseline. (D) Mice displaying ≥33% decreases in withdrawal threshold compared to their own baseline at each testing session. *P < 0.05 compared to Saline + FLU group by one-tailed Fisher's exact test; •P < 0.10 compared to Saline + FLU group.
RVVC does not have morphological or inflammatory effects
Visual inspection of hematoxylin and eosin (H&E)–stained sections obtained after the third infection revealed no edema and no obvious intergroup differences in inflammatory infiltrate (fig. S3). The presence of a small number of immune cells is typical of healthy vaginae, and a few basophils, macrophages, and mast cells were evident throughout the lamina propria and along blood vessel walls in all groups. Inflammatory cells did not penetrate the epithelial layer. No evidence of altered vulvar epithelial morphology was found in Saline + FLU or in Candida + FLU mice (allodynic or nonallodynic) after the resolution of the third infection (fig. S3). Epithelial thickness at the broad (F3,19 = 1.4, P = 0.29) and narrow (F3,19 = 0.8, P = 0.50) aspects of the epithelium did not differ between groups, and in all cases, the keratin layer was intact across the posterior surface of the vulva (table S1). Whereas fungal burden covaried with hypersensitivity during acute infection, leukocyte levels showed no such correlation (table S2).
Only allodynic RVVC mice show increases in vulvar innervation
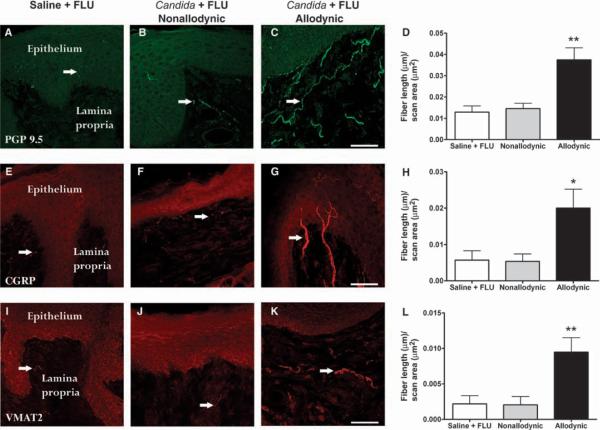
Immunohistochemical analyses after repeated infection in allodynic (n = 3 to 6) mice, after repeated infection in nonallodynic (n = 5) mice, and in fluconazole control (n = 4) mice were conducted on postmortem vulvar tissue. We observed an almost 300% increase in the density of nerve fibers [as detected by immunoreactivity (IR) for the pan-axonal marker protein gene product 9.5 (PGP 9.5)] throughout the lamina propria of vulvar tissue taken from allodynic compared to nonallodynic and control mice (F2,10 = 12.8, P = 0.002; Fig. 2, A to D). The increased density reflected both increased number of fibers and thicker, longer fibers. A significant, almost 400% increase in the density of peptidergic fibers, as assessed by calcitonin gene–related peptide (CGRP)–IR, was found in allodynic animals compared to the nonallodynic group (F2,14 = 4.6, P = 0.03; Fig. 2, E to H). In all groups, CGRP-IR fibers were observed throughout the lamina propria, but few fibers penetrated the basal cell layer of the epithelium. Allodynic mice displayed increased (more than four times higher) sympathetic innervation, as revealed by vesicular monoamine transporter 2 (VMAT2)–IR, compared to nonallodynic and control groups (F2,12 = 8.0, P < 0.01; Fig. 2, I to L). Sympathetic fibers in all mice were typically distributed in the deeper layers of the lamina propria; in allodynic mice, there was increased fiber density, with some thin processes seen to penetrate the lamina propria beneath the epithelium (Fig. 2K).
Fig. 2.
Allodynic mice previously infected with multiple rounds of vulvovaginal candidiasis have increased expression of total, peptidergic, and sympathetic fibers compared to nonallodynic mice and controls (n = 4 to 6 mice per group). (A, E, and I) Saline + FLU group. (B, F, and J) Non-allodynic Candida + FLU subgroup. (C, G, and K) Allodynic Candida + FLU subgroup. (D, H, and L) Bars represent mean ± SEM fiber length (μm) per unit area (μm2). Total nerve fiber density (top row; PGP 9.5–IR) is significantly increased in the allodynic Candida + FLU subgroup, with long fibers lining the lamina propria beneath the epithelium (C and D). Peptidergic nerve fibers immunoreactive for CGRP, which normally consist of fine processes throughout the lamina propria that occasionally penetrate the basal cell layer of the epithelium, are significantly increased in the allodynic Candida + FLU group (G and H) and represent about half of the total fiber population (compare y axes of D and H). Sympathetic nerve fibers immunoreactive for VMAT2 sparsely innervate the upper lamina propria in normal and nonallodynic mice, whereas a significant increase in innervation density is observed in the allodynic Candida + FLU group (K and L). *P < 0.05; **P < 0.01 compared to all other groups by one-way ANOVA followed by Tukey's post hoc test. Scale bars, 50 μm [(C), (G), and (K)]. Arrows point to fibers.
Extended primary fungal infection can cause vulvar allodynia
In a new experiment, mice were tested for vulvar and hind paw mechanical sensitivity throughout a single but extended-duration (14-day) vulvar C. albicans strain SC5314 infection (Fig. 3A). Vaginal fungal burden throughout the extended infection is shown in fig. S1B. At baseline, 96% of this cohort exhibited vulvar mechanical withdrawal thresholds exceeding 4.0 g, with no between-group differences (χ2 = 0.7, P = 0.41). The fluconazole control group continued to exhibit unchanged thresholds throughout the experiment (Fig. 3B). In contrast, a significant proportion of extended-infection mice became allodynic after the acute phase of SC5314 infection (day 14; χ2 = 8.1, P = 0.005) (Fig. 3C). This hypersensitivity persisted after completion of antifungal treatment (day 21; χ2 = 5.0, P < 0.05) and 3 weeks after infection resolution (day 42, χ2 = 6.9, P < 0.01). A large proportion of mice (86% exhibiting a >66% reduction from baseline threshold) continued to display allodynic behavior up to day 70, which was 7 weeks after the resolution of the SC5314 infection (χ2 = 14.4, P < 0.001) (Fig. 3C). No alterations in hind paw mechanical sensitivity were observed throughout the experiment (fig. S4).
Fig. 3.
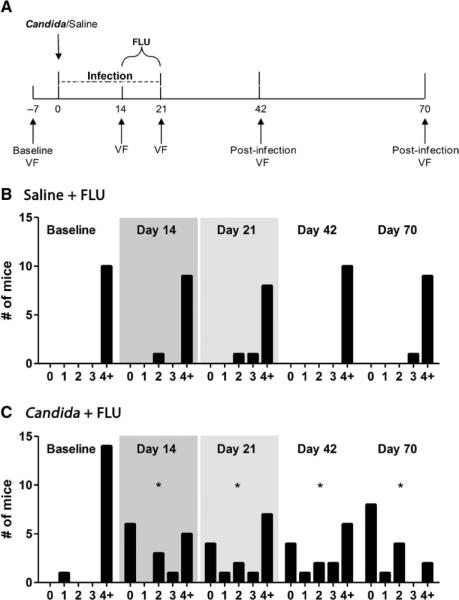
Development of vulvar mechanical allodynia in a subset of mice after a single, extended SC5314 infection. (A) Experimental time-line illustrating the experimental procedures. An inoculation of 5 × 104 SC5314 cells was given on day 0; fluconazole (FLU; 15 mg/kg, orally, once daily) treatment occurred from day 14 to day 21. Behavioral measurements of vulvar mechanical sensitivity [von Frey (VF)] were taken at baseline (day −7) and at 14, 21, 42, and 70 days after inoculation. (B and C) Frequency histograms showing the number of subjects (n = 10 to 15 per group) in the Saline + FLU (B) and Candida + FLU (C) groups displaying 50% withdrawal thresholds in five arbitrarily defined bins (0: 0 to 0.99 g; 1: 1.0 to 1.99 g; 2: 2.0 to 2.99 g; 3: 3.0 to 3.99 g; 4+: >4.0 g) at each testing session. Infection status is indicated by shading. *P < 0.05 by χ2 analysis compared to within-group baseline.
Repeated vulvar exposure to zymosan produces vulvar allodynia
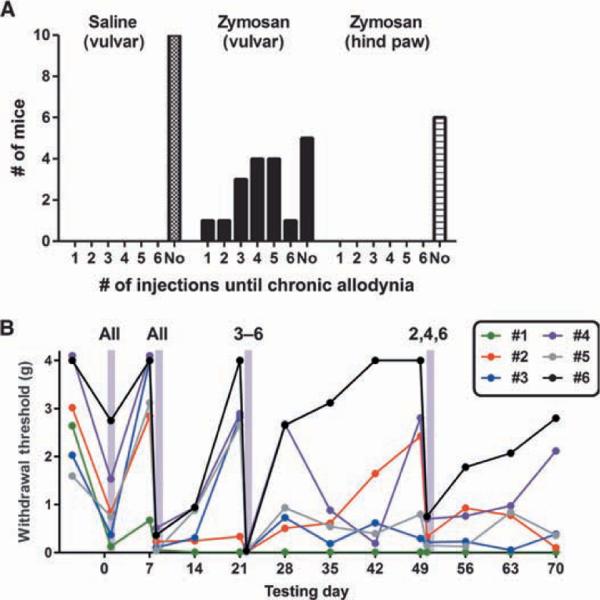
To determine whether the persistent vulvar allodynia observed with SC5314 infection required exposure to a live pathogen, we subjected a new cohort of mice to repeated vulvar injections of the yeast cell wall glucan zymosan (or saline, using baseline sensitivity–matched controls). Each mouse in the experimental group received two vulvar injections of zymosan, a week apart; each week thereafter, mice were reinjected only if they recovered to nonallodynic levels of mechanical sensitivity (>66% of baseline thresholds). This design allowed us to assess individual variability in the number of zymosan injections required to produce persistent vulvar allodynia. Figure 4A shows the frequency distribution of the full data set and the number of zymosan injections required to achieve chronic vulvar allodynia in the salin-eversus zymosan-treated groups (χ2 = 14.8, P < 0.001). We found considerable individual variation in the number of zymosan injections needed to induce persistent allodynia; data from the first six mice to be tested are shown in Fig. 4B to depict the range of patterns observed. Four hours after the first injection of vulvar zymosan, all mice displayed allodynia; for most mice, this allodynia completely resolved within a week. A single mouse (#1 in Fig. 4B) remained allodynic after the single inflammatory insult. Four hours after the second injection of zymosan, all mice showed robust vulvar allodynia; a week later, all mice remained allodynic. However, with each subsequent week, some mice maintained the allodynic state (#1 and #2 in Fig. 4B), whereas other mice returned to baseline and either required additional injections to achieve persistent allodynia (#2, #3, and #5) or never became persistently allodynic (#4 and #6). A separately performed experiment in which zymosan was injected into the hind paw revealed no evidence whatsoever of persistent allodynia development in any of the mice tested (Fig. 4A, right). In all cases, hind paw hypersensitivity resolved within a week and no chronic allodynia was ever observed even after repeated zymosan injections.
Fig. 4.
Development of vulvar mechanical allodynia in a subset of mice after repeated vulvar injections of zymosan. (A) Frequency histogram showing the proportion of female mice displaying chronic allodynia after one to six weekly (or less) injections of vulvar saline (left; n = 10), vulvar zymosan (middle; n = 19), or hind paw zymosan (right; n = 6). “No” indicates that chronic allodynia was never observed, even after six injections. (B) Representative patterns of vulvar mechanical sensitivity over time, using data from the first six mice to be tested (#1 to #6). Vulvar zymosan was injected into mice so indicated 4 hours before the data points highlighted in gray.
DISCUSSION
We have observed long-lasting mechanical vulvar hypersensitivity after repeated vulvovaginal infections with the yeast C. albicans. A single, 14- to 21-day-long, fully resolved infection with C. albicans strain SC5314 also induced mechanical allodynia that greatly outlasted the resolution of active inflammation. Finally, comparably long-lasting allodynia was observed in mice receiving multiple vulvar (but not hind paw) injections of zymosan, a mixture of fungal antigens. In all three experiments, only a subset of mice developed allodynia. In the RVVC experiment, the allodynic (but not the nonallodynic) mice displayed a significant increase in the density of vulvar nerve fibers, including identified peptidergic sensory and sympathetic fibers. Allodynia was not accompanied by gross morphological changes in the vulvar mucosa (for example, no reduced epithelial thickness or keratinization). Thus, repeated or extended infection with a common pathogen can induce a pathological pain state that persists long after the resolution of the infection.
As expected, we observed vulvar allodynia during and immediately after each active infection. The onset of vulvar allodynia during the acute infection corresponded with the peak of vaginal fungal burden, suggesting that acute inflammation during active infection can account for acute vulvar allodynia. Clinical reports of vulvovaginal pain during yeast infections are consistent with this finding (11). However, vulvar allodynia persisted despite reductions in fungal burden (day 11), indicating a dissociation between pain symptoms and fungal load. Despite the differences in vaginal and vulvar epithelium morphology (keratinization, thickness, and hormonal regulation), few differences have been identified in innate and/or adaptive immune responses throughout the lower genital tract (12). Innate immunity likely plays a dominant role in the acute response to C. albicans, given that changes associated with adaptive immunity are not observed after yeast infections in mice or women (13). C. albicans is recognized by the Toll-like receptor 2 (TLR-2) and TLR-4, which are expressed on immune and epithelial cells, and engages the innate immune response, including the up-regulation of a yeast-specific pattern of proinflammatory molecules through the nuclear factor κB pathway (14). Innate immune cells recruited during acute inflammation (macrophages, mast cells, and neutrophils) can interact directly with nerve endings to produce pain hypersensitivity and release inflammatory mediators that contribute to pain (15). The presence of TLR-4 on primary afferent endings (16) indicates another potential mechanism by which C. albicans activates nociceptors to induce behavioral hypersensitivity (17). Similarly, zymosan-induced vulvar allodynia may result from acute inflammation mediated by TLR-2 and TLR-6 and/or the direct sensitization of vulvar mechanoreceptors (18).
We have observed here that mechanical hypersensitivity can persist long after the resolution of the active infection. During the first and second rounds of candidiasis in the RVVC experiment, acute vulvar hypersensitivity resolved after antifungal treatment and was absent 21 days after infection resolution (day 32). By contrast, during the third round of infection, the vulvar hypersensitivity observed during the active stages of infection was maintained long after yeast were absent from the vaginal cavity. Moreover, chronic hypersensitivity was also evident after a single, extended infection with Candida, and the phenomenon could still be observed up to 7 weeks after infection resolution. Finally, some mice given as few as two zymosan injections exhibited allodynia lasting at least 11 weeks, indicating that the development of long-lasting hypersensitivity does not require a live pathogen and may be generalizable to fungi other than C. albicans (that is, Saccharomyces cerevisiae). The extended period of allodynia after the disappearance of detectable inflammation suggests that the chronic hypersensitivity that we observed is not inflammatory pain, at least as that term is generally understood. Given our observation of hyperinnervation, it is also problematic to characterize this phenomenon as neuropathic, which requires a neural lesion (19).
The fact that only a subset of infected mice developed mechanical allodynia mirrors the clinical situation, because only a minority of women with RVVC develop chronic vulvar pain. Even within the subset of (outbred) mice developing chronic allodynia after zymosan, there was considerable variability in the number of exposures required (see Fig. 4A), suggesting a classic gene-by-environment interaction between as yet unidentified genetic susceptibility factors and inflammatory exposures. Such interactions are well known in the animal pain genetics literature (20). Human genes suggested to be involved in the pathogenesis of vulvodynia include those coding for mannose-binding lectin codon 54, the melanocortin-1 receptor, and the interleukin-1 receptor antagonist (21). Of course, an unknown environmental factor may also be responsible for the susceptibility to chronic hypersensitivity in those that develop it after repeated inflammation. Given that repeated zymosan treatment to the hind paw failed to produce chronic allodynia in any subject, these phenomena may be unique to mucosal tissue (22) or the genital tract (23).
Vulvar hypersensitivity after the third infection in the RVVC model was accompanied by increased density of sensory afferents, including increased peptide-containing nerve fibers. Previous work has shown the presence of sensory hyperinnervation during an inflammatory response, for example, in the mucosa of the urinary bladder during the inflammatory responses evoked by cyclophosphamide (24), in the upper dermis during the inflammatory response evoked by complete Freund's adjuvant (25), and in bone during the inflammatory response evoked via inoculation of prostate cancer cells (26). Sensory hyperinnervation in the upper dermis has also been seen during the regeneration response after a traumatic nerve injury (27–29). Notably, in the current experiment, robust sensory hyperinnervation was seen in allodynic mice 3 weeks after resolution of the infection. This suggests that the pain that persists after the resolution of infection may be due to an abnormal persistence of the hyperinnervation evoked during the acute inflammatory response. The observations in the animal studies parallel findings of greatly increased vulvar nerve density and peptidergic innervation in the allodynic vulvar vestibular tissue of women with PVD (6, 7, 30, 31) and suggest that repeated infection is sufficient to alter innervation at the site of infection.
We also observed sympathetic (VMAT2-immunoreactive) hyper-innervation in the vulvae of allodynic mice 3 weeks after resolution of the infection. Sympathetic hyperinnervation has been observed in the skin during inflammation and after traumatic nerve injury (25, 27, 28, 32). For example, ectopic endometrial cyst growth, which becomes sympathetically innervated, correlates with vaginal hypersensitivity in a rat model of endometriosis (33, 34). Sprouting of free nerve endings (including those of peptidergic afferents) and sympathetic efferents suggest the presence of long-term physiological changes that may enhance nociceptive signaling of peripheral tactile input and promote spontaneous neuronal discharge of affected sensory fibers.
We have developed an etiologically valid and clinically relevant animal model of an idiopathic pain condition. This model will be useful in the investigation of mechanistic pathways of infection-induced pain, the evaluation of genetic and environmental risk factors, and the preclinical testing of the efficacy of new treatments for debilitating pain conditions secondary to infection. Because the most effective current treatment of PVD is surgical excision of the painful vulvar tissue (3), new and less invasive treatments are a clinical necessity.
MATERIALS AND METHODS
Subjects
Female, outbred CD-1 (ICR:Crl; Charles River) mice, 8 to 10 weeks of age, were housed in facilities equipped with Biohazard Level 2 containment. Mice were maintained on a 12:12-hour light/dark cycle (lights on at 07:00 hours) and received irradiated food (Harlan Teklad 8604) and autoclaved tap water ad libitum. All procedures, including inoculations, injections, and behavioral testing, were conducted within a class II biological safety cabinet. All procedures were approved by the McGill University animal care and use committee.
Microorganism
A strain of C. albicans isolated in a clinical setting (SC5314, a gift of M. Whiteway, National Research Council of Canada) was used for vulvovaginal inoculations. See the Supplementary Material for a rationale for selection of this strain. SC5314 was grown in a phytone peptone broth for 18 hours at 25°C on an orbital shaker at 7000 rpm. Stationary-phase blastoconidia were washed twice and adjusted to 5 × 104 cells/ml. Each inoculum solution was prepared from freshly subcultured SC5314 on the day of inoculation.
Vulvovaginal infection procedures and treatment
Mouse vaginal bacterial and fungal cultures were obtained before testing to ensure that no known pathogenic microorganisms were present. Under non–hormone-primed conditions, murine vaginal C. albicans infection resolves without antifungal treatment within 14 days (13). On day 0 of each infection, mice were lightly anesthetized with isoflurane/oxygen and inoculated vaginally with either 5 × 104 stationary-phase SC5314 blastoconidia in 20 μl of sterile phosphate-buffered saline (PBS) or saline only. The inoculum was gently pipetted into the vaginal opening, and the mouse was placed in the supine position to retain the inoculum in the vaginal cavity for 10 min. Post-inoculation vaginal lavages were collected daily until infection resolution was confirmed, and weekly thereafter. To minimize tissue irritation unrelated to infection, we took utmost care to minimize contact between the vulva and the pipette tip during lavages. Vaginal SC5314 burden and infection status were monitored with Gram- and Wright-Giemsa–stained smears prepared from vaginal lavage fluid, which were examined microscopically for the presence of polymorphonuclear leukocytes and Candida morphotypes (blastoconidia and pseudohyphae). Lavage fluid was serially diluted onto Sabouraud dextrose agar (Quelab) and incubated for 48 hours at 34°C, and colony-forming units (CFUs) were quantified. Loops of CFUs were submitted to two separate tests to ensure that the isolated yeast was indeed C. albicans: The isolated growth was submitted to a germ tube test and replated onto chromogenic agar specific to common Candida species (Candida CHROMagar, Hardy Diagnostics) for up to 1 week at 34°C. According to the manufacturer's guidelines, emerald green CFUs exhibiting growth characteristics consistent with C. albicans were considered positive.
Mice in both the SC5314-infected and the control groups were treated with fluconazole (15 mg/kg, once daily for 7 days; LKT Laboratories) administered via oral gavage. These doses are effective in eliminating C. albicans–induced vaginitis in mice (35). The treatment regimen was based on the broad use of fluconazole as a first-line treatment for RVVC in humans (11). The infection was considered to be resolved upon obtaining two successive negative vaginal cultures (see above), and weekly lavages were collected thereafter to ensure the absence of yeast.
Repeated vulvovaginal infection with C. albicans
To simulate RVVC, mice received three separate vulvovaginal infections with 5 × 104 C. albicans strain SC5314. Infections were allowed to last untreated for 4 days during each round of infection, followed by 7 days of fluconazole treatment (see above). For the second and third rounds of infection, mice were reinoculated with 5 × 104 SC5314 cells 4 weeks after clearence of the primary infection (1 week after post-infection behavior testing) to simulate RVVC. In humans, a new episode of candidiasis can begin from a few days to 3 months after a previous infection, and the 4-week interval between infection resolution and re-infection used here was deemed a valid analog of RVVC. Mice in the fluconazole control group received inoculations of saline and vaginal lavages concurrent to and in the same manner as infected mice.
Tests of baseline vulvar and hind paw thresholds were performed 1 week preceding initial SC5314 inoculation (day −7). During each round of infection, vulvar sensitivity was tested 4, 11, and 32 days after inoculation (see Fig. 1A). Hind paw sensitivity was measured after each infection, on day 33.
Extended primary vulvovaginal infection with C. albicans
The extended primary infection with 5 × 104 SC5314 blastoconidia was allowed to last untreated for 14 days, followed by 7 days of fluconazole treatment (see above). Mice in the fluconazole control group received saline and vaginal lavages concurrent to and in the same manner as infected mice.
Tests of baseline vulvar and hind paw thresholds were performed 1 week preceding initial SC5314 inoculation (day −7). Vulvar sensitivity was measured at 14, 21, 42, and 70 days after inoculation (see Fig. 4A). Hind paw sensitivity was measured on days 43 and 71.
Repeated inflammation with zymosan
A new cohort of mice was subjected to repeated subcutaneous injections of zymosan in the posterior vulva while lightly anesthetized with isoflurane/oxygen. Zymosan is prepared from S. cerevisiae yeast cell wall and produces sterile inflammation at the site of injection, leading to mechanical allodynia lasting up to 12 to 24 hours without the need for biohazard containment. The dose used (10 mg/ml in 10 μl of saline; 0.1 mg) was chosen on the basis of pilot experiments; lower doses produced inconsistent initial allodynia. Injections occurred no more frequently than weekly to allow acute inflammation to subside between successive injections. Mice received zymosan injections immediately after baseline behavior testing and were observed for evidence of vulvar allodynia (defined here as ≥33% reduction in mechanical threshold) 4 hours later, corresponding to the temporal peak of vulvar zymosan-induced mechanical allodynia as defined by our pilot experiments. One week later, each mouse was retested and reinjected with zymosan. Each week thereafter, vulvar von Frey measurements were obtained and additional injections were administered only if a mouse's vulvar sensitivity recovered to nonallodynic levels (defined as >66% of baseline threshold). If a mouse continued to show evidence of vulvar allodynia 1 week after zymosan injection, no injection was given, and the mouse was retested 1 week later. Mice were followed for a total of 11 weeks and received up to and including (but no more than) six zymosan injections. Mice that did not become persistently allodynic (that is, displaying a ≥33% reduction in threshold for 2 consecutive weeks) after six injections were classified as nonresponders. One control group received weekly vulvar saline injections, with timing matched to a zymosan-treated mouse with equivalent baseline vulvar sensitivity. In a separate experiment, six female mice received zymosan injections in a paradigm similar to that described above except that zymosan (0.25 mg/ml in 20 μl) was injected into the right mid-plantar hind paw. This dose was chosen because it produced equivalent levels of initial mechanical allodynia to the vulvar zymosan.
Mechanical (von Frey) sensitivity testing
On each day of behavioral testing, animals were allowed 3 hours (11:00 to 14:00 hours) to habituate to the testing environment. Each testing session consisted of two threshold determinations separated by 1 hour; these two thresholds were averaged. An observer blinded to experimental condition applied a calibrated series of von Frey filaments (Semmes-Weinstein monofilaments; Stoelting) to the target tissue using the up-down psychophysical method of Dixon (36), with pressure applied to each filament until it bowed, and held for 2 s. Across species, the vulva is defined as the external female genital organs; this includes the clitoral and preputial glands in the mouse, and accordingly, we stimulated the central, hairless posterior aspect of the mouse vulva. A series of eight von Frey filaments (0.06 to 3.9 g; filaments #4 to #11) were applied to the vulva beginning with the #7 filament. Hind paw mechanical sensitivity was also monitored in all experiments, as a control. For hind paw testing, a different series of eight filaments (0.015 to 1.3 g; filaments #2 to #9) was applied to the plantar aspect of the hind paw beginning with the #5 filament. Different ranges of fibers were used for vulva and hind paw testing because different amounts of force physically lift the stimulated area off the floor (which artificially imposes a ceiling value on testing). Any mouse showing continuous positive or negative responses was assigned ceiling and floor withdrawal threshold values of 4.0 and 0.025 g, respectively, for vulvar testing and 2.0 and 0.01 g, respectively, for hind paw testing. In all other cases, the 50% withdrawal threshold was calculated as described (36). Aiming accuracy in mouse vulvar stimulation (a 3-mm-diameter target) was maximized by shaving anogenital hair the day before testing to improve visibility. Von Frey filaments were disinfected with 70% ethanol between each testing session, and independent filament sets were used for each experimental condition to minimize risk of cross-contamination.
The nocifensive endpoint we adopted was a clear reflexive jump (all four paws lifted) in response to vulvar stimulation, chosen because it is most similar to the clear withdrawal response used in hind paw von Frey testing. Note, however, that even the strongest usable von Frey filament (3.9 g, with larger filaments lifting the mouse off the floor without bending) rarely produced this jumping response at baseline, with >75% of mice consistently not responding to the 3.9-g fiber. It is unclear whether “positive” (<4.0 g) responses at baseline for the remaining subjects represent measurement (or testing environment) artifacts or true biological variability.
For practical reasons, it was necessary to test all mice together, regardless of their estrous stage, on each scheduled testing day. A pilot experiment confirmed that vulvar mechanical thresholds were invariant of estrous stage. In addition, vaginal lavages taken during the experiment revealed that neither SC5314 nor fluconazole treatment altered normal 4- to 5-day estrous cyclicity. Thus, it is unlikely that the changes seen in SC5314-infected mice were produced by hormonal alterations.
Immunohistochemistry
After behavioral testing, mice were deeply anesthetized with sodium pentobarbital (≥50 mg/kg, intraperitoneally) and perfused transcardially with 5% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at room temperature. The vaginal canal (from the external vulva to the cervix) was excised and postfixed in 5% paraformaldehyde in phosphate buffer for 1 hour and then cryoprotected with 30% sucrose in phosphate buffer for 24 hours. Tissue was embedded in an optimum cutting temperature medium (Tissue Tek) and frozen at −80°C until cryosectioned. Twelve-micrometer-thick longitudinal sections were cut on a Leica CM3050 S cryostat at −25°C and placed directly on poly-l-lysine–treated slides. Slide-mounted sections were rinsed three times with 0.1 M PBS for 10 min, preincubated with 10% normal goat serum diluted with 0.3% Triton X-100 for 60 min, and then incubated for 24 hours at 4°C in one of three primary antibodies: anti–PGP 9.5 raised in rabbit (1:2000, Ultraclone), anti-CGRP (α isoform) raised in sheep (1:1000, Biomol), and anti-VMAT2 raised in rabbit (1:2000, Millipore). The next day, slides were washed three times with PBS for 10 min, incubated in Cy3 anti-rabbit and Cy2 anti-sheep secondary antibodies (1:500, Jackson ImmunoResearch Laboratories) in the dark for 2 hours, and washed three times for 10 min with PBS. For each reaction, negative controls (processed without the primary antibody) were included.
Quantitative analysis of RVVC immunohistochemistry
Immunohistochemical analysis of a subset of post–repeated-infection allodynic (n = 3 to 6, depending on the antibody), post-infection nonallodynic (n = 5), and fluconazole control (n = 4) animals (see below) was based on four randomly selected postmortem vulvar tissue sections per mouse, with a total of six nonconsecutive pictures per section (that is, 24 frames per mouse). Pictures were taken only of the lamina propria because very little innervation was observed in the epithelium. Images were acquired with a Zeiss Axioplan 2 imaging fluorescence microscope (lenses ranging from 40× to 60×) equipped with a Megaview II charge-coupled device (CCD) camera and processed with AnalySIS 5.0 software (Soft Imaging System). Images were saved in TIFF format and analyzed with an image analysis system (MCID Elite v.7, Imaging Research) by an observer blinded to condition. Fiber density was calculated with functions in the program configured to measure total fiber length per unit area (25).
Assessment of post-infection morphology
Slide-mounted 12-μm-thick sections were processed as described above and stained with H&E to identify gross vulvar morphology, epithelial thickness, and inflammation among allodynic (n = 4), nonallodynic (n = 6), and fluconazole controls (n = 5). Four nonconsecutive measurements were taken from the middle third of the posterior vulvar tissue, across six sections (24 measurements total). Sections were examined for signs of inflammatory infiltrate in the epithelium and lamina propria, as well as edema and plasma extravasation. Slides were digitally scanned with MIRAX Desk Scanner and visualized with MIRAX Viewer software using the 20× and 40× magnification functions for quantification (Zeiss).
Data analysis
Normally distributed hind paw threshold data were analyzed with repeated-measures analysis of variance (ANOVA). Vulvar threshold data were analyzed with nonparametric χ2 analysis, with five arbitrarily defined threshold categories (<1.0, 1.0 to 1.99, 2.0 to 2.99, 3.0 to 3.99, and >4.0 g). Data at particular testing sessions were compared to within-group baselines, but similar results were obtained when comparing between-group at each testing session. Analysis of percentage of allodynic mice was conducted with a one-tailed Fisher's exact test on the basis of the a priori hypothesis that previously infected mice would show more allodynia. Normally distributed immunohisto-chemical and epithelial thickness data were analyzed by one-way ANOVA followed by Tukey's post hoc test. In all cases, a criterion level of α = 0.05 was adopted. All statistical tests were two-tailed except as described above.
Supplementary Material
Acknowledgments
We thank P. L. Fidel Jr. for his procedural and theoretical guidance and L. S. Stone for use of her equipment and lab space.
Funding: Supported by grants from National Vulvodynia Association (M.A.F. and Y.M.B.), a National Research Service Award (F31NS062611) from the National Institute of Neurological Disorders and Stroke (M.A.F. and J.S.M.), the Canadian Institutes of Health Research (Y.M.B.), and the Louise and Alan Edwards Foundation (J.S.M.).
Footnotes
Author contributions: M.A.F. oversaw and participated in all data collection and wrote the initial manuscript draft. A.M.T., A.L.B., A.H.T., L.C.M., Z.E.M., and H.P.C. participated in data collection. G.J.B. and A.R.-d.-S. provided technical and interpretational advice and consultation, and edited the manuscript. The study was conceived and designed by M.A.F., Y.M.B., and J.S.M. All authors approved the manuscript.
Competing interests: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIAL www.sciencetranslationalmedicine.org/cgi/content/full/3/101/101ra91/DC1
Methods
Fig. S1. Increased vaginal fungal colonization during acute infection(s) after inoculation with C. albicans strain SC5314, and full clearance of yeast after fluconazole treatment.
Fig. S2. No effect of repeated vulvovaginal C. albicans strain SC5314 infection on hind paw mechanical sensitivity.
Fig. S3. No effect of repeated vulvovaginal C. albicans strain SC5314 infection on gross vulvar morphology.
Fig. S4. No effect of single, extended-duration vulvovaginal C. albicans strain SC5314 infection on hind paw mechanical sensitivity.
Table S1. No changes in epithelium thickness produced by repeated vulvovaginal C. albicans strain SC5314 infection.
Table S2. Vaginal leukocyte count was not associated with the presence of infection after SC5314 inoculation.
REFERENCES AND NOTES
- 1.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology. 2009;136:1979–1988. doi: 10.1053/j.gastro.2009.02.074. [DOI] [PubMed] [Google Scholar]
- 2.Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: Have we underestimated the prevalence of vulvodynia? J. Am. Med. Womens Assoc. 2003;58:82–88. [PubMed] [Google Scholar]
- 3.Goldstein AT, Burrows L. Vulvodynia. J. Sex. Med. 2008;5:5–14. doi: 10.1111/j.1743-6109.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 4.Meana M, Binik YM, Khalife S, Cohen DR. Biopsychosocial profile of women with dyspareunia. Obstet. Gynecol. 1997;90:583–589. doi: 10.1016/s0029-7844(98)80136-1. [DOI] [PubMed] [Google Scholar]
- 5.Pukall CF, Binik YM, Khalifé S, Amsel R, Abbott FV. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain. 2002;96:163–175. doi: 10.1016/s0304-3959(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 6.Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecol. Obstet. Invest. 1998;46:256–260. doi: 10.1159/000010045. [DOI] [PubMed] [Google Scholar]
- 7.Weström LV, Willén R. Vestibular nerve fiber proliferation in vulvar vestibulitis syndrome. Obstet. Gynecol. 1998;91:572–576. [PubMed] [Google Scholar]
- 8.Pukall CF, Strigo IA, Binik YM, Amsel R, Khalifé S, Bushnell MC. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005;115:118–127. doi: 10.1016/j.pain.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Bohm-Starke N, Hilliges M, Blomgren B, Falconer C, Rylander E. Increased blood flow and erythema in the posterior vestibular mucosa in vulvar vestibulitis. Obstet. Gynecol. 2001;98:1067–1074. doi: 10.1016/s0029-7844(01)01578-2. [DOI] [PubMed] [Google Scholar]
- 10.Witkin SS, Gerber S, Ledger WJ. Differential characterization of women with vulvar vestibulitis syndrome. Am. J. Obstet. Gynecol. 2002;187:589–594. doi: 10.1067/mob.2002.125889. [DOI] [PubMed] [Google Scholar]
- 11.Sobel JD. Vulvovaginal candidosis. Lancet. 2007;369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 12.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J. Reprod. Immunol. 2002;57:61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 13.Fidel PL., Jr. History and update on host defense against vaginal candidiasis. Am. J. Reprod. Immunol. 2007;57:2–12. doi: 10.1111/j.1600-0897.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 14.van der Graaf CA, Netea MG, Verschueren I, van der Meer JW, Kullberg BJ. Differential cytokine production and Toll-like receptor signaling pathways by Candida albicans blastoconidia and hyphae. Infect. Immun. 2005;73:7458–7464. doi: 10.1128/IAI.73.11.7458-7464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat. Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: A mechanism for pain due to infection. J. Dent. Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad. Sci. U.S.A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shinoda M, Feng B, Gebhart GF. Peripheral and central P2X3 receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137:2096–2104. doi: 10.1053/j.gastro.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 20.Mogil JS, Seltzer Z, Devor M. In: The Genetics of Pain: Progress in Pain Research and Management. Mogil JS, editor. vol. 28. IASP Press; Seattle, WA: 2004. chap. 13. [Google Scholar]
- 21.Foster DC, Sazenski TM, Stodgell CJ. Impact of genetic variation in interleukin-1 receptor antagonist and melanocortin-1 receptor genes on vulvar vestibulitis syndrome. J. Reprod. Med. 2004;49:503–509. [PubMed] [Google Scholar]
- 22.Richardson M, Rautemaa R. How the host fights against Candida infections. Front. Biosci. 2009;14:4363–4375. doi: 10.2741/3533. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki A. Antiviral immune responses in the genital tract: Clues for vaccines. Nat. Rev. Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickson A, Avelino A, Cruz F, Ribeiro-da-Silva A. Peptidergic sensory and parasympathetic fiber sprouting in the mucosa of the rat urinary bladder in a chronic model of cyclophosphamide-induced cystitis. Neuroscience. 2006;139:671–685. doi: 10.1016/j.neuroscience.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 25.Almarestani L, Longo G, Ribeiro-da-Silva A. Autonomic fiber sprouting in the skin in chronic inflammation. Mol. Pain. 2008;4(56) doi: 10.1186/1744-8069-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW. Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J. Neurosci. 2010;30:14649–14656. doi: 10.1523/JNEUROSCI.3300-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grelik C, Bennett GJ, Ribeiro-da-Silva A. Autonomic fibre sprouting and changes in nociceptive sensory innervation in the rat lower lip skin following chronic constriction injury. Eur. J. Neurosci. 2005;21:2475–2487. doi: 10.1111/j.1460-9568.2005.04089.x. [DOI] [PubMed] [Google Scholar]
- 28.Yen LD, Bennett GJ, Ribeiro-da-Silva A. Sympathetic sprouting and changes in nociceptive sensory innervation in the glabrous skin of the rat hind paw following partial peripheral nerve injury. J. Comp. Neurol. 2006;495:679–690. doi: 10.1002/cne.20899. [DOI] [PubMed] [Google Scholar]
- 29.Peleshok JC, Ribeiro-da-Silva A. Delayed reinnervation by nonpeptidergic nociceptive afferents of the glabrous skin of the rat hindpaw in a neuropathic pain model. J. Comp. Neurol. 2011;519:49–63. doi: 10.1002/cne.22500. [DOI] [PubMed] [Google Scholar]
- 30.Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Neurochemical characterization of the vestibular nerves in women with vulvar vestibulitis syndrome. Gynecol. Obstet. Invest. 1999;48:270–275. doi: 10.1159/000010198. [DOI] [PubMed] [Google Scholar]
- 31.Bornstein J, Goldschmid N, Sabo E. Hyperinnervation and mast cell activation may be used as histopathologic diagnostic criteria for vulvar vestibulitis. Gynecol. Obstet. Invest. 2004;58:171–178. doi: 10.1159/000079663. [DOI] [PubMed] [Google Scholar]
- 32.Ruocco I, Cuello AC, Ribeiro-da-Silva A. Peripheral nerve injury leads to the establishment of a novel pattern of sympathetic fibre innervation in the rat skin. J. Comp. Neurol. 2000;422:287–296. doi: 10.1002/(sici)1096-9861(20000626)422:2<287::aid-cne9>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.Cason AM, Samuelson CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm. Behav. 2003;44:123–131. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhang G, Dmitrieva N, Liu Y, McGinty KA, Berkley KJ. Endometriosis as a neurovascular condition: Estrous variations in innervation, vascularization, and growth factor content of ectopic endometrial cysts in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R162–R171. doi: 10.1152/ajpregu.00649.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fidel PL, Jr., Cutright JL, Sobel JD. Efficacy of D0870 treatment of experimental Candida vaginitis. Antimicrob. Agents Chemother. 1997;41:1455–1459. doi: 10.1128/aac.41.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.