Abstract
Although adults with Down syndrome (DS) show a decreased incidence of cancer as compared to individuals without DS, children with DS are at an increased risk of leukemia. Nearly half of these childhood leukemias are classified as acute megakaryoblastic leukemia (AMKL), a relatively rare subtype of acute myeloid leukemia (AML). Here, we summarize the clinical features of myeloid leukemia in DS, review recent research on the mechanisms of leukemogenesis, including the roles of GATA1 mutations and trisomy 21, and discuss treatment strategies. Given that trisomy 21 is a relatively common event in hematologic malignancies, greater knowledge of how the genes on chromosome 21 contribute to DS-AMKL will increase our understanding of a broader class of patients with leukemia.
Keywords: AMKL, leukemia, transient myeloproliferative disorder, trisomy 21, GATA-1, MPL
INTRODUCTION
Individuals with Down Syndrome (DS) display various developmental abnormalities, including craniofacial dysmorphy, cardiovascular defects and learning disabilities. Paradoxically, individuals with DS have a decreased frequency of solid tumors (epidemiological studies in Denmark, Finland, and Australia indicated an incidence ratio respectively of 0.50, 0.57, and 0.441–3), but a higher incidence of leukemia (10–20 fold).4 Even more strikingly, young children (<4 years) with DS have a 500-fold increased incidence of acute megakaryoblastic leukemia (AMKL, also known as ML-DS).5 The natural history of leukemia in children with DS suggests that trisomy 21 directly contributes to the malignant transformation of hematopoietic cells. In addition, somatic mutations of the GATA1 gene have been detected in nearly all DS AMKL cases and are notably absent in non-DS AMKL.6 In this review, we will highlight the clinical manifestations, outcomes and new observations related to signaling pathways aberrantly controlled by trisomy 21 or GATA1 mutations during DS-AMKL leukemogenesis.
CLINICAL FEATURES
There is a well-recognized preceding transient myeloproliferative disorder (TMD), aka transient leukemia (TL), occurring in the neonatal period in 10% of infants with DS.7–9 TMD is a clonal pre-leukemia characterized by an accumulation of immature megakaryoblasts in the fetal liver and peripheral blood.5 The incidence of TMD may be underestimated as not all cases come to medical attention. The median age of presentation of TMD, based on pooled data from > 200 neonates, is 3–7 days.10–12 The clinical presentation of neonates with TMD ranges from a healthy appearance to bruising, respiratory distress, fulminant hepatic failure, hydrops fetalis or even death in 15–20% of cases that have been diagnosed. Overall, though, the majority of cases resolve spontaneously with normal blood counts at a mean of 84 days.13 After a latency period of 1–4 years, a subset of these children (20–30%), develop acute megakaryoblastic leukemia.14 In a series of 112 patients with AMKL, the median age of DS patients was 1.8 years vs. approximately 8 years in non-DS cases.15–16 Patients with AMKL develop anemia, thrombocytopenia, myelofibrosis, organomegaly, extensive skeletal lesions,17–18 and leukocytosis although white blood counts are lower than in non-DS.19–20 CNS involvement is unusual.16
Diagnosis
Histological examination of the bone marrow in AMKL shows replacement with megakaryoblasts and reticulin deposition. Megakaryoblasts are identified by a positive platelet peroxidase reaction,21 and by immunophenotyping for glycoprotein IIb/IIIa or the von Willebrand factor protein.22 These blasts are non-reactive for myeloperoxidase and express stem/progenitor markers CD33, CD34, CD117, erythroid markers CD36 and glycophorin A, the lymphoid antigen CD7 and the megakaryocytic markers CD41 and CD42b.23–25 Of note, cytogenetic differences between DS and non-DS AMKL include the absence of the translocation t(1;22), and instead, the presence of trisomies involving chromosomes 8 and 1,7 as well as monosomy 7.26–27 Since Down syndrome is the most common cytogenetic abnormalities seen at birth (1/700), improved non-invasive prenatal diagnosis is an area of active research. Strategies are emerging based on screening differentially methylated regions (DMRs) of fetal DNA for chromosome 21 dosage assessment.28 Moreover, murine models of DS have helped identify differentially expressed genes in DS-fetal livers, some of which may represent potential chromosome 21 specific biomarkers.29
Prognosis
Prospective, multi-institutional studies in the US, Germany and Japan have examined the natural history of TMD in 264 infants.10–12 Early death occurred in up to 20% of infants and was significantly correlated with higher white blood cell count at diagnosis, increased bilirubin and liver enzymes, and a failure to normalize the blood count. Later development of leukemia occurred in 19% of infants at a mean of 20 months and was significantly correlated with karyotypic abnormalities in addition to trisomy 21, including trisomy 11, del 16q, der(14;21), t(5;13), and tetrasomy 21.10 In DS-AML age at diagnosis had independent prognostic significance, primarily a result of poor remission induction in older patients.30 Cytogenetic abnormalities such as monosomy 7 confer an adverse prognosis in non-DS and DS-AMKL in some studies.26
MECHANISMS
Pathogenesis
From trisomy 21 to TMD towards AMKL: an incremental process of leukemogenesis
If trisomy 21 is considered the first genetic event in DS-AMKL leukemogenesis, the second hit is a mutation of the X-linked gene GATA1, encoding a blood-specific transcription factor essential for development of the erythroid and megakaryocytic lineages. GATA1 mutations are present in nearly all TMD patient samples as early as 21 weeks gestation.31–34 Using the variable length of nucleotide insertions and deletions as a marker of individual TMD clones, sequential samples collected from the same patient during TMD, remission, and AMKL showed identical GATA1 mutations that disappeared during remission.33 This confirms the clonal nature of AMKL and its evolution from TMD.
TMD is a critical model to understand the natural history of AMKL, 20% of TMD cases evolve into AMKL either overtly, or following an apparent remission. AMKL and TMD blasts express erythroid markers such as gamma globulin and delta aminolevulinate synthase as well as GATA-1 and GATA-2 suggesting origin from the megakaryocyte-erythroid progenitor cells.35 Myeloid and erythroid dysplasia are common as well as the presence of karyotypic abnormalities in metaphases from CFU-GM and BFU-E mimicking those seen in megakaryoblasts.36
Fetal liver origin of leukemia initiating cell
GATA1 mutations most likely occur in utero, based on neonatal blood spot testing, and may precede disease development.37–38 Mice expressing a GATA-1 mutant ortholog of the one seen in human DS specimens display sustained proliferation of a yolk sac/early fetal liver megakaryocyte progenitor implicating this as the target cell for leukemic transformation in DS-AMKL and TMD.39–40 Moreover, GATA1 mutations were detected in 2 of 9 liver samples from terminated fetuses with DS (as early as 21 to 23 weeks of gestation) supporting the fetal liver origin of TMD.31
Role of trisomy 21
Second trimester DS fetal livers (FLs) show increased megakaryocyte-erythroid progenitor frequency and increased clonogenicity.41 Enhanced erythroid and megakaryocytic differentiation was seen in NOD/SCID mice transplanted with DS FL mononuclear cells.42 Those observations were obtained from 13 to 23 week trisomic FL, preceding the acquisition of any GATA1 mutation.
Through a high-resolution map of DS was generated using a panel of 30 individuals with rare segmental trisomies 21, Korbel et al. identified a critical region of 8.35 Mb (35–43.35) that is likely contributing to the risk increase for both TMD and AMKL. This region includes previously known oncogenes, such as RUNX1, ERG and ETS2. 43 Using mouse ES cells (ESC) bearing an extra copy of human chromosome 21 (Hsa21), disturbances in early hematopoietic differentiation were observed and related to increased expression of GATA-2, Tie-2 and c-kit. An siRNA silencing study implicated increased level of RUNX1 in abnormal Tie-2 and c-kit expression. Using a panel of ESCs partially trisomic mapped with tiling arrays, two non-overlapping regions of Hsa21 were correlated to abnormal hematopoiesis.44 The distal region contains RUNX1, DYRK1A,45 ETS2 and ERG while the pericentromeric region frequently harbors chromosome rearrangements and increased disomic homozygosity of DNA markers in DS-TMD and DS-AKML.46
Both ERG and ETS2 bind the hematopoietic enhancer of SCL/TAL1, a key regulator of hematopoietic stem cell and megakaryocytic development.47 Overexpression of ETS2 and ERG increase the megakaryocytic differentiation of GATA-1s progenitors, and immortalize Gata1s fetal liver progenitors in replating assays.48 Coexpression of ERG and GATA-1s in vivo results in leukemia with an immature megakaryocytic phenotype.49
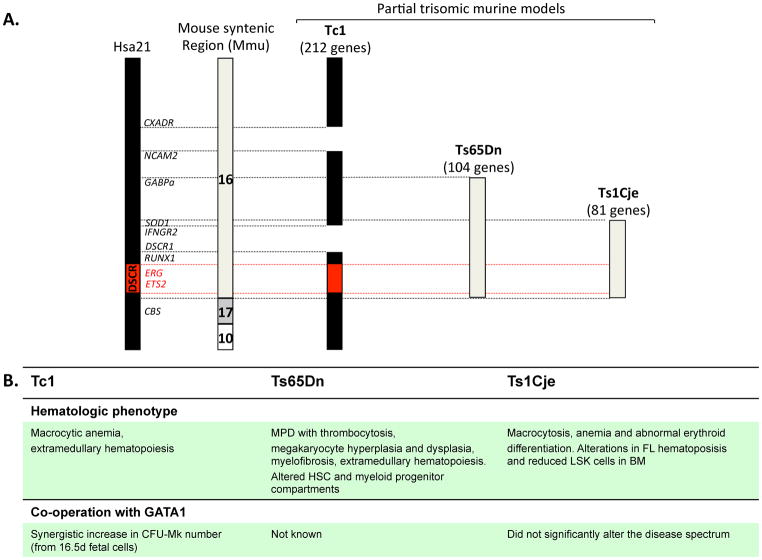
In parallel, several mouse models of DS have been developed to identify dosage-sensitive genes that contribute to specific hematopoietic phenotypes (see Figure 1). The Ts65Dn mouse, trisomic for 104 orthologous genes of human chromosome 21, develops a macrocytic anemia and a myeloproliferative disorder (MPD) associated with thrombocytosis.50 Interestingly, unlike trisomy of Runx1 in the Ts65Dn mouse model of DS, reduction to functional disomy of Erg using a loss-of-function allele, corrects the pathologic and hematologic features of myeloproliferation.51 Other segmental trisomy mouse models include the Tc1 mouse model (212 genes)52 and the Ts1Cje mouse (81 genes).53 Notably, none of these mouse strains develops TMD or AMKL alone or in cooperation with GATA-1s expression, suggesting undiscovered cooperating mutations.
Figure 1. Diagram of Hsa21 and the regions of trisomy in the various mouse models of DS.
A) Human chromosome 21 and specific genes in the DS critical region (DSCR) that may contribute to the development of leukemia are shown. The syntenic murine Mmu16 with varying degrees of trisomic representation in the different mouse models is depicted on the right. B) Summary of the hematopoietic phenotype of the murine models and the effect of coexpression of GATA-1s.
There are also 5 micro-RNAs encoded on chromosome 21, of which miR-125b2 is overexpressed in TMD and AMKL. In both fetal liver and human CD34+ cells, overexpression of miR-125b-2 led to hyperproliferation and enhanced self-renewal of megakaryocytic progenitors attributed to repression of DICER1 and the tumor suppressor ST18.54
Role of GATA-1
The first insight into the mechanism of DS-AMKL was the discovery of acquired mutations in the GATA1 gene. These mutations were restricted to the leukemic clones and were not found in normal remission samples.6 The mutation is not detectable in non-DS leukemia or other subtypes of DS leukemia,55 emphasizing the specific cooperation of GATA1 mutation with trisomy 21 in megakaryocytic leukemia. DS and non-DS-AMKL samples exhibit distinct gene expression profiles and a specific signature for DS-AMKL was identified with relatively increased expression of GATA-1 transcripts (as GATA-1s) and failure to down-regulate proliferation-promoting genes that are normally repressed by GATA-1.56–57 In almost all DS-AMKL and TMD samples, mutations in GATA1 are detectable in exon 2 producing a premature stop codon within the N terminal activation domain.55,58 These mutations prevent the generation of full-length GATA-1, but preserve the translation of GATA-1s, a truncated form of GATA-1 lacking the N terminal activation domain. Distinct regions in the GATA-1 N terminus are required for terminal megakaryocyte differentiation and controlling growth of immature precursors.59–60 Analysis of the mutational spectrum at GATA1 in DS TMD and AMKL blasts shows predominance of insertions/deletions, duplications (74%) and base substitutions (26%).61 A recent study concluded that the different classes of GATA1 mutations result in variable translation efficiency of GATA-1s, and further, that the level of GATA-1s protein correlates with risk of progression to leukemia.62 However, a subsequent study showed that the GATA1 mutational spectrum did not differ between TMD or AMKL, and that the type of GATA1 mutation was unable to predict evolution from TMD to AMKL.63
Mice with lineage-specific mutations of the GATA1 promoter show impaired maturation and dysregulated proliferation of megakaryocytes.64 Expression profiles of GATA-1s and full-length GATA-1 expressing murine fetal megakaryocytes have been contrasted and showed that GATA-1s fails to repress a number of transcription factor genes (including Gata2, Ikaros, Myb and Myc) that have “pro-proliferative” effect on hematopoietic cell growth.39, 60 Of note, in 2006, a family was discovered with a germline GATA1 mutation in which affected males generated only the GATA-1s isoform and exhibited anemia and trilineage dysplasia, but failed to develop leukemia.65 This observation established that trisomy 21 is necessary for leukemogenesis in the presence of mutated GATA1.
Cooperating mutations
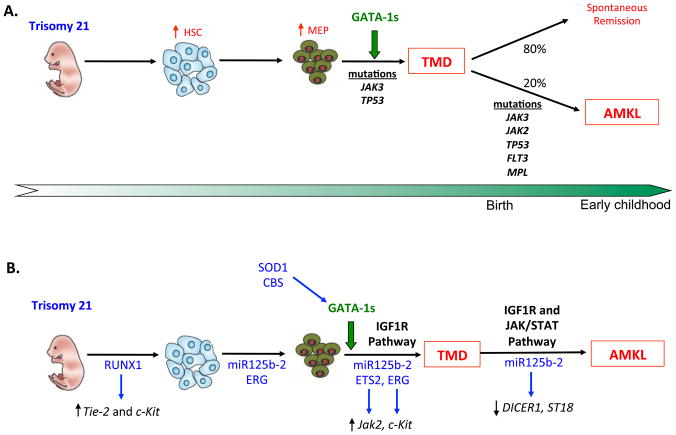
Mutations in the p53 tumor suppressor gene have been demonstrated in a proportion of patients after transformation from TMD to AMKL suggesting a role in disease evolution. To date, only a single case of a p53 mutation in TMD has been reported.66–67 Several activating mutations of the JAK3 gene have been identified in TMD, DS AMKL and non-DS AMKL patients as well as in DS-AMKL cell lines (CMK and CMY). These mutations result in constitutive JAK signaling13,68–69 and confer responsiveness to treatment with JAK3 inhibitors in vitro.70 Both JAK3 A572V and the recently identified JAK3 P132A68,71 mutants appear to be oncogenic in a murine models. However, recent data show that the purported activating JAK3 mutations are present in DNA samples from normal blood donors, at a frequency similar to that observed in patients with AML, suggesting that they may represent SNPs.71 Further study in this field is required to clarify the leukemogenic role of JAK3 mutations in DS-AMKL. In addition, activating mutations affecting FLT3, JAK2, and MPL genes were also identified within DS-AMKL.72–73 A summary of the stepwise acquisition of mutations is shown in Figure 2A.
Figure 2. Multi-step model of leukemogenesis in Down syndrome.
A) Sequential acquisition of known genetic abnormalities and their role in the evolution of DS-AMKL. B) Aberrant signaling pathways implicated in the pathogenesis of DS-AMKL. The chromosome 21 specific genes that appear to have a functional impact in these pathways are highlighted in blue.
Abberant signaling Pathways in DS-AMKL
Fetal liver hepatic stromal cells support hematopoietic stem cell (HSC) expansion by secreting insulin-like growth factor 2 (IGF-2).74 Constitutive activation of IGF signaling was demonstrated in DS-AMKL and TMD blast cells, as well as in DS-AMKL mouse model.75 Klusmann et al. showed that mutated GATA-1 fails to restrict IGF-mediated activation of the E2F transcription network. This aberrant response converges with overactive IGF signaling to promote enhanced proliferation and increased survival of DS fetal liver progenitors, and revealing a fetal stage-specific regulatory network (Figure 2B).
More than 20 genes involved in oxidative metabolism are localized to chromosome 21, including superoxide dismutase (SOD)76 and Cystathionine Beta Synthase (CBS). CBS overexpression in DS directs homocysteine to cystathionine synthesis and away from methionine remethylation, creating a folate trap and thymidylate imbalance. Perturbed folate metabolism in turn results in the accumuation of uracil and its misincorporation into DNA. This altered metabolism, when paired to oxidative stress caused by increased SOD1 activity seen in DS, has been implicated in a model linking chromosome 21 genes (CBS and SOD1) to the generation of mutations in the GATA1 gene.61 Additionally AMKL blasts, unlike TMD cells, have demonstrable telomerase activity, implicating telomerase with the malignant character of a leukemic proliferation.77
THERAPY
Treatment Options
One of the first clinical trials for this malignancy studied 12 children with DS-AML. These patients (POG8498) showed heightened sensitivity to high dose cytarabine and anthracycline based therapy with a significantly superior event-free survival compared to non-DS AML (3 yr EFS 100% in DS-AML vs 33% in non-DS AML).78 In subsequent trials, intensive induction showed unacceptable toxicity and increased mortality in DS-AML as did autologous and allogeneic transplant.15 AMKL has been treated on protocols involving either conventional (100–300mg/m2)26 or high dose cytosine arabinoside (3g/m2) with reported 3 yr OS>80%. However significant toxicity has been reported with the high dose Ara-C.16, 19, 30,79 Low dose subcutaneous Ara-C induced remission in almost all cases of AMKL and complicated TMD 80–81 with comparable 5 year EFS and OS to standard chemotherapy.82
There was a significant improvement in clinical trials survival outcomes in DS between 1993 and 1998 mainly due to reduction in treatment related mortality. This resulted from reduced anthracycline and cytarabine dosing and longer intervals of recovery between therapy.83 Due to the limitations of toxic deaths, infections, and cardiac toxicity in treating DS-AMKL, new, less-intensive protocols have been conducted in the United States, Japan and Europe.16, 84 In a single prospective study treatment of TMD with low dose cytarabine (0.5–1.5 mg/kg) improved 5 year EFS from 28% to 52% in children with risk factors for early death. Treatment of TMD did not alter risk of developing subsequent AMKL.11 The ML-DS prevention trial (EudraCT no. 2006-002962-20) is currently ongoing to assess if the progression from TL to ML-DS may be blocked by eradication of the GATA-1s cl one using low-dose cytarabine treatment and monitoring for minimal residual disease (MRD).
Chemosensitivity in DS-AMKL
The enhanced sensitivity of DS myeloblasts to Ara-C is due to greater extent of Ara-C incorporation into DNA, and increased relative numbers of double strand DNA strand breaks,85 attributed to dosage effect of genes localized to chromosome 21 including CBS. In vitro, DS myeloblasts generate higher concentrations of Ara-CTP, the active cytarabine metabolite. This is thought to be due to increased CBS expression and an elevated ratio of deoxycytidine kinase (CdK) to cytidine deaminase (CDA). CDA metabolizes Ara-C to the inactive metabolites uridine arabinoside (ara-U) and its levels are lower in DS-myeloblasts than non-DS myeloblasts. GATA-1 binding sites in the CDAsf promoter suggest the potential role of GATA-1 in regulating CDA transcription.86
Blast cells from DS patients are also significantly more sensitive to daunorubicin, melphalan, mitoxantrone, 4-hydroperoxy-cyclophosphamide, vincristine, etoposide, bleomycin, and pirarubicin than those from non-DS patients in MTT assays.87 Low levels of bone marrow stromal-cell antigen 2 (BST2) in DS megakaryoblasts may lead to decreased interaction of leukemia cells with bone marrow stroma, a mechanism of protection from chemotherapy-induced apoptosis. This may be explained by decreased stimulation of BST2 promoter activity by GATA-1s compared with the full-length protein.56 DS-AMKL and good prognosis non-DS AMKL blasts demonstrate high expression of CD36, the thrombospodin receptor. CD36 plays a role in fatty acid transport and may exacerbate drug-triggered apoptosis by intracellular lipid accumulation in AMKL.88 RUNX1 expression is lower in DS megakaryoblasts compared with non-DS megakaryoblasts.57 This suggests that RUNX1 may play a role in chemotherapy resistance and contribute to the poor outcomes in non DS-AMKL. Inhibition of RUNX1 may further chemosensitize leukemia cells by inhibition of the PI3 kinase survival pathway.89
CONCLUSIONS
It is clear that myeloid/megakaryocytic leukemia in DS is the result of a series of genetic events therefore representing a useful model to understand the role of the chromosome 21 in leukemia in general. A trisomic background results in oxidative stress and altered folate metabolism predisposing to the acquisition of GATA1 mutations, which then allow for the development of TMD. The discovery that mutated GATA-1 is unable to suppress E2F transcription in fetal liver cells may explain the cellular origin of TMD. Research to identify dosage-sensitive genes (or regulators) on chromosome 21 that contribute to megakaryocyte proliferation, implicate the ETS proteins ERG and ETS2. Recently, overactive IGF signaling and overexpression of miR-125b-2, which allow for dis-inhibition of tumor suppressor genes, have also been highlighted. Subsequent clonal selection and evolution to AMKL requires additional insults, including putative cooperating mutations in JAK3, FLT3, MPL or TP53. The multi-step progression to AMKL provides insight into the steps by which normal HSC/progenitors are transformed into leukemic cells. Moreover this is an excellent disease model to understand cell type–specific signaling pathways and their intersection with oncogenes during malignant transformation.
Acknowledgments
This review was funded in part by grants from the NCI (R01 CA101774) and the Leukemia and Lymphoma Society. IK is supported by the Clinical Oncology training grant (T32 CA079447) from the NCI. SM is a Fellow of the Leukemia and Lymphoma Society.
Abbreviations
- DS
Down syndrome
- AMKL
acute megakaryoblastic leukemia
- AML
acute myeloid leukemia
- ML-DS
myeloid leukemia Down syndrome
- DMR
differentially methylated region
- TMD
transient myeloproliferative disorder
- TL
transient leukemia
- CFU-GM
colony forming unit, granulocyte macrophage
- BFU-E
burst forming unit erythroid
- NOD/SCID
non-obese diabetic/severe combined immunodeficient
- FL
fetal liver
- ESC
ES cells
- Hsa21
human chromosome 21
- siRNA
small interfering RNA
- MPD
myeloproliferative disorder
- SNP
single nucleotide polymorphism
- HSC
hematopoietic stem cell
- IGF-2
insulin-like growth factor 2
- SOD
superoxide dismutase
- CBS
cystathionine beta synthase
- EFS
event fee survival
- OS
overall survival
- Ara-C
cytarabine
- MRD
minimal residual disease
- ara-U
arabinoside
- CDA
cytidine deaminase
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- BST2
bone marrow stromal-cell antigen 2
References
- 1.Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet. 2000 Jan 15;355(9199):165–9. doi: 10.1016/S0140-6736(99)05264-2. [DOI] [PubMed] [Google Scholar]
- 2.Patja K, Pukkala E, Sund R, Iivanainen M, Kaski M. Cancer incidence of persons with Down syndrome in Finland: a population-based study. Int J Cancer. 2006 Apr 1;118(7):1769–72. doi: 10.1002/ijc.21518. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan SG, Hussain R, Glasson EJ, Bittles AH. The profile and incidence of cancer in Down syndrome. J Intellect Disabil Res. 2007 Mar;51(Pt 3):228–31. doi: 10.1111/j.1365-2788.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 4.Mitelman F, Heim S, Mandahl N. Trisomy 21 in neoplastic cells. Am J Med Genet Suppl. 1990;7:262–6. doi: 10.1002/ajmg.1320370752. [DOI] [PubMed] [Google Scholar]
- 5.Lange B. The management of neoplastic disorders of haematopoiesis in children with Down’s syndrome. Br J Haematol. 2000 Sep;110(3):512–24. doi: 10.1046/j.1365-2141.2000.02027.x. [DOI] [PubMed] [Google Scholar]
- 6.Wechsler J, Greene M, McDevitt MA, Anastasi J, Karp JE, Le Beau MM, Crispino JD. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002 Sep;32(1):148–52. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 7.Creutzig U, Ritter J, Vormoor J, Ludwig WD, Niemeyer C, Reinisch I, Stollmann-Gibbels B, Zimmermann M, Harbott J. Myelodysplasia and acute myelogenous leukemia in Down’s syndrome. A report of 40 children of the AML-BFM Study Group. Leukemia. 1996 Nov;10(11):1677–86. [PubMed] [Google Scholar]
- 8.Okada H, Liu PI, Hoshino T, Yamamoto T, Yamaoka H, Murakami M. Down’s syndrome associated with a myeloproliferative disorder. Am J Dis Child. 1972 Jul;124(1):107–10. doi: 10.1001/archpedi.1972.02110130109018. [DOI] [PubMed] [Google Scholar]
- 9.Pine SR, Guo Q, Yin C, Jayabose S, Druschel CM, Sandoval C. Incidence and clinical implications of GATA1 mutations in newborns with Down syndrome. Blood. 2007 Sep 15;110(6):2128–31. doi: 10.1182/blood-2007-01-069542. [DOI] [PubMed] [Google Scholar]
- 10.Massey GV, Zipursky A, Chang MN, Doyle JJ, Nasim S, Taub JW, Ravindranath Y, Dahl G, Weinstein HJ. A prospective study of the natural history of transient leukemia (TL) in neonates with Down syndrome (DS): Children’s Oncology Group (COG) study POG-9481. Blood. 2006 Jun 15;107(12):4606–13. doi: 10.1182/blood-2005-06-2448. [DOI] [PubMed] [Google Scholar]
- 11.Klusmann JH, Creutzig U, Zimmermann M, Dworzak M, Jorch N, Langebrake C, Pekrun A, Macakova-Reinhardt K, Reinhardt D. Treatment and prognostic impact of transient leukemia in neonates with Down syndrome. Blood. 2008 Mar 15;111(6):2991–8. doi: 10.1182/blood-2007-10-118810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muramatsu H, Kato K, Watanabe N, Matsumoto K, Nakamura T, Horikoshi Y, Mimaya J, Suzuki C, Hayakawa M, Kojima S. Risk factors for early death in neonates with Down syndrome and transient leukaemia. Br J Haematol. 2008 Aug;142(4):610–5. doi: 10.1111/j.1365-2141.2008.07231.x. [DOI] [PubMed] [Google Scholar]
- 13.Massey GV. Transient leukemia in newborns with Down syndrome. Pediatr Blood Cancer. 2005 Jan;44(1):29–32. doi: 10.1002/pbc.20141. [DOI] [PubMed] [Google Scholar]
- 14.Zipursky A, Poon A, Doyle J. Leukemia in Down syndrome: a review. Pediatr Hematol Oncol. 1992 Apr-Jun;9(2):139–49. doi: 10.3109/08880019209018329. [DOI] [PubMed] [Google Scholar]
- 15.Lange BJ, Kobrinsky N, Barnard DR, Arthur DC, Buckley JD, Howells WB, Gold S, Sanders J, Neudorf S, Smith FO, Woods WG. Distinctive demography, biology, and outcome of acute myeloid leukemia and myelodysplastic syndrome in children with Down syndrome: Children’s Cancer Group Studies 2861 and 2891. Blood. 1998 Jan 15;91(2):608–15. [PubMed] [Google Scholar]
- 16.Creutzig U, Reinhardt D, Diekamp S, Dworzak M, Stary J, Zimmermann M. AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia. 2005 Aug;19(8):1355–60. doi: 10.1038/sj.leu.2403814. [DOI] [PubMed] [Google Scholar]
- 17.Moody A, Simpson E, Shaw D. Florid radiological appearance of megakaryoblastic leukaemia--an aid to earlier diagnosis. Pediatr Radiol. 1989;19(6–7):486–8. doi: 10.1007/BF02387668. [DOI] [PubMed] [Google Scholar]
- 18.Kozlowski K, Halimun E. Generalised bone disease with abundant periosteal reaction in megakaryocytic leukaemia. Eur J Pediatr. 1997 Nov;156(11):845–7. doi: 10.1007/s004310050726. [DOI] [PubMed] [Google Scholar]
- 19.Rao A, Hills RK, Stiller C, Gibson BE, de Graaf SS, Hann IM, O’Marcaigh A, Wheatley K, Webb DK. Treatment for myeloid leukaemia of Down syndrome: population-based experience in the UK and results from the Medical Research Council AML 10 and AML 12 trials. Br J Haematol. 2006 Mar;132(5):576–83. doi: 10.1111/j.1365-2141.2005.05906.x. [DOI] [PubMed] [Google Scholar]
- 20.Zeller B, Gustafsson G, Forestier E, Abrahamsson J, Clausen N, Heldrup J, Hovi L, Jonmundsson G, Lie SO, Glomstein A, Hasle H. Acute leukaemia in children with Down syndrome: a population-based Nordic study. Br J Haematol. 2005 Mar;128(6):797–804. doi: 10.1111/j.1365-2141.2005.05398.x. [DOI] [PubMed] [Google Scholar]
- 21.Bevan DRM, Greaves M. Leukaemia of platelet precursors: diverse features in four cases. Br J Haematol. 1982 May;51(1):147–64. doi: 10.1111/j.1365-2141.1982.tb07299.x. [DOI] [PubMed] [Google Scholar]
- 22.Windebank KP, Tefferi A, Smithson WA, Li CY, Solberg LA, Jr, Priest JR, Elliott SC, de Alarcon PA, Weinblatt ME, Burgert EO., Jr Acute megakaryocytic leukemia (M7) in children. Mayo Clin Proc. 1989 Nov;64(11):1339–51. doi: 10.1016/s0025-6196(12)65376-2. [DOI] [PubMed] [Google Scholar]
- 23.Yumura-Yagi K, Hara J, Kurahashi H, Nishiura T, Kaneyama Y, Osugi Y, Sakata N, Inoue M, Tawa A, Okada S. Mixed phenotype of blasts in acute megakaryocytic leukaemia and transient abnormal myelopoiesis in Down’s syndrome. Br J Haematol. 1992 Aug;81(4):520–5. doi: 10.1111/j.1365-2141.1992.tb02985.x. [DOI] [PubMed] [Google Scholar]
- 24.Langebrake C, Creutzig U, Reinhardt D. Immunophenotype of Down syndrome acute myeloid leukemia and transient myeloproliferative disease differs significantly from other diseases with morphologically identical or similar blasts. Klin Padiatr. 2005 May-Jun;217(3):126–34. doi: 10.1055/s-2005-836510. [DOI] [PubMed] [Google Scholar]
- 25.Litz CE, Davies S, Brunning RD, Kueck B, Parkin JL, Gajl Peczalska K, Arthur DC. Acute leukemia and the transient myeloproliferative disorder associated with Down syndrome: morphologic, immunophenotypic and cytogenetic manifestations. Leukemia. 1995 Sep;9(9):1432–9. [PubMed] [Google Scholar]
- 26.Kudo K, Kojima S, Tabuchi K, Yabe H, Tawa A, Imaizumi M, Hanada R, Hamamoto K, Kobayashi R, Morimoto A, Nakayama H, Tsuchida M, Horibe K, Kigasawa H, Tsukimoto I. Prospective study of a pirarubicin, intermediate-dose cytarabine, and etoposide regimen in children with Down syndrome and acute myeloid leukemia: the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2007 Dec 1;25(34):5442–7. doi: 10.1200/JCO.2007.12.3687. [DOI] [PubMed] [Google Scholar]
- 27.Forestier E, Izraeli S, Beverloo B, Haas O, Pession A, Michalova K, Stark B, Harrison CJ, Teigler-Schlegel A, Johansson B. Cytogenetic features of acute lymphoblastic and myeloid leukemias in pediatric patients with Down syndrome: an iBFM-SG study. Blood. 2008 Feb 1;111(3):1575–83. doi: 10.1182/blood-2007-09-114231. [DOI] [PubMed] [Google Scholar]
- 28.Papageorgiou EA, Karagrigoriou A, Tsaliki E, Velissariou V, Carter NP, Patsalis PC. Fetal-specific DNA methylation ratio permits noninvasive prenatal diagnosis of trisomy 21. Nat Med. 2011 Apr;17(4):510–3. doi: 10.1038/nm.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennings JL, Rodenburg W, Imholz S, Koster MP, van Oostrom CT, Breit TM, Schielen PC, de Vries A. Gene expression profiling in a mouse model identifies fetal liver- and placenta-derived potential biomarkers for Down Syndrome screening. PLoS One. 2011;6(4):e18866. doi: 10.1371/journal.pone.0018866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamis AS, Woods WG, Alonzo TA, Buxton A, Lange B, Barnard DR, Gold S, Smith FO. Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: a report from the Children’s Cancer Group Study 2891. J Clin Oncol. 2003 Sep 15;21(18):3415–22. doi: 10.1200/JCO.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 31.Taub JW, Mundschau G, Ge Y, Poulik JM, Qureshi F, Jensen T, James SJ, Matherly LH, Wechsler J, Crispino JD. Prenatal origin of GATA1 mutations may be an initiating step in the development of megakaryocytic leukemia in Down syndrome. Blood. 2004 Sep 1;104(5):1588–9. doi: 10.1182/blood-2004-04-1563. [DOI] [PubMed] [Google Scholar]
- 32.Mundschau G, Gurbuxani S, Gamis AS, Greene ME, Arceci RJ, Crispino JD. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood. 2003 Jun 1;101(11):4298–300. doi: 10.1182/blood-2002-12-3904. [DOI] [PubMed] [Google Scholar]
- 33.Hitzler JK, Cheung J, Li Y, Scherer SW, Zipursky A. GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of Down syndrome. Blood. 2003 Jun 1;101(11):4301–4. doi: 10.1182/blood-2003-01-0013. [DOI] [PubMed] [Google Scholar]
- 34.Groet J, McElwaine S, Spinelli M, Rinaldi A, Burtscher I, Mulligan C, Mensah A, Cavani S, Dagna-Bricarelli F, Basso G, Cotter FE, Nizetic D. Acquired mutations in GATA1 in neonates with Down’s syndrome with transient myeloid disorder. Lancet. 2003 May 10;361(9369):1617–20. doi: 10.1016/S0140-6736(03)13266-7. [DOI] [PubMed] [Google Scholar]
- 35.Ito E, Kasai M, Hayashi Y, Toki T, Arai K, Yokoyama S, Kato K, Tachibana N, Yamamoto M, Yokoyama M. Expression of erythroid-specific genes in acute megakaryoblastic leukaemia and transient myeloproliferative disorder in Down’s syndrome. Br J Haematol. 1995 Jul;90(3):607–14. doi: 10.1111/j.1365-2141.1995.tb05591.x. [DOI] [PubMed] [Google Scholar]
- 36.Koike T, Urushiyama M, Narita M, Saitoh H, Ishida F, Imashuku S, Morioka Y, Utsumi J, Ishizuka T, Tsuruta T. Target cell of leukemic transformation in acute megakaryoblastic leukemia. Am J Hematol. 1990 Aug;34(4):252–8. doi: 10.1002/ajh.2830340404. [DOI] [PubMed] [Google Scholar]
- 37.Shimada A, Xu G, Toki T, Kimura H, Hayashi Y, Ito E. Fetal origin of the GATA1 mutation in identical twins with transient myeloproliferative disorder and acute megakaryoblastic leukemia accompanying Down syndrome. Blood. 2004 Jan 1;103(1):366. doi: 10.1182/blood-2003-09-3219. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed M, Sternberg A, Hall G, Thomas A, Smith O, O’Marcaigh A, Wynn R, Stevens R, Addison M, King D, Stewart B, Gibson B, Roberts I, Vyas P. Natural history of GATA1 mutations in Down syndrome. Blood. 2004 Apr 1;103(7):2480–9. doi: 10.1182/blood-2003-10-3383. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Godinho FJ, Klusmann JH, Garriga-Canut M, Yu C, Orkin SH. Developmental stage-selective effect of somatically mutated leukemogenic transcription factor GATA1. Nat Genet. 2005 Jun;37(6):613–9. doi: 10.1038/ng1566. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu R, Kobayashi E, Engel JD, Yamamoto M. Induction of hyperproliferative fetal megakaryopoiesis by an N-terminally truncated GATA1 mutant. Genes Cells. 2009 Sep;14(9):1119–31. doi: 10.1111/j.1365-2443.2009.01338.x. [DOI] [PubMed] [Google Scholar]
- 41.Tunstall-Pedoe O, Roy A, Karadimitris A, de la Fuente J, Fisk NM, Bennett P, Norton A, Vyas P, Roberts I. Abnormalities in the myeloid progenitor compartment in Down syndrome fetal liver precede acquisition of GATA1 mutations. Blood. 2008 Dec 1;112(12):4507–11. doi: 10.1182/blood-2008-04-152967. [DOI] [PubMed] [Google Scholar]
- 42.Chou ST, Opalinska JB, Yao Y, Fernandes MA, Kalota A, Brooks JS, Choi JK, Gewirtz AM, Danet-Desnoyers GA, Nemiroff RL, Weiss MJ. Trisomy 21 enhances human fetal erythro-megakaryocytic development. Blood. 2008 Dec 1;112(12):4503–6. doi: 10.1182/blood-2008-05-157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korbel JO, Tirosh-Wagner T, Urban AE, Chen XN, Kasowski M, Dai L, Grubert F, Erdman C, Gao MC, Lange K, Sobel EM, Barlow GM, Aylsworth AS, Carpenter NJ, Clark RD, Cohen MY, Doran E, Falik-Zaccai T, Lewin SO, Lott IT, McGillivray BC, Moeschler JB, Pettenati MJ, Pueschel SM, Rao KW, Shaffer LG, Shohat M, Van Riper AJ, Warburton D, Weissman S, Gerstein MB, Snyder M, Korenberg JR. The genetic architecture of Down syndrome phenotypes revealed by high-resolution analysis of human segmental trisomies. Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):12031–6. doi: 10.1073/pnas.0813248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Vita S, Canzonetta C, Mulligan C, Delom F, Groet J, Baldo C, Vanes L, Dagna-Bricarelli F, Hoischen A, Veltman J, Fisher EM, Tybulewicz VL, Nizetic D. Trisomic dose of several chromosome 21 genes perturbs haematopoietic stem and progenitor cell differentiation in Down’s syndrome. Oncogene. 2010 Nov 18;29(46):6102–14. doi: 10.1038/onc.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Canzonetta C, Mulligan C, Deutsch S, Ruf S, O’Doherty A, Lyle R, Borel C, Lin-Marq N, Delom F, Groet J, Schnappauf F, De Vita S, Averill S, Priestley JV, Martin JE, Shipley J, Denyer G, Epstein CJ, Fillat C, Estivill X, Tybulewicz VL, Fisher EM, Antonarakis SE, Nizetic D. DYRK1A-dosage imbalance perturbs NRSF/REST levels, deregulating pluripotency and embryonic stem cell fate in Down syndrome. Am J Hum Genet. 2008 Sep;83(3):388–400. doi: 10.1016/j.ajhg.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen JJ, Williams BJ, Zipursky A, Doyle J, Sherman SL, Jacobs PA, Shugar AL, Soukup SW, Hassold TJ. Cytogenetic and molecular studies of Down syndrome individuals with leukemia. Am J Hum Genet. 1995 Apr;56(4):915–25. [PMC free article] [PubMed] [Google Scholar]
- 47.Rainis L, Toki T, Pimanda JE, Rosenthal E, Machol K, Strehl S, Gottgens B, Ito E, Izraeli S. The proto-oncogene ERG in megakaryoblastic leukemias. Cancer Res. 2005 Sep 1;65(17):7596–602. doi: 10.1158/0008-5472.CAN-05-0147. [DOI] [PubMed] [Google Scholar]
- 48.Stankiewicz MJ, Crispino JD. ETS2 and ERG promote megakaryopoiesis and synergize with alterations in GATA-1 to immortalize hematopoietic progenitor cells. Blood. 2009 Apr 2;113(14):3337–47. doi: 10.1182/blood-2008-08-174813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salek-Ardakani S, Smooha G, de Boer J, Sebire NJ, Morrow M, Rainis L, Lee S, Williams O, Izraeli S, Brady HJ. ERG is a megakaryocytic oncogene. Cancer Res. 2009 Jun 1;69(11):4665–73. doi: 10.1158/0008-5472.CAN-09-0075. [DOI] [PubMed] [Google Scholar]
- 50.Kirsammer G, Jilani S, Liu H, Davis E, Gurbuxani S, Le Beau MM, Crispino JD. Highly penetrant myeloproliferative disease in the Ts65Dn mouse model of Down syndrome. Blood. 2008 Jan 15;111(2):767–75. doi: 10.1182/blood-2007-04-085670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng AP, Hyland CD, Metcalf D, Carmichael CL, Loughran SJ, Di Rago L, Kile BT, Alexander WS. Trisomy of Erg is required for myeloproliferation in a mouse model of Down syndrome. Blood. 2010 May 13;115(19):3966–9. doi: 10.1182/blood-2009-09-242107. [DOI] [PubMed] [Google Scholar]
- 52.Alford KA, Slender A, Vanes L, Li Z, Fisher EM, Nizetic D, Orkin SH, Roberts I, Tybulewicz VL. Perturbed hematopoiesis in the Tc1 mouse model of Down syndrome. Blood. 2010 Apr 8;115(14):2928–37. doi: 10.1182/blood-2009-06-227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carmichael CL, Majewski IJ, Alexander WS, Metcalf D, Hilton DJ, Hewitt CA, Scott HS. Hematopoietic defects in the Ts1Cje mouse model of Down syndrome. Blood. 2009 Feb 26;113(9):1929–37. doi: 10.1182/blood-2008-06-161422. [DOI] [PubMed] [Google Scholar]
- 54.Klusmann JH, Li Z, Bohmer K, Maroz A, Koch ML, Emmrich S, Godinho FJ, Orkin SH, Reinhardt D. miR-125b-2 is a potential oncomiR on human chromosome 21 in megakaryoblastic leukemia. Genes Dev. 2010 Mar 1;24(5):478–90. doi: 10.1101/gad.1856210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greene ME, Mundschau G, Wechsler J, McDevitt M, Gamis A, Karp J, Gurbuxani S, Arceci R, Crispino JD. Mutations in GATA1 in both transient myeloproliferative disorder and acute megakaryoblastic leukemia of Down syndrome. Blood Cells Mol Dis. 2003 Nov-Dec;31(3):351–6. doi: 10.1016/j.bcmd.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 56.Ge Y, Dombkowski AA, LaFiura KM, Tatman D, Yedidi RS, Stout ML, Buck SA, Massey G, Becton DL, Weinstein HJ, Ravindranath Y, Matherly LH, Taub JW. Differential gene expression, GATA1 target genes, and the chemotherapy sensitivity of Down syndrome megakaryocytic leukemia. Blood. 2006 Feb 15;107(4):1570–81. doi: 10.1182/blood-2005-06-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bourquin JP, Subramanian A, Langebrake C, Reinhardt D, Bernard O, Ballerini P, Baruchel A, Cave H, Dastugue N, Hasle H, Kaspers GL, Lessard M, Michaux L, Vyas P, van Wering E, Zwaan CM, Golub TR, Orkin SH. Identification of distinct molecular phenotypes in acute megakaryoblastic leukemia by gene expression profiling. Proc Natl Acad Sci U S A. 2006 Feb 28;103(9):3339–44. doi: 10.1073/pnas.0511150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirose Y, Kudo K, Kiyoi H, Hayashi Y, Naoe T, Kojima S. Comprehensive analysis of gene alterations in acute megakaryoblastic leukemia of Down’s syndrome. Leukemia. 2003 Nov;17(11):2250–2. doi: 10.1038/sj.leu.2403121. [DOI] [PubMed] [Google Scholar]
- 59.Kuhl C, Atzberger A, Iborra F, Nieswandt B, Porcher C, Vyas P. GATA1-mediated megakaryocyte differentiation and growth control can be uncoupled and mapped to different domains in GATA1. Mol Cell Biol. 2005 Oct;25(19):8592–606. doi: 10.1128/MCB.25.19.8592-8606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muntean AG, Crispino JD. Differential requirements for the activation domain and FOG-interaction surface of GATA-1 in megakaryocyte gene expression and development. Blood. 2005 Aug 15;106(4):1223–31. doi: 10.1182/blood-2005-02-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cabelof DC, Patel HV, Chen Q, van Remmen H, Matherly LH, Ge Y, Taub JW. Mutational spectrum at GATA1 provides insights into mutagenesis and leukemogenesis in Down syndrome. Blood. 2009 Sep 24;114(13):2753–63. doi: 10.1182/blood-2008-11-190330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanezaki R, Toki T, Terui K, Xu G, Wang R, Shimada A, Hama A, Kanegane H, Kawakami K, Endo M, Hasegawa D, Kogawa K, Adachi S, Ikeda Y, Iwamoto S, Taga T, Kosaka Y, Kojima S, Hayashi Y, Ito E. Down syndrome and GATA1 mutations in transient abnormal myeloproliferative disorder: mutation classes correlate with progression to myeloid leukemia. Blood. 2010 Nov 25;116(22):4631–8. doi: 10.1182/blood-2010-05-282426. [DOI] [PubMed] [Google Scholar]
- 63.Alford KA, Reinhardt K, Garnett C, Norton A, Bohmer K, von Neuhoff C, Kolenova A, Marchi E, Klusmann JH, Roberts I, Hasle H, Reinhardt D, Vyas P. Analysis of GATA1 mutations in Down syndrome transient myeloproliferative disorder and myeloid leukemia. Blood. 2011 Jun 29; doi: 10.1182/blood-2011-03-342774. [DOI] [PubMed] [Google Scholar]
- 64.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997 Jul 1;16(13):3965–73. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollanda LM, Lima CS, Cunha AF, Albuquerque DM, Vassallo J, Ozelo MC, Joazeiro PP, Saad ST, Costa FF. An inherited mutation leading to production of only the short isoform of GATA-1 is associated with impaired erythropoiesis. Nat Genet. 2006 Jul;38(7):807–12. doi: 10.1038/ng1825. [DOI] [PubMed] [Google Scholar]
- 66.Malkin D, Brown EJ, Zipursky A. The role of p53 in megakaryocyte differentiation and the megakaryocytic leukemias of Down syndrome. Cancer Genet Cytogenet. 2000 Jan 1;116(1):1–5. doi: 10.1016/s0165-4608(99)00072-2. [DOI] [PubMed] [Google Scholar]
- 67.Kanezaki R, Toki T, Xu G, Narayanan R, Ito E. Cloning and characterization of the novel chimeric gene p53/FXR2 in the acute megakaryoblastic leukemia cell line CMK11-5. Tohoku J Exp Med. 2006 Jul;209(3):169–80. doi: 10.1620/tjem.209.169. [DOI] [PubMed] [Google Scholar]
- 68.Walters DK, Mercher T, Gu TL, O’Hare T, Tyner JW, Loriaux M, Goss VL, Lee KA, Eide CA, Wong MJ, Stoffregen EP, McGreevey L, Nardone J, Moore SA, Crispino J, Boggon TJ, Heinrich MC, Deininger MW, Polakiewicz RD, Gilliland DG, Druker BJ. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006 Jul;10(1):65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Kiyoi H, Yamaji S, Kojima S, Naoe T. JAK3 mutations occur in acute megakaryoblastic leukemia both in Down syndrome children and non-Down syndrome adults. Leukemia. 2007 Mar;21(3):574–6. doi: 10.1038/sj.leu.2404527. [DOI] [PubMed] [Google Scholar]
- 70.Sato T, Toki T, Kanezaki R, Xu G, Terui K, Kanegane H, Miura M, Adachi S, Migita M, Morinaga S, Nakano T, Endo M, Kojima S, Kiyoi H, Mano H, Ito E. Functional analysis of JAK3 mutations in transient myeloproliferative disorder and acute megakaryoblastic leukaemia accompanying Down syndrome. Br J Haematol. 2008 May;141(5):681–8. doi: 10.1111/j.1365-2141.2008.07081.x. [DOI] [PubMed] [Google Scholar]
- 71.Riera L, Lasorsa E, Bonello L, Sismondi F, Tondat F, Di Bello C, Di Celle PF, Chiarle R, Godio L, Pich A, Facchetti F, Ponzoni M, Marmont F, Zanon C, Bardelli A, Inghirami G. Description of a novel Janus kinase 3 P132A mutation in acute megakaryoblastic leukemia and demonstration of previously reported Janus kinase 3 mutations in normal subjects. Leuk Lymphoma. 2011 May 23; doi: 10.3109/10428194.2011.574757. [DOI] [PubMed] [Google Scholar]
- 72.Malinge S, Ragu C, Della-Valle V, Pisani D, Constantinescu SN, Perez C, Villeval JL, Reinhardt D, Landman-Parker J, Michaux L, Dastugue N, Baruchel A, Vainchenker W, Bourquin JP, Penard-Lacronique V, Bernard OA. Activating mutations in human acute megakaryoblastic leukemia. Blood. 2008 Nov 15;112(10):4220–6. doi: 10.1182/blood-2008-01-136366. [DOI] [PubMed] [Google Scholar]
- 73.Hussein K, Bock O, Theophile K, Schulz-Bischof K, Porwit A, Schlue J, Jonigk D, Kreipe H. MPLW515L mutation in acute megakaryoblastic leukaemia. Leukemia. 2009 May;23(5):852–5. doi: 10.1038/leu.2008.371. [DOI] [PubMed] [Google Scholar]
- 74.Chou S, Lodish HF. Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):7799–804. doi: 10.1073/pnas.1003586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klusmann JH, Godinho FJ, Heitmann K, Maroz A, Koch ML, Reinhardt D, Orkin SH, Li Z. Developmental stage-specific interplay of GATA1 and IGF signaling in fetal megakaryopoiesis and leukemogenesis. Genes Dev. 2010 Aug 1;24(15):1659–72. doi: 10.1101/gad.1903410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature. 1995 Dec 21–28;378(6559):776–9. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- 77.Holt SE, Brown EJ, Zipursky A. Telomerase and the benign and malignant megakaryoblastic leukemias of Down syndrome. J Pediatr Hematol Oncol. 2002 Jan;24(1):14–7. doi: 10.1097/00043426-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Ravindranath Y, Abella E, Krischer JP, Wiley J, Inoue S, Harris M, Chauvenet A, Alvarado CS, Dubowy R, Ritchey AK. Acute myeloid leukemia (AML) in Down’s syndrome is highly responsive to chemotherapy: experience on Pediatric Oncology Group AML Study 8498. Blood. 1992 Nov 1;80(9):2210–4. [PubMed] [Google Scholar]
- 79.Ravindranath YAE, Krischer S. AML in Down Syndrome; repsonse and toxicity with high-dose Ara-C. Proc ASCO. 1993;12:323a. [Google Scholar]
- 80.Tchernia G, Lejeune F, Boccara JF, Denavit MF, Dommergues JP, Bernaudin F. Erythroblastic and/or megakaryoblastic leukemia in Down syndrome: treatment with low-dose arabinosyl cytosine. J Pediatr Hematol Oncol. 1996 Feb;18(1):59–62. doi: 10.1097/00043426-199602000-00011. [DOI] [PubMed] [Google Scholar]
- 81.Al-Kasim F, Doyle JJ, Massey GV, Weinstein HJ, Zipursky A. Incidence and treatment of potentially lethal diseases in transient leukemia of Down syndrome: Pediatric Oncology Group Study. J Pediatr Hematol Oncol. 2002 Jan;24(1):9–13. doi: 10.1097/00043426-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Al-Ahmari A, Shah N, Sung L, Zipursky A, Hitzler J. Long-term results of an ultra low-dose cytarabine-based regimen for the treatment of acute megakaryoblastic leukaemia in children with Down syndrome. Br J Haematol. 2006 Jun;133(6):646–8. doi: 10.1111/j.1365-2141.2006.06097.x. [DOI] [PubMed] [Google Scholar]
- 83.Creutzig U, Zimmermann M, Ritter J, Reinhardt D, Hermann J, Henze G, Jurgens H, Kabisch H, Reiter A, Riehm H, Gadner H, Schellong G. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005 Dec;19(12):2030–42. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 84.Taga T, Shimomura Y, Horikoshi Y, Ogawa A, Itoh M, Okada M, Ueyama J, Higa T, Watanabe A, Kanegane H, Iwai A, Saiwakawa Y, Kogawa K, Yamanaka J, Tsurusawa M. Continuous and high-dose cytarabine combined chemotherapy in children with down syndrome and acute myeloid leukemia: Report from the Japanese children’s cancer and leukemia study group (JCCLSG) AML 9805 down study. Pediatr Blood Cancer. 2011 Jul 15;57(1):36–40. doi: 10.1002/pbc.22943. [DOI] [PubMed] [Google Scholar]
- 85.Taub JW, Matherly LH, Stout ML, Buck SA, Gurney JG, Ravindranath Y. Enhanced metabolism of 1-beta-D-arabinofuranosylcytosine in Down syndrome cells: a contributing factor to the superior event free survival of Down syndrome children with acute myeloid leukemia. Blood. 1996 Apr 15;87(8):3395–403. [PubMed] [Google Scholar]
- 86.Ge Y, Jensen TL, Stout ML, Flatley RM, Grohar PJ, Ravindranath Y, Matherly LH, Taub JW. The role of cytidine deaminase and GATA1 mutations in the increased cytosine arabinoside sensitivity of Down syndrome myeloblasts and leukemia cell lines. Cancer Res. 2004 Jan 15;64(2):728–35. doi: 10.1158/0008-5472.can-03-2456. [DOI] [PubMed] [Google Scholar]
- 87.Yamada S, Hongo T, Okada S, Watanabe C, Fujii Y, Hori H, Yazaki M, Hanada R, Horikoshi Y. Distinctive multidrug sensitivity and outcome of acute erythroblastic and megakaryoblastic leukemia in children with Down syndrome. Int J Hematol. 2001 Dec;74(4):428–36. doi: 10.1007/BF02982087. [DOI] [PubMed] [Google Scholar]
- 88.Savasan S, Buck S, Raimondi SC, Becton DL, Weinstein H, Chang M, Ravindranath Y. CD36 (thrombospondin receptor) expression in childhood acute megakaryoblastic leukemia: in vitro drug sensitivity and outcome. Leuk Lymphoma. 2006 Oct;47(10):2076–83. doi: 10.1080/10428190600773180. [DOI] [PubMed] [Google Scholar]
- 89.Edwards H, Xie C, LaFiura KM, Dombkowski AA, Buck SA, Boerner JL, Taub JW, Matherly LH, Ge Y. RUNX1 regulates phosphoinositide 3-kinase/AKT pathway: role in chemotherapy sensitivity in acute megakaryocytic leukemia. Blood. 2009 Sep 24;114(13):2744–52. doi: 10.1182/blood-2008-09-179812. [DOI] [PMC free article] [PubMed] [Google Scholar]