Summary
Background
Observational studies report reduced colorectal cancer in regular aspirin consumers. Randomised controlled trials have shown reduced risk of adenomas but none have employed prevention of colorectal cancer as a primary endpoint. The CAPP2 trial aimed to investigate the antineoplastic effects of aspirin and a resistant starch in carriers of Lynch syndrome, the major form of hereditary colorectal cancer; we now report long-term follow-up of participants randomly assigned to aspirin or placebo.
Methods
In the CAPP2 randomised trial, carriers of Lynch syndrome were randomly assigned in a two-by-two factorial design to 600 mg aspirin or aspirin placebo or 30 g resistant starch or starch placebo, for up to 4 years. Randomisation was in blocks of 16 with provision for optional single-agent randomisation and extended postintervention double-blind follow-up; participants and investigators were masked to treatment allocation. The primary endpoint was development of colorectal cancer. Analysis was by intention to treat and per protocol. This trial is registered, ISRCTN59521990.
Results
861 participants were randomly assigned to aspirin or aspirin placebo. At a mean follow-up of 55·7 months, 48 participants had developed 53 primary colorectal cancers (18 of 427 randomly assigned to aspirin, 30 of 434 to aspirin placebo). Intention-to-treat analysis of time to first colorectal cancer showed a hazard ratio (HR) of 0·63 (95% CI 0·35–1·13, p=0·12). Poisson regression taking account of multiple primary events gave an incidence rate ratio (IRR) of 0·56 (95% CI 0·32–0·99, p=0·05). For participants completing 2 years of intervention (258 aspirin, 250 aspirin placebo), per-protocol analysis yielded an HR of 0·41 (0·19–0·86, p=0·02) and an IRR of 0·37 (0·18–0·78, p=0·008). No data for adverse events were available postintervention; during the intervention, adverse events did not differ between aspirin and placebo groups.
Interpretation
600 mg aspirin per day for a mean of 25 months substantially reduced cancer incidence after 55·7 months in carriers of hereditary colorectal cancer. Further studies are needed to establish the optimum dose and duration of aspirin treatment.
Funding
European Union; Cancer Research UK; Bayer Corporation; National Starch and Chemical Co; UK Medical Research Council; Newcastle Hospitals trustees; Cancer Council of Victoria Australia; THRIPP South Africa; The Finnish Cancer Foundation; SIAK Switzerland; Bayer Pharma.
Introduction
People with monogenic predisposition to cancer offer an ideal focus for chemoprevention trials; the high probability of early tumours provides statistical power, and knowledge of genetic basis reduces heterogeneity while providing data relevant to patients whose sporadic cancers involve the same molecular pathway. Existing planned surveillance reduces cost and the relevance to family members encourages patient compliance. The Colorectal Adenoma/carcinoma Prevention Programme (CAPP) was launched in 1990. CAPP1 investigated 200 young people with familial adenomatous polyposis. CAPP2, the first large-scale genetically targeted chemoprevention trial, focused on 1000 people with Lynch syndrome (also known as hereditary non-polyposis colon cancer or HNPCC), most carrying pathological DNA mismatch repair (MMR) gene variants, plus previously affected patients within families meeting the Amsterdam criteria.1
Both trials used a factorial two-by-two design to assess two agents, aspirin and resistant starch, thought to protect against colorectal cancer. CAPP1 revealed a weakly significant effect of aspirin on size of largest observed polyp and a significant reduction in crypt length in participants given resistant starch.2 In CAPP2 over 6 years, 937 people from 43 international centres commenced intervention.3 After intervention, mean 29 months, there was no evidence that either agent affected development of colonic neoplasia, with most lesions being adenomas.3 In view of cohort and case-control evidence of a protective effect of aspirin against colorectal cancer only after long-term exposure,4 the original design of the CAPP2 study included double-blind post-intervention follow-up for at least 10 years.
At the end of the intervention period,3 128 participants had developed at least one adenoma and 23 had developed colorectal cancer. These were pooled for analysis as neoplasia since the primary endpoint of colorectal cancer was judged unlikely to be affected within 4 years in a population under colonoscopic surveillance. We now report the effect of aspirin on the incidence of colorectal cancer, the primary CAPP2 outcome, and other Lynch syndrome cancers as secondary outcomes. The baseline population of 861 participants (randomly assigned to aspirin or aspirin placebo in the randomised controlled trial) differs from our first report, which was confined to those with an exit colonoscopy.
Methods
Trial design and participants
Between January, 1999, and March, 2005, 937 carriers of Lynch syndrome started intervention in the CAPP2 study3,5 and 746 were included in the end-of-intervention analysis (mean 29 months). Randomisation was in blocks of 16 in a two-by-two factorial design to aspirin (600 mg), aspirin placebo, resistant starch (30 g; Novelose, National Starch and Chemical Co, NJ, USA), and resistant starch placebo. Of the 937 participants, 427 were randomly assigned to aspirin, 434 to aspirin placebo, and the remaining recruits were not randomly assigned for the aspirin intervention, having opted not to participate in this part of the study (n=76; almost all due to perceived aspirin sensitivity or history of peptic ulceration). All participants who refused randomisation to the aspirin groups were randomly assigned to the resistant starch or resistant starch placebo intervention only (figure 1; webappendix p 1). Participants and investigators were masked to treatment allocation; one participant asked to be informed of her randomisation status after leaving the study. The study had a preplanned design for 10 years' follow-up; at the time of this analysis, the earliest enrolled participants had reached the 10-year threshold. All participants consented to long-term follow-up at recruitment and more detailed consent was obtained in the later stages of the study to ensure continued support.
Figure 1.
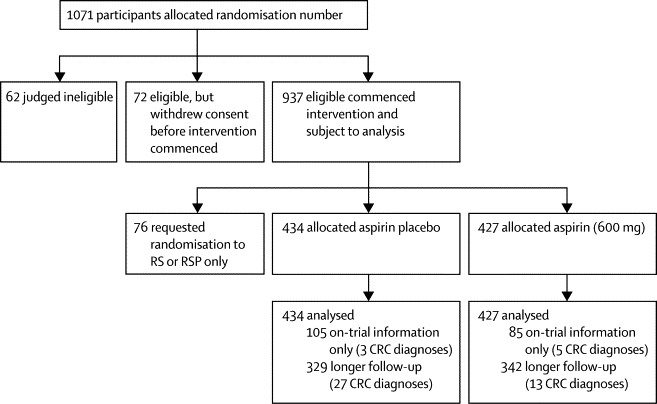
Trial profile
RS=resistant starch. RSP=resistant starch placebo. CRC=colorectal cancer.
The primary outcome of CAPP2 was development of colorectal cancer; the secondary outcomes were development of colorectal adenomas or the development of other Lynch syndrome-related cancers, or both. This analysis focused on 861 CAPP2 participants randomly assigned to aspirin or aspirin placebo from entry until the latest date for which the recruiters had information about cancer diagnosis—a timepoint usually corresponding to the date of last surveillance attendance. Our analysis included Lynch syndrome cancers that were included in the earlier report,3 those that occurred subsequent to exit from the intervention phase, and all cancers that occurred in people without an exit colonoscopy, which excluded them from the statistical analysis in our earlier report.3 As a result of dispersed international recruitment and because routine surveillance was provided by local health-care teams, records of adenoma occurrence in CAPP2 participants subsequent to the intervention phase are incomplete. Similarly, no details of adverse events were available postintervention; during the intervention phase, adverse events in the aspirin and placebo groups were similar (webappendix p 2).3 There was also no significant difference in compliance (ie, proportion of scheduled tablets not taken during the intervention phase) between the aspirin and aspirin placebo groups for participants with complete intervention phase data (χ2(1)=1·27, p=0·20).3
Statistical analysis
This analysis was designed to test the primary hypothesis that aspirin would reduce the development of colorectal cancer (as primary outcome) and Lynch syndrome cancers (as secondary outcome) in 861 participants randomly assigned to aspirin (n=427) or aspirin placebo (n=434). The original protocol invited participants to continue with the original intervention for a further 2-year cycle after the initial 2 years. Two analytical approaches were taken: first, time to first occurrence of colorectal cancer (our original focus), which was examined with life-table methods and Cox proportional hazards; and second, Poisson regression modelling to investigate primary cancers at multiple anatomical sites, a feature of Lynch syndrome. Poisson regression analysis took into account the complete cancer history of the participant since randomisation, by contrast with the more restricted time-to-first-event analysis.
For life-table analysis, end of follow-up was determined as the time of first diagnosis of colorectal cancer, if the participant was affected, or the last recorded date at which clinical status was known. Analyses included Cox proportional hazards models to estimate sex-adjusted hazard ratios (HRs) and 95% CIs, and Kaplan-Meier curves to assess non-parametrically the outcome differences between the aspirin and aspirin placebo interventions. The assumption of proportional hazard was tested to assess compliance. For the Poisson regression analysis, incidence rate ratios (IRRs) for the effect of aspirin adjusted for sex were estimated from log-linear models for the number of primary cancers diagnosed after randomisation; exposure time was from randomisation until date of last known clinical status.
All analyses used Stata (version 10). Analyses were undertaken on an intention-to-treat basis (ie, intervention assigned at randomisation) and per protocol (restricting consideration to those taking aspirin [or aspirin placebo] for at least 2 years). A secondary planned analysis addressed incidence of Lynch syndrome cancer, including new cancers thought to result from the underlying genetic defect. Designation of Lynch syndrome cancer spectrum was a clinical assessment, masked to intervention, and based on a review of the Lynch syndrome phenotype;6 endometrial, ovarian, pancreatic, small bowel, gall bladder, ureter, stomach, and kidney cancers and cancer of the brain were included. A final analysis examined the total burden of Lynch syndrome-related cancers in participants who had been on intervention for at least 2 years (per protocol). All p values reported are two-sided (in keeping with the original sample-size calculation).
This trial is registered, ISRCTN59521990.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Recruitment ran from Jan 1, 1999, to March 10, 2005. The mean observation period was 55·7 months (range 1–128) and eight (1%) recruits were 10 years or more from randomisation by the time of the present analysis (table 1). Times are measured from the date of randomisation. For 671 participants, we report both data for during the intervention and for longer follow-up, whereas for 190 we report on-intervention information only (webappendix p 3). Demographic data show no differences between those traced and not traced in this follow-up analysis with respect to age, sex, randomisation category, or geographical location (data not shown), although the development of a cancer could make follow-up reporting more complete. There were no significant regional differences in incidence of colorectal cancer (data not shown; χ2(2)=5·03, p=0·08).
Table 1.
Study population
| Aspirin (n=427) | Aspirin placebo (n=434) | Total (n=861) | ||
|---|---|---|---|---|
| Time in CAPP2 intervention study (months) | 25·0 (12·5; 0·8–60·6) | 25·4 (14·2; 1·1–74·4) | 25·2 (13·4; 0·8–74·4) | |
| Time since study entry (months) | 56·6 (30·9; 0·8–125·4) | 54·8 (31·8; 1·6–128·0) | 55·7 (31·4; 0·8–128·0) | |
| Participants with first colorectal cancer | ||||
| Since randomisation | 18 | 30 | 48 | |
| Within 2 years of randomisation | 10 | 10 | 20 | |
| More than 2 years from randomisation | 8 | 20 | 28 | |
| Participants with other Lynch syndrome cancers* | ||||
| Since randomisation | 16 | 24 | 40 | |
| Within 2 years of randomisation | 5 | 9 | 14 | |
| More than 2 years from randomisation | 11 | 15 | 26 | |
| Participants with one or more Lynch syndrome cancer (including colorectal) | ||||
| Since randomisation | 34 | 52 | 86 | |
| Within 2 years of randomisation | 15 | 19 | 34 | |
| More than 2 years from randomisation | 19 | 33 | 52 | |
| Participants with non-Lynch syndrome cancers | 19 | 19 | 38 | |
Data are mean (SD; range) or n.
Two participants in the placebo group each had a colorectal cancer and another Lynch syndrome cancer; these participants were counted in the rows relating to both colorectal and other Lynch syndrome cancers; in the row relating to all Lynch syndrome cancers, these participants were counted only once.
Table 1 summarises the cancer burden in the study population. Overall, 40 people with postintervention information were diagnosed with colorectal cancer (13 of 342 allocated aspirin and 27 of 329 allocated aspirin placebo). Colorectal cancer occurred in a further eight people among 190 individuals with information about the intervention phase only (five of 85 patients on aspirin and three of 105 on aspirin placebo; figure 1; webappendix p 4).
Evidence has emerged of delayed protection by aspirin against cancer. For the whole postrandomisation period, the HR for colorectal cancer was 0·63 (95% CI 0·35–1·13, p=0·12), favouring protection in the aspirin group (table 2; figure 2). Five of 48 people who developed colorectal cancer each had two primary colon cancers. Of these, one had received aspirin and four aspirin placebo. Although the intention-to-treat time-to-event analysis showed a non-significant protective effect of aspirin, the Poisson regression taking into account the five participants with multiple primary colorectal cancer (53 cancers) indicated a protective effect (IRR 0·56, 95% CI 0·32–0·99, p=0·05). We re-estimated this protective effect with a per-protocol analysis and obtained similar results.
Table 2.
Cox proportional hazards analysis and Poisson regression for colorectal cancer, non-colorectal Lynch syndrome cancers, and all Lynch syndrome cancers (adjusted for sex) in participants randomly assigned to aspirin or aspirin placebo
|
Hazard ratio |
Incidence rate ratio† |
||||
|---|---|---|---|---|---|
| 95% CI* | p value | 95% CI | p value | ||
| Colorectal cancer | |||||
| Intention-to-treat analysis | |||||
| Aspirin versus aspirin placebo | 0·63 (0·35–1·13) | 0·12 | 0·56 (0·32–0·99) | 0·05 | |
| Per-protocol analysis | |||||
| ≥2 years' aspirin placebo‡ | 1·0 | .. | 1·0 | .. | |
| <2 years' aspirin placebo‡ | 0·62 (0·25–1·52) | 0·30 | 0·72 (0·32–1·59) | 0·41 | |
| <2 years' aspirin‡ | 1·07 (0·47–2·41) | 0·87 | 0·90 (0·42–1·91) | 0·77 | |
| ≥2 years' aspirin‡ | 0·41 (0·19–0·86) | 0·02 | 0·37 (0·18–0·78) | 0·008 | |
| Cumulative aspirin dose | |||||
| Units of 100 aspirin§ | 0·97 (0·94–1·00) | 0·06 | 0·97 (0·94–1·00) | 0·03 | |
| Non-colorectal Lynch syndrome cancers | |||||
| Intention-to-treat analysis | |||||
| Aspirin versus aspirin placebo | 0·63 (0·34–1·19) | 0·16 | 0·63 (0·34–1·16) | 0·14 | |
| Per-protocol analysis | |||||
| ≥2 years' aspirin placebo‡ | 1·0 | .. | 1·0 | .. | |
| <2 years' aspirin placebo‡ | 0·96 (0·40–2·34) | 0·94 | 0·82 (0·35–1·96) | 0·66 | |
| <2 years' aspirin‡ | 1·11 (0·46–2·68) | 0·82 | 0·90 (0·38–2·14) | 0·81 | |
| ≥2 years' aspirin‡ | 0·47 (0·21–1·06) | 0·07 | 0·49 (0·23–1·05) | 0·07 | |
| Cumulative aspirin dose | |||||
| Units of 100 aspirin§ | 0·96 (0·93–1·00) | 0·03 | 0·96 (0·93–1·00) | 0·03 | |
| All Lynch syndrome cancers | |||||
| Intention-to-treat analysis | |||||
| Aspirin versus aspirin placebo | 0·65 (0·42–1·00) | 0·05 | 0·59 (0·39–0·90) | 0·01 | |
| Per-protocol analysis | |||||
| ≥2 years' aspirin placebo‡ | 1·0 | .. | 1·0 | .. | |
| <2 years' aspirin placebo‡ | 0·79 (0·42–1·49) | 0·47 | 0·76 (0·43–1·37) | 0·36 | |
| <2 years' aspirin‡ | 1·13 (0·62–2·06) | 0·69 | 0·90 (0·51–1·59) | 0·71 | |
| ≥2 years' aspirin‡ | 0·45 (0·26–0·79) | 0·005 | 0·42 (0·25–0·72) | 0·001 | |
| Cumulative aspirin dose | |||||
| Units of 100 aspirin§ | 0·97 (0·95–0·99) | 0·007 | 0·96 (0·94–0·99) | 0·002 | |
Cox proportional Hazards analysis based on 48 participants with colorectal cancer (including 53 cancer diagnoses), 40 cases of non-colorectal Lynch syndrome cancer, and 86 participants with Lynch syndrome cancers (93 cancer diagnoses).
Incidence rate ratio from Poisson regression.
The threshold for 2 years' intervention was consumption of more than 1400 aspirin tablets; rounded down from a 2-year total of 1461 tablets to allow for early scheduling of the exit colonoscopy or occasional missed dosage.
Units of 100 aspirin=total number of aspirin taken divided by 100.
Figure 2.
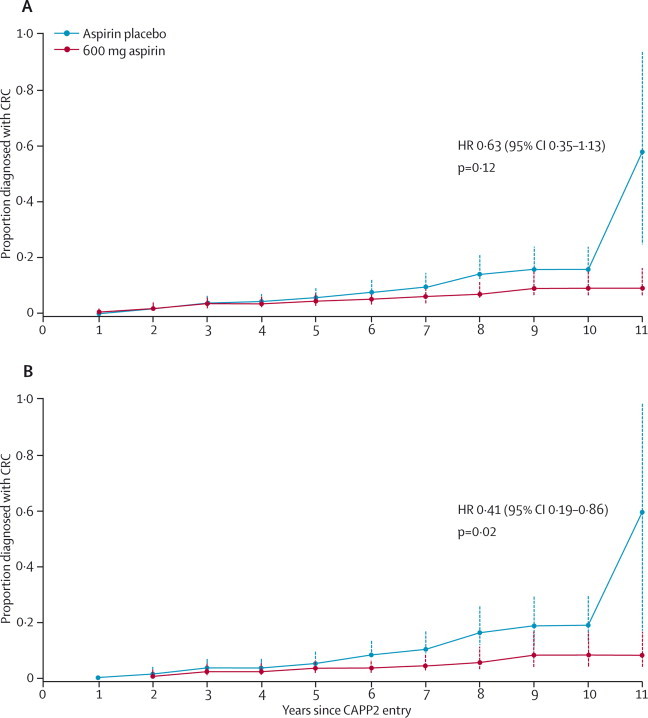
Time to first colorectal cancer in participants randomly assigned to aspirin compared with those assigned to aspirin placebo
(A) Kaplan-Meier analysis, adjusted for sex. (B) Kaplan-Meier analysis restricted to participants who had taken the intervention for 2 years or more, adjusted for sex. Each point on the plots shows the estimated cumulative incidence by years of follow-up; error bars show 95% CIs. HR=hazard ratio. CRC=colorectal cancer.
We examined outcomes in participants who took aspirin (or aspirin placebo) for a minimum of 2 years, defined as consumption of 1400 (300 mg) tablets (rounded down from a 2-year total [1461 tablets] to allow for early scheduling of the exit colonoscopy or occasional missed dosage). On the basis of this definition, 258 (60%) of those on aspirin and 250 (58%) of those on aspirin placebo were treated for 2 years or longer. The HR for those taking aspirin for 2 years or longer was 0·41 (95% CI 0·19–0·86, p=0·02; table 2, figure 2) and the IRR was 0·37 (95% CI 0·18–0·78, p=0·008). These results are similar to those for Poisson regression in the intention-to-treat analysis.
We explored the effect of compliance on outcome (which is important because non-compliance could be related to factors that also affect risk of colorectal cancer) using per-protocol analysis, and found that participants who took aspirin for 2 years or more had an incidence rate of 0·06 per 100 person-years compared with 0·13 per 100 person-years in those who took aspirin for less than 2 years. A similar analysis within the placebo group showed no significant difference in incidence of colorectal cancer between participants who took aspirin placebo for 2 years or more (0·14 per 100 person years) compared with those who took aspirin placebo for less than 2 years (0·10 per 100 person years; data not shown).
We also undertook a planned secondary analysis with other Lynch syndrome cancers as the outcome. 18 participants developed endometrial cancer, of whom five were randomly assigned to aspirin and 13 to aspirin placebo; in total, 38 participants developed cancer at a site other than the colorectum (additionally, two participants had colorectal and another Lynch syndrome cancer) of whom 16 were randomly assigned to aspirin and 22 to aspirin placebo (webappendix p 5). The HR for those randomly assigned to aspirin was 0·63 (95% CI 0·34–1·19 p=0·16, table 2; webappendix p 6) and the IRR was 0·63 (95% CI 0·34–1·16 p=0·14) compared with the aspirin placebo group. Per-protocol analysis showed an HR for those who had taken aspirin for 2 years or more of 0·47 (95% CI 0·21–1·06, p=0·07) with an IRR of 0·49 (95% CI 0·23–1·05 p=0·07; table 2).
Table 2 shows the combined analysis of all Lynch syndrome cancers including colorectal cancer. On intention-to-treat analysis, the HR was 0·65 (95% CI 0·42–1·00, p=0·05) and IRR was 0·59 (95% CI 0·39–0·90, p=0·01), and in the per-protocol analysis the HR was 0·45 (0·26–0·79, p=0·005; figure 3) and the IRR was 0·42 (0·25–0·72, p=0·001), supporting the protective effect of aspirin. Cox proportional hazards model analysis by cumulative aspirin consumption suggested a dose-response effect, which was significant for non-colorectal Lynch syndrome cancers (p=0·03) and Lynch syndrome cancers overall (p=0·007), but not for colorectal cancer (p=0·06; table 2). Corresponding outcomes from the Poisson regression analysis were also significant (p=0·03 for non-colorectal Lynch syndrome cancers, p=0·002 for Lynch syndrome cancers overall, and p=0·03 for colorectal cancer).
Figure 3.
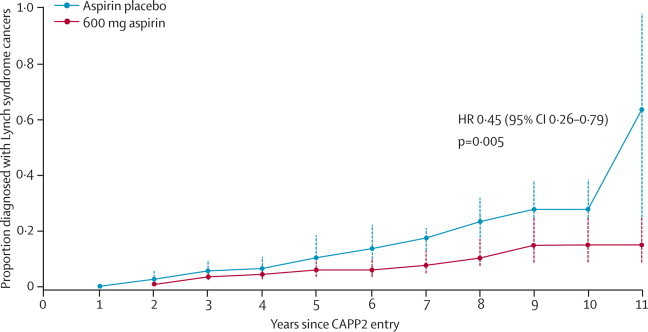
Time to first Lynch syndrome cancer in participants randomly assigned to aspirin compared with those assigned to aspirin placebo
Kaplan-Meier analysis restricted to participants who had taken the intervention for 2 years or more, adjusted for sex. Each point on the plot shows the estimated cumulative incidence by years of follow-up; error bars show 95% CIs. HR=hazard ratio.
The CAPP2 study included a group of participants who chose not to be randomly assigned for aspirin and who were randomly assigned for the resistant starch intervention only. To establish whether the apparent protective effect of aspirin might be attributable to unexpectedly high numbers of cancers in the aspirin placebo group, we tested the risk of colorectal cancer for the non-randomised group (resistant starch or resistant starch placebo only) compared with the aspirin placebo group. The HR for colorectal cancer in this group was 1·4 times higher compared with the aspirin placebo group (p=0·4). This finding supports the protective effect of aspirin.
Where possible, details of adenoma development were obtained in the postintervention period. Although incomplete, these data, gathered by masked contributors, revealed no apparent effect of aspirin on numbers of participants who developed adenomas subsequent to the intervention phase—ie, 51 and 48 in the aspirin and aspirin placebo groups, respectively.
The data were analysed according to the underlying MMR gene defect; colorectal cancer was reported with equal frequency in participants carrying MLH1 and MSH2 mutations (6·0% and 7·0%, respectively), and none of the MSH6 mutation carriers developed colorectal cancer, in keeping with the anticipated milder phenotype (webappendix p 7). The remaining 163 recruits were diagnosed on the basis of Amsterdam Criteria1 and had been treated for a Lynch syndrome-related neoplasia. Of these, seven (4%) developed colorectal cancer. Overall, there was no evidence of difference in incidence of colorectal cancer by presence of proven germ-line mutation (χ2(2)=3·1, p=0·38).
18 (34%) of 53 colorectal cancers diagnosed in aspirin or aspirin placebo groups were Dukes stage A, 21 (40%) Dukes B, ten (19%) had Dukes C and D, and four (8%) were unknown. 27 (51%) tumours were located in the ascending colon, transverse colon, and splenic flexure, six (11%) in the descending colon, 12 (23%) in the sigmoid and rectum, and eight (15%) were unknown. There was no significant difference in staging (χ2(3)=2·92, p=0·40) and tumour location (χ2(3)=0·08, p=0·99) between aspirin and aspirin placebo groups.
Discussion
The CAPP2 study was the first double-blind randomised controlled trial of aspirin chemoprevention with cancer as the primary endpoint. The outcome is consistent with more than two decades of observational data showing that risk of colorectal cancer is halved in regular aspirin consumers7 and recent long-term follow-up of aspirin trials for cardiovascular disease prevention showing that dosing with 75 mg or more of aspirin per day for several years reduced deaths from gastrointestinal cancers, particularly involving the proximal colon.8,9 This concept of delayed cancer chemoprevention was apparent in observational studies, in which protection against cancer in regular aspirin users took about 10 years to emerge.4,7 This effect was presumed to be dependent on continued aspirin exposure, but in the cardiovascular disease trials, treatment ended at a mean of 6 years. Analysis of cancer-related death in eight trials10 revealed significant protection in participants allocated aspirin for 4 years or more, but only when followed up for a further 5 years. Our findings support the hypothesis of a delayed effect of aspirin on colorectal cancer by showing that aspirin reduced incidence of colorectal cancer with the effect becoming apparent after 3–4 years from the start of aspirin intervention, a difference consistent with faster cancer development in those with Lynch syndrome (panel).11,12
Panel. Research in context.
Systematic review
We have accessed, using PubMed and our professional networks, all publications related to use of aspirin as a cancer chemopreventive agent and we have reviewed all research studies addressing therapeutic and preventive interventions in hereditary colorectal cancer in general and Lynch syndrome in particular. Search terms used were “hereditary colorectal cancer”, “Lynch syndrome”, or “HNPCC”, and “therapeutic”, “chemoprevention”, “prevention”, “aspirin”, or “NSAID”. There is extensive support to the effect that regular aspirin use reduces the cancer burden from observational studies7 and a meta-analysis of follow-up registry data derived from participants in randomised controlled trials of aspirin as a means of preventing occlusive vascular disease.10 Randomised trials based on prevention of colorectal adenomas as a biomarker of cancer showed an equivocal effect, but meta-analysis revealed a small but significant benefit.13
Interpretation
CAPP2 is the first randomised trial of aspirin as a chemopreventive agent with cancer as the primary endpoint. In the context of the published literature, the trial provides clear evidence that aspirin is an effective chemopreventive agent in hereditary cancer with an effect equivalent to that achieved with surveillance colonoscopy. The case for prescription of aspirin to this high-risk group is clear. The mechanism of this delayed action and, consequently, the optimum dose and duration of treatment remain to be established. A worldwide dose inferiority study is planned, but will take several years since adenomas are not a reliable biomarker of effect. In the meantime, clinicians should consider aspirin prescription for all individuals judged to be at high risk of colorectal cancer, but taking appropriate measures to minimise adverse effects. Indirect evidence suggests that a lower dose of aspirin than was used here will have a protective effect and its use would not compromise future involvement in a masked comparison of low-dose aspirin with 600 mg. CAPP3 will compare the effect of different aspirin doses in Lynch syndrome.
In intention-to-treat analysis, Poisson regression analysis, which incorporates more of the follow-up information than the time-to-event analysis (ie, total number of cancers in follow-up period vs time to first cancer), showed similar estimates of the protective effect but, as anticipated, greater statistical significance. The per-protocol analysis showed a similar effect.
In keeping with the effect of aspirin on non-colonic Lynch syndrome cancers (endometrial cancer, ovarian cancer, pancreatic cancer, and cancer of the brain, small bowel, gall bladder, ureter, stomach, and kidney) in our trial, Rothwell and colleagues10 reported that aspirin treatment reduced risk of death from several non-colonic solid cancers including oesophageal, pancreatic, brain, lung, stomach, and prostate cancer. Whether Lynch syndrome cancers are more responsive to aspirin is unclear, although in CAPP2 non-Lynch syndrome extracolonic cancers seemed unaffected by aspirin intervention. A weakness of our international study was the inability to collect a comprehensive series of tumour blocks to confirm that tumour development was related to the germline MMR mutation.
Our discovery of substantial protection by aspirin against colorectal cancer and other Lynch syndrome cancers is in striking contrast with our earlier report3 of no effect of aspirin on large-bowel neoplasia. Taken together, these findings might help to explain the marked disparity between the 50% cancer reduction reported in observational studies and the outcomes of randomised adenoma prevention trials, which have shown at best a small reduction effect; meta-analysis revealed a pooled risk ratio of any adenoma for any dose of aspirin versus placebo of 0·83 (95% CI 0·72–0·96).13 Our recent CAPP1 report2 in carriers of familial adenomatous polyposis revealed a small effect of aspirin on adenoma progression, but no demonstrable effect on polyp number, albeit using insensitive methods of analysis. In view of the CAPP2 findings, revisiting the CAPP1 participants to see whether aspirin has long-term effects on their disease progression will be interesting.
Several important questions remain: (1) whether aspirin targets the minority of adenomas with the greatest malignant potential; (2) whether some Lynch syndrome colorectal cancers arise from lesions other than adenomas;14 and (3) why do some tumours seem to be resistant to the effects of aspirin? The mechanism by which aspirin suppresses cancer development long after cessation of exposure to the drug is unclear. The assumed primary action of anti-inflammatory drugs on COX2 in colonic tumours15 is unlikely to be the primary mechanism. The rapid progression from adenoma to carcinoma in Lynch syndrome12 makes it likely that many screen-detected cancers would have begun to develop after aspirin intervention ended. Aspirin might be proapoptotic at early stages of colorectal cancer development, perhaps preceding adenoma formation. Ruschoff and colleagues16,17 reported reduced microsatellite instability and increased apoptosis in MMR-deficient cells exposed to aspirin and argued that aspirin might induce genetic selection for microsatellite stability in a subset of MMR-deficient cells. Aspirin might delete those aberrant stem cells most likely to progress rapidly to cancer. Analysis of the conditional MSH2 knockout mouse, reported recently to survive significantly longer when exposed to aspirin,18 might shed light on the mechanism.
Despite regular colonoscopy, almost one in 14 participants not taking aspirin in CAPP2 developed colorectal cancer in less than 5 years, emphasising the need for additional prevention strategies. Our results, taken in conjunction with recent research, provide a basis for recommendation of aspirin chemoprevention in Lynch syndrome as standard of care. CAPP3 will seek to establish the optimum dose and duration of aspirin treatment.
Acknowledgments
Acknowledgments
The CAPP2 study is an academic collaboration. Funding was initially provided by a European Union award supplemented by Programme funding in Newcastle and Leeds from Cancer Research UK (C588/A10589). Following completion of design and choice of interventions, Bayer Corporation and National Starch and Chemical Co were approached for support. Both provided free intervention including the cost of packaging and made donations to Newcastle University to help cover the cost of administration and distribution. They had no influence on design, conduct, or analysis of the study. The contracts associated with their donations required that they be given sight of the results before submission with up to 90 days for evaluation. The UK Medical Research Council (MRC) approved the trial in 2002 and became the primary funder. MRC steering and data monitoring committees were established. Financial contributions were also made to local sites by the Newcastle Hospitals trustees, Cancer Council of Victoria Australia, THRIPP South Africa, The Finnish Cancer Foundation and SIAK Switzerland. When renewal requests were declined by MRC and Cancer Research UK, follow-up analysis 2009-11 was supported by a donation from Bayer Schering Pharma to Newcastle University. CAPP2 has been made possible by all the participants who agreed to be randomised and take daily treatments for up to 4 years. Special mention must also be given to Pascale Ives (Melbourne, Australia) and Su Werner (Dusseldorf, Germany) for their exceptional recruitment achievements and to Pam Chapman, the project manager in the early stages of the CAPP2 study. Other core staff were Paul Adamson, Olive Armstrong, Julie Coaker, Jonathan Coxhead, Joanne Gascoyne, John Gilroy, Louise Lynagh, Lynn Reed, and Rachel Toes. Recruitment depended on a large number of colleagues around the world. Clinical collaborators not listed as authors are: Jan Ball, Lauren Baxter, Alex Boussioutas, Nicola Bradshaw, Carole Brewer, Mary Broughton, Barbara Bulman, Monica Castiglione, Sue Clark, Rowena Ching, Carol Chu, Susanne Cina, Jackie Cook, Carole Cummings, Rhodri Davies, Tadeusz Debniak, Celine de Moncuit, Sarah Drummond, Tony Ellis, Paulo Fidalgo, Steve Gallinger, Sheila Goff, Paul A Goldberg, Selina Goodman, Chris Harocopos, Pierre Hutter, Lisa Jeffers, Sheila Jordan, Pip Killick, Christian Krauss, Jørgen Kristensen, Caroline Langman, Julio Leite, Annelie Liljegren, Cristina Oliani, Christopher Marks, Veronique Membrez-Antonioli, Julie Miller, Tony Miles, Pedro Perez Segura, Gabriella Pichert, Elize Pietersen, Giovanni Rossi, Paola Sala, Julian Sampson, Beverly Schmocker, Joan Shaw, Allan Spigelman, Alfonso Tempesta, Mary Velthuizen, and Ian Walpole. We are also indebted to the trial steering committee (David Kerr [Chair], Sarah Perkins [MRC], Jack Cuzick, Lynn Faulds Wood, Robert Steele) and the data monitoring committee (Doug Altman [Chair], Chris Paraskeva, Wendy Atkin, Mark Hull). We are grateful to the International Society for Gastrointestinal Hereditary Tumours (InSiGHT) for its support, since its formation in 2003, in sustaining the CAPP consortium.
Contributors
JB, FM, J-PM, GM, LB, HFV, RF, HL, JCM, and DTB contributed to study design. JB, A-MG, FM, J-PM, GM, SO, DE, DGE, ERM, LB, M-LB, MD, JWCH, SVH, AL, JL, PJM, VM, RR, LS, RJS, HJWT, HFV, GC, and KP were responsible for recruitment and clinical follow-up. JB, GB, JTW, RF, JCM, and DTB provided trial management and support. A-MG, FE, MM, and DTB contributed to randomisation, data processing, and statistical analysis. FM, J-PM, GM, LB, HFV, and HTL provided international oversight. JB, A-MG, MM, JCM, and DTB were the writing team.
Conflicts of interest
The chief investigator received a fee as a speaker at a Bayer workshop in 2010. None of the co-authors have any financial relationship with either of the commercial sponsors. We declare that we have no conflicts of interest.
Web Extra Material
References
- 1.Vasen HFA, Watson P, Mecklin J-P, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch Syndrome) proposed by the International Collaborative Group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 2.Burn J, Bishop DT, Chapman PD. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila) 2011;4:655–665. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burn J, Bishop DT, Mecklin J-P. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008;359:2567–2578. doi: 10.1056/NEJMoa0801297. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E, Egan KM, Hunter DJ. Aspirin and the risk of colorectal cancer in women. N Engl J Med. 1995;333:609–614. doi: 10.1056/NEJM199509073331001. [DOI] [PubMed] [Google Scholar]
- 5.Liljegren A, Barker G, Elliott F. Prevalence of adenomas and hyperplastic polyps in mismatch repair mutation carriers among CAPP2 participants: report by the Colorectal Adenoma/Carcinoma Prevention Programme 2. J Clin Oncol. 2008;26:3434–3439. doi: 10.1200/JCO.2007.13.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasen HF, Moslein G, Alonso A. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer) J Med Genet. 2007;44:353–362. doi: 10.1136/jmg.2007.048991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuzick J, Otto F, Baron JA. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 8.Flossmann E, Rothwell PM, on behalf of the British Doctors Aspirin Trial and the UK-TIA Aspirin Trial Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 9.Rothwell PM, Wilson M, Elwin CE. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 11.Lynch HT, Smyrk T, Jass JR. Hereditary nonpolyposis colorectal cancer and colonic adenomas: aggressive adenomas? Semin Surg Oncol. 1995;11:406–410. doi: 10.1002/ssu.2980110607. [DOI] [PubMed] [Google Scholar]
- 12.Vasen HF. Can the identification of high risk groups increase the effectiveness of colon cancer screening programmes? Z Gastroenterol. 2008;46(suppl 1):S41–S42. doi: 10.1055/s-2007-963483. [DOI] [PubMed] [Google Scholar]
- 13.Cole BF, Logan RF, Halabi S. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256–266. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jass JR, Walsh MD, Barker M, Simms LA, Young J, Leggett BA. Distinction between familial and sporadic forms of colorectal cancer showing DNA microsatellite instability. Eur J Cancer. 2002;38:858–866. doi: 10.1016/s0959-8049(02)00041-2. [DOI] [PubMed] [Google Scholar]
- 15.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 16.Ruschoff J, Wallinger S, Dietmaier W. Aspirin suppresses the mutator phenotype associated with hereditary nonpolyposis colorectal cancer by genetic selection. Proc Natl Acad Sci USA. 1998;95:11301–11306. doi: 10.1073/pnas.95.19.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIlhatton MA, Tyler J, Burkholder S. Nitric oxide-donating aspirin derivatives suppress microsatellite instability in mismatch repair-deficient and hereditary nonpolyposis colorectal cancer cells. Cancer Res. 2007;67:10966–10975. doi: 10.1158/0008-5472.CAN-07-2562. [DOI] [PubMed] [Google Scholar]
- 18.McIlhatton MA, Tyler J, Kerepesi LA. Aspirin and low dose nitric oxide-donating aspirin increase life span in a Lynch syndrome mouse model. Cancer Prev Res (Phila) 2011;4:684–693. doi: 10.1158/1940-6207.CAPR-10-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


