Abstract
Although physical activity (PA) has been inversely associated with depressive symptoms, it is not clear whether regular PA and television watching are associated with clinical depression risk. The authors conducted a prospective analysis involving 49,821 US women from the Nurses’ Health Study who were free from depressive symptoms at baseline (1996). Information on PA was obtained from validated questionnaires completed in 1992, 1994, 1996, 1998, and 2000; analyses were conducted using the cumulative average of PA (minutes/day) with a 2-year latency period applied. Participants were asked about television-watching habits in 1992. Cox proportional hazards regression models adjusted for multiple risk factors were used to estimate relative risks of clinical depression (self-reported physician-diagnosed depression or use of antidepressants). During 10 years of follow-up (1996–2006), 6,505 incident cases of depression were documented. Higher levels of PA were associated with lower depression risk. The multivariate relative risk comparing the highest level of PA (≥90 minutes/day) with the lowest (<10 minutes/day) was 0.80 (95% confidence interval: 0.70, 0.92; Ptrend < 0.001). In contrast, the risk of depression increased with increasing television-watching time. The multivariate relative risk comparing women who spent 21 hours/week or more watching television with those who spent 0–1 hour/week was 1.13 (95% confidence interval: 1.00, 1.27; Ptrend = 0.01). Analyses simultaneously considering PA and television watching suggested that both contributed independently to depression risk.
Keywords: aged, cohort studies, depression, motor activity, television, walking, women
Major depressive disorder is a chronic and recurrent illness that affects 2 times more women than men (1); in the United States, about 20% of the women will be affected during their lifetimes (2). The economic consequences of major depressive disorder are considerable (3) because of not only the increased use of health-care services and the worse health outcomes associated with this disease but also the loss of productivity due to absenteeism and inefficiency at work (4). Thus, depression prevention is a major health priority and new strategies for dealing with it are needed.
Since Hippocrates’ time, physical activity (PA) has been recommended by physicians to prevent depression (5). Despite dissimilarities in the designs of the 21 longitudinal studies that analyzed the relation between PA and depression symptoms, 17 found significant inverse associations (6–22), whereas only 4 did not (23–26). Of these 21 studies, only 5 (8, 11, 20, 23, 25) used Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, depression criteria as an outcome, and of these, 3 found significant inverse associations (8, 11, 20). Most of these studies, however, had several limitations, including a relatively small study population, a short follow-up time, a lack of repeated measurements of the exposure and outcome, and no exclusion of depressed participants at baseline. A recent Cochrane review of randomized trials concluded that PA interventions had only a modest impact on depressive symptoms in patients who had been diagnosed with major depressive disorder (27).
Although lack of regular PA is often associated with depressive symptoms, few studies have analyzed the joint association between sedentary behavior and PA in relation to clinical depression, and only 1 study has considered the relation between television watching and depression (25). In participants of the Nurses’ Health Study, we examined the relation of PA and television watching, separately and jointly, and incident clinical depression risk while assessing any potential dose-response relation.
MATERIALS AND METHODS
Study population
The Nurses’ Health Study is a prospective cohort study of 121,700 US female registered nurses aged 30–55 years at enrollment in 1976. Every 2 years, participants provide updated information via mailed questionnaires about lifestyle, medical history, and newly diagnosed medical illnesses. For the present analysis, the follow-up period began at the return of the 1996 questionnaire and ended in June of 2006. Women were first asked to report their use of antidepressants in 1996 and to report their history of physician-diagnosed depression in 2000. To prospectively examine the relation between PA and depression, we excluded from the baseline population women who reported using antidepressants in 1996 or had a physician-diagnosed episode of depression in 1996 or earlier; women who had severe depressive symptoms on the 1992 or 1996 Mental Health Index (MHI-5) questionnaire (score ≤52) (28–30), a 5-item subscale of the Short-Form Health Status Survey, were also excluded. Women who did not report their depressive status in 1996, 1998, or 2000 or did not return or answer the 1992 or 1996 MHI-5 questionnaire were excluded because their depression history by 1996 could not be ascertained. By 2000, a total of 60,833 women were alive and had completed the 1996–2000 questionnaires; of these, 50,605 were considered depression-free in 1996 and comprised the baseline population for the present analyses. As in a previous study (31), we excluded women who reported more than 4 hours/day of PA on any returned questionnaire (n = 784). The final 1996 baseline population comprised 49,821 women. The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and the Harvard School of Public Health.
Assessment of PA and television watching
In 1992, 1994, 1996, 1998, and 2000, participants reported the average amount of time they had spent per week in each of the following 9 recreational activities in the past year: walking, jogging (<10 minutes/mile), running (≥10 minutes/mile), bicycling, playing tennis/squash/racquetball, lap swimming, performing calisthenics/aerobics/aerobic dance or using a rowing machine, doing yoga/stretching/toning, and lawn mowing. For each activity, women chose one of 11 duration categories, which ranged from 0 minutes/week to 11 hours/week or more. Women also reported their usual walking pace in miles per hour as easy (<2 mph), average (2–2.9 mph), brisk (3–3.9 mph), very brisk/striding (≥4 mph), or unable to walk. Because only 2% of the women reported a very brisk/striding pace, brisk and very brisk pace were combined into 1 category. Moreover, women reported the average number of flights of stairs they climbed daily. Time spent stair climbing in minutes per day was then estimated. Total PA (minutes/day) was considered the sum of the duration reported for each of the 9 activities plus stair climbing. In the present analyses, 5 categories of total PA were coded (<10, 10–29, 30–59, 60–89, and ≥90 minutes/day). The reproducibility and validity of the PA questionnaire have been described elsewhere (32). Briefly, in a random representative sample of Nurses’ Health Study II participants (n = 147), using past-week activity recalls and 7-day activity diaries as the referent methods, the correlation between activity reported on questionnaires and that of recalls was 0.79, and that reported on diaries was 0.62. In 1992, participants were asked to report their average weekly time spent sitting at home watching television. The responses included 9 categories (ranging from 0 hours/week to >90 hours/week). In the current analyses, 5 categories of television watching were coded (0–1, 2–5, 6–10, 11–20, and ≥21 hours/week).
Case ascertainment
Having clinical depression was defined as reporting either a new physician’s diagnosis of depression or beginning regular use of antidepressant medication. Because antidepressants can be used for indications other than depression, our main analyses were repeated using a stricter definition of depression that required both a physician’s diagnosis and the use of antidepressants (33). Because a significant percentage of depressed cases might have never received treatment or might have received treatments other than antidepressants, we repeated our main analyses using a broader definition of depression that required a physician’s diagnosis or use of antidepressants or severe depressive symptoms (score ≤52 on the MHI-5 in 2000 or score ≥10 on the Center for Epidemiologic Studies Depression Scale in 2004) (34) (Appendix Table 1). In 2000, participants were asked to report the year of their first physician diagnosis of depression (1996 or before, 1997, 1998, 1999, or 2000). Thereafter, this information was updated biennially through 2006. The question about regular antidepressant medication use was first asked in 1996, and information was updated biennially through 2006. Hence, the 1996 questionnaire cycle was considered to be the baseline.
Covariates
Data on demographic factors, lifestyle, and comorbidity were collected by using the standardized questionnaires mailed to the nurses. In the baseline questionnaire (1996), we requested information about age, weight, smoking, menopausal status, use of postmenopausal hormone therapy, and previously diagnosed medical conditions (including diabetes mellitus, cancer, myocardial infarction or angina, high blood pressure, rheumatoid arthritis, asthma, and emphysema). This information has been updated in the biennial follow-up questionnaires. Information on marital status, osteoarthritis, and social or community group involvement (determined by answers to the question, “How many hours each week do you participate in any church, volunteer, or other community group?”) was obtained at baseline (1996) and updated in 2000 and 2004. Dietary variables were assessed using a validated semiquantitative food frequency questionnaire (35). Neighborhood socioeconomic status summary scores (36), which include information from the US Census about wealth and income, educational level, and occupation, were determined at baseline. Participants’ employment status (retired, homemaker, or working part- or full-time) was determined at baseline, whereas educational level (registered nurse, bachelor’s degree, master’s degree, or doctorate) was measured in 1992. Physical function and mental health were assessed using the 36-Item Short Form Health Survey in the 1992 questionnaire. The physical functioning score was dichotomized by physical limitation, which was defined as more than a little limitation on moderate activities or more than moderate limitations on more demanding physical performance. MHI-5 score was categorized (score = 86–100, 76–85, or 53–75) as in a previous study (37).
Statistical analysis
To reduce random measurement error and optimally represent long-term effects, we used cumulative average PA instead of a single measurement. To minimize the possibility of reverse causation (i.e., depression leading to decreased PA), we applied a 2-year latency period. For example, the cumulative average of PA information from 1992 and 1994 was used to predict clinical depression in 1996–1998, and the cumulative average of PA information from 1992, 1994, and 1996 was used to predict clinical depression in 1998–2000. Moreover, PA information in the cumulative average estimate was not updated after a new diagnosis of cardiovascular disease, diabetes mellitus, or cancer because participants could have changed their PA level after such a diagnosis. Additionally, we conducted 2 sensitivity analyses, one in which we did not stop updating PA after disease diagnosis and one in which we also censored women upon disease diagnosis. Because walking is by far the most prevalent PA among older adults (38), we also examined the relation between walking pace and risk of clinical depression.
Person-years of follow-up were calculated from the date of return of the 1996 questionnaire to the earliest of the first questionnaire on which depression was reported, death, the end of follow-up (June 1, 2006), or return of the last questionnaire. Cox proportional hazards models, stratified on age in months and questionnaire cycle, were used to estimate the relative risks of developing clinical depression. Initially, the relative risks in model 1 were adjusted for known and putative risk factors for depression, including current postmenopausal hormonal use (binary), body mass index (defined as weight (kg)/height (m)2; <25, 25–29.9, or ≥30), marital status (married/in a partnership, widowed, or separated/divorced/single), social or community group involvement (binary), smoking (never smoker, past smoker, or currently smoking 1–14 cigarettes/day, 15–24 cigarettes/day, or ≥25 cigarettes/day), total energy intake (kcal/day; continuous), coffee intake (never or <1 time/month, <2 times/day, or ≥2 times/day), diabetes mellitus (binary), cancer (binary), myocardial infarction or angina (binary), high blood pressure (binary), rheumatoid arthritis (binary), osteoarthritis (binary), asthma (binary), and emphysema (binary). A dummy category was used for missing data. In multivariate analyses, PA and television watching were included simultaneously in the same model to estimate their independent contributions to depression risk. Model 2 included further adjustment for physical limitations (binary) and MHI-5 score (86–100, 76–85, or 53–75) in 1992. We also fitted model 2 considering as cases only women who reported both a diagnosis of depression and use of antidepressants. All analyses were performed with SAS software, version 9.1 (SAS Institute, Inc., Cary, North Carolina). All P values reported are 2-sided.
RESULTS
The distributions of selected age-standardized characteristics according to categories of PA are outlined in Table 1. Compared with less active women, physically active women were less likely to be current smokers, to have physical limitations, to have a worse MHI-5 score, or to spend 21 hours/week or more watching television; they also had lower body mass indexes, consumed more calories and alcohol, and were more likely to be involved in social or community groups and to have the lowest prevalence of diagnosed diseases. The mean PA level was 36 minutes/day and the median was 27 minutes/day. In this cohort, walking was the most frequent activity, contributing to 52.8% of the total minutes reported daily. In comparison, women who were excluded from the analysis were less physically active smoked more, and had higher disease prevalence at baseline.
Table 1.
Age-Standardized Characteristicsa of the Study Cohort According to Daily Amount of Physical Activity, Nurses’ Health Study, 1992–2006
| Physical Activity (1992–2000), minutes/day |
||||||||||
| <10 (5.4)b (n = 9,358) |
10–29 (18.9) (n = 17,322) |
30–59 (42.4) (n = 14,243) |
60–89 (71.9) (n = 5,609) |
≥90 (110.4) (n = 3,289) |
||||||
| Mean | % | Mean | % | Mean | % | Mean | % | Mean | % | |
| Age, years | 62.9 | 62.8 | 63.1 | 63.5 | 63.8 | |||||
| Body mass indexc | 27.6 | 26.5 | 25.8 | 25.3 | 24.8 | |||||
| <25 | 37.8 | 43.9 | 50.7 | 54.6 | 59.2 | |||||
| 25–29.9 | 33.5 | 35.5 | 33.7 | 32.5 | 30.0 | |||||
| ≥30 | 28.7 | 20.7 | 15.6 | 12.9 | 10.9 | |||||
| Total energy intake (1994), kcal/day | 1,669 | 1,714 | 1,745 | 1,786 | 1,843 | |||||
| Alcohol intake (1994), g/day | 4.8 | 4.9 | 5.3 | 5.7 | 6.0 | |||||
| Television-watching (1992), hours/week | ||||||||||
| 0–1 | 6.3 | 6.1 | 6.6 | 6.8 | 8.3 | |||||
| 2–5 | 24.1 | 23.2 | 24.0 | 24.0 | 25.7 | |||||
| 6–10 | 24.0 | 25.5 | 25.9 | 26.1 | 25.4 | |||||
| 11–20 | 26.1 | 27.7 | 26.6 | 26.7 | 26.7 | |||||
| ≥21 | 17.9 | 16.4 | 15.5 | 14.8 | 12.7 | |||||
| Coffee intake (1994) | ||||||||||
| Never or <1 time/month | 27.8 | 28.4 | 29.2 | 29.0 | 30.0 | |||||
| <2 times/day | 28.4 | 29.4 | 29.1 | 29.4 | 28.1 | |||||
| ≥2 times/day | 43.8 | 42.1 | 41.7 | 41.6 | 41.8 | |||||
| Physical limitations (1992)d | 52.8 | 40.6 | 31.7 | 27.1 | 22.1 | |||||
| Mental Health Index-5 score (1992)e | ||||||||||
| ≥86 | 30.7 | 32.9 | 35.8 | 38.0 | 39.0 | |||||
| 76–85 | 38.0 | 40.2 | 40.4 | 39.3 | 40.1 | |||||
| 53–75 | 31.3 | 26.9 | 23.8 | 22.7 | 20.9 | |||||
| White race | 97.6 | 98.1 | 98.3 | 98.1 | 98.2 | |||||
| Not involved in social or community group | 45.6 | 37.2 | 34.1 | 34.5 | 32.8 | |||||
| Marital status | ||||||||||
| Married/in a partnership | 78.7 | 79.7 | 79.7 | 80.2 | 78.9 | |||||
| Widowed | 14.3 | 13.6 | 13.2 | 12.3 | 12.7 | |||||
| Separated/divorced/single | 7.1 | 6.6 | 7.1 | 7.5 | 8.4 | |||||
| Current menopausal hormone use | 36.4 | 41.3 | 42.4 | 42.8 | 41.3 | |||||
| Reported diagnosis of: | ||||||||||
| Myocardial infarction or angina | 5.1 | 3.6 | 3.2 | 3.1 | 3.4 | |||||
| High blood pressure | 33.8 | 30.4 | 28.1 | 26.5 | 23.9 | |||||
| Cancer | 8.1 | 6.3 | 5.9 | 6.2 | 6.8 | |||||
| Arthritis | 4.9 | 4.3 | 3.7 | 3.9 | 4.0 | |||||
| Osteoarthritis | 22.1 | 21.0 | 20.4 | 19.3 | 19.8 | |||||
| Emphysema | 3.3 | 2.4 | 2.0 | 1.8 | 1.5 | |||||
| Asthma | 5.6 | 4.9 | 4.8 | 3.9 | 4.6 | |||||
| Current smoking | 13.5 | 10.3 | 8.6 | 8.9 | 9.7 | |||||
All values were from 1996 unless otherwise indicated. All characteristics were age-standardized, with the exception of age. Physical activity was computed as the cumulative average of physical activity between 1992 and 2000. A 2-year latency period was used to compute physical activity exposure. For example, physical activity information from 1992 and 1994 was used to compute exposure for 1996–1998, the cumulative average of 1992, 1994, and 1996 physical activity information was used to predict clinical depression in 1998–2000, and so on. We also stopped updating physical activity information in the cumulative average estimate after new diagnoses of nonfatal myocardial infarction, angina, nonfatal stroke, diabetes mellitus, and cancer.
Median value.
Weight (kg)/height (m)2.
Based on 1992 answers through physical function items of the 36-Item Short-Form Health Status Survey. The physical functioning score was dichotomized by physical limitation, which was defined as reporting more than a little limitation on moderate activities or more than moderate limitations on more demanding physical performance.
Mental Health Index-5 score measured in 1992. A higher score denoted better mental health.
In the 49,821 women who were free from depressive symptoms at baseline, we documented 6,505 incident cases of clinical depression during the 10-year (450,968-person-year) follow-up period (1996–2006). There was an inverse age-adjusted dose-response relation between duration of PA and depression risk (Ptrend < 0.001; Table 2). This inverse gradient remained statistically significant after adjusting for all covariates in the 2 different multivariate models (Table 2) and when PA level was updated after new disease diagnosis (data not shown). These results did not materially change when we censored women who developed diseases during follow-up (data not shown). Further adjustment for socioeconomic status did not materially affect the results. For cases who reported both depression diagnosis and use of antidepressants (n = 2,576), the multivariate-adjusted relative risk comparing those with the highest level of PA (≥90 minutes/day) with those with the lowest level (<10 minutes/day) was 0.80 (95% confidence interval (CI): 0.65, 0.99). However, the trend was not statistically significant. All the categories of time spent watching television were positively associated with risk of depression in the age-adjusted analysis (Ptrend < 0.001; Table 2). After further adjustment for all covariates (Table 2, model 2), none of the categories of time spent watching television were significantly associated with risk of depression, but the overall trend remained significant (Ptrend = 0.01). We also observed similar risk estimates that were somewhat stronger for PA and television watching when we utilized the broader definition of depression that required a physician diagnosis, use of antidepressants, or severe depressive symptoms (Appendix Table 1).
Table 2.
Relative Risk of Clinical Depressiona According to Levels of Physical Activity and Television Watching, Nurses’ Health Study, 1992–2006
| Physical Activity (1992–2000), minutes/day |
Ptrend | |||||||||||||||
| <10 |
10–29 |
30–59 |
60–89 |
≥90 |
||||||||||||
| No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | ||
| No. of cases | 1,378 | 2,374 | 1,869 | 602 | 282 | |||||||||||
| Person-years of follow-up | 77,472 | 163,430 | 135,748 | 49,558 | 24,761 | |||||||||||
| Age-adjusted RRb | 1.00 | 0.82 | 0.76, 0.87 | 0.78 | 0.72, 0.83 | 0.69 | 0.63, 0.76 | 0.65 | 0.57, 0.74 | <0.001 | ||||||
| Multivariate model 1c | 1.00 | 0.86 | 0.80, 0.92 | 0.83 | 0.77, 0.89 | 0.75 | 0.68, 0.83 | 0.71 | 0.62, 0.80 | <0.001 | ||||||
| Multivariate model 2d | 1.00 | 0.90 | 0.84, 0.96 | 0.91 | 0.84, 0.97 | 0.84 | 0.76, 0.92 | 0.80 | 0.70, 0.92 | <0.001 | ||||||
| Cases who reported both depression diagnosis and use of antidepressants | ||||||||||||||||
| No. of cases | 534 | 907 | 775 | 252 | 108 | |||||||||||
| Person-years of follow-up | 78,301 | 164,859 | 136,820 | 49,906 | 24,933 | |||||||||||
| Multivariate model 2d | 1.00 | 0.89 | 0.80, 0.99 | 0.98 | 0.88, 1.10 | 0.91 | 0.78, 1.07 | 0.80 | 0.65, 0.99 | 0.24 | ||||||
| Television Watching (1992), hours/week | PTrend | |||||||||||||||
| 0–1 | 2–5 | 6–10 | 11–20 | ≥ 21 | ||||||||||||
| No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | ||
| No. of cases | 368 | 1,503 | 1,604 | 1,789 | 1,143 | |||||||||||
| Person-years of follow-up | 29,525 | 107,715 | 114,980 | 121,346 | 71,131 | |||||||||||
| Age-adjusted RRb | 1.00 | 1.14 | 1.01, 1.27 | 1.14 | 1.02, 1.28 | 1.21 | 1.08, 1.36 | 1.34 | 1.19, 1.51 | <0.001 | ||||||
| Multivariate model 1c | 1.00 | 1.08 | 0.97, 1.22 | 1.08 | 0.96, 1.21 | 1.12 | 1.00, 1.25 | 1.19 | 1.06, 1.34 | 0.001 | ||||||
| Multivariate model 2d | 1.00 | 1.05 | 0.94, 1.18 | 1.04 | 0.92, 1.16 | 1.08 | 0.97, 1.21 | 1.13 | 1.00, 1.27 | 0.01 | ||||||
| Cases who reported both depression diagnosis and use of antidepressants | ||||||||||||||||
| No. of cases | 142 | 597 | 647 | 693 | 461 | |||||||||||
| Person-years of follow-up | 29,749 | 108,597 | 115,924 | 122,419 | 71,797 | |||||||||||
| Multivariate model 2d | 1.00 | 1.08 | 0.90, 1.30 | 1.08 | 0.90, 1.30 | 1.09 | 0.91, 1.31 | 1.21 | 1.00, 1.47 | 0.04 | ||||||
Abbreviations: CI, confidence interval; RR, relative risk.
Clinical depression was defined as antidepressant medication use or physician-diagnosed depression (1996–2006). Physical activity was computed as the cumulative average of physical activity between 1992 and 2000. A 2-year latency was used to compute physical activity exposure. For example, physical activity information from 1992 and 1994 was used to compute exposure for 1996–1998, the cumulative average of 1992, 1994, and 1996 physical activity information was used to predict clinical depression in 1998–2000, and so on. We also stopped updating physical activity information in the cumulative average estimate after new diagnoses of nonfatal myocardial infarction, angina, nonfatal stroke, diabetes mellitus, and cancer.
Adjusted for age (continuous) and time interval.
Adjusted for current postmenopausal hormonal use (binary), body mass index (weight (kg)/height (m)2; <25, 25–29.9, or ≥30), marital status (married/in a partnership, widowed, or separated/divorced/single), involvement in a social or community group (binary), smoking status (never smoker, past smoker, or current smoker (1–14 cigarettes/day, 15–24 cigarettes/day, or ≥25 cigarettes/day)), total energy intake (continuous), coffee intake (never or <1 time/month, <2 times/day, or ≥2 times/day), and reported diagnosis of diabetes mellitus (binary), cancer (binary), myocardial infarction or angina (binary), high blood pressure (binary), rheumatoid arthritis (binary), osteoarthritis (binary), asthma (binary), and emphysema (binary). Multivariate model 2 for physical activity was further adjusted for categories of television watching. Multivariate model 2 for television watching was further adjusted for categories of physical activity.
Additional adjustment for physical limitations in 1992 (binary) and for 5-item Mental Health Index score (86–100, 76–85, or 53–75) in 1992.
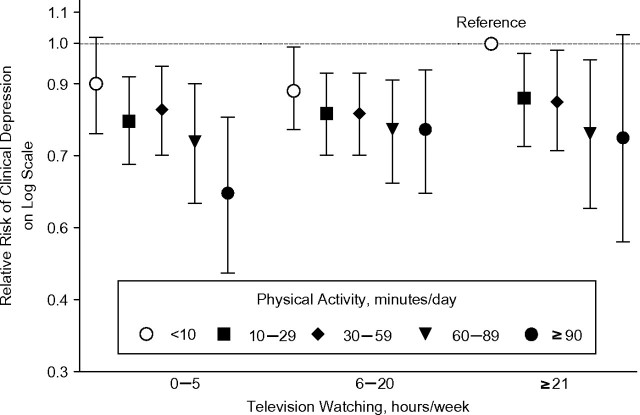
In fully adjusted analyses of the joint influence of television watching and PA on cases who reported both depression diagnosis and use of antidepressants, those who were the most active (≥90 minutes/day) and spent the least amount of time watching television (0–5 hours/week) had the strongest significant inverse association with depression (relative risk = 0.62, 95% CI: 0.48, 0.79) when compared with women with the least activity (<10 minutes/day) and the most television watching (≥21 hours/week) (Figure 1). In multivariate analyses, depression risk decreased with increasing time spent walking at an average pace or brisk/very brisk pace but was not related to time spent walking at an easy pace (Table 3). However, increased time spent walking, regardless of the pace, was not significantly associated with risk of depression when cases were defined as both having a depression diagnosis and using antidepressants (data not shown). The multivariate-adjusted relative risk for walking 40 minutes/day or more at an average pace was 0.90 (95% CI: 0.73, 1.11), whereas the relative risk for walking 40 minutes/day or more at a brisk/very brisk pace was 0.89 (95% CI: 0.74, 1.08) in comparison with walking less frequently (<30 minutes/week) at either pace (data not shown).
Figure 1.
Multivariate-adjusted relative risk of clinical depression according to levels of physical activity and television watching. Results were adjusted for the same covariates as in model 2 in Table 2. Cases of clinical depression were women who reported both a physician’s diagnosis of depression and use of antidepressants. The reference group for relative risks was women with 21 hours/week or more of television watching and less than 10 minutes/day of physical activity. Bars, 95% confidence interval.
Table 3.
Relative Risk of Clinical Depressiona According to Usual Walking Time and Walking Pace, Nurses’ Health Study, 1992–2006
| Walking Time |
Ptrend | ||||||||||||
| <30 minutes/week |
<20 minutes/day |
20–40 minutes/day |
≥40 minutes/day |
||||||||||
| No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | ||
| Easy pace (<2 miles/hour) | |||||||||||||
| No. of cases | 6,125 | 310 | 51 | 19 | |||||||||
| Person-years of follow-up | 430,979 | 16,449 | 2,509 | 1,032 | |||||||||
| Age-adjusted RRb | 1.00 | 1.16 | 1.03, 1.31 | 1.28 | 0.97, 1.69 | 1.11 | 0.70, 1.76 | 0.02 | |||||
| Multivariate model 1c | 1.00 | 1.05 | 0.93, 1.18 | 1.15 | 0.87, 1.52 | 1.01 | 0.64, 1.59 | 0.39 | |||||
| Multivariate model 2d | 1.00 | 0.97 | 0.86, 1.10 | 1.07 | 0.81, 1.41 | 0.94 | 0.59, 1.49 | 0.89 | |||||
| Average pace (2–2.9 miles/hour) | |||||||||||||
| No. of cases | 3,601 | 2,075 | 604 | 225 | |||||||||
| Person-years of follow-up | 243,662 | 144,563 | 43,953 | 18,790 | |||||||||
| Age-adjusted RRb | 1.00 | 0.90 | 0.85, 0.95 | 0.87 | 0.80, 0.96 | 0.75 | 0.66, 0.87 | <0.001 | |||||
| Multivariate model 1c | 1.00 | 0.93 | 0.87, 0.98 | 0.90 | 0.83, 0.99 | 0.76 | 0.66, 0.88 | <0.001 | |||||
| Multivariate model 2d | 1.00 | 0.94 | 0.88, 0.99 | 0.94 | 0.85, 1.02 | 0.80 | 0.70, 0.92 | 0.001 | |||||
| Brisk/very brisk pace (≥3 miles/hour) | |||||||||||||
| No. of cases | 4,357 | 1,231 | 609 | 308 | |||||||||
| Person-years of follow-up | 279,451 | 92,589 | 50,614 | 28,314 | |||||||||
| Age-adjusted RRb | 1.00 | 0.83 | 0.78, 0.89 | 0.75 | 0.69, 0.83 | 0.69 | 0.61, 0.78 | <0.001 | |||||
| Multivariate model 1c | 1.00 | 0.89 | 0.83, 0.95 | 0.81 | 0.74, 0.89 | 0.75 | 0.66, 0.85 | <0.001 | |||||
| Multivariate model 2d | 1.00 | 0.95 | 0.88, 1.02 | 0.88 | 0.80, 0.97 | 0.83 | 0.73, 0.94 | <0.001 | |||||
Abbreviations: CI, confidence interval; RR, relative risk.
Clinical depression was defined as antidepressant medication use or physician-diagnosed depression (1996–2006). Physical activity was computed as the cumulative average of physical activity between 1992 and 2000. A 2-year latency was used to compute physical activity exposure. For example, physical activity information from 1992 and 1994 was used to compute exposure for 1996–1998, the cumulative average of 1992, 1994, and 1996 physical activity information was used to predict clinical depression in 1998–2000, and so on. We also stopped updating physical activity information in the cumulative average estimate after new diagnoses of nonfatal myocardial infarction, angina, nonfatal stroke, diabetes mellitus, and cancer.
Adjusted for age (continuous), time interval, and other categories of physical activity level (<30 minutes/week, 30 minutes/week–20 minutes/day, 20–40 minutes/day, 40–60 minutes/day, and ≥60 minutes/day).
Adjusted for current postmenopausal hormonal use (binary), body mass index (weight (kg)/height (m)2; <25, 25–29.9, or ≥30), marital status (married/in a partnership, widowed, or separated/divorced/single), involvement in a social or community group (binary), smoking status (never smoker, past smoker, or current smoker (1–14 cigarettes/day, 15–24 cigarettes/day, or ≥25 cigarettes/day)), total energy intake (continuous), coffee intake (never or <1 time/month, <2 times/day, or ≥2 times/day), reported diagnosis of diabetes mellitus (binary), cancer (binary), myocardial infarction or angina (binary), high blood pressure (binary), rheumatoid arthritis (binary), osteoarthritis (binary), asthma (binary), emphysema (binary), and television watching (0–1, 2–5, 6–10, 11–20, and ≥21 hours/week).
Additional adjustment for physical limitations in 1992 (binary) and for 5-item Mental Health Index score (86–100, 76–85, or 53–75) in 1992.
DISCUSSION
In this large, prospective cohort study of women who were free from clinical depression or severe depressive symptoms at baseline, increased time spent in daily PA was associated with a reduced risk of clinical depression, whereas increased television watching was associated with a trend toward an increased risk. The decreased risk of depression was the strongest when assessing the joint influence of high PA level (≥90 minutes/day) and low amount of time spent watching television (0–5 hours/week). Additionally, walking at an average or brisk/very brisk pace, but not slow walking, was associated with a reduced risk of depression. Although previous longitudinal studies have addressed the link between PA and depression, most of these studies examined the relation between PA and prevalent depressive symptoms. The unique contribution of our study is that it actually examined risk of incident depression with an additional advantage of using long-term follow-up, multiple assessments of PA and depression, and a large sample size.
Our results are consistent with those of previous longitudinal studies that reported inverse associations between PA and depressive symptoms in men and women (11, 16–22, 39). Among the 6 studies that analyzed women separately (7, 13, 15, 18, 20, 24), 5 reported a protective association between PA and depression symptoms (7, 13, 15, 18, 20), whereas 1 study found no association (24). In our cohort, we noted a relative risk of 0.80 for clinical depression when we compared the highest level of PA (≥90 minutes/day) with the lowest level (<10 minutes/day). Similarly, in the Copenhagen City Heart Study, which comprised 5,937 women with a 26-year follow-up period, Mikkelsen et al. (20) noted a higher risk of clinical depression (multivariate relative risk = 1.80, 95% CI: 1.29, 2.51) when comparing women with a low level of PA (<2 hours/week) with those with a high level of PA (≥4 hours/week of light PA or ≥2–4 hours/week of vigorous PA). In another cohort of 9,207 middle-aged Australian women in whom those with high habitual PA levels (>300 minutes of moderate-intensity PA/week) were compared with those with very low levels (<60 minutes of moderate-intensity PA/week), Brown et al. (13) noted an inverse association with depressive symptoms (multivariate odds ratio (OR) = 0.62 for an MHI-5 score ≤52 and OR = 0.62 for a Center for Epidemiologic Studies Depression Scale score ≥10). An effect of similar magnitude to our results was noted in the US Black Women’s Health Study (n = 35,224) (15). Compared with those who engaged in no vigorous activity (running, aerobics, basketball, or swimming), women with ≥7 hours/week of vigorous activity had a multivariate odds ratio for depressive symptoms (20-item Center for Epidemiologic Studies Depression Scale score ≥16) of 0.75 (95% CI: 0.65, 0.87). Despite some variations in PA type and duration, study design, and analysis across studies in women, a consistent inverse dose-response relation between PA duration and clinical depression risk was evident in all of these studies. Although the US Black Women (15) and Danish (20) cohorts, as well as our own, excluded women with physician-diagnosed depression at baseline, other studies did not (7, 13, 15, 18, 20, 24). A subtle difference from our results was observed in the US Black Women’s cohort (15), in which the inverse dose-response relation ceased beyond 3–4 hours/week of vigorous PA (equivalent to 26–34 minutes/day).
In the present analyses, walking at an average or brisk/very brisk pace, but not slow walking, was associated with a significantly reduced risk of clinical depression, indicating that PA has to reach a certain intensity level for clinical depression risk reduction. However, this association was attenuated and no longer significant in analyses in which we used the stricter definition of depression, and it is thus of uncertain interpretation. Among the 4 cohorts in which the effect of walking was analyzed prospectively, 3 studies found an inverse association with depressive symptoms (9, 10, 21) and 1 study did not find any association (15); however, none of the published articles provided information on walking pace.
We also noted that the risk of depression increased with increasing television watching (Ptrend = 0.01). A marginally significant (P = 0.05) increased depression risk of about 13% was noted among women who spent 21 hours/week or more watching television compared with those who did so for 0–1 hour/week. Similarly, in the Seguimiento University of Navarra (SUN) cohort (25), comprising 10,381 men and women, participants with the highest sedentary index score were found to be at increased risk (OR = 1.31, 95% CI: 1.01, 1.68) of mental disorders (self-reported physician’s diagnosis of depression, bipolar disorder, anxiety, or stress or use of antidepressant medication or tranquilizers) when compared with those with the lowest score. However, when depression (self-reported physician-diagnosed or use of antidepressants) was analyzed separately, the association with the sedentary index score lost significance (for the highest category vs. the lowest category, OR = 1.35, 95% CI: 0.94, 1.94).
The main hypothetical reason for the positive association we noted between television watching and depression is that television watching typically displaces PA. In our cohort, women who spent more time watching television tended to exercise less. A joint influence of television watching and PA on depression risk was noted (Figure 1). In the SUN cohort (25), the only other longitudinal study that evaluated prospectively the joint association between sedentary behavior and PA and mental disorders, the combination of PA (above the median) and sedentary index (below the median) was associated with 25% decreased odds of mental disorders (OR = 0.75, 95% CI: 0.60, 0.93) when compared with the reference group (PA below the median and sedentary index above the median). Therefore, the combinations of PA and sedentary behavior, such as television watching, could help to identify subjects at higher risk.
Several mechanisms have been proposed to explain the impact that increased PA has on depressive mood, such as increased sense of self-esteem, diversion from negative thoughts, perception of control and mastery (40, 41), increased circulating beta-endorphin (42) and monoamine (43, 44) levels, alterations in hypothalamic-pituitary-adrenal axis (45) and brain plasticity, and neurogenesis enhancement (46). Although PA has beneficial effects on cardiovascular health, in our analyses, the inverse relation between PA and depression seemed independent of other beneficial effects on cardiovascular health. In their recommendation for older adults, the American College of Sports Medicine and the American Heart Association indicated that all adults aged 65 years or older need moderate-intensity aerobic PA for a minimum of 30 minutes on 5 days each week or vigorous-intensity aerobic activity for a minimum of 20 minutes on 3 days each week (47). About 42.8% of our participants in 2004 did not met these American College of Sports Medicine/American Heart Association recommendations, whereas this rate was 47.9% for all US women and 63.7% for those older than 65 years of age (in 2005) (48). A sedentary lifestyle has become pervasive in US society, as reflected by the 2009 Nielsen’s Three Screen Report (49), which indicated that the amounts of time spent watching television and Internet and mobile video were escalating among Americans. On average, Americans watch television at home approximately 153 hours/month (approximately 5 hours/day) (49). Given the relation observed between television watching and depression risk, public health campaigns should promote replacement of these sedentary behaviors with PA. Substantial health benefits can be gained by even convenient activities such as walking at an average pace or higher. When conducting future research in this area, investigators should consider taking repeated measurements of both sedentary behavior (television watching and computer use) and PA.
The major strengths of the present study included its large sample size, prospective design, and repeated measures of PA and other covariates. The use of our validated PA questionnaire in 5 assessments over a period of 8 years was another unique strength of our study. This study also had limitations, and the results should be interpreted with caution. Some outcome misclassification bias was inevitable because of a combination of errors in reporting depression or antidepressant intake, low depression recognition by physicians (50), undertreatment of depression (51), and prescription of antidepressants for indications other than depression (e.g., neuropathic pain (52), premenstrual syndrome (53), and hot flushes (54)). Bias could have resulted if this misclassification was related to PA or television watching. For example, the inverse association between PA and depression could have been underestimated if women with lower socioeconomic status were both less active and, if depressed, less likely to be diagnosed with depression. The robustness of the results after adjustment for socioeconomic status, however, suggests that bias from this source is likely to be modest. Exposure misclassification could also be likely, particularly for television watching, which was assessed only in 1992 and might not accurately represent the total television-watching time during follow-up. Because women with subclinical depression might be more prone to develop clinical depression, a spurious inverse association between PA and the risk of depression could also be observed if women with subclinical depression reduced their PA level. Moreover, because of lack of information on depression history, we were not able to discern whether our incident cases were first onsets, as late-onset depression mainly occurs among people who have already been diagnosed with other disorders (55). Further, our results might not be generalizable to younger women or men, who were not included in this study. Our incidence is not directly comparable to that observed in unselected populations because to minimize reverse causation, we excluded women with severe depressive symptoms at baseline, thus artificially selecting a healthier population and eliminating a group of women at higher risk of depression. Last but not least, the observational nature of the present study cannot prove a causal relation between television watching or PA and depression. For example, genes involved in regulation of brain monoamides (dopaminergic monoamides, norepinephrine, and serotonin) have been suggested as likely candidates to affect both voluntary leisure-time regular PA and depressive mood (26, 56, 57). In conclusion, our results in this large cohort of older women indicate that regular PA and lower television-watching time may contribute to a reduced risk of depression.
Acknowledgments
Author affiliations: Department of Nutrition, Harvard School of Public Health, Boston, Massachusetts (Michel Lucas, Rania Mekary, An Pan, Fariba Mirzaei, Éilis J. O’Reilly, Walter C. Willett, Alberto Ascherio); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Rania Mekary, Fariba Mirzaei, Walter C. Willett, Karestan Koenen, Olivia I. Okereke, Alberto Ascherio); Department of Society, Human Development, and Health, Harvard School of Public Health, Boston, Massachusetts (Karestan Koenen); Channing Laboratory, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Olivia I. Okereke, Walter C. Willett, Alberto Ascherio); Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Olivia I. Okereke); and Department of Psychiatry, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts (Olivia I. Okereke).
The study was supported by National Institutes of Health grant DK58845. Dr. Ascherio received a grant from the National Alliance for Research on Schizophrenia and Depression (project ID 5048070-01). Dr. Lucas received a postdoctoral fellowship from the Fonds de Recherche en Santé du Québec.
The funding sources were not involved in data collection, data analysis, manuscript writing, or publication.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- MHI
Mental Health Index
- OR
odds ratio
- PA
physical activity
- SUN
Seguimiento University of Navarra
Appendix Table 1.
Relative Risk of Severe Depressive Symptomsa According to Levels of Physical Activity and Television Watching, Nurses’ Health Study, 1992–2006
| Physical Activity (1992–2000), minutes/day |
Ptrend | |||||||||||||||
| <10 |
10–29 |
30–59 |
60–89 |
≥90 |
||||||||||||
| No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | ||
| No. of cases | 2,229 | 3,830 | 2,981 | 979 | 444 | |||||||||||
| Person-years of follow-up | 75,372 | 159,903 | 133,125 | 48,649 | 24,381 | |||||||||||
| Age-adjusted RRb | 1.00 | 0.80 | 0.75, 0.84 | 0.75 | 0.70, 0.79 | 0.68 | 0.63, 0.74 | 0.64 | 0.58, 0.71 | <0.001 | ||||||
| Multivariate model 1c | 1.00 | 0.84 | 0.80, 0.88 | 0.80 | 0.76, 0.85 | 0.75 | 0.69, 0.81 | 0.70 | 0.63, 0.78 | <0.001 | ||||||
| Multivariate model 2d | 1.00 | 0.89 | 0.84, 0.94 | 0.89 | 0.84, 0.95 | 0.86 | 0.80, 0.93 | 0.82 | 0.74, 0.91 | <0.001 | ||||||
| Television Watching (1992), hours/week | PTrend | |||||||||||||||
| 0–1 | 2–5 | 6–10 | 11–20 | ≥21 | ||||||||||||
| No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | No. | RR | 95% CI | ||
| No. of cases | 539 | 2,326 | 2,547 | 2,913 | 1,983 | |||||||||||
| Person-years of follow-up | 29,093 | 105,656 | 112,611 | 118,771 | 69,175 | |||||||||||
| Age-adjusted RRb | 1.00 | 1.17 | 1.07, 1.29 | 1.20 | 1.09, 1.32 | 1.30 | 1.19, 1.43 | 1.49 | 1.35, 1.64 | <0.001 | ||||||
| Multivariate model 1c | 1.00 | 1.12 | 1.02, 1.23 | 1.14 | 1.03, 1.25 | 1.20 | 1.10, 1.32 | 1.33 | 1.20, 1.46 | <0.001 | ||||||
| Multivariate model 2d | 1.00 | 1.08 | 0.98, 1.19 | 1.08 | 0.99, 1.19 | 1.15 | 1.05, 1.26 | 1.24 | 1.12, 1.36 | <0.001 | ||||||
Abbreviations: CI, confidence interval; RR, relative risk.
Severe depressive symptoms were defined as antidepressant medication use or physician-diagnosed depression (1996–2006) or severe depressive symptoms (5-item Mental Health Index score in 2000 ≤52 or Center for Epidemiologic Studies Depression Scale 10 score in 2004 ≥10). Physical activity was computed as the cumulative average of physical activity between 1992 and 2000. A 2-year latency period was used to compute physical activity exposure. For example, physical activity information from 1992 and 1994 was used to compute exposure for 1996–1998, the cumulative average of 1992, 1994, and 1996 physical activity information was used to predict clinical depression in 1998–2000, and so on. We also stopped updating physical activity information in the cumulative average estimate after new diagnoses of nonfatal myocardial infarction, angina, nonfatal stroke, diabetes mellitus, and cancer.
Adjusted for age (continuous) and time interval.
Adjusted for current postmenopausal hormonal use (binary), body mass index (weight (kg)/height (m)2; <25, 25–29.9, or ≥30), marital status (married/in a partnership, widowed, or separated/divorced/single), involvement in a social or community group (binary), smoking status (never smoker, past smoker, or current smoker (1–14 cigarettes/day, 15–24 cigarettes/day, or ≥25 cigarettes/day)), total energy intake (continuous), coffee intake (never or <1 time/month, <2 times/day, or ≥2 times/day), reported diagnosis of diabetes mellitus (binary), cancer (binary), myocardial infarction or angina (binary), high blood pressure (binary), rheumatoid arthritis (binary), osteoarthritis (binary), asthma (binary), and emphysema (binary). Multivariate model 2 for physical activity was further adjusted for categories of television watching. Multivariate model 2 for television watching was further adjusted for categories of physical activity.
Additional adjustment for physical limitations in 1992 (binary) and for 5-item Mental Health Index score (86–100, 76–85, or 53–75) in 1992.
References
- 1.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Bloom BS. Prevalence and economic effects of depression. Manag Care. 2004;13(6):9–16. [PubMed] [Google Scholar]
- 4.Kessler RC, Akiskal HS, Ames M, et al. Prevalence and effects of mood disorders on work performance in a nationally representative sample of U.S. workers. Am J Psychiatry. 2006;163(9):1561–1568. doi: 10.1176/appi.ajp.163.9.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckworth J, Dishman RK. In: Exercise Psychology. Champaign, IL: Human Kinetics; 2002. Depression; pp. 131–154. [Google Scholar]
- 6.Farmer ME, Locke BZ, Mościcki EK, et al. Physical activity and depressive symptoms: the NHANES I Epidemiologic Follow-up Study. Am J Epidemiol. 1988;128(6):1340–1351. doi: 10.1093/oxfordjournals.aje.a115087. [DOI] [PubMed] [Google Scholar]
- 7.Camacho TC, Roberts RE, Lazarus NB, et al. Physical activity and depression: evidence from the Alameda County Study. Am J Epidemiol. 1991;134(2):220–231. doi: 10.1093/oxfordjournals.aje.a116074. [DOI] [PubMed] [Google Scholar]
- 8.Paffenbarger RS, Jr, Lee IM, Leung R. Physical activity and personal characteristics associated with depression and suicide in American college men. Acta Psychiatr Scand Suppl. 1994;377:16–22. doi: 10.1111/j.1600-0447.1994.tb05796.x. [DOI] [PubMed] [Google Scholar]
- 9.Mobily KE, Rubenstein LM, Lemke JH, et al. Walking and depression in a cohort of older adults: the Iowa 65+ Rural Health Study. J Aging Phys Act. 1996;4(2):119–135. [Google Scholar]
- 10.Lampinen P, Heikkinen RL, Ruoppila I. Changes in intensity of physical exercise as predictors of depressive symptoms among older adults: an eight-year follow-up. Prev Med. 2000;30(5):371–380. doi: 10.1006/pmed.2000.0641. [DOI] [PubMed] [Google Scholar]
- 11.Strawbridge WJ, Deleger S, Roberts RE, et al. Physical activity reduces the risk of subsequent depression for older adults. Am J Epidemiol. 2002;156(4):328–334. doi: 10.1093/aje/kwf047. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Russell A. Effects of physical activity on emotional well-being among older Australian women: cross-sectional and longitudinal analyses. J Psychosom Res. 2003;54(2):155–160. doi: 10.1016/s0022-3999(02)00414-2. [DOI] [PubMed] [Google Scholar]
- 13.Brown WJ, Ford JH, Burton NW, et al. Prospective study of physical activity and depressive symptoms in middle-aged women. Am J Prev Med. 2005;29(4):265–272. doi: 10.1016/j.amepre.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Bernaards CM, Jans MP, van den Heuvel SG, et al. Can strenuous leisure time physical activity prevent psychological complaints in a working population? Occup Environ Med. 2006;63(1):10–16. doi: 10.1136/oem.2004.017541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise LA, Adams-Campbell LL, Palmer JR, et al. Leisure time physical activity in relation to depressive symptoms in the Black Women’s Health Study. Ann Behav Med. 2006;32(1):68–76. doi: 10.1207/s15324796abm3201_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Gool CH, Kempen GI, Bosma H, et al. Associations between lifestyle and depressed mood: longitudinal results from the Maastricht Aging Study. Am J Public Health. 2007;97(5):887–894. doi: 10.2105/AJPH.2004.053199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiles NJ, Haase AM, Gallacher J, et al. Physical activity and common mental disorder: results from the Caerphilly Study. Am J Epidemiol. 2007;165(8):946–954. doi: 10.1093/aje/kwk070. [DOI] [PubMed] [Google Scholar]
- 18.Augestad LB, Slettemoen RP, Flanders WD. Physical activity and depressive symptoms among Norwegian adults aged 20–50. Public Health Nurs. 2008;25(6):536–545. doi: 10.1111/j.1525-1446.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 19.Ku PW, Fox KR, Chen LJ. Physical activity and depressive symptoms in Taiwanese older adults: a seven-year follow-up study. Prev Med. 2009;48(3):250–255. doi: 10.1016/j.ypmed.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Mikkelsen SS, Tolstrup JS, Flachs EM, et al. A cohort study of leisure time physical activity and depression. Prev Med. 2010;51(6):471–475. doi: 10.1016/j.ypmed.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Smith TL, Masaki KH, Fong K, et al. Effect of walking distance on 8-year incident depressive symptoms in elderly men with and without chronic disease: the Honolulu-Asia Aging Study. J Am Geriatr Soc. 2010;58(8):1447–1452. doi: 10.1111/j.1532-5415.2010.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamer M, Molloy GJ, de Oliveira C, et al. Leisure time physical activity, risk of depressive symptoms, and inflammatory mediators: the English Longitudinal Study of Ageing. Psychoneuroendocrinology. 2009;34(7):1050–1055. doi: 10.1016/j.psyneuen.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Cooper-Patrick L, Ford DE, Mead LA, et al. Exercise and depression in midlife: a prospective study. Am J Public Health. 1997;87(4):670–673. doi: 10.2105/ajph.87.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kritz-Silverstein D, Barrett-Connor E, Corbeau C. Cross-sectional and prospective study of exercise and depressed mood in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2001;153(6):596–603. doi: 10.1093/aje/153.6.596. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Villegas A, Ara I, Guillén-Grima F, et al. Physical activity, sedentary index, and mental disorders in the SUN cohort study. Med Sci Sports Exerc. 2008;40(5):827–834. doi: 10.1249/MSS.0b013e31816348b9. [DOI] [PubMed] [Google Scholar]
- 26.De Moor MH, Boomsma DI, Stubbe JH, et al. Testing causality in the association between regular exercise and symptoms of anxiety and depression. Arch Gen Psychiatry. 2008;65(8):897–905. doi: 10.1001/archpsyc.65.8.897. [DOI] [PubMed] [Google Scholar]
- 27.Mead GE, Morley W, Campbell P, et al. Exercise for depression. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD004366.pub4. (3):CD004366. (doi:10.1002/14651858.CD004366.pub4) [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Vol 8. Boston, MA: The Health Institute, New England Medical Center; 1994. [Google Scholar]
- 29.Yamazaki S, Fukuhara S, Green J. Usefulness of five-item and three-item Mental Health Inventories to screen for depressive symptoms in the general population of Japan. Health Qual Life Outcomes. 2005;3:48. doi: 10.1186/1477-7525-3-48. (doi:10.1186/1477-7525-3-48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berwick DM, Murphy JM, Goldman PA, et al. Performance of a five-item mental health screening test. Med Care. 1991;29(2):169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Mekary RA, Willett WC, Hu FB, et al. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol. 2009;170(4):519–527. doi: 10.1093/aje/kwp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 33.Lucas M, Mirzaei F, O’Reilly EJ, et al. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. 2011;93(6):1337–1343. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 35.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 36.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 37.Kroenke CH, Bennett GG, Fuchs C, et al. Depressive symptoms and prospective incidence of colorectal cancer in women. Am J Epidemiol. 2005;162(9):839–848. doi: 10.1093/aje/kwi302. [DOI] [PubMed] [Google Scholar]
- 38.Yusuf HR, Croft JB, Giles WH, et al. Leisure-time physical activity among older adults.United States, 1990. Arch Intern Med. 1996;156(12):1321–1326. [PubMed] [Google Scholar]
- 39.Teychenne M, Ball K, Salmon J. Physical activity and likelihood of depression in adults: a review. Prev Med. 2008;46(5):397–411. doi: 10.1016/j.ypmed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Lepore SJ. Expressive writing moderates the relation between intrusive thoughts and depressive symptoms. J Pers Soc Psychol. 1997;73(5):1030–1037. doi: 10.1037//0022-3514.73.5.1030. [DOI] [PubMed] [Google Scholar]
- 41.Paluska SA, Schwenk TL. Physical activity and mental health: current concepts. Sports Med. 2000;29(3):167–180. doi: 10.2165/00007256-200029030-00003. [DOI] [PubMed] [Google Scholar]
- 42.Goldfarb AH, Jamurtas AZ, Kamimori GH, et al. Gender effect on beta-endorphin response to exercise. Med Sci Sports Exerc. 1998;30(12):1672–1676. doi: 10.1097/00005768-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Dunn AL, Dishman RK. Exercise and the neurobiology of depression. Exerc Sport Sci Rev. 1991;19(1):41–98. [PubMed] [Google Scholar]
- 44.Meeusen R, Thorré K, Chaouloff F, et al. Effects of tryptophan and/or acute running on extracellular 5-HT and 5-HIAA levels in the hippocampus of food-deprived rats. Brain Res. 1996;740(1-2):245–252. doi: 10.1016/s0006-8993(96)00872-4. [DOI] [PubMed] [Google Scholar]
- 45.Hill EE, Zack E, Battaglini C, et al. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31(7):587–591. doi: 10.1007/BF03345606. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen BK, Pedersen M, Krabbe KS, et al. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol. 2009;94(12):1153–1160. doi: 10.1113/expphysiol.2009.048561. [DOI] [PubMed] [Google Scholar]
- 47.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 48.Prevalence of regular physical activity among adults—United States, 2001 and 2005. MMWR Morb Mortal Wkly Rep. 2007;56(46):1209–1212. [PubMed] [Google Scholar]
- 49.The Nielsen Company. Three Screen Report: Television, Internet and Mobile Usage in the U.S. Vol 7. New York, NY: The Nielsen Company; 2010. ( http://in.nielsen.com/site/documents/3Screens_4Q09_US_rpt.pdf). (Accessed April 3, 2011) [Google Scholar]
- 50.Löwe B, Spitzer RL, Gräfe K, et al. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians’ diagnoses. J Affect Disord. 2004;78(2):131–140. doi: 10.1016/s0165-0327(02)00237-9. [DOI] [PubMed] [Google Scholar]
- 51.Demyttenaere K, Bruffaerts R, Posada-Villa J, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291(21):2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- 52.Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848–856. doi: 10.1001/archgenpsychiatry.2009.81. [DOI] [PubMed] [Google Scholar]
- 53.Brown J, O’Brien PM, Marjoribanks J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD001396.pub2. (2):CD001396. (doi:10.1002/14651858.CD001396.pub2) [DOI] [PubMed] [Google Scholar]
- 54.Stearns V, Ullmer L, López JF, et al. Hot flushes. Lancet. 2002;360(9348):1851–1861. doi: 10.1016/s0140-6736(02)11774-0. [DOI] [PubMed] [Google Scholar]
- 55.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 56.Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29(1):63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Chaouloff F. Effects of acute physical exercise on central serotonergic systems. Med Sci Sports Exerc. 1997;29(1):58–62. doi: 10.1097/00005768-199701000-00009. [DOI] [PubMed] [Google Scholar]