Abstract
Diabetic retinopathy is a chronic inflammatory disease characterized by vascular damage and neuronal degeneration. Previously we reported that activated retinal pericytes secret high levels of pro-inflammatory cytokines, such as macrophage chemoattractant protein 1 (MCP-1), and may play a pivotal role in macrophage recruitment and inflammatory retinal damage. However, the mechanism underlying diabetes-induced pericyte inflammation remains poorly understood. In the present study, we evaluated the effects of constant and intermittent high glucose on inflammatory cytokine production in human retinal pericytes (HRP) and explored the role of endoplasmic reticulum (ER) stress in pericyte inflammation. We found that intermittent high glucose, but not constant high glucose, increases MCP-1 secretion and expression of activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP), key mediators of ER stress-associated inflammation and cell death. Inhibition of ER stress by chemical chaperones successfully prevented glucose fluctuation-induced ATF4/CHOP activation and inflammatory cytokine production. Our results suggest that activation of ER stress by glucose fluctuation may play a causal role in pericyte injury and inflammation in diabetic retinopathy.
XX.1 Introduction
Increased inflammatory cytokines in the retina are closely associated with retinal pathologies in diabetic retinopathy (Li et al., 2009b). Pericytes, along with endothelial cells, are the major cell components of retinal capillaries. Pericytes activated by oxidized lipids secret high levels of inflammatory cytokines, such as macrophage chemoattractant protein 1 (MCP-1) (Zhang et al., 2008). In addition, pericyte injury and cell death are considered as a hallmark pathological change in diabetic retinopathy. Although the mechanisms underlying diabetes-induced pericyte injury are not fully understood, studies suggest that fluctuating glucose, when compared to constantly increased glucose concentration, is more detrimental to vascular cells, including pericytes (Quagliaro et al., 2003; Beltramo et al., 2009). In addition, fluctuating glucose stimulates a greater increase in inflammatory cytokine production from endothelial cells than stable high glucose (Piconi et al., 2004). However, it is unclear whether glucose fluctuation influences inflammatory mediators in pericytes. Moreover, we recently demonstrated that endoplasmic reticulum (ER) stress is implicated in retinal inflammation during diabetes (Li et al., 2009a). In the present study, we evaluated the effects of intermittent and constant high glucose and the role of ER stress in inflammatory factor production in retinal pericytes.
XX.2 Materials and Methods
XX.2.1 Materials
Sodium 4-phenyl butyrate and tauroursodeoxycholic acid were purchased from Calbiochem (San Diego, CA). Anti-VEGF, anti-ATF4 and anti-CHOP antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-nucleoporin p62 antibody was from BD Biosciences Pharmingen (San Diego, CA). Anti-KDEL (for detection of GRP78) and anti-β-actin antibodies were obtained from Abcam (Cambridge, MA). horse-radish peroxidase-conjugated secondary antibodies were obtained from Vector Laboratories (Burlingame, CA).
XX.2.2 Cell culture
Primary human retinal pericytes (HRP) were purchased from Clonetics, Inc. (Walkersville, MD). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% antibiotic/antimycotic. After reaching 50% of confluence, cells were exposed to the following experimental conditions for 8 days with medium replaced every 2 days: 1) normal glucose (5mM); 2) constant high glucose (HG, 25mM); 3) normal and high glucose alternating every 48 h. On day eight, cells were quiescent in DMEM with 1% FBS for 24 h. Medium were collected and cells harvested for analysis.
XX.2.3 Western blot analysis
Western blot analysis was performed as described previously(Li et al., 2009a). Briefly, cells were lysed in radioimmunoprecipitation assay lysis buffer. Nuclear and cytoplasmic extracts were prepared using a Nuclear Extract Kit (Active Motif, Carlsbad, CA) following manufacturer’s instructions. Twenty-five micrograms of protein were dissolved by SDS-PAGE. Primary antibodies used for blotting include: anti-KDEL (1:5000), anti-phospho-eIF2α (1:1000), anti-ATF4 (1:500), anti-GADD153 (1:500), anti-VEGF (1:500), anti-β-actin (1:5,000) and anti-nucleoporin p62 (1:2000) antibodies.
XX.2.4 Quantification of MCP-1 secretion in pericytes
MCP-1 secreted into the medium was measured using the DuoSet ELISA kit for human MCP-1 (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions as described previously (Zhang et al., 2008).
XX.2.5 Statistical analysis
Data were expressed as mean ± SD. Statistical analysis was performed using Student t-test when comparing two groups, or ANOVA with Bonferroni’s post hoc test when comparing 3 or more groups. Statistical significance was accepted as P <0.05.
XX.3 Results
XX.3.1 High glucose suppresses GRP78 expression in HRP
Glucose regulated protein (GRP78), also known as heat shock 70 kDa protein 5 (hsp70-5 or hspA5) or immunoglobulin heavy chain-binding protein (BiP), is a prominent ER chaperone that promotes appropriate protein folding. Pharmaceutical induction of GRP78 expression or over-expression of GRP78 gene in the retina protects retinal ganglion cells and photoreceptors from ER stress-induced apoptosis and cell death, suggesting that GRP78 is a cyto-protective factor in retinal cells (Gorbatyuk et al.; Inokuchi et al., 2008). In the present study, we examined expression of GRP78 in HRP after exposure to constant or intermittent high glucose for 8 days. We found that GRP78 level was decreased in both constant and intermittent high glucose-treated cells when compared to cells exposed to normal glucose. Intermittent glucose induced a more remarkable decrease in GRP78 expression when compared to constant high glucose (Fig. 1A-1C), indicative of an inhibitory effect of high glucose on GRP78 expression.
Fig. XX.1. Effects of intermittent and constant high glucose on ER stress and MCP-1 secretion.
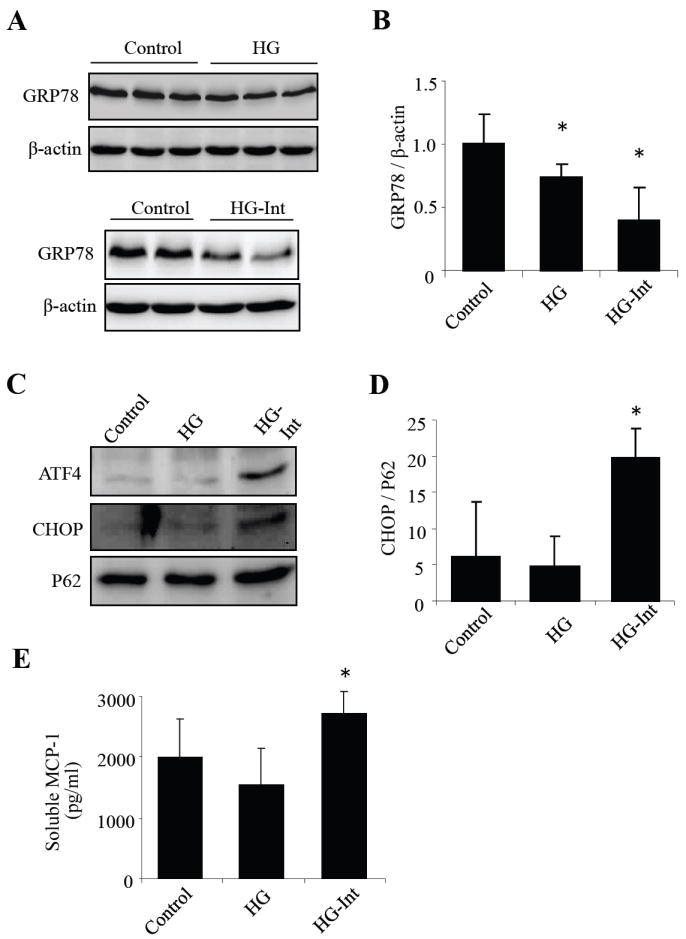
A: GRP78 expression, increases MCP-1 level and activates ER stress-mediated apoptosis in human retinal pericytes. HRP were exposed to stable high glucose (HG, 25 mM), intermittent high glucose (HG-Int, intermittent exposure to 25 mM glucose at 48h intervals) or normal glucose (5 mM) for 8 days. (A-B) Expression of GRP78 was detected by Western blot analysis in whole cell lysates. (C) Protein level of GRP78 was quantified by densitometry (mean ± SD, n=3). (D) Expression of ATF4 and CHOP was determined by Western blot analysis in nuclear extracts. (F) Protein level of CHOP was semiquantified by densitometry (mean ± SD, n=3). (F) Soluble MCP-1 secreted into the medium was measured using ELISA (mean ± SD, n=3). *P<0.05 vs. control.
XX.3.2 Intermittent but not constant high glucose activates ATF4/CHOP and increases MCP-1 secretion in HRP
Activating transcription factor 4 (ATF4) and its target gene C/EBP homologous protein (CHOP) are important ER stress response genes that trigger inflammatory and apoptotic cascades (Endo et al., 2006). We next determined the effect of high glucose on ATF4 and CHOP expression. As both ATF4 and CHOP are transcription factors, which translocate into the nucleus when activated, we thus measured the level of ATF4 and CHOP protein in nuclear extract from HRP. We found that ATF4 and CHOP are expressed at very low level in cells cultured in normal glucose. Exposure to constant high glucose did not alter ATF4 or CHOP expression. In contrast, intermittent high glucose induced a significant increase in nuclear levels of ATF4 and CHOP, suggesting an activation of ER stress in HRP (Fig. 1C, 1D). To determine if induction of ER stress by glucose fluctuation is associated with increased inflammation, we measured MCP-1 secretion from HRP after treatment with constant or intermittent high glucose for 8 days. We found that intermittent high glucose induced a significant increase in MCP-1 secretion when compared to normal glucose and constant high glucose, while constant high glucose had no effect on MCP-1 secretion (Fig. 1E). These results corroborate the changes in ATF4 and CHOP expression, suggesting that only intermittent, but not constant, high glucose, induces ER stress and inflammatory mediators in HRP.
XX.3.3 Inhibition of ER stress by chemical chaperones alleviates inflammatory cytokine expression in HRP exposed to intermittent high glucose
Sodium 4-phenyl butyrate (PBA) and tauroursodeoxycholic acid (TUDCA) are small molecule chaperones that suppress the induction of ER stress(Li et al., 2009a). To investigate if ER stress plays a role in glucose fluctuation-induced inflammation in pericytes, HRP were pretreated with TUDCA or PBA for 8 h, followed by incubation with intermittent high glucose for 8 days. MCP-1 secreted into the medium was measured by ELISA. We found that TUDCA and PBA dose-dependently decreased intermittent high glucose-induced MCP-1 secretion from HRP. Vascular endothelial growth factor (VEGF) is a key pro-inflammatory cytokine in the pathogenesis of vascular leakage and retina neovascularization in diabetic retinopathy(Li et al., 2009a). We also measured VEGF expression in HRP by Western blot analysis. The results show that VEGF expression was markedly increased by intermittent high glucose, and the increase was largely abolished by ER stress inhibitor TUDCA and PBA. As CHOP is key mediator of ER stress-induced inflammatory response and apoptosis, we further examined CHOP expression in HRP treated with TUDAC or PBA. We found that TUDCA and PBA effectively suppressed the induction of CHOP expression by intermittent high glucose. These results collectively suggest that ER stress plays a critical role in glucose fluctuation-induced inflammation in HRP.
XX.4 Discussion
The endoplasmic reticulum (ER) has long been recognized as a cellular factory for protein processing. Intriguingly, emerging evidence suggests that the ER also acts as a principal stress sensor that initiates numerous intracellular signaling pathways implicated in pathological conditions, such as inflammation and apoptosis. C/EBP homologous protein (CHOP), a target gene of ATF4, is a key mediator of ER stress-associated inflammatory and apoptotic processes. Inhibition of CHOP expression attenuates inflammation and prevents caspase activation and apoptotic cascade in cells exposed to LPS or diabetic stressors(Song et al., 2008). Our recent study shows that CHOP and ATF4 expression is significantly elevated, accompanied by increased retinal inflammation and vascular leakage, in the retina in diabetic animals (Li et al., 2009a). Induction of ER stress is sufficient to induce inflammatory cytokine expression in the retina. Conversely, suppression of ER stress using small molecule ER chaperones significantly alleviates diabetes- and ischemia-induced retinal inflammation, suggesting ER stress is a potential mediator of inflammatory damage of retinal cells in diabetic retinopathy. In the present study, we further addressed the role of ER stress in high glucose-induced inflammation in pericytes. We found that exposure of human retinal pericytes to intermittent high glucose induces ATF4 and CHOP activation and inflammatory cytokine expression. Moreover, inhibition of ATF4 and CHOP by chemical chaperones largely reversed fluctuating high glucose-induced VEGF and MCP-1 expression. These results suggest that ATF4 and CHOP activation secondary to ER stress contributes to pericyte inflammation in diabetes.
Although we have shown that glucose fluctuation induces ER stress in pericytes, the mechanisms underlying the activation of ATF4/CHOP pathway remains poorly understood. GRP78 is recognized as a protective factor against ER stress-induced inflammation and cell damage. We found that persistent high glucose for 8 days suppressed GRP78 expression, but did not induce ATF4 and CHOP activation. In contrast, intermittent exposure to high glucose caused a more marked decrease in GRP78 expression, accompanied by increased ATF4 and CHOP expression. The association between decreased GRP78 expression and activation of ATF4 and CHOP remains to be elucidated. In addition, Ikesugi and associates reported glucose deprivation, but not high glucose, elicits ER stress in rat retinal pericytes(Ikesugi et al., 2006). It is possible that the repetitive shift from high glucose to normal glucose during glucose fluctuation induces ER stress, while GRP78 suppression compromises the protein folding capacity of the ER, resulting in exaggerated ATF4/CHOP activation and inflammation in retinal pericytes. In addition, increased ATF4/CHOP activation may also contribute to pericyte apoptosis induced by glucose fluctuation. Future studies are warranted to investigate how ER stress-associated apoptotic pathway is implicated in retinal cell death in diabetic retinopathy.
Fig. XX.2. ER stress mediates intermittent high glucose induced inflammation and apoptosis in human retinal pericytes.
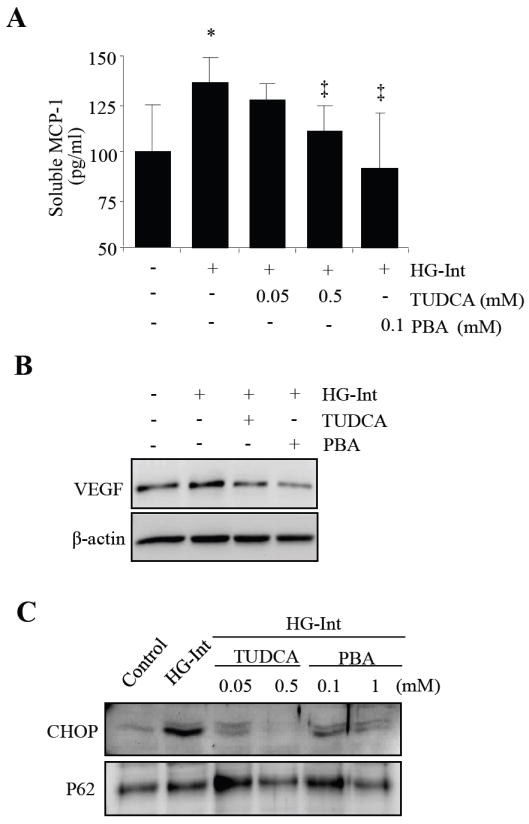
(A) HRP were treated with ER stress inducer, tunicamycin (TM) or thapsigargin (TG) for 24 h. Expression of VEGF was determined by Western blot analysis. (B) HRP were exposed to intermittent high glucose (HG-Int) with or without the chemical chaperon, TUDCA (0.5 mM) or PBA (0.1 mM) for 8 days, expression of VEGF in the cytoplasm was determined by Western blot analysis. (C and D) HRP were treated with intermittent high glucose with or without TUDCA and PBA for 8 days. (C) Soluble MCP-1 level in the culture medium was measured by ELISA (mean ± SD, n=4). *P<0.05 vs. control. ‡P<0.05 vs. HG-Int. (D) Expression of ATF4 and CHOP was determined by Western blot analysis in nuclear extracts. And protein level of p62 was used as an internal control of nuclear extracts.
Acknowledgments
This work was supported by National Institutes of Health grant EY019949; Research Award 5-2009-475 from Juvenile Diabetes Research Foundation; Research Grants HR07-167 and HR10-060 from Oklahoma Center for the Advancement of Science and Technology; Research Grant M2010088 from American Health Assistance Foundation; and Dr. William Talley Research Award from Harold Hamm Oklahoma Diabetes Center.
References
- Beltramo E, Berrone E, Tarallo S, Porta M. Different apoptotic responses of human and bovine pericytes to fluctuating glucose levels and protective role of thiamine. Diabetes/Metabolism Research and Reviews. 2009;25:566–576. doi: 10.1002/dmrr.996. [DOI] [PubMed] [Google Scholar]
- Endo M, Mori M, Akira S, Gotoh T. C/EBP Homologous Protein (CHOP) Is Crucial for the Induction of Caspase-11 and the Pathogenesis of Lipopolysaccharide-Induced Inflammatio. J Immunol. 2006;176:6245–6253. doi: 10.4049/jimmunol.176.10.6245. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW, Lin JH, Muzyczka N, Lewin AS. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proceedings of the National Academy of Sciences. 107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikesugi K, Mulhern ML, Madson CJ, Hosoya K, Terasaki T, Kador PF, Shinohara T. Induction of endoplasmic reticulum stress in retinal pericytes by glucose deprivation. Curr Eye Res. 2006;31:947–953. doi: 10.1080/02713680600966785. [DOI] [PubMed] [Google Scholar]
- Inokuchi Y, Nakajima Y, Shimazawa M, Kurita T, Kubo M, Saito A, Sajiki H, Kudo T, Aihara M, Imaizumi K, Araie M, Hara H. Inducer of BiP, an Endoplasmic Reticulum (ER)-resident Protein, Limits Retinal Cell Death. Invest Ophthalmol Vis Sci. 2008 Aug 29; doi: 10.1167/iovs.08-2123. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Li J, Wang JJ, Yu Q, Wang M, Zhang SX. Endoplasmic Reticulum Stress is implicated in Retinal Inflammation and Diabetic Retinopathy. FEBS Lett 2009. 2009a Apr 10; doi: 10.1016/j.febslet.2009.04.007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res 2009. 2009b Feb 28; doi: 10.1016/j.exer.2009.02.013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconi L, Quagliaro L, Da Ros R, Assaloni R, Giugliano D, Esposito K, Szabó C, Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly(ADP-ribose) polymerase. Journal of Thrombosis and Haemostasis. 2004;2:1453–1459. doi: 10.1111/j.1538-7836.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent High Glucose Enhances Apoptosis Related to Oxidative Stress in Human Umbilical Vein Endothelial Cells. Diabetes. 2003;52:2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Dashti A, Wilson K, Szweda LI, Zou MH, Ma JX, Lyons TJ. Pigment epithelium-derived factor (PEDF) mitigates inflammation and oxidative stress in retinal pericytes exposed to oxidized-LDL. J Mol Endocrinol. 2008;41:135–143. doi: 10.1677/JME-08-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]


