Abstract
We have evaluated “NMEGylation”—the covalent attachment of a oligo-N-methoxyethylglycine (NMEG) chain—as a new form of peptide/protein modification to enhance the bioavailability of short peptides. OligoNMEGs are hydrophilic PEG-like molecules made by solid-phase synthesis, typically up to 40 monomers in length. They have been studied as non-fouling surface coatings, and as monodisperse mobility modifiers for free-solution conjugate capillary electrophoresis. However, polyNMEGs have not been demonstrated prior to this work as modifiers of therapeutic proteins. In prior published work, we identified a short peptide, “C20”, as a potential extracellular inhibitor of the fusion of human respiratory syncytial virus (hRSV) with mammalian cells. The present study was aimed at improving the C20 peptide's stability and solubility. To this end we synthesized and studied a series of NMEGylated C20 peptide-peptoid bioconjugates comprising different numbers of NMEGs at either the N- or C- terminus of C20. NMEGylation was found to greatly improve this peptide's solubility and serum stability; however, longer polyNMEGs (n > 3) deleteriously affected peptide binding to the target protein. By incorporating just one NMEG monomer, along with a glycine monomer as a flexible spacer, at C20's N-terminus (NMEG-Gly-C20), we increased both solubility and serum stability greatly, while recovering a binding affinity comparable to that of unmodified C20 peptide. Our results suggest that NMEGylation with an optimized number of NMEG monomers and a proper linker could be useful, more broadly, as a novel modification to enhance bioavailability and efficacy of therapeutic peptides.
Keywords: NMEGylation, bioavailability, peptide-based therapeutics
Introduction
With the monumental advances in biotechnology over the past few decades, peptides and proteins have become key players in the drug market, as therapeutic candidates with undeniably high specificity and low toxicity compared to conventional synthetic small-molecule drugs, resulting in more than 60 biotherapeutics, which have sales reaching US $40 billion.1,2 However, clinical uses, manufacturing, and storage of peptide-based medicines are challenged by several unfavorable biophysical properties. One major obstacle to peptide-based therapeutics is that they are susceptible to proteolytic degradation, which can result in rapid renal clearance and short in vivo circulation half-lives (∼ 2-5 min). Efforts have been made to increase the half-lives of biotherapeutics without sacrificing efficacy, but these efforts have yielded only a few notable successes.3-8
PEGylation, the conjugation of PEG (polyethylene glycol) chains to druggable materials, has been studied intensively and clinically proven and is an acceptable method of modifying peptide/protein-based medicines. The consequent increase in proteolytic stability reduces the rate of renal clearance and enhances therapeutic efficacy. PEGylation is known to improve the physical and thermal stability and the solubility of biopharmaceuticals, which makes this modification suitable for applications in drug delivery and formulation.9-12 Since Adagen® (PEGylated adenosine deaminase) was introduced as the first FDA-approved PEGylated therapeutic agent in 1990, several PEGylated protein-based drugs have appeared, including PEGasys® (PEGylated α -interferons, for Hepatitis C treatment) and Cimzia® (PEGylated Anti-TNF Fab, for rheumatoid arthritis and Crohn's disease).13 However, PEGylation has limitations, such as the heterogeneity of PEG (lack of complete monodispersity). As a result, detailed bioanalytical aspects of the resultant “mixtures” of PEGylated products are an issue in the FDA approval of and clinical uses of PEGylated drugs.14 The avoidance of variable extents of PEGylation requires a complicated, multi-step manufacturing process, which increases costs.15 In addition to other approaches to improve specificity and homogeneity in PEGylation,16,17 efforts to develop alternatives to PEG are also being pursued, so far resulting in limited but promising outcomes.18-20
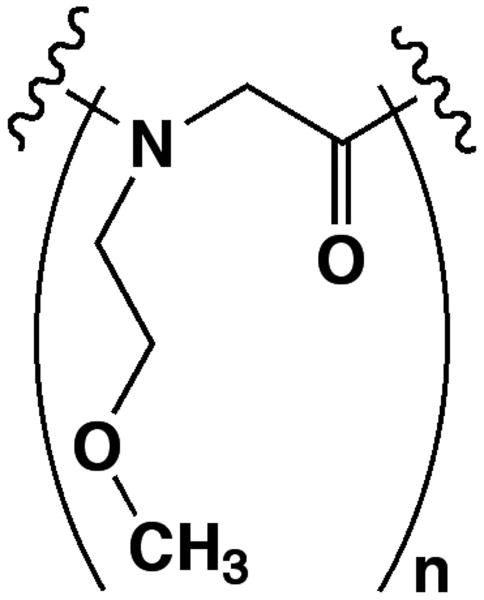
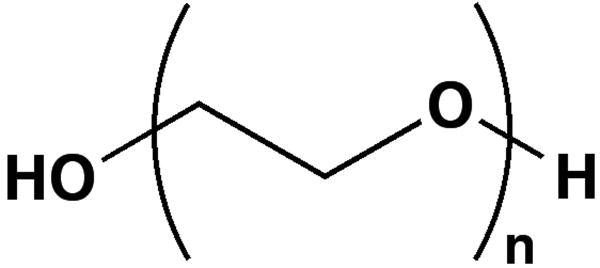
N-methoxyethylglycine (NMEG) is a hydrophilic peptoid monomer that bears a similar chemical side-chain moiety (an ethylene oxide unit) to the PEG monomer (Figure 1). Peptoids are peptidomimetics based on a peptide backbone, and resist proteolysis because their side chains are attached to backbone nitrogens rather than to the α-carbons.21 Since 1998, peptoids have been demonstrated for uses in gene delivery,22 drug delivery,23 and as a scaffold for the design of novel protein ligands with improved binding affinities.24-26 Virtually any primary amine can be incorporated into oligopeptoids as a side chain using the “sub-monomer” approach, enabling the use and exploration of a diverse set of biomimetic monomers in peptoid research.27,28
FIGURE 1.
Structural comparison of (A) N-methoxyethylglycine (NMEG) and (B) polyethylene glycol (PEG).
Previously, the Barron group has reported that oligoNMEGs (with n = 10, 20 and 30) are promising as (1) antifouling agents, which prevent non-specific protein and cell binding to surfaces29, and (2) mobility modifiers enabling free-solution DNA separations by bioconjugate capillary electrophoresis.30 Although NMEGylation, i.e, covalent attachment of an oligoNMEG, should be anticipated to enhance protease resistance,31,32 this has not yet been investigated. Thus, we have explored oligoNMEG as a PEG-like but more homogeneous modification.
We have previously identified a particular 20mer polypeptide (“C20”) as a potential new antiviral agent using a protein-based (five-helix bundle, 5HB) fluorescence polarization (FP) screening assay, as part of an effort to identify potential viral entry inhibitors that could prevent human respiratory syncytial virus (hRSV) infections.33 As a lead compound, the C20 peptide shows low micromolar binding affinity to the 5HB; however, neither the solubility nor the stability of this peptide is ideal. Therefore, using C20 as the parent peptide, we prepared a series of NMEGylated C20 analogues, all completely monodisperse and comprising different numbers of NMEG monomers, and evaluated the biophysical properties and biological activity of each bioconjugate. We were able to identify interesting new compounds, and thus believe that oligopeptoids, oligoNMEGs in particular, are promising as novel modifiers of biotherapeutics, and an approach that could have a broad usefulness in biological and medical research.
Methods and Materials
General materials
Reagents for peptide and peptoid synthesis were purchased from Applied Biosystems (Foster city, CA, USA) or Sigma-Aldrich (Milwaukee, WI, USA). Resins and Fmoc-protected amino acids were purchased from NovaBioChem (San Diego, CA, USA) or Anaspec (San Jose, CA, USA). Solvents for analytical and preparation RP-HPLC were purchased from Fisher Scientific (Pittsburgh, PA, USA). All other chemicals were purchased from Sigma-Aldrich (Milwaukee, WI, USA) and were used without additional purification.
NMEGylated therapeutics and C20 peptide synthesis
The C20 peptide and its NMEGylated analogues were synthesized using standard Fmoc chemistry and submonomer peptoid synthesis28, each in a single synthesis, using an automated ABI 433A peptide synthesizer (Life Technologies, Foster city, CA USA). Final purities of synthetic peptides and therapeutics were confirmed by analytical RP-HPLC, and the molar masses of the purified products were confirmed by electrospray mass spectrometry (ESI) at the Stanford University Mass Spectrometry (SUMS) facility.
Percent acetonitrile at RP-HPLC eluation as a relative measure of bioconjugate hydrophilicity
The relative hydrophilicities (the percent acetonitrile at elution in RP-HPLC) of NMEGylated peptides were compared. Previously this has been reported as a reliable method for determining the contribution of the hydrophilicity of constituent amino acids of a polypeptide, based on the retention time of peptides.34,35 The purified C20 peptide and its NMEGylated analogues were analyzed using RP-HPLC on a Phenomenex (Torrance, CA, USA) C18 column (250 mm × 2.00 mm) with 5 μm resin size using a linear gradient of 5-95% solvent B over 30 or 60 min (solvent A is water with 0.1% (v/v) TFA and solvent B is acetonitrile with 0.1% (v/v) TFA).
Serum stability assay36
Peptides (∼ 0.4 mg) were dissolved in PBS pH 7.6 (400 μL, containing 0.1% v/v benzyl alcohol as internal standard). Equal volumes of 50% human serum plasma (Sigma-Aldrich, Milwaukee, WI, USA) and peptide/bioconjugate solutions were combined, and incubated at 37°C. Samples were taken in triplicate (3 × 50 μL) at 0, 30, and 240 min and placed on ice. Each sample (including the t = 0 time point) was prepared for HPLC analysis in the following way. First, 20 μL of 0.5M lysine monohydrochloride was added, followed by 65 μL of acetonitrile. The samples were cooled on ice for 10 min and subsequently centrifuged for 10 min at 3000 rpm. A 50 μL aliquot of resulting samples was taken from the supernatant, diluted with 100 μL of water, and analyzed by analytical RP-HPLC. The ratio of the disappearing peptide or bioconjugate peak area to the benzyl alcohol peak area was calculated for each time point, and normalized against the ratio obtained at the t = 0 time point (representing 100% peptide remaining) as a percentage peptide remaining.
Competitive fluorescence polarization (FP) binding assay
Competitive FP binding assays of NMEGylated C20 therapeutics were carried out as previously reported. Briefly, using a 5-helix bundle (5HB) protein construct and a fluorescently labeled 35-amino acid-long polypeptide (Fl-C35: YDPLVFPSDEFDASISQVNEKINQSLAFIRKSDEL) as a target and tracer, respectively, the binding affinities of NMEGylated therapeutics were each evaluated individually. Both the C20 and C35 peptide sequences are derived from HRB domain of hRSV fusion (F) protein, are meant to mimic the 6-helix bundle formation of hRSV F protein by occupying the one empty binding site on the 5HB protein. The binding of the Fl-C35 peptide to the 5HB in the absence of test ligands provided us with our relative “100% 5HB·Fl-C35 bound” for use as a positive control. Each well in a black 96-well plate (Corning Inc. Lowell, MA, USA) contained an aliquot of 20 nM 5HB solution, and increasing concentrations of each NMEGylated bioconjugate in FP buffer (20 mM PBS at pH 7.4, 500 mM NaCl, 0.01% (v/v) Tween-20, and 0.05 mg/ml bovine gamma globulin) in a final volume of 185 μL with 1 hr incubation at room temperature. Fl-C35 was then added to a final concentration of 5 nM followed by 30 min incubation at room temperature. FP response was monitored in a Synergy4 (Biotek, Winooski, VT, USA) plate reader with an excitation wavelength of 485 nm and emission wavelength of 530 nm. All experimental data were obtained in duplicate and the percentage of 5HB·Fl-C35 bound was calculated using the following equation: % of 5HB·Fl-C35 bound = 100×[1-(mP-mPf)/(mPb-mPf)], where unbound Fl-C35 (mPf), bound Fl-C35 (mPb) and the bound inhibitor to the 5HB (mP) are used accordingly.
Results and discussion
Synthesis, purification and characterization of NMEGylated C20 therapeutics
The C20 peptide was previously identified from peptide truncation studies as having low-micromolar binding affinity to a 5-helix bundle (5HB) protein, a genetically engineered protein construct derived from the hRSV fusion protein (F); however, because of its short length and unstructured conformation,37 the C20 peptide ligand has high susceptibility to proteases and moreover, because of its amino acid sequence, poor solubility in aqueous solution. Although PEGylation might also improve the desirable biophysical features of the C20 peptide, any attempt to PEGylate the C20 peptide was discouraged by the following concerns: the large increase in molecular weight of PEGylated C20 products and potential steric shielding would likely impede the binding of C20 to the 5HB. (Monodisperse oligoethylene glycols available from Quanta BioSciences would likely also “work”, but are expensive and thus were not tested here.) Instead we decided to explore NMEG as a potential alternative to PEG, because NMEG is hydrophilic, like conventional PEG, but after HPLC purification is completely monodisperse, as compared with other commercially available PEG derivatives (even low-polydispersity PEGs, activated for reaction with amines, are expensive). Importantly, because N-substituted glycines such as the, oligoNMEGs cannot be recognized or cleaved by proteases38, we anticipated that the peptoid-peptide hybrids (therapeutics) formed by NMEGylation would be less susceptible to proteolytic degradation. As shown in Table I, a series of NMEGylated C20 therapeutics were prepared by attaching NMEG oligomers (n = 1 – 10) to either the N- or C- terminus of the C20. The resulting NMEGylated therapeutics were found to be extremely soluble in aqueous buffer (up to > 10 mg/mL) as compared to C20 (< 2 mg/mL). The relative hydrophilicity of NMEGylated C20 analogues, based on percent acetonitrile at elution, was evaluated by RP-HPLC (see Table I).34,35 A decrease in the percent acetonitrile at which modified peptides eluted demonstrates that NMEGylation, even with just a few monomers, dramatically enhances the hydrophilicity of the C20 peptide.
TABLE I. NMEGylated C20 bioconjugate sequences tested in this study with molar mass, purity, solubility, hydrophilicity and their IC50 values.
| Compounds | Sequences (amino to carboxy) | Molar mass (Da) Calc./Observ. | Purity (%) | % AcN at elutiona | IC50b |
|---|---|---|---|---|---|
| C20 | ISQVNEKINWSLAFIRKSDE | 2319.6/2319.0 | > 95 | 78 | 36 ± 1 |
| NMEG3-C20 | NMEG3-ISQVNEKINWSLAFIRKSDE | 2664.9/2664.4 | > 95 | 60 | > 200 |
| NMEG10-C20 | NMEG5-ISQVNEKINWSLAFIRKSDE | 3470.3/3471.8 | > 95 | 57 | > 200 |
| C20-NMEG10 | ISQVNEKINWSLAFIRKSDE-NMEG10 | 3468.9/3469.8 | > 95 | 57 | > 200 |
| C20-NMEG5 | ISQVNEKINWSLAFIRKSDE-NMEG5 | 2894.2/2894.1 | > 95 | 54 | > 200 |
| C20-NMEG3 | ISQVNEKINWSLAFIRKSDE-NMEG3 | 2663.9/2663.7 | > 95 | 52 | > 200 |
| NMEG1-C20 | NMEG1-ISQVNEKINWSLAFIRKSDE | 2434.7/2334.0 | > 95 | 52 | > 200 |
| C20-NMEG1 | ISQVNEKINWSLAFIRKSDE-NMEG1 | 2433.7/2432.9 | > 95 | 52 | > 200 |
| NMEG1-C20-NMEG1 | NMEG1-ISQVNEKINWSLAFIRKSDENMEG1 | 2548.8/2547.9 | > 95 | 55 | > 150 |
| NMEG1-Gly-C20 | NMEG1-G-ISQVNEKINWSLAFIRKSDE | 2491.7/2491.1 | > 90 | 55 | 81 ± 2 |
% AcN = percent acetonitrile at RP-HPLC elution of each compound.
The mean of IC50 with standard error in μM.
Biological activities of NMEGylated C20 peptides
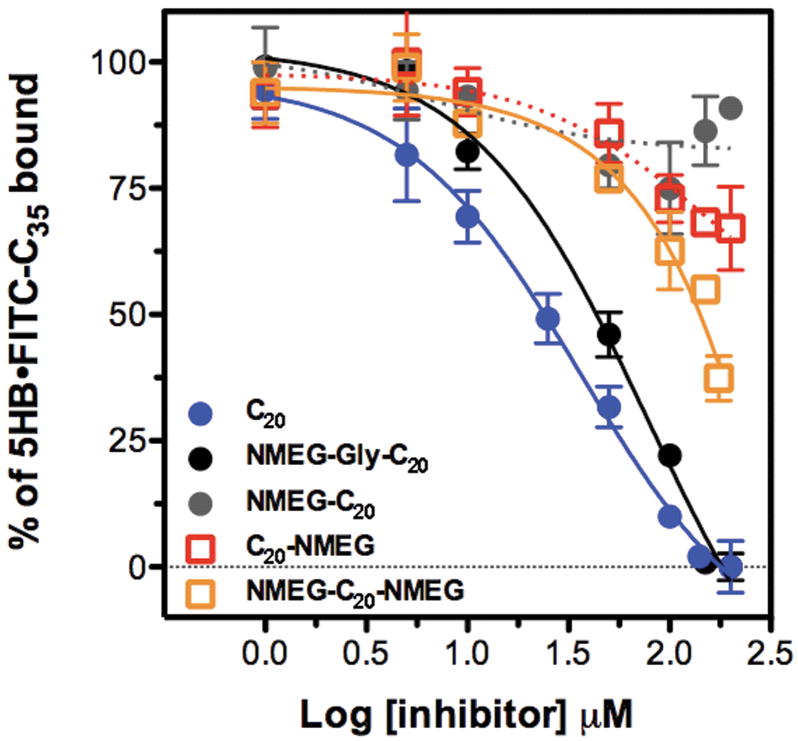
While greatly increasing peptide hydrophilicity and solubility, NMEGylation of C20 with greater numbers of NMEG monomers (n = 3, 5, and 10) led to a significant loss of binding affinity to the 5HB target protein, as determined with the use of our previously reported fluorescence polarization (FP)-based assay.39 Since the binding of the C20 peptide to the 5HB protein involves a large interface, steric hindrance of binding could be caused by the NMEG oligomers, suggesting a need to optimize the precise number of NMEG oligomers incorporated. NMEGylated C20 peptides comprising just one NMEG at either, or both, termini were prepared, and their biophysical properties and binding affinities toward the 5HB construct were evaluated. The addition of even a single NMEG to the C20 peptide greatly improved its solubility and hydrophilicity. However, the binding affinity was significantly diminished, even by just one or two terminal NMEG monomers (Figure 2 and Table I).
FIGURE 2. Date resulting from competitive FP assays.
The binding affinities of NMEGylated C20 peptide to the 5HB were determined by the use of a competitive FP assay. The “% 5HB·Fl-C35 bound” represents the capability of NMEGylated bioconjugates to displace a tracer (Fl-C35) peptide that binds to the target protein (5HB), and is presented as a function of the concentration of NMEGylated C20 peptides.
To recover the biological activity of NMEGylated C20 peptides, we decided to use an NMEG modifier attached through a flexible linker, to allow C20 enough “space” to properly form its required α-helical conformation upon binding to the 5HB. On the other hand, a linker region that is too flexible might lose too much entropy upon binding and consequently might also deleteriously affect the binding affinity. Glycine—one or a few—was chosen, because we anticipated that one glycine would provide the needed flexibility while maintaining a minimally sized peptide structure. NMEG-glycine-C20 (NMEG-Gly-C20) showed significantly tighter binding affinity to the 5HB than the rest of NMEGylated C20 analogues, with greatly improved solubility and hydrophilicity (Table I, Figure 2, and Supporting Information). Because glycine is known to be a helix breaker,40,41 it is possible that the longer glycine linker might impede the binding affinities of therapeutics to the 5HB by increasing the flexibility of the peptide backbone structure (Figure S1 and S2, Supporting Information). The benefits of NMEGylating potential biotherapeutics should increase when the NMEG attachment is “pointed” directly away from the likely binding interface. Since the C20 peptide likely forms a α-helix upon binding to the helical bundle (5HB),42 it makes sense that the direct attachment of N-substituted glycine (peptoid) monomers could disrupt the α-helix due to the presence of the N-methoxyethyl side chain.43
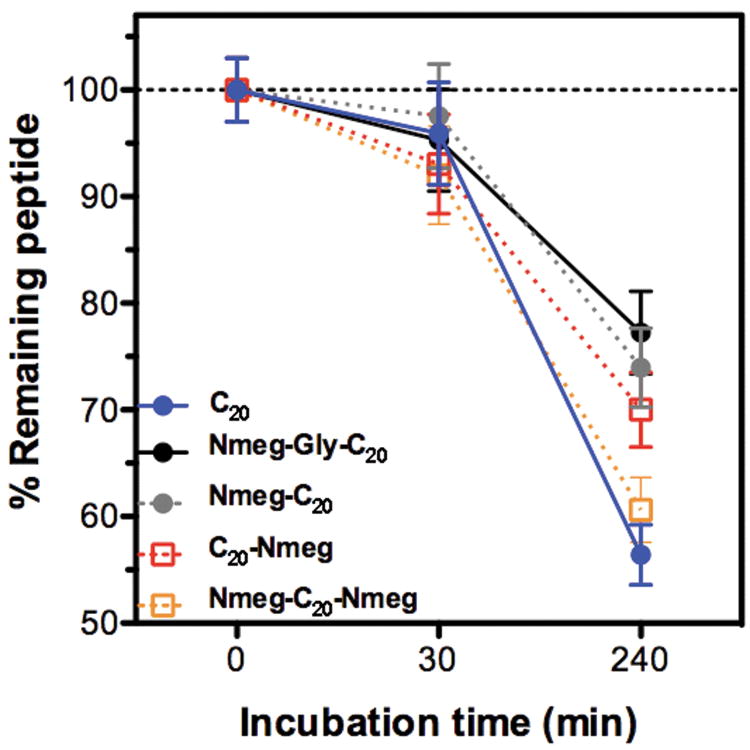
Serum stability of NMEGylated C20 peptides, and unexpected results
As discussed earlier, PEGylation is known to prolong the half-life of biotherapeutics, thereby improving in vivo efficacy. We therefore examined whether NMEGylation can provide a similar beneficial effect, focusing on singly-NMEGylated therapeutics with the added glycine spacer; this small modification proved to be sufficient to greatly improve the C20 peptide's aqueous solubility. NMEGylated C20 peptides were incubated with human serum, which contains many proteases such as trypsin and elastase, at room temperature, and then analyzed by RP-HPLC to determine the amount of remaining intact C20 peptide. Our resulting data (Figure 3), indicate that NMEGylation indeed enhances the serum stability of NMEGylated peptides as compared to the unmodified peptide. Interestingly, the protease stability of NMEG-Gly-C20 was most greatly improved, suggesting that NMEG-Gly-C20 could present comparable therapeutic efficacy to the C20 peptide, but with a prolonged half-life.
FIGURE 3. Bioconjugate stability in the presence of serum.
NMEGylated therapeutics were incubated in human plasma at 37 °C and sampled at certain time points, followed by RP-HPLC analysis. The amount of remaining peptide or peptide-peptoid conjugate at each time point was quantified against an enzyme-stable internal organic standard (benzyl alcohol).
Conclusions
In this study, we demonstrate that NMEGylation potentially enhances multiple therapeutically desirable properties including the water solubility, hydrophilicity and serum stability of a polypeptide therapeutic. Our data also suggest that if this NMEGylation approach is taken, a minor modification of the target peptide, C20, changing its molar mass by less than < 5%, can be sufficient to achieve quite favorable biophysical properties. Furthermore, optimizing the length of the mono- or oligoNMEG modifier and the inclusion of glycine linker enabled the NMEGylated bioconjugate to substantially recover the binding affinity of the unmodified peptide to its biological target. While PEGylation is still a useful technique for large therapeutic proteins, it requires post-synthetic conjugation and multiple, subsequent purification steps to remove the undesired polydisperse PEGylated products.13 By contrast, NMEGylation can be used to modify short therapeutic peptides as well as proteins, quickly yielding highly monodisperse products at lower costs. Since NMEGylation is intrinsically and quite simply compatible with solid-phase peptide/peptoid synthesis, this approach has the advantage of low cost, negligible extension of the synthesis and purification time for the peptide, and of course, site-specific incorporation as part of the synthesis protocols. NMEGylated peptide/peptoid libraries could be designed at an early stage of molecular optimization by varying both the number and position of NMEG monomers and glycine or other flexible linker units. Based on these finding, we propose that NMEGylation may prove to be a broadly useful modification of peptide and protein therapeutics.
Supplementary Material
Acknowledgments
We are grateful to the National Institutes of Health (R01-AI072666 to A.E.B. and R01-GM61050 to T.S.J.) and the Bio-X Interdisciplinary Initiatives Program at Stanford University for support. M.P. thanks Dr. Modi Wetzler (Stanford University) for insightful comments on the manuscript and Dr. Ronald N. Zuckermann (Lawrence Berkeley National Laboratory) for valuable advice.
References
- 1.Otvos L., Jr Methods Mol Biol. 2008;494:1–8. doi: 10.1007/978-1-59745-419-3_1. [DOI] [PubMed] [Google Scholar]
- 2.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Drug Discov Today. 2010;15:40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Werle M, Bernkop-Schnurch A. Amino Acids. 2006;30:351–367. doi: 10.1007/s00726-005-0289-3. [DOI] [PubMed] [Google Scholar]
- 4.Watt PM. Nat Biotechnol. 2006;24:177–183. doi: 10.1038/nbt1190. [DOI] [PubMed] [Google Scholar]
- 5.Sato AK, Viswanathan M, Kent RB, Wood CR. Curr Opin Biotechnol. 2006;17:638–642. doi: 10.1016/j.copbio.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Lien S, Lowman HB. Trends Biotechnol. 2003;21:556–562. doi: 10.1016/j.tibtech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Latham PW. Nat Biotechnol. 1999;17:755–757. doi: 10.1038/11686. [DOI] [PubMed] [Google Scholar]
- 8.Antosova Z, Mackova M, Kral V, Macek T. Trends Biotechnol. 2009;27:628–635. doi: 10.1016/j.tibtech.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Harris JM, Chess RB. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 10.Kozlowski A, Harris JM. J Control Release. 2001;72:217–224. doi: 10.1016/s0168-3659(01)00277-2. [DOI] [PubMed] [Google Scholar]
- 11.Harris JM, Martin NE, Modi M. Clin Pharmacokinet. 2001;40:539–551. doi: 10.2165/00003088-200140070-00005. [DOI] [PubMed] [Google Scholar]
- 12.Joralemon MJ, McRae S, Emrick T. Chem Commun (Camb) 2010;46:1377–1393. doi: 10.1039/b920570p. [DOI] [PubMed] [Google Scholar]
- 13.Jevsevar S, Kunstelj M, Porekar VG. Biotechnol J. 2010;5:113–128. doi: 10.1002/biot.200900218. [DOI] [PubMed] [Google Scholar]
- 14.Fee CJ, Van Alstine JM. Chemical engineering and science. 2006;61:924–939. [Google Scholar]
- 15.Grace MJ, Lee S, Bradshaw S, Chapman J, Spond J, Cox S, Delorenzo M, Brassard D, Wylie D, Cannon-Carlson S, Cullen C, Indelicato S, Voloch M, Bordens R. J Biol Chem. 2005;280:6327–6336. doi: 10.1074/jbc.M412134200. [DOI] [PubMed] [Google Scholar]
- 16.Shaunak S, Godwin A, Choi JW, Balan S, Pedone E, Vijayarangam D, Heidelberger S, Teo I, Zloh M, Brocchini S. Nat Chem Biol. 2006;2:312–313. doi: 10.1038/nchembio786. [DOI] [PubMed] [Google Scholar]
- 17.Brocchini S, Godwin A, Balan S, Choi JW, Zloh M, Shaunak S. Adv Drug Deliv Rev. 2008;60:3–12. doi: 10.1016/j.addr.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Perrino C, Lee S, Choi SW, Maruyama A, Spencer ND. Langmuir. 2008;24:8850–8856. doi: 10.1021/la800947z. [DOI] [PubMed] [Google Scholar]
- 19.Han JO, Joo MK, Jang JH, Park MH, Jeong B. Macromolecules. 2009;42:6710–6715. [Google Scholar]
- 20.Schellenberger V, Wang CW, Geething NC, Spink BJ, Campbell A, To W, Scholle MD, Yin Y, Yao Y, Bogin O, Cleland JL, Silverman J, Stemmer WP. Nat Biotechnol. 2009;27:1186–1190. doi: 10.1038/nbt.1588. [DOI] [PubMed] [Google Scholar]
- 21.Patch JA, Kirshenbaum K, Seurynck SL, Zuckermann RN, Barron AE. Pseudopeptides in Drug Development. Wiley-VCH; Weinheim, Germany: 2004. [Google Scholar]
- 22.Murphy JE, Uno T, Hamer JD, Cohen FE, Dwarki V, Zuckermann RN. Proc Natl Acad Sci U S A. 1998;95:1517–1522. doi: 10.1073/pnas.95.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. Proc Natl Acad Sci U S A. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen JT, Porter M, Amoui M, Miller WT, Zuckermann RN, Lim WA. Chem Biol. 2000;7:463–473. doi: 10.1016/s1074-5521(00)00130-7. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen JT, Turck CW, Cohen FE, Zuckermann RN, Lim WA. Science. 1998;282:2088–2092. doi: 10.1126/science.282.5396.2088. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann J, Kuhne R, Volkmer-Engert R, Jarchau T, Walter U, Oschkinat H, Ball LJ. J Biol Chem. 2003;278:36810–36818. doi: 10.1074/jbc.M305934200. [DOI] [PubMed] [Google Scholar]
- 27.Burkoth TS, Fafarman AT, Charych DH, Connolly MD, Zuckermann RN. J Am Chem Soc. 2003;125:8841–8845. doi: 10.1021/ja0352101. [DOI] [PubMed] [Google Scholar]
- 28.Zuckermann RN, Kerr JM, Kent SBH, Moos WH. J Am Chem Soc. 1992;114:10646–10647. [Google Scholar]
- 29.Statz AR, Meagher RJ, Barron AE, Messersmith PB. J Am Chem Soc. 2005;127:7972–7973. doi: 10.1021/ja0522534. [DOI] [PubMed] [Google Scholar]
- 30.Haynes RD, Meagher RJ, Won JI, Bogdan FM, Barron AE. Bioconjug Chem. 2005;16:929–938. doi: 10.1021/bc0496915. [DOI] [PubMed] [Google Scholar]
- 31.Miller SM, Simon RJ, Ng S, Zuckermann RN, Kerr JM, Moos WH. Drug Dev Res. 1995;35:20–32. [Google Scholar]
- 32.Miller SM, Simon RJ, Ng S, Zuckermann RN, Kerr JM, Moos WH. Bioorg Med Chem Lett. 1994;4:2657–2662. [Google Scholar]
- 33.Park M, Matsuura H, Lamb RA, Barron AE, Jardetzky TS. Analytical Biochemistry. 2011;409:195–201. doi: 10.1016/j.ab.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker JM, Guo D, Hodges RS. Biochemistry. 1986;25:5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- 35.Sereda TJ, Mant CT, Sonnichsen FD, Hodges RS. J Chromatogr A. 1994;676:139–153. doi: 10.1016/0021-9673(94)00371-8. [DOI] [PubMed] [Google Scholar]
- 36.Scanlon D, Harris KS, Coley AM, Karas JA, Casey JL, Hughes AB, Foley M. International Journal of Peptide Research and Therapeutics. 2008;14:381–386. [Google Scholar]
- 37.Matthews JM, Young TF, Tucker SP, Mackay JP. J Virol. 2000;74:5911–5920. doi: 10.1128/jvi.74.13.5911-5920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller SM, Simon RJ, Ng S, Zuckermann RN, Kerr JM, Moos WM. Drug Dev Res. 1995:20–32. [Google Scholar]
- 39.Park M, Matsuura H, Lamb RA, Barron AE, Jardetzky TS. Analytical Biochemistry. 2010;409:195–201. doi: 10.1016/j.ab.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakrabartty A, Kortemme T, Baldwin RL. Protein Sci. 1994;3:843–852. doi: 10.1002/pro.5560030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Javadpour MM, Eilers M, Groesbeek M, Smith SO. Biophys J. 1999;77:1609–1618. doi: 10.1016/S0006-3495(99)77009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao X, Singh M, Malashkevich VN, Kim PS. Proc Natl Acad Sci U S A. 2000;97:14172–14177. doi: 10.1073/pnas.260499197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fowler SA, Blackwell HE. Org Biomol Chem. 2009;7:1508–1524. doi: 10.1039/b817980h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.