Abstract
Integrin-linked kinase 1 (ILK1) is a serine/threonine kinase that plays important roles in a variety of cellular functions including cell survival, migration and angiogenesis. ILK1 is normally expressed in numerous tissues and activated by growth factors, cytokines and hormones. Dysregulation of ILK1 expression or function is found in several hormonal tumors including breast, ovary and prostate. Emerging evidence suggests that ILK overexpression promotes cellular transformation, cell survival, epithelial mesenchymal transition (EMT), and metastasis of hormonal cancer cells while inhibition of ILK1 reduces tumor growth and progression. The recent development of ILK1 inhibitors has provided novel mechanisms for blocking ILK1 signaling to curb metastasis and therapy resistance of hormonal tumors. This review will focus on recent advances made towards understanding the role of ILK signaling axis in progression of hormonal cancer.
Keywords: Estrogen receptor, hormonal signaling, therapy resistance, integrin linked kinase, EMT, metastasis, AKT, ErbB2
2. INTRODUCTION
ILK1 is a 59 kDa cytoplasmic protein that contains three distinct domains: (a) a phosphoinositide phospholipid-binding domain that mediates phosphoinositide binding, (b) an N-terminal ankyrin repeat domain that facilitates protein interactions and (c) a C-terminal serine/threonine protein kinase domain (1). ILK1 is a serine/threonine kinase that was first discovered as an integrin-binding protein in a yeast two-hybrid screen (2). It is able to directly activate several signaling pathways downstream of integrins and to participate in integrin signaling crosstalk with growth factors and hormones (3, 4). Substrates of the ILK1 include integrin β1 (2), myosin light chain (MLC) (5), protein kinase B /Akt (AKT) and Glycogen synthase kinase 3 (GSK-3) (6). ILK1 is a unique kinase because it also functions as an intracellular adaptor protein, coupling a wide variety of signaling proteins to integrin and growth factor signaling. ILK interacting proteins include Pinch (7), Paxillin (8), Parvins (9), Affixin (10), ILK BP (11), p21 activated kinase 1 (Pak1) (12) and estrogen receptor (ER) (13). Physiological signals including growth factors (6), cytokines (14, 15) estrogen (16), and the Wnt pathway (17) can activate ILK. Direct regulators of ILK include phosphoinositide 3-kinase (PI3K) (6), phosphatase and tensin homolog (PTEN) (18), protein phosphatase 2C (19), ILKAP (20) and secreted protein acidic and rich in cysteine (SPARC) (21). Accumulating evidence implicates ILK1 as a potential oncogene modulating several signaling pathways for cancer cell survival and tumor progression (22). Here, we will summarize key evidence for ILK signaling in hormonal tumor progression and discuss the possibility of the ILK1 axis as a possible therapeutic target for hormonal cancers.
3. DISCUSSION
3.1. ILK1 signaling and tumorigenesis
ILK1 signaling axis is implicated in many key signaling pathways that are activated in tumor cells promoting anchorage independence, motility, apoptosis, angiogenesis, EMT and tumor progression (22). Overexpression of ILK in epithelial cells enables anchorage-independent growth and survival of tumor cells (22–24) and tumorigenicity in nude mice (25). ILK1 overexpression in prostate cancer cells can suppress anoikis, promote anchorage-independence, and induce tumorigenesis (22). Accordingly, inhibition of ILK1 in prostatic adenocarcinoma (CaP) cells elicits cell cycle arrest and induces apoptosis (26).
Compared to normal cells, breast cancer cells appear to be preferentially dependent on the ILK1 signaling for survival (27). ILK1 function is also required for cytokine osteopontin (OPN)-induced AKT activation and for prostate cancer cell survival (28). Rictor, a known regulator of cytoskeletal dynamics, interacts with ILK1 to promote AKT phosphorylation leading to cell survival in cancer cells (29, 30). Transgenic mice expressing ILK1 in the mammary epithelium (MMTV-ILK1) develop a hyperplastic mammary phenotype and focal mammary tumors (31). These results provide strong evidence for an in vivo oncogenic role for ILK1 (31). Given the focal nature and long latency of the tumors, additional genetic events are likely required for tumor induction in MMTV-ILK1 mice.
ILK1-mediated AKT Ser473 phosphorylation may be celltype and context dependent. For example, genetic studies in Drosophila Melanogaster and Caenorhabditis elegans show AKT phosphorylation on Ser473 was not affected in ILK 1mutants (32, 33) and levels of Ser473 phosphorylation on AKT were equivalent in ILK1-null and wild-type mouse fibroblasts (34). The majority of analyses using tumor cells indicate that AKT Ser473 phosphorylation is dependent on a functional ILK1 axis; hence, the ILK1 pathway may be important during epithelial tumor progression presumably by promoting cell survival. In breast cancer cells, inhibition of ILK1 activity results in a decrease in AKT Ser473 phosphorylation and induction of apoptosis; whereas inhibition of ILK1 in normal cells has no such effects. These findings suggest that ILK1 promotes survival function uniquely in breast cancer cells. ILK1 targeted treatment using specific ILK1 inhibitors may therefore have potential to reduce side effects in cancer patients (27).
Evidence also implicates ILK1 in regulating tumor angiogenesis; ILK1 increases vascular endothelial growth factor (VEGF), modulate levels of hypoxia inducible factor (HIF1α) and promote cell migration, blood-vessel formation and tumor growth of VEGF-treated endothelial cells (35, 36). In ovarian cancer cells, ILK1 serves as a key mediator in transforming growth factor (TGF) β1 regulation of uPA/PAI-1 system, which is critical for the invasiveness of human ovarian cancer cells (37). ILK1 promotes epithelial to mesochymal transformation (EMT) of cancer cells by modulating β-catenin/TCF, Snail and TGFβ pathways (38–40). Collectively, these evolving findings indicate ILK1 signaling has the potential to activate multiple signaling pathways that contribute to the growth advantage of cancer cells.
3.2. Expression of ILK in hormonal cancers
While ILK1 is normally expressed in many hormonal tissues, emerging evidence implicates dysregulation of ILK1 expression and/or activity in many cancers including those of the breast, prostate and ovary (22). ILK1 expression increases as ovarian tumor grade and its expression can be sustained by peritoneal tumor fluid (PTF). PTF-induced over-expression of ILK correlates with the activation of the AKT pathway (41). Thus, ILK1 has potential to serve as a biological marker for early detection and a therapeutic target for ovarian cancer (41).
One study found that serum from ovarian cancer patients contains cell-free immunoreactive ILK1 at statistically elevated levels compared to controls without ovarian cancer (42); ILK1 was present at elevated levels in both the serum and PTF of ovarian cancer patients. The correlation between ILK1 expression with CA125 concentration in these biological fluids suggests a potential role of ILK1 as a serological ovarian tumor marker for early detection and treatment monitoring (43). Integrin alphavbeta3 upregulates ILK1 expression in human ovarian cancer cells via enhancement of ILK1 gene transcription. Mechanistic studies show that transcription factor Ets contributes to alphavbeta3-mediated ILK1 upregulation. By increasing ILK1 as an important integrin-proximal kinase, alphavbeta3 may promote its intracellular signaling and tumor biological processes (42).
ILK1 mRNA is upregulated in prostate adenocarcinoma cells compared to normal epithelial cells and therefore, can be a useful internal reference gene marker (44). ILK1 expression also increases with prostate tumor grade and is specifically associated with the increased proliferative index that typifies CaP progression. Further, enhanced ILK1 expression is inversely related to 5-year patient survival linking increased ILK1 expression in prostate tumor progression (26). b-parvin (ParvB) is an adaptor protein that binds to the ILK1. Expression studies indicated ParvB expression was down regulated in breast tumors compared to ParvB expression in patient-matched normal mammary gland tissue. These results suggest that loss of ParvB expression could be a mechanism for upregulating ILK1 activity in tumors (9).
3.3. Role of ILK1 in cancer cell metastasis
Metastasis is a frequent and fatal culmination to hormone-sensitive cancers, particularly of ovarian origin. Acquisition of invasive and migratory characteristics in cancer cells results primarily from adopting an EMT phenotype. This phenotype is supported by various prometastatic factors. Emerging studies have unraveled the role of ILK1 in governing metastatic features in various cancers, predominantly for acquiring the mesenchymal phenotype and promoting cell invasion through increased expression of various matrix-degrading proteases.
Increased expression of ILK1 correlates significantly with higher grade of ovarian tumors (41, 43). ILK1 played a predominant role in endothelin-1 (ET-1/ETAR)–induced EMT and in the development of the invasive phenotype in ovarian cancer by increasing levels of Snail, stabilizing beta-catenin and suppressing E-cadherin expression through a PI3K-dependent signaling pathway. Also, enhanced expression and activity of matrix metalloproteinases (MMP-2 and MMP-9) mediated by ET-1 correlated with increased ILK expression (45). In addition, PI3K-ILK1 axis played a critical role in TGF β1-mediated invasive phenotype in ovarian epithelial cancer cells via up-regulation of urokinase-type plasminogen activator (uPA) and PA inhibitor 1 (PAI-1). It is worthwhile to note that expression of uPA and PAI-1 were reported to correlate with advanced stages of ovarian cancer (46). Unlike ET-1, the TGF β1-mediated increase in MMP-2 expression was found to be independent of ILK1 signaling (37). Similarly, Y-box-binding protein 1 (YB-1), a known, poor prognostic marker of ovarian cancer, localized in the nucleus and enhanced CXCR4 expression for acquiring the malignant phenotype (47). Interestingly, siRNA depletion of ILK1 and AKT affects both nuclear translocation of YB-1 and expression of CXCR4 in ovarian cancer cells, suggesting that disrupting ILK1-AKT pathway can be used to block YB-1–mediated metastasis (48).
One of the earliest insights into the potential of ILK1 to govern the metastatic phenotype came from the Dedhar lab. They demonstrated that stable overexpression of ILK1 in scp2 murine mammary gland epithelial cell lines induced the classic EMT phenotype including reduction in E-cadherin along with translocation of β-catenin and formation of β-catenin/LEF complex inside the nucleus and thus upregulating expression of various mesenchymal genes (49). Similarly, Somasiri et al., found that forced exogenous expression of wild type ILK1 but not the dominant negative kinase-dead version of ILK in scp2 murine mammary epithelial cell lines induced the EMT phenotype via reduction in E-Cadherins and acquisition of vimentin filaments (39). Suppression of anoikis, a unique process of apoptosis resulting from insufficient cell-matrix adhesion, appears to be an important event in the development of metastasis (50). Studies using both scp2 murine mammary cell lines and human breast cancer cell lines implicated ILK1 as a suppressor of anoikis (24). Inhibition of cell death/anoikis by ILK overexpression supports the idea that ILK is a predominant player in regulating the emergence of the metastatic phenotype. Subsequent to these studies, ILK-mediated induction of the invasive phenotype in mammary epithelial cells was found to be associated with increased MMP-9 expression. This increase in MMP-9 proteins was attributed to ILK-mediated activation of GSK-3beta and AP-1 transcription factor (51). The ILK1-AP1 axis was further shown to contribute to the invasive phenotype in mammary gland epithelial cells mediated by osteopontin (OPN), a metastasis-associated glyco-phosphoprotein. Using the murine metastatic mammary epithelial cells 4T1, Mi et al., demonstrated that an OPN-mediated increase in expression of MMP2 and uPA can be attributed to ILK1-dependent AP-1 activation (14). Transgenic mice specifically expressing ILK in mammary glands had increased mesenchymal-like cell populations within their tumors, suggesting that stand-alone ILK1 overexpression can initiate the EMT phenotype (31). Another piece of evidence connecting ILK1 to anoikis is from a study that demonstrated the role of the tumor suppressor DOC-2/hDab-2 in the induction of anoikis in breast cancer cells. DOC-2 was shown to induce anoikis by down regulating ILK activity but this activity was found to be independent of the PI3K/AKT and MAPK pathways, suggesting that ILK1 may utilize alternate pathways to suppress anoikis and promote anchorage independence (52). Estrogen-mediated extranuclear functions are also shown to activate ILK1. Since PELP1 expression is upregulated in metastatic breast cancer (53); modulation of the ILK1 pathway by PELP1 may represent a potential mechanism by which estrogen signaling promotes metastasis in breast cancer cells (54).
Relatively fewer studies have been done to elucidate the role of ILK1 in metastatic prostate, cervical and endometrial cancers. A recent study using prostate cancer cell lines implicated ILK1 as a downstream effector for talin1-mediated resistance to anoikis (55). It is worthy to note that talin1 was found to be overexpressed in metastatic prostate cancer and tumors with a high Gleason score when compared to its expression in normal Gleason scores and benign tumors (55). PI3K/AKT-dependent anoikis has also been found in endometrial cancer cell lines but the role of ILK1 in governing this anoikis has not yet been demonstrated (56). Similarly, Notch1-Rhoc axis and anoikis are reported to regulate the metastatic potential in cervical cancer progression but the role of ILK1 in these cancers remains elusive (57).
3.4. ILK and ER signaling crosstalk
Several lines of evidence implicate ILK1 axis crosstalk with ER signaling. One of the lacunae in our understanding of the mechanistic details of the metastatic evolution of these hormonal cancers and how hormones like estrogen and their respective steroid receptors regulate ILK1 pathway. Dr. Kumar’s group provided first evidence of estrogen receptor (ER)-ILK crosstalk. They showed a direct interaction of ER with ILK1 and that the interaction occurred through the nuclear receptor box (i.e., LXXLL) located between the ILK1 pleckstrin homology–like domain and the ankyrin repeats (58). In addition, we recently identified ILK1 as a novel interacting protein of PELP1, an ER-coregulator protein (54). Our study demonstrated that ILK functions as a downstream effector of ER extranuclear signaling, leading to cytoskeleton reorganization. These extranuclear actions of estrogen facilitated activation of the ILK enzyme via the PI3K pathway and inhibition of ILK functions significantly affected the estrogen-mediated cell migratory potential. The proposed signaling pathway is E2>PELP1>PI3K>ILK>CDC42 and it may contribute to estrogen mediated cytoskeleton changes (54). Earlier evidence suggests that the ILK1 axis is a major signaling node that links integrins and growth factor signaling to a variety of cellular responses. The ability of ILK1 to interact with ER and growth factor/integrin signaling components suggests that deregulation of ILK has the potential to promote ER growth factor crosstalk and thus a potential to contribute to therapy resistance.
3.5. ILK1 and hormonal therapy resistance
Deregulation of human epidermal growth factor receptor 2 (ErbB2) expression and /or signaling has emerged as the most significant factor in the development of hormonal resistance (59). ErbB2 is an oncogene that has been shown to be over expressed, amplified, or both, in several human malignancies including breast tumors. ER expression occurs in ~50% ErbB2 positive breast cancers and crosstalk between the ER and ErbB2 pathways promotes endocrine therapy resistance (58, 59). Disruption of ILK expression by siRNA or inhibition of ILK1 function in ErbB2-expressing cells with a small molecule inhibitor resulted in a profound block in invasive properties resulting from the induction of apoptotic cell death. These observations support the concept of ILK1 having a critical role in the initiation phase of ErbB2 tumor induction (61).
AKT signaling plays an important role in the development of hormonal therapy resistance (62). Many hormonal tumors exhibit an increase in constitutively active AKT; however, mutations in AKT are rare in breast tumors (62). Therefore, proteins contributing to AKT activation may play a role in the development of therapy resistance. In this context, several lines of evidence indicate that ILK is a receptor-proximal effector for the PI3K-dependent, extracellular matrix- and growth factor-mediated activation of PKB/AKT and inhibition of GSK-3 (6). Since ILK1 expression is deregulated in hormonal cancer, increases in ILK1 signaling has the potential to contribute to therapy resistance.
Nuclear localization of PAK1, a proto-oncogene (63), is associated with the progressive limitation of tamoxifen sensitivity and implicated in development hormonal therapy resistance (64). ILK1 is a PAK1 substrate, and undergoes phosphorylation-dependent shuttling between the cell nucleus and cytoplasm, and interacts with gene-regulatory chromatin, thus ILK1-PAK1 interactions may have a role in therapy resistance (12).
Cyclin D1 overexpression commonly occurs in breast cancer. The level of cyclin D1 expression and activated STAT3 are important markers to predict response to tamoxifen treatment (65). ILK1 signaling increases cyclin D1 protein levels (65). Mechanistic studies showed that ILK-induced CREB transactivation and CREB binding to the cyclin D1 promoter CRE led to cyclin D activation. Wnt-1, an oncogene implicated in mammary tumorigenesis also induced cyclin D1 mRNA via ILK pathway (65).
ER-coregulators play an essential role in hormonal therapy responsiveness and cancer progression (67). Recent findings suggest that ILK1 interacts with ER-coregulator PELP1 (54) and that such interactions enhance ILK1-kinase activity. Since PELP1 expression is commonly deregulated in many hormone-responsive tissues (16), the PELP1-ILK1 interaction is likely to have significant implications in tumor cell survival and therapy resistance.
3.6. Therapeutic potential of targeting ILK in hormonal cancers
Current strategies to block the ILK1-mediated phenotype using in vitro systems include the usage of small molecular inhibitors (like KP-392/KP-SD-1 and QLT-0267) (27, 57, 68) and ILK-targeted siRNAs and antisense oligonucleotides (69). Also, the use of a dominant negative ILK (ILK-E359K) has been proposed. Initial work towards generating potent small molecule inhibitors against ILK1 was accomplished by Dedhar and colleagues in collaboration with Kinetek Pharmaceuticals (now a part of QLT Inc). As a result the KP-392/KP-SD-1 and KP-SD-2 compounds were identified and shown to inhibit ILK-mediated AKT activation and the EMT phenotype when tested using in vitro model cells. The use of KP-SD-1 was highly encouraging when tested on human colon carcinoma cells using an in vivo xenograft transplantation assay (70). QLT-0267 is a second-generation ILK1 inhibitor that is more potent with increased sensitivity over the parental KP-392 compound (27). Interestingly, QLT-2067 induces apoptosis in the breast cancer cell lines MDA-MB-231, MDA-MB-435, BT-549, and MDA-MB-468, but not in normal human breast epithelial cells at a concentration of 10 µmol/L or less.
Some evidence indicates blockage of ILK1 signaling along with conventional chemotherapy may be beneficial. QLT-0267 in combination with docetaxel exhibited synergistic effects on reducing the viability of various breast cancer cells (68). A remarkable observation made in this study was that the ErbB2 status of cells has a definitive effect on this combinatorial treatment. Low ErbB2-expressing cells were more sensitive to this combination when reduction of phospho-AKT was used as endpoint for assessing the efficacy. Further, this combination was found to be more effective than the single treatment in reducing the tumor burden and prolonging survival in an orthotopic breast cancer model using transplanted LCC6 cells, which have reduced ErbB2 expression. Similarly, the ILK1 inhibitor KP-307-2, an analog of KP-392, was found to suppress tumorigenesis in xenograft tumor models using the prostate cancer cell line PC3. Surprisingly, a novel feedback mechanism between ILK and VEGF expression was also observed in this study and therefore treatment with ILK1 inhibitor causes a ‘double jeopardy’ situation in the cells by causing inhibition of tumorigenesis and suppressing angiogenesis (35).
The second generation ILK1 inhibitor QLT-0267 may be useful in radiosensitizing cancer cells, particularly squamous cell carcinoma cells of head and neck and also, engenders the possibility of similar effect on gynecologic cervical carcinoma that is predominantly squamous cell carcinoma upon histological type (71). Overall, it appears that ILK1 inhibitors, although at various levels of development, have the potential to down regulate ILK1 activity, the ILK1-mediated EMT phenotype and tumorigenesis when tested using in vitro and various preclinical mouse models.
4. FUTURE PERSPECTIVES
In summary, the data reviewed herein provides support for the following conclusions:(a) deregulation of ILK1 expression and/or functions occurs in human hormonal cancers; (b) inhibition of ILK1 correlates with delayed tumor growth in preclinical models; (c) ILK1 can modulate key signaling pathways including cell survival, tumor growth, angiogenesis, EMT and metastasis; and (d) ILK1 crosstalk with various signaling pathways that are commonly deregulated in hormonal cancers including ErbB2, PAK1 and ER (Figure 1). Thus, these data strongly support a role of ILK1 in the hormonal cancer progression. The recent availability of drugs that specifically target ILK1 has begun to open up new avenues for targeting hormonal tumors. Most interesting is the difference in sensitivity to the effects of ILK inhibition between normal breast epithelial and breast cancer cells, provide a potential for the use of ILK1 inhibitors in patient therapy. Future studies using combination of ILK inhibitors with drugs that target hormone therapy could possibly be done to achieve significant reduction in various hormonal cancers. Future studies are warranted to identify the signaling pathways that regulate ILK1 expression in hormonal cancers and to examine the prognostic / diagnostic significance of ILK1 using larger number of tumor samples. A better understanding of the ILK1 signaling and its crosstalk with hormonal signaling components is expected to assist in the development of an integrated model for targeting ILKs in the management of hormone-driven tumors.
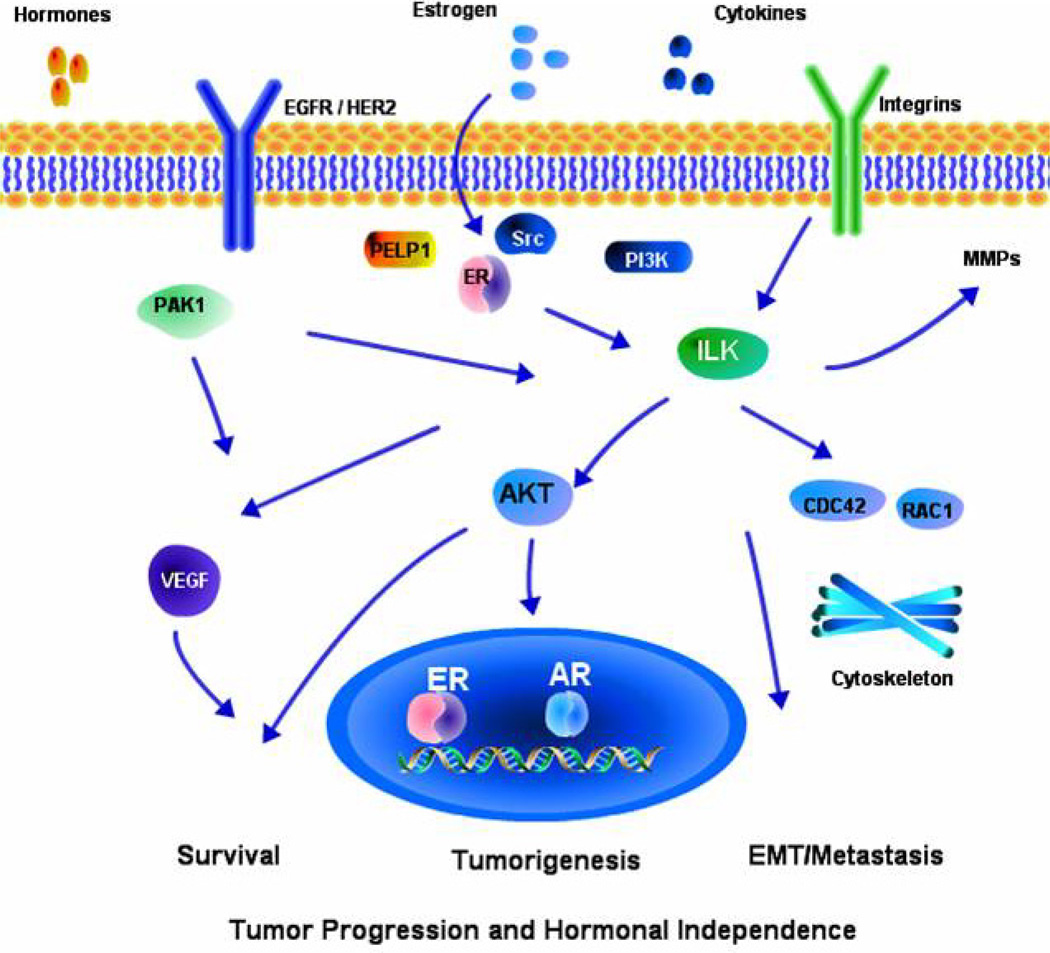
Figure 1.
Schematic representation of the current understanding of ILK1 signaling crosstalk with pathways that are commonly deregulated in hormonal cancers. Crosstalk of ILK axis with the estrogen receptor, ERBB2, Pak1 signaling pathways suggest that deregulation of ILK expression and/or function is likely to contribute to the hormonal cancer progression and development of therapy resistance.
ACKNOWLEDGEMENTS
This work was supported by grants from the DOD W81XWH-08-1-0604 (RKV), NIH pre-doctoral fellowship CA095681 (VC), and DOD Pre-doctoral Fellowship W81XWH-09-1-0010 (BCN), Susan G. Komen post-doctoral fellow ship KG091267 (DC).
Abbreviations
- AKT
protein kinase B
- EMT
epithelial mesenchymal transition
- ErbB2
epidermal growth factor receptor2
- ER
estrogen receptor
- ILK1
integrin linked kinase 1
- Pak1
p21 activated kinase 1
- PI3K
phosphotidyl inositol 3 kinase
- TGF
transforming frowth factor
REFERENCES
- 1.Persad S, Dedhar S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 2003;22:375–384. doi: 10.1023/a:1023777013659. [DOI] [PubMed] [Google Scholar]
- 2.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 3.Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth-factor signalling. Trends Cell Biol. 1999;9:319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- 4.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase--essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 5.Deng JT, Van Lierop JE, Sutherland C, Walsh MP. Ca2+-independent smooth muscle contraction. a novel function for integrin-linked kinase. J Biol Chem. 2001;276:16365–16373. doi: 10.1074/jbc.M011634200. [DOI] [PubMed] [Google Scholar]
- 6.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu Y, Li F, Goicoechea S, Wu C. The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol. 1999;19:2425–2434. doi: 10.1128/mcb.19.3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolopoulos SN, Turner CE. Integrin-linked kinase (ILK) binding to paxillin LD1 motif regulates ILK localization to focal adhesions. J Biol Chem. 2001;276:23499–23505. doi: 10.1074/jbc.M102163200. [DOI] [PubMed] [Google Scholar]
- 9.Mongroo PS, Johnstone CN, Naruszewicz I, Leung-Hagesteijn C, Sung RK, Carnio L, Rustgi AK, Hannigan GE. Beta-parvin inhibits integrin-linked kinase signaling and is downregulated in breast cancer. Oncogene. 2004;23:8959–8970. doi: 10.1038/sj.onc.1208112. [DOI] [PubMed] [Google Scholar]
- 10.Yamaji S, Suzuki A, Sugiyama Y, Koide Y, Yoshida M, Kanamori H, Mohri H, Ohno S, Ishigatsubo Y. A novel integrin-linked kinase-binding protein, affixin, is involved in the early stage of cell-substrate interaction. J Cell Biol. 2001;153:1251–1264. doi: 10.1083/jcb.153.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tu Y, Huang Y, Zhang Y, Hua Y, Wu C. A new focal adhesion protein that interacts with integrin-linked kinase and regulates cell adhesion and spreading. J Cell Biol. 2001;153:585–598. doi: 10.1083/jcb.153.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acconcia F, Barnes CJ, Singh RR, Talukder AH, Kumar R. Phosphorylation-dependent regulation of nuclear localization and functions of integrin-linked kinase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6782–6787. doi: 10.1073/pnas.0701999104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acconcia F, Manavathi B, Mascarenhas J, Talukder AH, Mills G, Kumar R. An inherent role of integrin-linked kinase-estrogen receptor alpha interaction in cell migration. Cancer Res. 2006;66:11030–11038. doi: 10.1158/0008-5472.CAN-06-2676. [DOI] [PubMed] [Google Scholar]
- 14.Mi Z, Guo H, Wai PY, Gao C, Kuo PV. Integrin-linked kinase regulates osteopontin-dependent MMP-2 and uPA expression to convey metastatic function in murine mammary epithelial cancer cells. Carcinogenesis. 2006;27:1134–1145. doi: 10.1093/carcin/bgi352. [DOI] [PubMed] [Google Scholar]
- 15.Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H, Manabe T. Integrin-linked kinase activity is associated with interleukin-1 alpha-induced progressive behavior of pancreatic cancer and poor patient survival. Oncogene. 2006;25:3237–3246. doi: 10.1038/sj.onc.1209356. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarty D, Tekmal RR, Vadlamudi RK. PELP1: A novel therapeutic target for hormonal cancers. IUBMB Life. 2010;62:162–169. doi: 10.1002/iub.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oloumi A, Syam S, Dedhar S. Modulation of Wnt3a-mediated nuclear beta-catenin accumulation and activation by integrin-linked kinase in mammalian cells. Oncogene. 2006;25:7747–7757. doi: 10.1038/sj.onc.1209752. [DOI] [PubMed] [Google Scholar]
- 18.Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J, Dedhar S. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA. 2000;97:3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung-Hagesteijn C, Mahendra A, Naruszewicz I, Hannigan GE. Modulation of integrin signal transduction by ILKAP, a protein phosphatase 2C associating with the integrin-linked kinase, ILK1. EMBO J. 2001;20:2160–2170. doi: 10.1093/emboj/20.9.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar AS, Naruszewicz I, Wang P, Leung-Hagesteijn C, Hannigan GE. ILKAP regulates ILK signaling and inhibits anchorage-independent growth. Oncogene. 2004;23:3454–3461. doi: 10.1038/sj.onc.1207473. [DOI] [PubMed] [Google Scholar]
- 21.Weaver MS, Workman G, Sage EH. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin beta1 and activation of integrin-linked kinase. J Biol Chem. 2008;283:22826–22837. doi: 10.1074/jbc.M706563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 23.Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J Biol Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- 24.Attwell S, Roskelley C, Dedhar S. The integrin-linked kinase (ILK) suppresses anoikis. Oncogene. 2000;19:3811–3815. doi: 10.1038/sj.onc.1203711. [DOI] [PubMed] [Google Scholar]
- 25.Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA, Dedhar S. Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression, and tumorigenicity. J Biol Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- 26.Graff JR, Deddens JA, Konicek BW, Colligan BM, Hurst BM, Carter HW, Carter JH. Integrin-linked kinase expression increases with prostate tumor grade. Clin Cancer Res. 2001;7:1987–1991. [PubMed] [Google Scholar]
- 27.Troussard AA, McDonald PC, Wederell ED, Mawji NM, Filipenko NR, Gelmon KA, Kucab JE, Dunn SE, Emerman JT, Bally MB, Dedhar S. Preferential dependence of breast cancer cells versus normal cells on integrin-linked kinase for protein kinase B/Akt activation and cell survival. Cancer Res. 2006;66:393–403. doi: 10.1158/0008-5472.CAN-05-2304. [DOI] [PubMed] [Google Scholar]
- 28.Robertson BW, Chellaiah MA. Osteopontin induces beta-catenin signaling through activation of Akt in prostate cancer cells. Exp. Cell Res. 2010;316:1–11. doi: 10.1016/j.yexcr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 30.McDonald PC, Oloumi A, Mills J, Dobreva I, Maidan M, Gray V, Wederell ED, Bally MB, Foster LJ, Dedhar S. Rictor and integrin-linked kinase interact and regulate Akt phosphorylation and cancer cell survival. Cancer Res. 2008;68:1618–1624. doi: 10.1158/0008-5472.CAN-07-5869. [DOI] [PubMed] [Google Scholar]
- 31.White DE, Cardiff RD, Dedhar S, Muller WJ. Mammary epithelial-specific expression of the integrin-linked kinase (ILK) results in the induction of mammary gland hyperplasias and tumors in transgenic mice. Oncogene. 2001;20:7064–7072. doi: 10.1038/sj.onc.1204910. [DOI] [PubMed] [Google Scholar]
- 32.Zervas CG, Gregory SL, Brown NH. Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J Cell Biol. 2001;152:1007–1018. doi: 10.1083/jcb.152.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackinnon AC, Qadota H, Norman KR, Moerman DG, Williams BD. C. elegans PAT-4/ILK functions as an adaptor protein within integrin adhesion complexes. Curr Biol. 2002;12:787–797. doi: 10.1016/s0960-9822(02)00810-2. [DOI] [PubMed] [Google Scholar]
- 34.Sakai T, Li S, Docheva D, Grashoff C, Sakai K, Kostka G, Braun A, Pfeifer A, Yurchenco PD, Fassler R. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan C, Cruet-Hennequart S, Troussard A, Fazli L, Costello P, Sutton K, Wheeler J, Gleave M, Sanghera J, Dedhar S. Regulation of tumor angiogenesis by integrin-linked kinase (ILK) Cancer Cell. 2004;5:79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko Y, Kitazato K, Basaki Y. Integrin-linked kinase regulates vascular morphogenesis induced by vascular endothelial growth factor. J. Cell Sci. 2004;117:407–415. doi: 10.1242/jcs.00871. [DOI] [PubMed] [Google Scholar]
- 37.Lin SW, Ke FC, Hsiao PW, Lee PP, Lee MT, Hwang JJ. Critical involvement of ILK in TGFbeta1-stimulated invasion/migration of human ovarian cancer cells is associated with urokinase plasminogen activator system. Exp Cell Res. 2007;313:602–613. doi: 10.1016/j.yexcr.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Barbera MJ, Puig I, Dominguez D, Julien-Grille S, Guaita-Esteruelas S, Peiro S, Baulida J, Franci C, Dedhar S, Larue L, Garcia de HA. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 39.Somasiri A, Howarth A, Goswami D, Dedhar S, Roskelley CD. Overexpression of the integrin-linked kinase mesenchymally transforms mammary epithelial cells. J Cell Sci. 2001;114:1125–1136. doi: 10.1242/jcs.114.6.1125. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed N, Riley C, Oliva K, Stutt E, Rice GE, Quinn MA. Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J Pathol. 2003;201:229–237. doi: 10.1002/path.1441. [DOI] [PubMed] [Google Scholar]
- 42.Lossner D, Abou-Ajram C, Benge A, Aumercier M, Schmitt M, Reuning U. Integrin alphavbeta3 upregulates integrin-linked kinase expression in human ovarian cancer cells via enhancement of ILK gene transcription. J. Cell Physiol. 2009;220:367–375. doi: 10.1002/jcp.21774. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed N, Oliva K, Rice GE, Quinn MA. Cell-free 59 kDa immunoreactive integrin-linked kinase: a novel marker for ovarian carcinoma. Clin Cancer Res. 2004;10:2415–2420. doi: 10.1158/1078-0432.ccr-03-0042. [DOI] [PubMed] [Google Scholar]
- 44.Kieffer N, Schmitz M, Plancon S, Margue C, Huselstein F, Grignard G, Dippel W, Nathan M, Giacchi S, Scheiden R. ILK as a potential marker gene to ascertain specific adenocarcinoma cell mRNA isolation from frozen prostate biopsy tissue sections. Int J Oncol. 2005;26:1549–1558. [PubMed] [Google Scholar]
- 45.Rosano L, Spinella F, Di C, Dedhar S, Nicotra MR, Natali PG, Bagnato A. Integrin-linked kinase functions as a downstream mediator of endothelin-1 to promote invasive behavior in ovarian carcinoma. Mol. Cancer Ther. 2006;5:833–842. doi: 10.1158/1535-7163.MCT-05-0523. [DOI] [PubMed] [Google Scholar]
- 46.Hoffmann G, Pollow K, Weikel W, Strittmatter HJ, Bach J, Schaffrath M, Knapstein P, Melchert F, Pollow B. Urokinase and plasminogen activator-inhibitor (PAI-1) status in primary ovarian carcinomas and ovarian metastases compared to benign ovarian tumors as a function of histopathological parameters. Clin Chem Lab Med. 1999;37:47–54. doi: 10.1515/CCLM.1999.007. [DOI] [PubMed] [Google Scholar]
- 47.Oda Y, Ohishi Y, Basaki Y, Kobayashi H, Hirakawa T, Wake N, Ono M, Nishio K, Kuwano M, Tsuneyoshi M. Prognostic implications of the nuclear localization of Y-box-binding protein-1 and CXCR4 expression in ovarian cancer: their correlation with activated Akt, LRP/MVP and P-glycoprotein expression. Cancer Sci. 2007;98:1020–1026. doi: 10.1111/j.1349-7006.2007.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basaki Y, Hosoi F, Oda Y, Fotovati A, Maruyama Y, Oie S, Ono M, Izumi H, Kohno K, Sakai K, Shimoyama T, Nishio K, Kuwano M. Akt-dependent nuclear localization of Y-box-binding protein 1 in acquisition of malignant characteristics by human ovarian cancer cells. Oncogene. 2007;26:2736–2746. doi: 10.1038/sj.onc.1210084. [DOI] [PubMed] [Google Scholar]
- 49.Novak A, Hsu SC, Leung-Hagesteijn C, Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R, Dedhar S. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways. Proc Natl Acad Sci USA. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frisch SM, Ruoslahti,E E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 51.Troussard AA, Costello P, Yoganathan TN, Kumagai S, Roskelley CD, Dedhar S. The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9) Oncogene. 2000;19:5444–5452. doi: 10.1038/sj.onc.1203928. [DOI] [PubMed] [Google Scholar]
- 52.Wang SC, Makino K, Xia W, Kim JS, Im SA, Peng H, Mok SC, Singletary SE, Hung MC. DOC-2/hDab-2 inhibits ILK activity and induces anoikis in breast cancer cells through an Akt-independent pathway. Oncogene. 2001;20:6960–6964. doi: 10.1038/sj.onc.1204873. [DOI] [PubMed] [Google Scholar]
- 53.Habashy HO, Powe DG, Rakha EA, Ball G, Macmillan RD, Green AR, Ellis IO. The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat. 2009;120:603–612. doi: 10.1007/s10549-009-0419-9. [DOI] [PubMed] [Google Scholar]
- 54.Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, Bandyopadhyay A, Agyin JK, Brann D, Sun LZ, Yeh IT, Lee FY, Tekmal RR, Kumar R, Vadlamudi RK. Extranuclear Functions of ER Impact Invasive Migration and Metastasis by Breast Cancer Cells. Cancer Res. 2010;70:4092–4101. doi: 10.1158/0008-5472.CAN-09-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakamoto S, McCann RO, Dhir R, Kyprianou N. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 2010;70:1885–1895. doi: 10.1158/0008-5472.CAN-09-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanayama S, Yamada Y, Kawaguchi R, Tsuji Y, Haruta S, Kobayashi H. Hepatocyte growth factor induces anoikis resistance by up-regulation of cyclooxygenase-2 expression in uterine endometrial cancer cells. Oncol Rep. 2008;19:117–122. [PubMed] [Google Scholar]
- 57.Srivastava S, Ramdass B, Nagarajan S, Rehman M, Mukherjee G, Krishna S. Notch1 regulates the functional contribution of RhoC to cervical carcinoma progression. Br J Cancer. 2010;102:196–205. doi: 10.1038/sj.bjc.6605451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Acconcia F, Kumar,R R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2005;238:1–14. doi: 10.1016/j.canlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 59.Schiff R, Massarweh SA, Shou J, Bharwani L, Arpino G, Rimawi M, Osborne CK. Advanced concepts in estrogen receptor biology and breast cancer endocrine resistance: implicated role of growth factor signaling and estrogen receptor coregulators. Cancer Chemother Pharmacol. 2005;56 Suppl 1:10–20. doi: 10.1007/s00280-005-0108-2. 10–20. [DOI] [PubMed] [Google Scholar]
- 60.Marcom PK, Isaacs C, Harris L, Long ZW, Kommarreddy A, Novielli N, Mann G, Tao Y, Ellis MJ. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res Treat. 2007;102:43–49. doi: 10.1007/s10549-006-9307-8. [DOI] [PubMed] [Google Scholar]
- 61.Pontier SM, Huck L, White DE, Rayment J, Sanguin-Gendreau V, Hennessy B, Zuo D, St-Arnaud R, Mills GB, Dedhar S, Marshall CJ, Muller WJ. Integrin-linked kinase has a critical role in ErbB2 mammary tumor progression: implications for human breast cancer. Oncogene. 2010 doi: 10.1038/onc.2010.86. [DOI] [PubMed] [Google Scholar]
- 62.Tokunaga E, Kataoka A, Kimura Y, Oki E, Mashino K, Nishida K, Koga T, Morita M, Kakeji Y, Baba H, Ohno S, Maehara Y. The association between Akt activation and resistance to hormone therapy in metastatic breast cancer. Eur J Cancer. 2006;42:629–635. doi: 10.1016/j.ejca.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 63.Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene. 2009;28:2545–2555. doi: 10.1038/onc.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rayala SK, Molli PR, Kumar R. Nuclear p21-activated kinase 1 in breast cancer packs off tamoxifen sensitivity. Cancer Res. 2006;66:5985–5988. doi: 10.1158/0008-5472.CAN-06-0978. [DOI] [PubMed] [Google Scholar]
- 65.Ishii Y, Waxman S, Germain D. Tamoxifen stimulates the growth of cyclin D1-overexpressing breast cancer cells by promoting the activation of signal transducer and activator of transcription 3. Cancer Res. 2008;68:852–860. doi: 10.1158/0008-5472.CAN-07-2879. [DOI] [PubMed] [Google Scholar]
- 66.D'Amico M, Hulit J, Amanatullah DF, Zafonte BT, Albanese C, Bouzahzah B, Fu M, Augenlicht LH, Donehower LA, Takemaru K, Moon RT, Davis R, Lisanti MP, Shtutman M, Zhurinsky J, Ben-Ze'ev A, Troussard AA, Dedhar S, Pestell RG. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3beta and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem. 2000;20:275, 32649–32657. doi: 10.1074/jbc.M000643200. [DOI] [PubMed] [Google Scholar]
- 67.Kumar R, Zhang H, Holm C, Vadlamudi RK, Landberg G, Rayala SK. Extranuclear coactivator signaling confers insensitivity to tamoxifen. Clin Cancer Res. 2009;15:4123–4130. doi: 10.1158/1078-0432.CCR-08-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalra J, Warburton C, Fang K, Edwards L, Daynard T, Waterhouse D, Dragowska W, Sutherland BW, Dedhar S, Gelmon K, Bally M. QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK), and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outcomes in an orthotopic breast cancer model. Breast Cancer Res. 2009;11:R25. doi: 10.1186/bcr2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Q, Zhang YH, Li PL, Pei JR. Inhibitory effect of antisense oligonucleotide of integrin-linked kinase on cell proliferation of human epithelial ovarian cancer. Zhonghua Fu Chan Ke. Za Zhi. 2008;43:218–222. [PubMed] [Google Scholar]
- 70.Tan C, Costello P, Sanghera J, Dominguez D, Baulida J, de Herreros AG, Dedhar S. Inhibition of integrin linked kinase (ILK) suppresses beta-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin repressor, snail, in APC−/− human colon carcinoma cells. Oncogene. 2001;20:133–140. doi: 10.1038/sj.onc.1204052. [DOI] [PubMed] [Google Scholar]
- 71.Eke I, Leonhardt F, Storch K, Hehlgans S, Cordes N. The small molecule inhibitor QLT0267 Radiosensitizes squamous cell carcinoma cells of the head and neck. PLoS One. 2009;4:e6434. doi: 10.1371/journal.pone.0006434. [DOI] [PMC free article] [PubMed] [Google Scholar]