Abstract
Background
Phenylephrine (PHE), an α1 adrenergic receptor agonist, increases phospholipase D (PLD) activity, independent of classical and novel protein kinase C (PKC) isoforms, in rat-1 fibroblasts expressing α1A adrenergic receptors. The aim of this study was to determine the contribution of atypical PKCζ to PLD activation in response to PHE in these cells.
Results
PHE stimulated a PLD activity as demonstrated by phosphatidylethanol production. PHE increased PKCζ translocation to the particulate cell fraction in parallel with a time-dependent decrease in its activity. PKCζ activity was reduced at 2 and 5 min and returned to a sub-basal level within 10–15 min. Ectopic expression of kinase-dead PKCζ, but not constitutively active PKCζ, potentiated PLD activation elicited by PHE. A cell-permeable pseudosubstrate inhibitor of PKCζ reduced basal PKCζ activity and abolished PHE-induced PLD activation.
Conclusion
α1A adrenergic receptor stimulation promotes the activation of a PLD activity by a mechanism dependent on PKCζ; Our data also suggest that catalytic activation of PKCζ is not required for PLD stimulation.
Background
Phospholipase D (PLD) is widely distributed in mammalian cells and has been shown to be involved in signal transduction, protein trafficking, cell proliferation, differentiation and apoptosis [1-3]. PLD catalyzes the hydrolysis of phosphatidylcholine to phosphatidic acid and choline. Activation of PLD by various agents has been shown to involve small G-proteins of the Arf and Rho families, protein kinase C (PKC) and phosphatidylinositol 4,5-biphosphate (PtdIns(4,5)P2) [1-3]. Two PLD isoforms have been cloned in humans and rats. PLD1 exhibits a low basal activity and is activated by Arf, RhoA and PKC [4,5]. PLD2 has a high basal activity, requires PtdIns(4,5)P2, and is not or is less responsive to Arf, Rho or PKC than PLD1 [6,7].
Stimulation of α1 adrenergic receptors (AR) increases PLD activity in rat tail artery [8] and MDCK cells [9]. In rat-1 fibroblasts expressing different subtypes of α1 AR, α1A AR is more effectively coupled to PLD activation than other α1 AR subtypes [10,11].
The involvement of PKC in PLD regulation has been documented both in vivo and in vitro [1-3]. PKC isoforms are classified on the basis of their protein sequences and biochemical properties [12]. The classical PKC isoforms (α, β1,β2 and γ) are activated by phosphatidylserine and diacylglycerol (DAG) or phorbol esters in a calcium-dependent manner. The novel PKC isoforms (δ, ε, Η and θ) are activated by DAG or phorbol esters in the presence of phosphatidylserine and in the absence of calcium. Classical and novel PKCs play a critical role in cell proliferation, differentiation, tumorigenesis, and apoptosis and have a multitude of cellular substrates with broadly overlapping specificity [12,13]. The atypical PKC isoforms (ι/λ and ζ) are both calcium- and DAG-independent [13]. PKCζ is a critical mediator of mitogenic signaling in many cell types [13-16]. The activation of PI3-kinase by growth factors induces a moderate activation of PKCζ that is mediated by phosphorylation at its T-loop site by PDK1 followed by a subsequent autophosphorylation [17,18]. The activity of PKCζ is reversibly regulated by an autoinhibitory pseudosubstrate region in the regulatory domain, which blocks the active site of the enzyme in the absence of activators, a feature common to all PKCs [19]. In addition, the PKCζ pseudosubstrate is able to interact with tubulin and p62/ZIP protein [20,21]. PKCζ is activated by nonselective binding of acidic lipids such as polyphosphoinositides and phosphatidic acid, unsaturated fatty acids such as arachidonic acid [12], and acidic proteins such as 14-3-3 proteins [22]. In rat-1 fibroblasts, PKCζ mediates the activation of ERK and the increase in mitogenesis elicited by PDGF [15]. However, in rat-1 fibroblasts expressing the α1A AR subtype, norepinephrine does not activate ERK [23].
Classical PKC subtypes have been implicated in PLD activation in vitro or in cells overexpressing classical PKCs [3,24]. However, there are reports indicating receptor-mediated PLD activation that is independent of classical PKCs [9,25]. PLD activation by classical PKCs in vitro does not involve a phosphorylation mechanism [26]. It is currently unclear if the non-catalytic mechanism by which PKCα and β activate PLD1 in vitro accounts for PKC-dependent increases in PLD activity in intact cells [1-3]. We have previously reported that α1A adrenergic stimulation of PLD in rat-1 fibroblasts is independent of classical or novel PKCs [25]. Three recent articles have placed activation of atypical PKCs downstream of PLD, presumably through phosphatidic acid generation [27-29]. On the other hand, PKCζ mediates norepinephrine-induced PLD activation in rabbit vascular smooth muscle cells (VSMC) [30]. The present study was conducted to investigate the relationship between PKCζ and PLD activation in response to PHE in rat-1 fibroblasts expressing the α1A AR subtype. Our study demonstrates that PHE stimulates a PLD activity in rat-1 fibroblasts by a mechanism dependent on the inactivation of PKCζ activity and suggests a role for the pseudosubstrate domain.
Results
PHE and PMA stimulate PLD activity in rat-1 fibroblasts
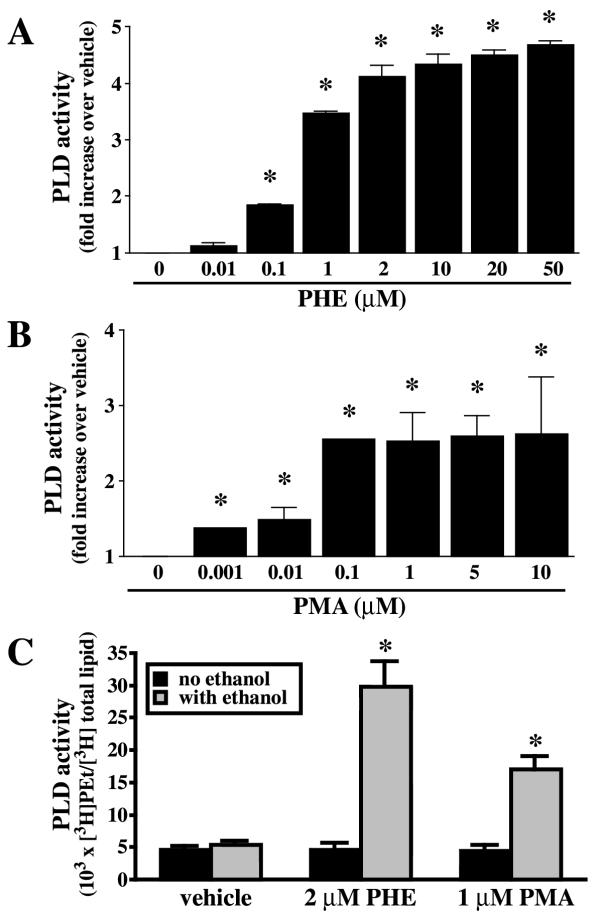
Rat-1 fibroblasts stably transfected with α1A AR expressed 288 ± 2 fmol/mg protein of receptors [10]. PLD activity was measured by the production of [3H]phosphatidylethanol (PEt) in cells pre-labeled with [3H]oleic acid and in the presence of ethanol. Basal PLD activity was determined in serum-starved cells in the presence of ethanol and in the absence of any agonists. PLD activation in response to PHE is characterized by a rapid initial rise (30 sec) followed by a slower rate of PEt formation [11]. PLD activity was measured at 15 min and represents the accumulation of PEt formed in vehicle or PHE-treated cells. PHE elicited a concentration-dependent increase in PLD activity (Fig. 1A). On the other hand, the phorbol ester PMA produced a maximal increase at 100 nM (Fig. 1B), to a significantly lesser extent than PHE. In rat-1 fibroblasts expressing α1A AR, [3H]PEt/ [3H]total lipids ratio was almost identical in the presence or absence of ethanol (5.43 ± 0.52 vs. 4.53 ± 0.63 × 103 × [3H]PEt/ [3H]total lipids, n = 8). Therefore, [3H]PEt/ [3H]total lipids ratio measured in the absence of ethanol may be accounted by non-PLD pathways, as recently described [31]. PLD activity was stimulated 4–5 times above basal by 2 μM PHE and 2.5 times by 1 μM PMA (Fig. 1C), concentrations chosen for our experiments. Furthermore, when basal PEt formation was calculated by subtracting the residual radioactive background found in the absence of ethanol by non-PLD pathways (5.43-4.53 = 0.90 × 103 × [3H]PEt/ [3H]total lipids), the magnitude of PLD activation with PHE was increased from 4–5 to 30 times. It may be significant that, in VSMC, basal PEt formation in untreated cells accounts for half of the measured PLD activity [30], typical of a constitutive PLD2-like activity in these cells [32], whereas, in rat-1 fibroblasts, the presence of an inducible isoform is most probable. The PLD isoform sensitive to α1A AR stimulation in rat-1 fibroblasts is inhibited by cAMP through a negative feedback mechanism [10]. On the other hand, in VSMC, norepinephrine-induced PLD activity (64.95 ± 0.49 % over basal), which is mainly dependent on the activation of PLD2 [32], is instead potentiated by the adenylyl cyclase activator forksolin (91.64 ± 11.70 % above basal). These results support our contention that the PLD isoform activated by PHE in rat-1 fibroblasts may be distinct from PLD2.
Figure 1.
Phospholipase D (PLD) activity is stimulated by phenylephrine (PHE) and PMA in rat-1 fibroblasts. Cells in serum-free DMEM were incubated overnight with 1 μCi/ml [3H]oleic acid, pretreated with 200 mM ethanol (A, B) or with or without ethanol (C) for the measurement of phosphatidylethanol (PEt) formation, and finally treated with different concentration of PHE (A, basal = 7.09 ± 2.68 × 103 × [3H]PEt / [3H]total lipids) or PMA (B, basal = 6.81 ± 2.07 × 103 × [3H]PEt / [3H]total lipids) for 15 min. Data are expressed as the ratio of [3H]PEt over [3H]total lipids. Values are the mean ± S.E. of three independent experiments performed in duplicate. * Value significantly different from vehicle, p < .05.
PKCζ is inactivated in response to α1A-adrenergic stimulation
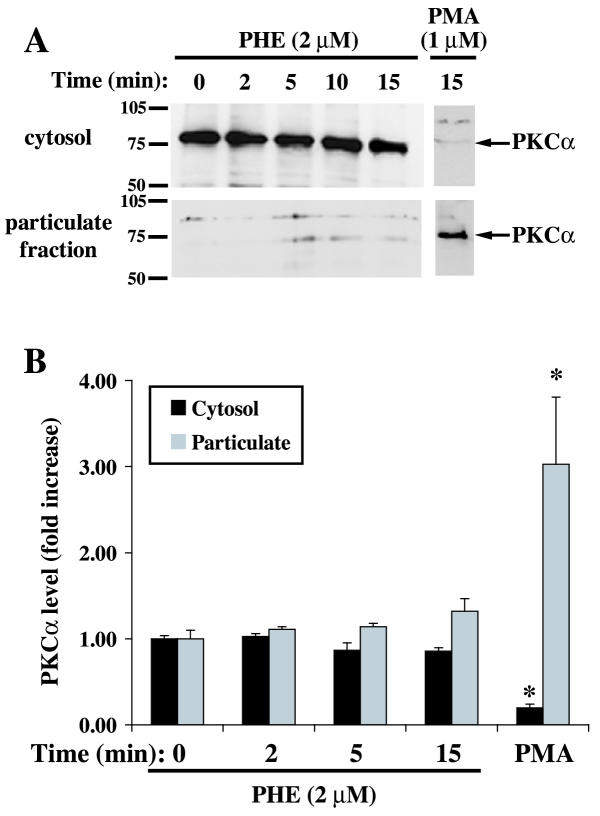
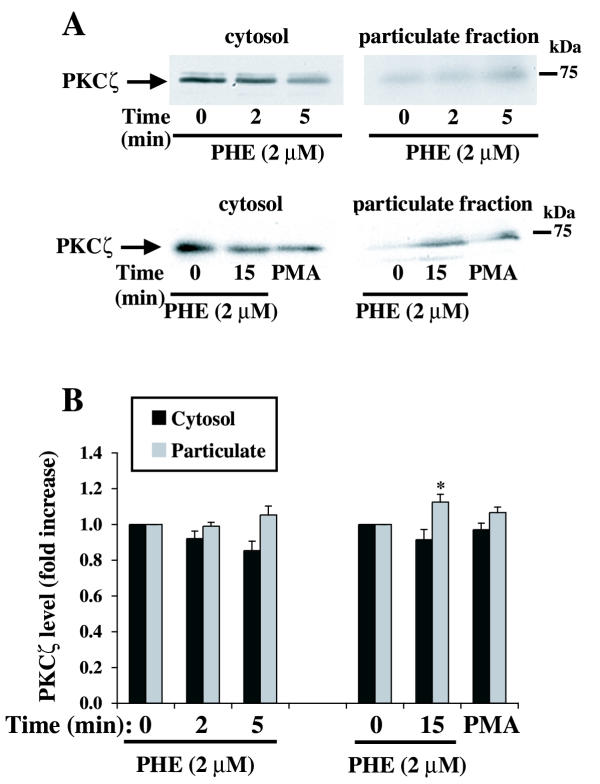
To gain more insight into the effect of PHE on these PKC isoforms, we measured the distribution of PKCα and PKCζ between the cytosolic and particulate cellular fractions by Western blot analysis. PKCα localization, measured at different times after PHE stimulation, was not significantly altered despite a slight decrease in cytosolic fraction and a corresponding increase in particulate fraction (Fig. 2). PMA, included as positive control, promoted PKCα translocation from the cytosol (-80%) to the particulate (+200%) (cytoskeleton/membrane) fraction (Fig. 2). We have previously reported the lack of change in classical PKC activity in response to 2 μM PHE in membrane/cytosolic fractions in these cells [25]. The location of PKCζ was examined under the same conditions. A small amount of PKCζ was translocated to the particulate fraction following treatment with PHE, reaching statistical significance only at 15 min of treatment (Fig. 3). PMA did not significantly increase PKCζ translocation. Therefore, based on the present study and previous work by others [11] and ourselves [25], we conclude that the translocation of PKCs as an index of their activation by PHE is sufficient for PKCα, but PKCζ requires different methods.
Figure 2.
Effect of PHE and PMA on PKCα localization in rat-1 fibroblasts. Serum-deprived cells in DMEM were treated with 2 μM PHE or 1 μM PMA for 2, 5, 10 and 15 min, lysed and cytosol and particulate fractions were subsequently prepared as described in methods. A representative Western blot is shown in A. The bar graph (B) represents quantitation of the PKCα protein bands by densitometric analysis of blots from five different experiments. * Value significantly different from time = 0 (cytosol or particulate), p < .05.
Figure 3.
Effect of PHE and PMA on PKCζ localization in rat-1 fibroblasts. Serum-deprived cells in DMEM were treated with 2 μM PHE or 1 μM PMA for 2, 5, 10 and 15 min, lysed and cytosol and particulate fractions were subsequently prepared as described in methods. A representative Western blot is shown in A. The bar graph (B) represents quantitation of the PKCζ protein bands by densitometric analysis of blots from five different experiments. * Value significantly different from time = 0 (cytosol or particulate), p < .05.
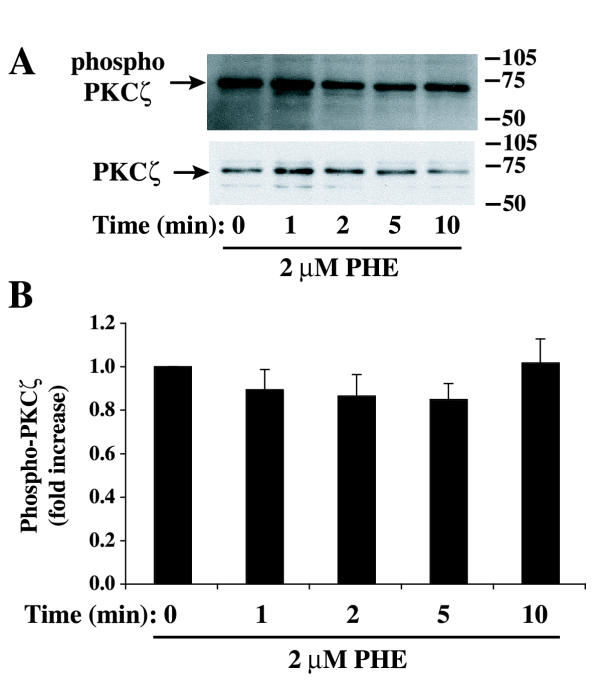
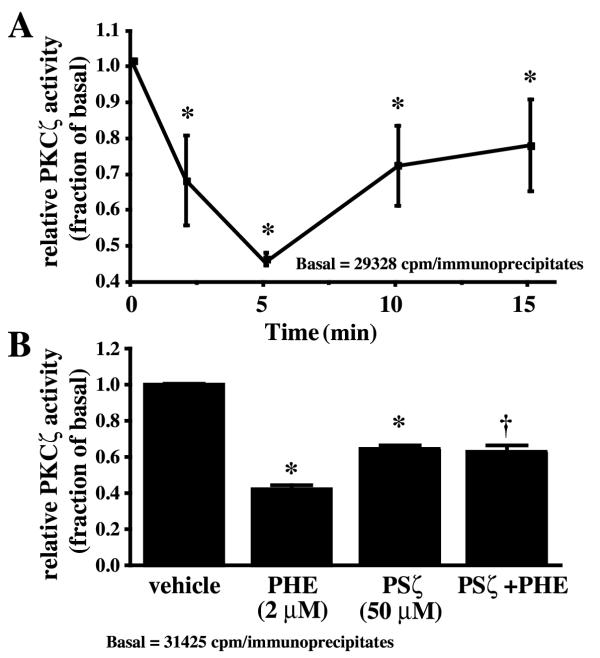
The phosphorylation of PKCζ on Thr 410, a PDK1-dependent phosphorylation site, is required for PKCζ activation [17]. However, PKCζ phosphorylation at Thr 410 was already detected in serum-deprived rat-1 fibroblasts and remained essentially unchanged in the presence of PHE (Fig. 4). In contrast, we have previously shown that norepinephrine promoted an increase of Thr 410 phosphorylation in a time-dependent manner in parallel with an increase in PKCζ activity in VSMC [30]. The other atypical isoform, PKCλ/ι, is not expressed in rat-1 fibroblasts [15]. Since only a small fraction of PKCζ was translocated to the particulate fraction and the Thr 410 phosphorylation was not altered, we measured the change in kinase activity of PKCζ in response to PHE. PKCζ activity was measured by the ability of immunoprecipitated PKCζ to phosphorylate a selective substrate in the presence of [γ32P]ATP. We have previously shown PKCζ activation in response to norepinephrine in rat VSMC using the same method [30]. In contrast to the increase in PLD activity, PHE decreased PKCζ activity at 2 and 5 min; activity returned to sub-basal level within 10–15 min (Fig. 5A). In conclusion, the decrease in PKCζ activity did not correlate with a decrease in the phosphorylation of PKCζ at Thr 410. These results suggest that although phosphorylation at Thr 410 and subsequent autophosphorylation of Thr 560 is a prerequisite for PKCζ activation, as shown in other cell systems [17,18], dephosphorylation of Thr 410 is not a prerequisite for its inactivation.
Figure 4.
Effect of PHE on PKCζ phosphorylation at Thr 410. Cells were arrested for 48 hours and incubated for different time with 2 μM PHE. Samples were prepared for Western blot analysis and incubated with a phospho-PKCζ/ (Thr 410/403) antibody (A, top) as described in Methods. The same membranes were stripped off and reprobed with PKCζ antibody (A, bottom). The bar graph (B) represents quantitation of the ratio phospho-PKCζ/ PKCζ protein bands by densitometric analysis of blots from three different experiments. No statistically significant differences were found.
(Thr 410/403) antibody (A, top) as described in Methods. The same membranes were stripped off and reprobed with PKCζ antibody (A, bottom). The bar graph (B) represents quantitation of the ratio phospho-PKCζ/ PKCζ protein bands by densitometric analysis of blots from three different experiments. No statistically significant differences were found.
Figure 5.
PHE decreases PKCζ activity in rat-1 fibroblasts. A, Cells were treated with 2 μM PHE for 0, 2, 5, 10 and 15 min, lysed and immunoprecipitated with PKCζ antibody for a kinase assay using [γ-32P]ATP and a selective peptide substrate as described in Methods. Relative PKCζ activity was expressed as the fold (increase or decrease) of basal. Values are the mean ± S.E. of four independent experiments. * Value significantly different from the basal, p < .05. B, Effect of myristoylated PSζ on PKCζ activity. Cells were pretreated with 50 μM PSζ for 1 hour followed by a 5 min treatment with 2 μM PHE or its vehicle. PKCζ activity was measured as previously described. Values are the mean ± S.E. of four independent experiments. * Value significantly different from vehicle, p < .001. †, Value significantly different from PHE alone, p < .01.
To gain more insight into the mechanism of PKCζ regulation by PHE, we measured PKCζ activity in immunoprecipitates from cells pretreated with myristoylated PKCζ peptide inhibitor (PSζ) [33]. Myristoylated PSζ reduced basal PKCζ activity and prevented its further decrease by PHE (Fig. 5B), confirming that PKCζ is active in untreated cells arrested in serum-free medium. It should be noted that PHE produced a significantly greater decrease in PKCζ activity than myristoylated PSζ, alone or with PHE, pointing to a closely regulated mechanism of PHE-induced PKCζ inactivation. Together, these results show that PHE inhibits PKCζ and simultaneously activates PLD in rat-1 fibroblasts.
Effect of PKCζ on PLD activity
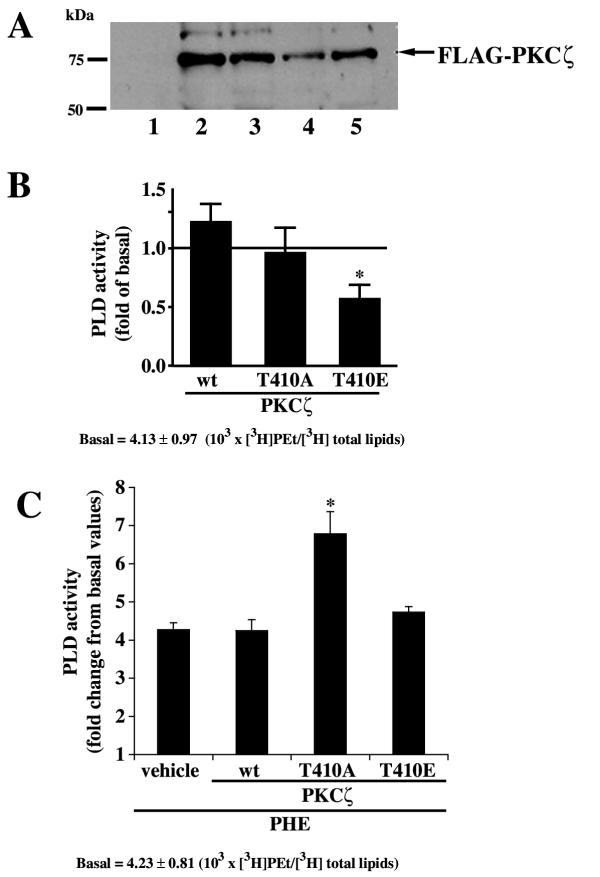
Since 1) PKCζ activity was decreased by PHE, 2) PLD activity was increased by PHE and 3) previous results showed the lack of classical and new PKC contribution to PHE-induced PLD activation [25], we tested the hypothesis that PKCζ regulates PLD activity in rat-1 fibroblasts. The contribution of PKCζ to PHE-induced PLD activation was determined with transient expression of wild type (wt), kinase deficient (T410A) and constitutively active (T410E) PKCζ. The efficiency of FLAG-PKCζ transfection after 48 hours was assessed by Western blot analysis (Fig. 6A). Overexpression of constitutively active T410E PKCζ caused a statistically significant decrease in basal PLD activity (Fig. 6B) whereas wt and T410A PKCζ did not alter PLD activity. However, the apparent inhibition of basal PLD activity observed with T410E PKCζ may be an artifact since Fig. 1C clearly shows that PLD activity (PEt formation) is not significantly reduced in the absence of ethanol in vehicle. Therefore, it appears that T410E PKCζ decreases the non-PLD product co-migrating with phosphatidylethanol in rat-1 fibroblasts. This observation raises the possibility that T410E PKCζ does not decrease PLD activity but only the non-PLD part. We therefore calculated a PHE-induced PLD activity corrected for each basal PLD activity observed in presence of the different PKCζ constructs (Fig. 6C). Figure 6, panel C clearly shows the absence of effect of T410E PKCζ on the extent of PHE-induced PLD activity. On the other hand, we observe a significant potentiation of PHE-induced PLD activity with kinase-deficient T410A PKCζ. Thus, inactivation of the catalytic activity of PKCζ potentiated PHE-induced PLD activity suggesting a negative correlation between PKCζ and PLD activities. This is compatible with the decrease in PKCζ activity (Fig. 5A) elicited by PHE together with the simultaneous increase in PLD activity. These results raise the possibility that PKCζ catalytic activity is a negative regulator of α1A AR-stimulated PLD activity in rat-1 fibroblasts.
Figure 6.
Effect of catalytically active and inactive PKCζ mutants on PLD activity. A, Representative Western blot showing the transfection of pCMV-FLAG-PKCζ constructs using an anti-FLAG antibody. Samples were immunoprecipitated with anti-FLAG antibody and probed with the same antibody; 1: untransfected, 2: pCMV-FLAG wt PKCζ, 3: pCMV-FLAG T410A PKCζ, 4 and 5: pCMV-FLAG T410E PKCζ. B, Cells were transiently transfected with wt PKCζ, kinase-deficient T410A PKCζ and constitutively active T410E PKCζ for 48 h and PLD activity was determined as described in Methods. Data are expressed as the change in PLD activity as a fraction of basal activity (non transfected cells). Values are the mean ± S.E. of three independent experiments performed in duplicate on different batches of cells. * Value significantly different from basal p < .01. C, PHE-induced increase in PLD activity was adjusted for basal variations in the absence of PHE (shown in panel B) for each treatment (vehicle, wt, T410A, T410E). PLD activity was calculated as [PKCζ construct + PHE / [PKCζ construct alone]. This data presentation allows the elimination of non specific variations of basal PLD activity due to the overexpression of the PKCζ constructs. Values are the mean ± S.E. of three independent experiments performed in duplicate on different batches of cells. * Value significantly different from vehicle, p < .01.
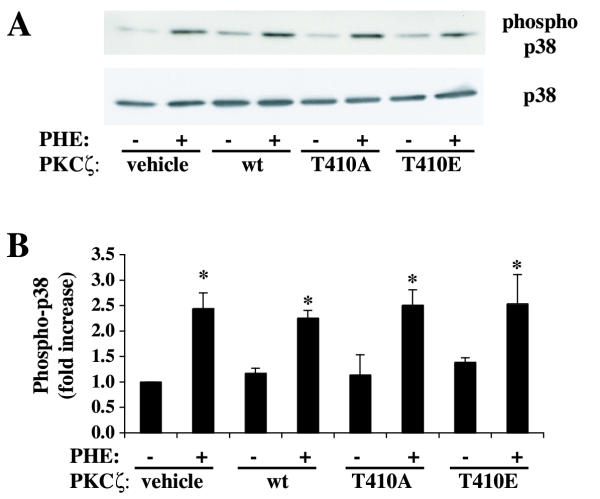
PHE stimulates p38 MAP kinase activation in rat-1 fibroblasts expressing α1A AR [23]. We have also measured p38 phosphorylation in response to PHE in cells transfected with wild-type, kinase inactive (T410A) or constitutively active (T410E) PKCζ to rule out an effect of heterologous PKCζ overexpression on α1A AR signaling or function (Fig. 7). The results show that heterologous expression of PKCζ did not alter p38 phosphorylation in response to 2 μM PHE. Therefore, α1A AR expression and/or function are not altered by transfection of PKCζ mutants. These results also indicate the lack of deleterious effect or toxicity of PKCζ overexpression on the α1A adrenergic signaling pathway.
Figure 7.
Effect of PKCζ mutants on p38 phosphorylation. A, Cells were transiently transfected with wt PKCζ, kinase-deficient T410A PKCζ and constitutively active T410E PKCζ for 48 h and treated with or without PHE for 5 min. Phospho-p38 and p38 protein levels in cell lysates were determined by western blot analysis as described in Methods. The bar graph (B) represents quantification of the ratio phospho-p38 / 38 protein bands by densitometric analysis of blots from four different experiments. * denotes value significantly different from vehicle (nontransfected and lipofectamine-treated cells), p < .01.
A PKCζ inhibitor blocks PHE-induced PLD activity
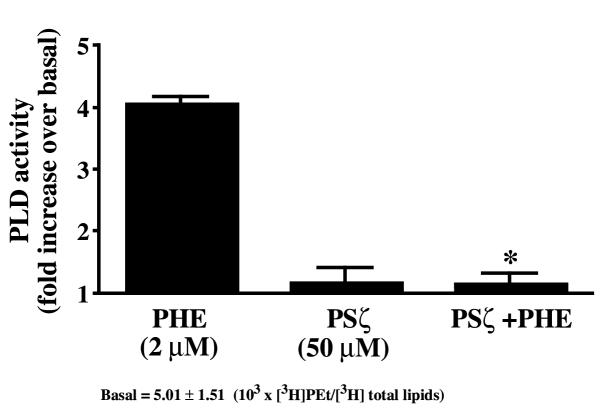
The specific PKCζ inhibitor, PSζ [33], was used to selectively inhibit PKCζ activity (Fig. 5B). Surprisingly, PHE-induced PLD activation was blocked with 50 μM PSζ (Fig. 8), suggesting a specific role for the PKCζ pseudosubstrate domain in the regulation of PLD activity. Myristoylated PKC(20–28) peptide inhibitor (50 μM), a selective inhibitor of classical PKCs such as PKCα or PKCβ1/2, did not alter PLD activity (4.24 ± 0.34 fold increase with 2 μM PHE versus 4.09 ± 0.03 fold increase with PHE + PKC(20–28) peptide inhibitor).
Figure 8.
Effect of the pseudosubstrate peptide inhibitor of PKCζ (PSζ) on PLD activity. Cells were pretreated with 50 μM myristoylated PSζ for 1 hour before the addition of 2 μM PHE for 15 min. PLD activity was then measured as described in Methods. Data are expressed as the fold increase in PLD activity above basal (untreated cells). * Value significantly different from PHE alone, p < .05.
Discussion
This study confirms that stimulation of α1A AR with PHE in rat-1 fibroblasts promotes activation of a PLD activity [10,11,25]. Moreover, it demonstrates that PHE selectively decreases PKCζ activity and that PLD activity is regulated by a mechanism involving PKCζ. Furthermore, the pseudosubstrate domain of PKCζ appears to play an important role in the regulation of PLD. Our previous study has ruled out the involvement of classical or novel PKC isoforms in α1A AR-stimulated PLD activation [25].
In rat-1 fibroblasts expressing the α1A AR subtype, PHE causes a transient Ca2+ increase, cAMP accumulation and activation of PKA, p38 mitogen-activated protein kinase, p70 S6 kinase and PLD activation [10,11,23,34]. In contrast, PHE inhibits basal levels or agonist-induced activation of ERK, PI 3-kinase and Akt in these cells [11,34]. In addition, PHE slightly decreases basal PtdInsP2 and PtdInsP3 levels [34]. Despite these negative effects on proliferation (ERK) and survival (PI 3-kinase, Akt) pathways in rat-1 fibroblasts, α1A AR stimulation does not significantly promote apoptosis [34]. It should be noted that in these cells, PLD activity is extremely low both in serum-deprived or serum-treated cells; PLD activation by PHE is not affected by the presence of serum and PHE decreases cell proliferation (Parmentier JH, Saeed AE and Malik KU, our unpublished observation). These observations, together with our finding that PHE-induced decrease in PKCζ activity is associated with an increase in PLD activity, support an anti-proliferative effect of α1A AR stimulation in rat-1 fibroblasts, involving PLD activation. In contrast, in VSMC expressing high levels of the PLD2 isoform [30,32], norepinephrine-induced cell proliferation was dependent on PLD activation. In addition, norepinephrine selectively stimulates the PLD2 isoform in VSMC [32] through the catalytic activation of PKCζ [30], in contrast to the effect of PHE in rat-1 fibroblasts. The cell phenotype, VSMC vs. rat-1 fibroblasts, or the adrenergic receptor subtype, α1A AR in rat-1 vs. several α1 and α2 AR subtypes in VSMC, may be responsible for this difference.
To our knowledge, this is the first report demonstrating inhibition of PKCζ activity in response to receptor stimulation. However, treatment with PHE decreases the activity of many mitogenic indices in rat-1 fibroblasts, including DNA synthesis, ERK, phosphatidylinositol 3-kinase and Akt [11,34]. A noteworthy fact is that there is elevated mitogenic signaling in rat-1 fibroblasts expressing α1A AR cultured in serum-free medium. The decrease in PKCζ activity elicited by PHE could result from a physical interaction between PKCζ and K10 keratin, causing sequestration of PKCζ within the cytoskeleton and preventing its intracellular translocation, thus impairing its activation, as reported by Paramio et al. [35], although translocation is often associated with activation for other PKC isoforms. In addition, a recent study shows that a small pool of PKCζ is constitutively active and bound to 14-3-3 zeta in the brain [22], a mechanism that could account for the persistent activation of PKCζ in rat-1 fibroblasts. PI 3-kinase, an upstream activator required for PKCζ activation, is also inhibited by PHE in rat-1 fibroblasts [34]. Recently, it has been demonstrated that atypical PKC activation is dependent on PLD activity [27-29]. However, our data show that PLD activation with PHE was associated with a decrease in PKCζ activity in rat-1 fibroblasts.
Our data also shows that the decrease in PKCζ activity elicited by PHE is not matched by a decrease in the phosphorylation of Thr 410. PKCζ is maintained in an inactive state by direct binding of the N-terminal pseudosubstrate domain to the C-terminal catalytic domain [13]. Phosphorylation of the activation loop at Thr 410 is necessary and sufficient to activate the kinase function of PKCζ after autophosphorylation of Thr 560 [36-38]. In VSMC, we observed a simultaneous increase in PKCζ phosphorylation and activity in response to norepinephrine [30]. However, at 5 min of stimulation, PKCζ phosphorylation continued to increase whereas PKCζ activity was already decreasing [30]. Therefore, although phosphorylation at Thr 410 is a prerequisite for PKCζ activation as shown by different studies [36-38], it is not clear if dephosphorylation of Thr 410 is a prerequisite for its inactivation. It has been recently reported that in fact phosphorylation of Thr 410 and subsequent autophosphorylation of Thr 560 targets PKCζ towards proteosomal degradation [39]. Moreover, proteins that bind to PKCζ may directly inhibit its activity. For example, PAR-4, product of a gene induced during apoptosis, inhibits atypical PKCζ and PKCλ/ι activity through direct protein-protein interaction [40]. This mechanism of inhibition of PKCζ activity may not require dephosphorylation of the enzyme. Thus, phosphorylation at Thr 410 seems to be required for PKCζ activation whereas inhibition of PKCζ activity seems to be independent of Thr 410 dephosphorylation and may involve other proteins. Since PKCζ immunoprecipitation and assay were carried out in non-denaturing conditions, it is likely that the interaction of PKCζ with other proteins, such as with an endogenous inhibitor, is conserved during the assay.
The activation of PLD elicited by PHE may be independent of PKCζ catalytic activity since constitutively active T410E PKCζ did not alter the extent of PHE-induced PLD activation. It is widely accepted that PKCζ activates downstream targets through a phosphorylation-dependent mechanism. However, it has been reported that atypical PKCζ may also directly stimulate MEK5/ERK5 pathway through its N-terminal regulatory domain (containing the pseudosubstrate site), independent of its catalytic activity [41]. Therefore, PKCζ may stimulate downstream targets independent of its activity and phosphorylation state. Moreover, the actual proposed mechanism of PLD1 activation by the classical isoform PKCα is independent of the catalytic activity of PKC [26]. In contrast, the fact that catalytically inactive PKCζ potentiated PLD activity and PKCζ activity was decreased after PHE stimulation indicate that an initial decrease in PKCζ activity may be required for a non-catalytic PKCζ-dependent activation of PLD activity. On the other hand, the decrease in PKCζ activity could be a consequence and not a prerequisite for this potential mechanism of action.
Regulation of PLD activity by PKCζ may involve the pseudosubstrate domain or a regulatory domain of PKCζ. PSζ is utilized to inhibit PKCζ activity [14,30]. However, we show that PHE is a better inhibitor than PSζ and PSζ further blocks the decrease in PKCζ activity elicited by PHE. These data and the lack of decrease in PKCζ phosphorylation suggest that other proteins activated by PHE may be involved in reducing PKCζ activity. In addition, PSζ blocked PHE-induced PLD activation. Therefore, PSζ may alter PKCζ function(s) through two different actions. First, it inhibits kinase activity through binding to the catalytic site, which mimics the endogenous pseudosubstrate domain [14,33]. Second, it may act a competitive inhibitor of the interaction of the regulatory N-terminal domains (PB1, PS, C1) [42,43] of PKCζ with other effectors, such as PAR, MEK5, p62/ZIP, tubulin or other proteins [20,21,40,41,44,45]. PKCζ directly stimulates the MEK5/ERK5 pathway through its N-terminal regulatory domain independently of its catalytic activity [41]. PSζ may competitively inhibit the interaction of the regulatory domain with a protein containing an aPKC interaction domain, such as MEK-5 or the scaffold protein p62, in addition to its effect on catalytic activity. This mechanism of action would explain the effect of PSζ on PLD activity and PKCζ activity. It should be noted that a PKCα pseudosubstrate inhibitor did not alter PHE-induced PLD activity (Fig. 8), underscoring the selectivity of myristoylated PSζ.
An alternative hypothesis involves a PKCζ-mediated PLD phosphorylation that would keep PLD inactive when PKCζ is active. However, our data do not support this, since kinase inactive PKCζ or myristoylated PSζ did not stimulate PLD activity in the absence of PHE stimulation. Moreover, PLD phosphorylation is probably not involved in its mechanism of activation [3].
PKCα, the only classical isoform found in rat-1 fibroblasts expressing α1A AR, was not activated, as previously shown in these cells [24]. A previous study by Taguchi et al. [11] showed an increase in PKCα translocation in response to PHE in the same cell model. The main difference between the two studies resides in the PHE concentration, 2 μM PHE in our work vs. 100 μM PHE [11], indicating that PHE is able to significantly stimulate PKCα translocation at concentration higher than 2 μM.
Conclusions
Our data indicate that a non-constitutively active PLD isoform expressed in rat-1 fibroblasts is regulated by a PKCζ-dependent mechanism following α1A AR stimulation by PHE. The exact mechanism of PLD activation by PKCζ is still unknown and may be independent of the catalytic activity. Our data show the possible regulation of PLD by a mechanism involving the pseudosubstrate or the regulatory domain of PKCζ. Further characterization of the mechanism of PLD activation and the possible interaction of PLD isoforms with PKCζ should provide important information about its regulation and its functional significance in rat-1 fibroblasts.
Methods
Materials
Phenylephrine was obtained from Sigma (St. Louis, MO); phorbol-12-myristate-13-acetate (PMA) from Calbiochem (San Diego, CA); myristoylated PKCζ and PKCα/β (20–28) peptide inhibitors from Biomol (Plymouth Meeting, PA); [3H]oleic acid (50 Ci/mmol) from American Radiolabeled Chemicals (St. Louis, MO); [γ-32P]ATP (3000 Ci/mmol) from Amersham (Arlington Heights, IL). Antibodies to PKCα, PKCζ and p38 were from Santa-Cruz Biotechnology (Santa Cruz, CA); phospho-PKCζ/λ and phospho-p38 antibodies were from Cell Signaling Technology (Beverly, MA).
Cell culture
Rat-1 fibroblasts were stably transfected with bovine α1A AR (a kind gift from Drs. L.F. Allen and R.J. Lefkowitz (Howard Hughes Medical Institute, Duke University Medical Center, Durham, NC). Cells were maintained under 5% CO2 at 37°C in Dulbecco's modified Eagle's medium (DMEM) containing 50 units of penicillin, 50 μg of streptomycin per ml, and 10% fetal bovine serum (FBS). Selection and maintenance of stably transfected cells were carried out with 400 μg/ml G418 (Invitrogen, San Diego, CA).
Transient transfection
Rat-1 fibroblasts were transiently transfected with pCMV-FLAG-wt-PKCζ, pCMV-FLAG-T410A-PKCζ (kinase inactive), pCMV-FLAG-T410E-PKCζ (constitutively active) vectors (gift from Dr. A. Toker, BBRI, Boston, MA and R. Farese, USF, Tampa, FL) using Lipofectamine PLUS (Invitrogen). Efficiency of transfection was determined by Western blot analysis using an anti-FLAG antibody (Sigma). Transfection of rat-1 fibroblasts was performed for 2 days before assays with cells washed 8 hours after transfection to avoid lipofectamine-induced cytotoxicity.
Phospholipase D assay
PLD activity was quantitated by the formation of phosphatidylethanol according to a method previously described for rat-1 fibroblast [10]. Briefly, serum-starved rat-1 fibroblasts in 6-well plates were labeled overnight with [3H]oleic acid (1 μCi/ml) in DMEM. Cells were then incubated with inhibitors and exposed to PHE (2 μM) for 15 minutes in the presence of 200 mM ethanol. Rat-1 fibroblasts were scraped into 2 ml ice-cold methanol and HCl solution, and 1 ml chloroform was added. Lipids were separated by chloroform extraction. A 40 μl aliquot was removed from the chloroform phase to determine the content of radioactivity in the total lipid fraction. The chloroform phase (0.8 ml) was evaporated under nitrogen and redissolved in 50 μl chloroform/methanol (9:1) containing phosphatidylethanol standard. Samples were spotted onto a silica gel thin-layer chromatography plate, and lipids were separated with the solvent system of chloroform/acetone/methanol/HCl/water (50:20:12.5:10:7.5). Phosphatidylethanol was identified by the mobility of authentic standard visualized with iodine vapor. Lanes containing phosphatidylethanol were scraped, and radioactivity was measured by scintillation spectroscopy. Data were measured as the ratio of [3H]phosphatidylethanol to [3H]total lipids.
PKCζ assay
The activity of PKCζ was determined according to the method described [14,24]. Rat-1 fibroblasts in 100 mm dish were washed with PBS and scraped in 1 ml RIPA buffer [50 mM Tris.Cl, pH 7.4; 150 mM NaCl, 1% IGEPAL, 1 mM EDTA] containing protease and phosphatase inhibitors [1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM PMSF, 1 mM Na orthovanadate, 1 mg/ml p-nitrophenyl-phosphate]. Total cell lysates were incubated with rabbit polyclonal PKCζ antibody (Santa Cruz Biotechnology, CA) for three hours and the immunocomplex was captured with a 50% slurry of protein A agarose beads. PKCζ immunoprecipitates were washed twice with high salt [50 mM Tris.Cl, pH 7.5; 10 mM MgCl2; 0.5 M LiCl] and low salt [50 mM Tris.Cl, pH 7.5; 10 mM MgCl2] buffers, and incubated with a kinase buffer [50 mM Tris.Cl, pH 7.5; 10 mM MgCl2; 0.2 mM EGTA, 50 μM ATP] containing 50 μM ε-peptide and 3 μCi [γ-32P]ATP at 30°C. The reaction was stopped by the addition of 200 mM EDTA and the proteins were precipitated by the addition of 25% TCA. The solutions were centrifuged for one minute at 14,000 rpm, and supernatants spotted onto p81 phosphocellulose filters. Filters were washed with 1% (v/v) orthophosphoric acid and analyzed by Cerenkov counting. PKCζ activity was calculated from the amount of 32P incorporated into the ε-peptide.
Western blot Analysis
Total cell lysate was prepared in modified RIPA buffer containing protease and phosphatase inhibitors and protein concentration was determined by the Bradford method. Proteins in 50 μg of lysate were separated by SDS-PAGE and blotted onto nitrocellulose membranes. Blots were incubated with antibodies at dilutions recommended by the manufacturers (Cell Signaling Technology and Santa-Cruz Biotechnology). The blots were visualized with the ECL plus detection system (Amersham Pharmacia, Piscataway, NJ). For PKCζ phosphorylation at Thr 410, an indicator of PKCζ kinase activity, a phospho-PKCζ/λ antibody was used. Blots were also stripped off with a stripping buffer [100 mM β-mercaptoethanol; 62.5 mM Tris.Cl, pH 6.7, 2% SDS] for 30 min at 50°C, washed twice with TBST, and reprobed with nPKCζ or p38 antibody.
Cell Fractionation
For experiments involving cell fractionation, cells cultured in 150 mm dishes (two per treatment) were scraped in fractionation buffer (10 mM Tris-HCl [pH 7.5], 10 mM NaCl, 0.5 mM EDTA, 0.5 mM EGTA, 1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM sodium orthovanadate, 10 μg/ml trypsin inhibitor), subjected to 25 passes in a Potter-type teflon-on-glass homogenizer with a loose fitting and centrifuged at 700 × g for 5 min. The supernatant was centrifuged at 100,000 × g for 30 min, and the cytosolic fraction was stored at -80°C. The particulate fraction was resuspended in 1% Triton-RIPA buffer, sonicated, and stored at -80°C. 50 μg aliquots of each fraction were analyzed by Western-blot.
Data analysis
The results are expressed as mean ± SE. The data were analyzed by one-way ANOVA. The unpaired Student's t-test was applied to determine the difference between two groups, and the Newman-Keuls' a posteriori test to determine the difference between multiple groups. A value of P = 0.05 was considered significant. PLD activity was expressed as either 1000 × [3H]phosphatidylethanol / [3H]total lipids, or as a fraction of the basal or as the fold increase over vehicle. The phosphoprotein and protein level were estimated by densitometric analysis of the Western blots and performed on the indicated number of blots using NIH Image software, and expressed as a fold increase or decrease (mean ± SE) of the control, arbitrarily chosen as 1.
Author's contributions
Jean-Hugues Parmentier carried out PLD assays, fractionation and Western blot studies, and transfection experiments. Gautam K. Gandhi performed most of the PKCζ assay and some Western blot analysis. Monique T. Wiggins prepared the PKCζ constructs. Abdelwahab E. Saeed performed some PLD activity assays. Sylvain G. Bourgoin assisted in the design of the study and analysis of the data. Kafait U. Malik coordinated the study. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This research was supported by NIH-NHLBI grant 19134-29. JHP was supported by a grant of the American Heart Association (Southeast Affiliates). GKG was supported by UT Graduate Health Science grant. AES was supported by a NIH Minority Postdoctoral Fellowship, supplement to NIH-NHLBI grant 19134-29. We thank Anne Estes for her technical assistance and Dr. Lauren Cagen for editorial comments. We gratefully acknowledge Dr. Alex Toker (BBRI, Boston, MA) and Dr. Robert Farese (University of South Florida, Tampa, FL) for generously supplying us with the PKCζ plasmids.
Contributor Information
Jean-Hugues Parmentier, Email: jparmentier@utmem.edu.
Gautam K Gandhi, Email: GandhiGautamK@uams.edu.
Monique T Wiggins, Email: twiggins@utmem.edu.
Abdelwahab E Saeed, Email: asaeed@utmem.edu.
Sylvain G Bourgoin, Email: sylvain.bourgoin@crchul.ulaval.ca.
Kafait U Malik, Email: kmalik@utmem.edu.
References
- Frohman MA, Morris AJ. Phospholipase D structure and regulation. Chem Phys Lipids. 1999;98:127–140. doi: 10.1016/S0009-3084(99)00025-0. [DOI] [PubMed] [Google Scholar]
- Liscovitch M, Czarny M, Fiucci G, Tang X. Phospholipase D: molecular and cell biology of a novel gene family. Biochem J. 2000;345:401–415. doi: 10.1042/0264-6021:3450401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton JH. Regulation of phospholipase D. FEBS Lett. 2002;531:58–61. doi: 10.1016/S0014-5793(02)03405-1. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Jenco JM, Nakashima S, Cadwallader K, Gu Q, Cook S, Nozawa Y, Prestwich GD, Frohman MA, Morris AJ. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-alpha. J Biol Chem. 1997;272:3860–3868. doi: 10.1074/jbc.272.6.3860. [DOI] [PubMed] [Google Scholar]
- Park SK, Provost JJ, Bae CD, Ho WT, Exton JH. Cloning and characterization of phospholipase D from rat brain. J Biol Chem. 1997;272:29263–29271. doi: 10.1074/jbc.272.46.29263. [DOI] [PubMed] [Google Scholar]
- Colley WC, Sung TC, Roll R, Jenco J, Hammond SM, Altshuller Y, Bar-Sagi D, Morris AJ, Frohman MA. Phospholipase D2, a distinct phospholipase D isoform with novel regulatory properties that provokes cytoskeletal reorganization. Curr Biol. 1997;7:191–201. doi: 10.1016/s0960-9822(97)70090-3. [DOI] [PubMed] [Google Scholar]
- Kodaki T, Yamashita S. Cloning, expression, and characterization of a novel phospholipase D complementary DNA from rat brain. J Biol Chem. 1997;272:11408–11418. doi: 10.1074/jbc.272.17.11408. [DOI] [PubMed] [Google Scholar]
- Labelle EF, Fulbright RM, Barsotti RJ, Gu H, Polyak E. Phospholipase D is activated by G protein and not by calcium ions in vascular smooth muscle. Am J Physiol. 1996;270:H1031–H1037. doi: 10.1152/ajpheart.1996.270.3.H1031. [DOI] [PubMed] [Google Scholar]
- Balboa MA, Insel PA. Stimulation of phospholipase D via alpha1-adrenergic receptors in Madin-Darby canine kidney cells is independent of PKC alpha and -epsilon activation. Mol Pharmacol. 1998;53:221–227. doi: 10.1124/mol.53.2.221. [DOI] [PubMed] [Google Scholar]
- Ruan Y, Kan H, Parmentier JH, Fatima S, Allen LF, Malik KU. Alpha-1A adrenergic receptor stimulation with phenylephrine promotes arachidonic acid release by activation of phospholipase D in rat-1 fibroblasts: inhibition by protein kinase A. J Pharmacol Exp Ther. 1998;284:576–585. [PubMed] [Google Scholar]
- Taguchi K, Yang M, Goepel M, Michel MC. Comparison of human alpha1-adrenoceptor subtype coupling to protein kinase C activation and related signaling pathways. Naunyn-Schmiedeberg's Arch Pharmacol. 1998;357:100–110. doi: 10.1007/pl00005143. [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. Signaling through protein kinase C. Front Biosci. 1998;3:1134–1147. doi: 10.2741/a350. [DOI] [PubMed] [Google Scholar]
- Liao DF, Monia B, Dean N, Berk B. Protein kinase C-zeta mediates angiotensin II activation of ERK1/2 in vascular smooth muscle cells. J Biol Chem. 1997;272:6146–6150. doi: 10.1074/jbc.272.10.6146. [DOI] [PubMed] [Google Scholar]
- Van Dijk MC, Muriana JG, Van der Hoeven PCJ, De Widt J, Schaap D, Moolenar WH, Van Blitterswijk WJ. Diacylglycerol generated by exogenous phospholipase C activates the mitogen-activated protein kinase pathway independent of Ras- and phorbol ester-sensitive protein kinase C: dependence on protein kinase C-zeta. Biochem J. 1997;323:693–699. doi: 10.1042/bj3230693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajan MP, Standaert ML, Bandyopadhyay G, Quon MJ, Burke TR, Farese RV. Protein kinase C-zeta and phosphoinositide-dependent protein kinase-1 are required for insulin-induced activation of ERK in rat adipocytes. J Biol Chem. 1999;274:30495–30500. doi: 10.1074/jbc.274.43.30495. [DOI] [PubMed] [Google Scholar]
- Balendran A, Biondi RM, Cheung PCF, Casamayor A, Deak M, Alessi DR. A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase C zeta (PKC zeta) and PKC-related kinase 2 by PDK1. J Biol Chem. 2000;275:20806–20813. doi: 10.1074/jbc.M000421200. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Perez L, Price D, Galloway L, Poklepovic A, Sajan MP, Cenni V, Sirri A, Moscat J, Toker A, Farese RV. Insulin activates protein kinases C-zeta and C-lambda by an autophosphorylation-dependent mechanism and stimulates their translocation to GLUT4 vesicles and other membrane fractions in rat adipocytes. J Biol Chem. 1999;274:25308–25316. doi: 10.1074/jbc.274.36.25308. [DOI] [PubMed] [Google Scholar]
- Dutil EM, Newton AC. Dual role of pseudosubstrate in the coordinated regulation of protein kinase C by phosphorylation and diacylglycerol. J Biol Chem. 2000;275:10697–10701. doi: 10.1074/jbc.275.14.10697. [DOI] [PubMed] [Google Scholar]
- Garcia-Rocha M, Avila J, Lozano J. The zeta isozyme of protein kinase C binds to tubulin through the pseudosubstrate domain. Exp Cell Res. 1997;230:1–8. doi: 10.1006/excr.1996.3364. [DOI] [PubMed] [Google Scholar]
- Puls A, Schmidt S, Grawe F, Stabel S. Interaction of protein kinase C zeta with ZIP, a novel protein kinase C-binding protein. Proc Natl Acad Sci USA. 1997;94:6191–6196. doi: 10.1073/pnas.94.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai JG, Murakami K. Constitutively and autonomously active protein kinase C associated with 14-3-3 zeta in the rodent brain. J Neurochem. 2003;84:23–34. doi: 10.1046/j.1471-4159.2003.01254.x. [DOI] [PubMed] [Google Scholar]
- Alexandrov A, Keffel S, Goepel M, Michel MC. Stimulation of alpha1A-adrenoceptors in Rat-1 cells inhibits extracellular signal-regulated kinase by activating p38 mitogen-activated protein kinase. Mol Pharmacol. 1998;54:755–760. doi: 10.1124/mol.54.5.755. [DOI] [PubMed] [Google Scholar]
- Mukherjee JJ, Chung T, Ways DK, Kiss Z. Protein kinase C alpha is a major mediator of the stimulatory effect of phorbol ester on phospholipase D-mediated hydrolysis of phosphatidylethanolamine. J Biol Chem. 1996;271:28912–28917. doi: 10.1074/jbc.271.46.28912. [DOI] [PubMed] [Google Scholar]
- Parmentier JH, Ahmed A, Ruan Y, Gandhi GK, Saeed AE, Malik KU. Calcium and protein kinase C (PKC)-related kinase mediate alpha 1A-adrenergic receptor-stimulated activation of phospholipase D in rat-1 cells, independent of PKC. J Pharmacol Exp Ther. 2002;303:1206–1215. doi: 10.1124/jpet.102.041384. [DOI] [PubMed] [Google Scholar]
- Singer WD, Brown HA, Jiang X, Sternweis PC. Regulation of phospholipase D by protein kinase C is synergistic with ADP-ribosylation factor and independent of protein kinase activity. J Biol Chem. 1996;271:4504–4510. doi: 10.1074/jbc.271.13.7412. [DOI] [PubMed] [Google Scholar]
- Bandhyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Reed BC, Dikic I, Farese RV. Glucose activates protein kinase C-zeta/lambda through proline-rich tyrosine kinase-2, extracellular signal-regulated kinase, and phospholipase D: a novel mechanism for activating glucose transporter translocation. J Biol Chem. 2001;276:35537–35545. doi: 10.1074/jbc.M106042200. [DOI] [PubMed] [Google Scholar]
- Melendez AJ, Harnett MM, Allen JM. Crosstalk between ARF6 and protein kinase C alpha in Fc(gamma)RI-mediated activation of phospholipase D1. Curr Biol. 2001;11:869–874. doi: 10.1016/S0960-9822(01)00260-3. [DOI] [PubMed] [Google Scholar]
- Mwanjewe J, Spitaler M, Ebner M, Windegger M, Geiger M, Kampfer S, Hofmann J, Uberall F, Grunicke HH. Regulation of phospholipase D isoenzymes by transforming Ras and atypical protein kinase C-iota. Biochem J. 2001;359:211–217. doi: 10.1042/0264-6021:3590211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier JH, Smelcer P, Pavicevic Z, Basic E, Idrizovic A, Estes A, Malik KU. PKC-ζ mediates norepinephrine-induced phospholipase D activation and cell proliferation in VSMC. Hypertension. 2003;41:794–800. doi: 10.1161/01.HYP.0000047873.76255.0B. [DOI] [PubMed] [Google Scholar]
- Pettitt TR, McDermott M, Saqib KM, Shimwell N, Wakelam MJO. Phospholipase D1b and D2a generate structurally identical phosphatidic acid species in mammalian cells. Biochem J. 2001;360:707–715. doi: 10.1042/0264-6021:3600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier JH, Muthalif MM, Saeed AE, Malik KU. Phospholipase D activation by norepinephrine is mediated by 12(s)-, 15(s)-, and 20-hydroxyeicosatetraenoic acids generated by stimulation of cytosolic phospholipase A2. tyrosine phosphorylation of phospholipase D2 in response to norepinephrine. J Biol Chem. 2001;276:15704–15711. doi: 10.1074/jbc.M011473200. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- Ballou LM, Cross ME, Huang S, McReynolds EM, Zhang BX, Lin R. Differential regulation of the phosphatidylinositol 3-kinase/Akt and p70 S6 kinase pathways by the alpha(1A)-adrenergic receptor in rat-1 fibroblasts. J Biol Chem. 2000;275:4803–4809. doi: 10.1074/jbc.275.7.4803. [DOI] [PubMed] [Google Scholar]
- Paramio JM, Segrelles C, Ruiz S, Jorcano JL. Inhibition of protein kinase B (PKB) and PKC zeta mediates keratin K10-induced cell cycle arrest. Mol Cell Biol. 2001;21:7449–7459. doi: 10.1128/MCB.21.21.7449-7459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou MM, Hou W, Johnson J, Graham LK, Lee MH, Chen CS, Newton AC, Schaffhausen BS, Toker A. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–77. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–5. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- Smith L, Smith JB. Lack of constitutive activity of the free kinase domain of protein kinase C zeta. Dependence on transphosphorylation of the activation loop. J Biol Chem. 2002;277:45866–45873. doi: 10.1074/jbc.M206420200. [DOI] [PubMed] [Google Scholar]
- Le Good JA, Brindley DN. Molecular mechanisms regulating PKC-ζ turnover and cellular transformation. Biochem J. 2003. [DOI] [PMC free article] [PubMed]
- Diaz-Meco MT, Municio MM, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–786. doi: 10.1016/S0092-8674(00)80152-X. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco MT, Moscat J. MEK5: a new target of the atypical protein kinase C isoforms in mitogenic signaling. Mol Cell Biol. 2001;21:1218–1227. doi: 10.1128/MCB.21.4.1218-1227.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000;1:399–403. doi: 10.1093/embo-reports/kvd098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki A, Akimoto K, Ohno S. Protein kinase C λ/ι (PKCλ/ι): A PKC isotype essential for the development of multicellular organisms. J Biochem. 2003;133:9–16. doi: 10.1093/jb/mvg018. [DOI] [PubMed] [Google Scholar]
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]