Summary
Objective
The periaqueductal gray matter (PAG), a known modulator of somatic pain transmission, shows evidence of interictal functional and structural abnormalities in migraineurs, which may contribute to hyperexcitability along spinal and trigeminal nociceptive pathways, and lead to the migraine attack. The aim of this study was to examine functional connectivity of the PAG in migraine.
Methods
Using resting-state functional MRI, we compared functional connectivity between PAG and a subset of brain areas involved in nociceptive/somatosensory processing and pain modulation in 17 subjects with migraine, during a pain free state, versus 17 gender- and age-matched controls. We also assessed the relation between intrinsic resting-state correlations within PAG networks and the average monthly frequency of migraine attacks, as well as allodynia.
Results
Our findings show stronger connectivity between the PAG and several brain areas within nociceptive and somatosensory processing pathways in migraineurs versus controls. In addition, as the monthly frequency of migraine attacks worsens, the strength of the connectivity in some areas within these pathways increases while a significant decrease in functional resting-state connectivity between the PAG and brain regions with a predominant role in pain modulation (prefrontal cortex, anterior cingulate, amygdala) can be evidenced. Finally, migraineurs with a history of allodynia exhibit significantly reduced connectivity between PAG, prefrontal regions and anterior cingulate compared to migraineurs without allodynia.
Conclusions
These data reveal interictal dysfunctional dynamics within pain pathways in migraine manifested as an impairment of the descending pain modulatory, likely leading to loss of pain inhibition, and hyperexcitability primarily in nociceptive areas.
Keywords: periaqueductal gray, connectivity, resting-state, functional MRI, migraine
Background
Current concepts of migraine suggest that a broad sensory processing dysfunction characterizes this common neurological disorder, and identify the periaqueductal gray matter (PAG), a known modulator of somatic pain transmission, as one of the key areas in the pathophysiology of migraine headache. The relevance of the PAG involved in the pathogenesis of migraine is further supported by reports of subjects without headache who developed migraine-like episodes after stereotactic placement of electrodes in this area of brainstem1, 2. Abnormalities in the PAG of migraineurs are often paralleled by structural changes in functionally and/or anatomically connected brain regions involved in pain processing and modulation3-9.
A prevailing theory in the pathogenesis of the migraine attack is that hyperexcitability develops along the trigeminovascular pathway, probably facilitated by a dysfunction of the descending pain modulatory circuits. Functional MRI (fMRI) studies of the interictal phase of migraine show hypofunction of pain modulatory circuits to thermal stimuli, and a specific involvement of the nucleus cuneiformis (NCF) in the brainstem10, a structure extensively connected to the PAG and sharing its modulatory effects on pain perception. Interictal altered dynamics within pain pathways might therefore contribute to the development of the migraine attack. Task-fMRI findings, however, reflect task-related increases of neuronal metabolism that are small, less than 5% compared to the metabolism of the brain at rest11. Thus, it is critical to take into account brain activity that occurs in the absence of external stimulation in order to understand better how pain networks function and adapt in migraineurs. Clarifying this aspect will have important implications for understanding the pathogenesis of migraine, and for disclosing similarities and potential differences with other chronic pain conditions.
During migraine attacks, in about two-thirds of patients, allodynia, the perception of pain to normally innocuous stimulation, may manifest12. Migraineurs with allodynia show a significant drop in pain thresholds to mechanical and thermal stimulation of cephalic and extracephalic skin, a phenomenon not observed in the absence of migraine12, 13. Experimental studies have demonstrated that the neurophysiological correlate of allodynia is sensitization along the trigemino-vascular pathway, an increased afferent barrage for an unchanged peripheral stimulus14, 15. Speculations exist whether this sensitization can be indirectly activated through pain modulatory neurons in the brainstem.
We used resting-state fMRI, a technique that allows to identify correlations during rest between remote brain areas (functional connectivity) through their highly correlated low frequency spontaneous fluctuations16, to investigate intrinsic connectivity within the PAG in 17 patients with episodic migraine during a pain free period (i.e. the interictal phase) and in 17 gender- and age-matched healthy controls. Given that connectivity within pain pathways may be dysfunctional outside episodes of migraine pain, even in the absence of external pain stimulation, and that accumulating evidence suggests that this might lead to the development of the migraine attack, we focused our approach on a subset of brain areas involved in the processing and modulation of somatosensory and pain signals. We tested the hypotheses that: 1) intrinsic connectivity within the PAG and either somatosensory/pain processing and modulatory pathways would be dysfunctional in migraineurs relative to age-matched healthy subjects; and that 2) these alterations would be associated with disease severity as measured by the frequency of migraine attacks per month. A further aim was to assess functional connectivity differences within PAG circuits between patients who reported allodynia during the migraine attack and patients without allodynia.
Methods
Subjects
Demographic and clinical characteristics of migraineurs are shown in Table 1. Seventeen subjects with migraine (15 females, mean±SD age= 32.4±8.2 years, median, range disease duration= 16.6, 1-30 years), eight with migraine with aura (MWA), and nine with migraine without aura (MWoA), as defined by the IHS diagnostic criteria17, were included in the study. All patients were migraine free for at least 72 hours at the time of MRI, had no other medical or psychiatric condition than migraine, no history of medications overuse, and without any prophylactic or chronic medication for at least 1 year prior to study entry. All migraineurs included in the study had a normal neurological examination.
Table 1.
Demographic and clinical characteristics of migraineurs.
| Patient# | Age (years) | Gender | Disease duration (years) | Migraines frequency (per month) | Aura | Allodynia |
|---|---|---|---|---|---|---|
| MIG113 | 18 | Female | 8 | 3 | No | No |
| MIG108 | 23 | Female | 17 | 3 | Yes | No |
| MIG110 | 24 | Female | 13 | 3 | No | No |
| MIG116 | 24 | Female | 2.5 | 4 | Yes | No |
| MIG118 | 24 | Female | 12 | 1 | No | No |
| MIG117 | 29 | Female | 12 | 1 | Yes | Yes |
| MIG105 | 30 | Female | 25 | 4 | No | No |
| MIG119 | 31 | Female | 16 | 5 | No | Yes |
| MIG107 | 33 | Female | 20 | 5 | Yes | Yes |
| MIG111 | 34 | Female | 20 | 1 | Yes | No |
| MIG112 | 34 | Female | 8.5 | 3 | No | No |
| MIG109 | 37 | Female | 30 | 1 | No | No |
| MIG104 | 38 | Male | 22 | 1 | Yes | Yes |
| MIG106 | 39 | Female | 1 | 3 | Yes | No |
| MIG101 | 42 | Female | 15 | 2 | No | No |
| MIG102 | 44 | Male | 30 | 15 | No | No |
| MIG120 | 47 | Female | 30 | 3 | Yes | Yes |
Seventeen gender- and age-matched healthy subjects (15 females, mean±SD age= 31.9±8.45 years) with no history of migraine or other headache types, and free from any chronic medical condition and any chronic treatment were included as controls.
The local ethics committee of our Institution approved all experimental procedures of the study, and written informed consent was obtained from each study participant.
Acquisition
All subjects underwent anatomical and functional scanning on a 3 Tesla Tim Trio scanner (Siemens, Erlangen) with a 32-channel Siemens head-coil. Single-shot EPI images (47, 3×3×3mm3 slices, TR/TE=3000/30ms, flip angle 90°, 160 volumes) were acquired for functional resting state analysis. During resting state all subjects were asked to keep their eyes closed. Anatomical data consisted of a high-resolution structural 3D scan with a magnetization-prepared rapid acquisition with multiple gradient echoes (MEMPR) sequence resolution (0.9 × 0.9 × 0.9 mm3, TI=1200 ms, TR=2530 ms, flip angle=7°, TE, 1.7, 3.58, 5.46 and 7.34 milliseconds, FoV=230 mm, bandwidth=651 Hz/px). We also acquired in each control and in 16 out of 17 migraineurs T2 turbo-spin-echo (TSE) weighted images (TR/TE=11810/95 ms, flip angle=120°, matrix size= 320×328, 63 2 mm thick slices, 2 averages) for identification of white matter (WM) lesions. None of the subjects enrolled in the study showed brain WM hyperintensities. Finally, none of the patients developed a migraine during the entire scan and in the following hour during which they underwent neurological examination.
Post-processing
Images were processed using FMRIB software library (FSL, http://www.fmrib.ox.ac.uk/fsl/). Data were motion corrected to the middle frame in each series using MCFLIRT 18 and spatially smoothed with a 6mm FWHM 3D Gaussian kernel using FSL’s SUSAN. Non-brain areas of the scan were removed using BET 19. Grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor was performed, as well as high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=60.0s). Each time series was prewhitened 20. Independent Component Analysis (ICA) using MELODIC was run to remove structured noise from the data. Registration of functional and anatomical data was performed using FLIRT. Nonlinear registration using FNIRT was applied between the subject’s structural and the standard space (the MNI_2mm brain). Seeds were manually selected in standard space based on the anatomy. The right and left PAG were selected as regions of interest (peak MNI coordinates: left PAG= -2; -28; -6; right PAG= 4; -28; -6, with 3 mm radius). We chose these seed locations based on our previous findings of interictal fractional anisotropy changes in the PAG in another population of migraineurs. Average time courses were extracted from these seeds using FSL’s featquery function. The preprocessed time series were then fitted with a linear model consisting of regressor representing the extracted time courses. A temporal derivative of that regressor was entered into the model, to allow the general linear model (GLM) to compensate for latency offsets in the hemodynamic response of different brain regions, and to compensate for slice timing differences in the acquisition. In addition, motion correction parameters were used as distractor regressors. The spatially normalized effect size and standard error volumes served as input to a mixed effects group analysis in FSL’s feat based on the method described by Beckman et al., 21. The modeled group effect size and standard error were then divided to produce a volume whose voxels were T scores, subsequently transformed to Z scores. Images were thresholded using clusters determined by Z>2.3 and a corrected cluster significance threshold of p=0.05, including at least 20 contiguous voxels 22.
The thresholded Z maps were then sampled on the cortical surface of an individual subject using the inverse coordinate transformation between this individual’s native space and the group MNI space. Within- and between-group comparisons of correlation effect size were performed using one sample t-test in each group and unpaired t-test in controls versus migraineurs. Correlation analysis with the monthly frequency of migraine was performed by including the mean number of migraine attacks of each patient as a regressor. Since we did not detect laterality differences between the two seeds used, results for left and right PAG were grouped together.
Results
Results of intrinsic functional connectivity of the PAG in migraineurs and controls are shown in Table A - Supplemental data and Figure 1. In both groups, we found predominantly positive correlations between PAG and nearby structures including the brainstem (mostly the midbrain), thalamus, putamen, parahippocampus, amygdala (only in controls), cerebellum, and with distant regions including the anterior cingulate, frontal and temporal cortex, and left insula (only in controls), although correlation with these cortical areas was more prevalent in migraineurs than in controls. Migraineurs showed additional positive correlations in the precentral and postcentral cortex, and angular gyri. There were no negative correlations in migraineurs, while controls showed significant negative correlations between the PAG and the caudal brainstem and central opercular cortex.
Figure 1.
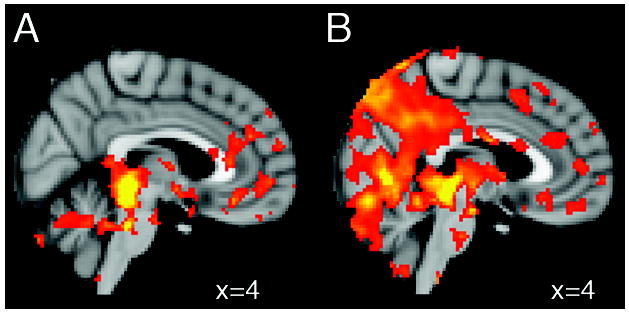
Sagittal sections of the MNI brain illustrating representative brain regions that show positive functional resting state connectivity with the periaqueductal gray in (A) 17 healthy subjects and in (B) 17 age- and gender-matched patients with migraine, interictally. Statistical threshold corresponds to Z>5.0
The comparison between migraineurs and controls revealed in patients significantly increased functional resting-state correlations (Z>2.3, corrected cluster p=0.05) between the PAG and several cortical regions primarily involved in nociception and somatosensory processing compared to age-matched controls (Table 2, Figure A – Supplemental data). Seeds with a 3 mm radius were selected in a subset of these areas using the peak MNI coordinates in Table 2. Average time courses were extracted from each seed using FSL’s featquery function and correlation analysis with both right and left PAG time courses was performed using linear regression. The resulting r-values were entered into single sample t-tests with the null hypothesis of 0 for the mean r-value of the group using Bonferroni correction for multiple comparisons (Table B – Supplemental data). We did not find any increased intrinsic connectivity between PAG and other pain regions in controls compared to migraineurs.
Table 2.
Peak locations and significance of brain areas with increased connectivity with the periaqueductal gray (PAG) during resting state fMRI in 17 patients with migraine compared to 17 gender- and age-matched controls.
| Location | PAG
|
|
|---|---|---|
| MNI coordinates (x, y, z) | Z | |
| R Ventrolateral prefrontal cortex | 28, 58, -16 | 2.49 |
| R Supramarginal gyrus | 56, -44, 34 | 2.93 |
| 64, -36, 44 | 2.83 | |
| 52, -40, 26 | 2.73 | |
| R Anterior insula | 40, 14, -10 | 2.3 |
| R Precentral gyrus (M1) | 42, 0, 56 | 2.9 |
| R Postcentral gyrus (S1) | 46, -34, 52 | 2.45 |
| R Thalamus | 10, -14, 4 | 2.55 |
| L Angular gyrus | -56, -52, 44 | 3.24 |
| L Supramarginal gyrus/Parietal operculum | -58, 52, 44 | 3.17 |
| (S2) | -10, 10, 52 | 3.0 |
| L Precentral gyrus (M1) | -44, 8, 28 | 2.53 |
| -44, 6, 28 | 2.32 | |
R=right; L=left; M1=primary motor cortex; S1=primary somatosensory cortex; S2=secondary somatosensory cortex Z>2.3, corrected cluster significance of p=0.05 corresponding to at least 20 contiguous voxels.
In patients, we found that the higher the monthly frequency, the greater the connectivity between the PAG and some of the brain areas that showed significantly higher correlations compared to controls (Table 3, Fig.2). We observed a similar positive correlation between the monthly frequency of migraine episodes and resting state correlations between the PAG the nucleus NCF and the hypothalamus. (Fig.2)
Table 3.
Brain areas where resting state connectivity with the periaqueductal gray (PAG) positively correlates with the frequency of migraine attacks in 17 patients with migraine.
| Location | PAG
|
|
|---|---|---|
| MNI coordinates (x, y, z) | Z | |
| R Supramarginal gyrus | 68, -38, 38 | 3.03 |
| 60, -44, 50 | 2.84 | |
| R Ventral prefrontal cortex | 20, 64, -16 | 3.14 |
| R Anterior Insula | 42, 16, 0 | 3 |
| L Supramarginal gyrus / Parietal operculum (S2) | -56, 44, 26 | 2.78 |
| -54, -44, 28 | 2.6 | |
| L Nucleus Cuneiformis | -4, -38, -22 | 2.47 |
| -6, -38, 20 | 2.83 | |
| Hypothalamus | 2, -18, -14 | 2.34 |
R=right; L=left; S2=secondary somatosensory cortex; PAG= periaqueductal gray; Z>2.3, corrected cluster significance of p=0.05 corresponding to at least 20 contiguous voxels.
Figure 2.
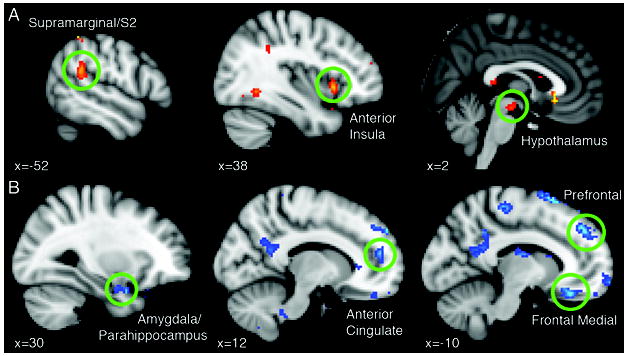
Sagittal sections of the MNI brain showing in 17 patients with migraine, interictally, A) brain areas where increased functional resting-state connectivity with the periaqueductal gray (PAG) positively correlates with the frequency of migraine attacks; B) brain regions where decreased functional connectivity with the PAG is associated with an increased frequency of migraine attacks. Statistical threshold corresponds to Z>2.3 and a corrected cluster significance of p=0.05, including clusters with at least 20 contiguous voxels.
Interestingly, an increased monthly frequency of migraine attacks was associated with reduced functional connectivity between the PAG and several regions of the prefrontal cortex, anterior cingulate, amygdala, medial thalamus (Table 4, Fig. 2).
Table 4.
Brain areas where resting state connectivity with the periaqueductal gray (PAG) negatively correlates with the frequency of migraine attacks in 17 patients with migraine.
| Location | PAG
|
|
|---|---|---|
| MNI coordinates (x, y, z) | Z | |
| R Dorsomedial prefrontal cortex | 16, 56, 34 | -3.29 |
| R Postcentral gyrus (S1) | 36, -24, 52 | -2.79 |
| R Precentral gyrus (M1) | -8, -28, 58 | -2.99 |
| R Anterior Cingulate | 6, 52, 16 | -2.83 |
| R Parahippocampal gyrus/Amygdala | 16, 2, -28 | -3.08 |
| L Dorsomedial prefrontal cortex | -14, 48, 42 | -3.91 |
| L Medial prefrontal cortex | -10, 68, -10 | -2.61 |
| L Precentral gyrus (M1) | -50, -4, 38 | -3.14 |
| L Angular gyrus | -40, -52, 14 | 4.13 |
| L Medial Thalamus | -8, -8, 16 | 2.54 |
R=right; L=left; M1=primary motor cortex; S1=primary somatosensory cortex; Z>2.3, corrected cluster significance of p=0.05 corresponding to at least 20 contiguous voxels.
Subjects with migraine were asked to fill out a questionnaire to determine if they usually developed allodynia during the migraine attack. Five subjects reported a history of either cephalic or extra-cephalic allodynia during migraines. The comparison of functional resting-state connectivity in these patients (mean±SD age= 35.6±7.2 years) versus five gender- ad age-matched migraineurs with no history of allodynia (mean±SD age= 36.8±5.9 years) showed that migraineurs who develop pain to normally innocuous stimulation have significantly reduced connectivity between PAG, prefrontal regions, anterior cingulate, and anterior insula compared to subjects without allodynia (Table 5, Fig. 3).
Table 5.
Brain areas showing significantly decreased connectivity with the periaqueductal gray (PAG) during resting state fMRI in five patients with allodynia compared to age-matched patients without allodynia.
| Location | PAG
|
|
|---|---|---|
| MNI coordinates (x, y, z) | Z | |
| R Dorsolateral prefrontal cortex | 36, 50, 6 | -2.8 |
| R Lateral prefrontal cortex | 44, 46, -14 | -2.61 |
| R Anterior Cingulate | 12, 36, 24 | -2.54 |
| L Dorsomedial prefrontal cortex | -18, 62, 20 | -2.55 |
| L Medial prefrontal cortex | -12, 62, -22 | -3.00 |
| L Anterior Insula | -34, 16, 10 | -2.15 |
R=right; L=left; Z>2.3, corrected cluster significance of p=0.05 corresponding to at least 20 contiguous voxels.
Figure 3.
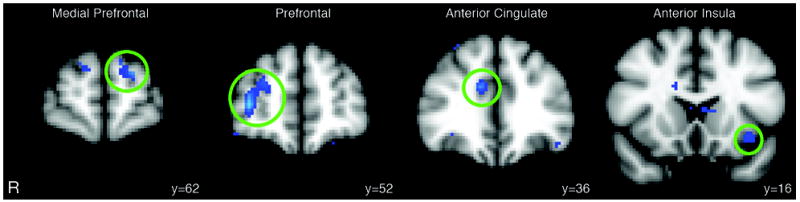
Coronal views of the MNI showing brain areas (Z>2.3 and a corrected cluster significance of p=0.05 corresponding to at least 20 contiguous voxels) of reduced functional resting-state connectivity with the periaqueductal gray in five migraineurs with a history of allodynia compared to five age- and gendermatched migraineurs without any history of allodynia development during the migraine attack. R= right.
Discussion
Our findings demonstrate an interictal increase in resting-state intrinsic connectivity between the PAG and both nociceptive and sensory processing pathways in migraineurs relative to age and gender- matched controls. Connectivity in some of these pathways was stronger as the monthly frequency of migraine attacks increased. Conversely, the greater the number of attacks was, the lower the functional resting-state connectivity appeared to be between the PAG and several brain regions with a predominant role in pain modulation (prefrontal cortex, anterior cingulate, amygdala). Since the occurrence of the next migraine attack was not recorded, whether the observed association between migraine frequency and changes in PAG connectivity reflects either an adaptive mechanism or an abnormal status in cortical excitability that predisposes to a migraine attack needs to be further clarified. Excitability in the visual cortex has been shown to increase interictally in pediatric MWoA, although it tends to decrease or normalize in the peri-ictal period23.
Previous neuroimaging findings indicate that the brainstem covers a crucial role in acute migraine24, and positron emission tomography (PET) studies report metabolic changes in either the dorsal rostral brainstem or in the locus coeruleus and dorsal raphe nucleus of migraineurs25, 26. Dysfunction of the regulation of the brainstem pain-inhibiting circuitry, of which the PAG is best known, may provide an explanation for many of the facets of migraine headache.
Compared to healthy subjects, migraineurs showed, in the absence of any pain, significantly greater intrinsic connectivity between PAG in several brain regions including thalamus, posterior parietal cortex (angular and supramarginal gyri), anterior insula, S1, M1, and partly S2. While S1, S2, anterior insula, and thalamus are part of the fundamental core network found active by several neuroimaging studies in different pain conditions activity in other regions including the posterior parietal cortex and M1 has also been reported in nociception27. These findings could reflect a condition of hyperexcitability of pain pathways within the central nervous system (CNS), which is thought to represent a crucial event in the pathophysiology of migraine. Transcranial magnetic stimulation (TMS), and magneto-encephalography (MEG) studies have shown generalized interictal changes of excitability in the cerebral cortex in migraine with alteration of excitability in the motor and visual cortices 28-33. In addition, in patients with unilateral migraine, in a headache-free condition, diffuse hypersensitivity of the peripheral nerves has been measured, leading to further evidence to the presence of a state of hyperexcitability of the CNS of migraineurs34. Interestingly, central hyperexcitability does not seem to be a feature of chronic either tension type or cervicogenic headaches, which are characterized by different pathogenetic and clinical features than migraine35.
A systematic investigation of intrinsic connectivity patterns of the PAG by resting-state fMRI in a large cohort of healthy subjects reports that PAG activity is positively correlated with surrounding subcortical brain regions including midbrain tegmentum, substantia nigra, raphe nucleus, thalamus, and hypothalamus, as well as with cortical regions including the anterior cingulate, and anterior insula36. In our study, in some of these areas (anterior insula, NCF in the midbrain, and hypothalamus) the strength of intrinsic connectivity with PAG positively correlated with disease severity as measured by the number of migraine episodes per month. Additional areas involved in nociception and somatosensory processing including S2 and the posterior parietal cortex also showed increased resting-state connectivity with PAG with increasing in the monthly frequency of migraine attacks.
The finding that hypothalamic intrinsic correlations with PAG are related to the frequency of migraine attacks extends previous PET data that have first described activation of the hypothalamus in migraineurs during the headache phase, which persisted after headache remission with sumatriptan37. While specific hypothalamic activations have been consistently observed in cluster headache, and linked to the autonomic manifestations of trigeminal autonomic cephalalgias, the role of the hypothalamus in migraine has not been clarified yet24. The hypothalamus is integrated in the hypothalamic-pituitary-adrenal (HPA) axis, which is also involved in mood disorders such as stress and anxiety. In addition to pain modulation, the PAG is well known to participate in mood and emotion regulation, which may be carried out through a pathway involving the HPA axis also36. In this context, although subjects with depression were excluded from our study, an increase in the intrinsic correlations between PAG and hypothalamus in relation to the frequency of migraine attacks can be viewed as a part of a stress/anxiety response of the brain to worsening of the disease. Behavioral data assessing anxiety levels in patients with migraine may help to clarify this aspect.
In migraineurs, we also found that the greater the frequency of migraine episodes was, the lower the resting-state functional connectivity appeared to be between PAG and several brain foci, mostly located in the bilateral prefrontal (medial, dorsomedial) cortex, but also in the anterior cingulate and amygdala, both involved in descending pain modulation and nociception27, and in somatosensory processing regions including the angular gyrus (posterior parietal cortex), right S1, and M1. The pattern, however, was clearly dominated by a widespread reduced connectivity between PAG and prefrontal cortex that correlated with the increase in migraine attacks frequency. Pain studies have emphasized the role of prefrontal areas in controlling the functional interactions among key nociceptive brain regions in order to modify the perceptual correlates of pain, specifically by driving endogenous pain-inhibitory mechanisms27, 38. Our findings could therefore indicate an interictal dysfunction of the descending inhibitory system that in turns contributes to the development of migraines. TMS and PET imaging demonstrated that in patients with chronic migraine increase in visual cortical excitability is accompanied by brainstem activation and inhibition of specific cortical areas including the medial frontal and parietal cortex, and somatosensory area, suggesting a potential dysfunction of inhibitory pathways39. Interestingly, this pattern of significantly reduced connectivity between PAG, prefrontal cortex, and anterior cingulate was also observed in migraineurs with allodynia compared to migraineurs without allodynia, corroborating the hypothesis that pain modulatory neurons might be involved in the development of allodynia.
Taken together, our data demonstrate the presence of interictal dysfunctional dynamics within PAG networks in episodic migraine. Since resting brain activity is either increased or decreased within multiple networks in other chronic pain conditions including fibromyalgia40 and diabethic neuropatic pain41, future studies may clarify whether the changes that we observed within the PAG networks can be interpreted as a “brain signature” of migraine; or rather they are shared by other chronic pain conditions.
Supplementary Material
Figure A. Sagittal sections of the MNI brain illustrating brain regions of significantly increased functional resting-state connectivity with the periaqueductal gray in 17 subjects with migraine, interictally, relative to 17 age- and gender-matched healthy controls. Statistical threshold corresponded to Z>2.3 and a corrected cluster significance of p=0.05, including clusters with at least 20 contiguous voxels.
Acknowledgments
We thank Nils Rettby for his help with MRI data collection. This work was supported by NIH grant 5P01 NS 35611-09.
References
- 1.Raskin NH, Hosobuchi Y, Lamb S. Headache may arise from perturbation of brain. Headache. 1987;27:416–420. doi: 10.1111/j.1526-4610.1987.hed2708416.x. [DOI] [PubMed] [Google Scholar]
- 2.Veloso F, Kumar K, Toth C. Headache secondary to deep brain implantation. Headache. 1998;38:507–515. doi: 10.1046/j.1526-4610.1998.3807507.x. [DOI] [PubMed] [Google Scholar]
- 3.Rocca MA, Ceccarelli A, Falini A, et al. Diffusion tensor magnetic resonance imaging at 3.0 tesla shows subtle cerebral grey matter abnormalities in patients with migraine. J Neurol Neurosurg Psychiatry. 2006;77:686–689. doi: 10.1136/jnnp.2005.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DaSilva AF, Granziera C, Tuch DS, et al. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport. 2007;18:301–305. doi: 10.1097/WNR.0b013e32801776bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69:1990–1995. doi: 10.1212/01.wnl.0000291618.32247.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Suh SI, Seol HY, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28:598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Wilcke T, Ganssbauer S, Neuner T, et al. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28:1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz N, Admiraal-Behloul F, Arkink EB, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008;48:1044–1055. doi: 10.1111/j.1526-4610.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- 9.Valfre W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48:109–117. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 10.Moulton EA, Burstein R, Tully S, et al. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3:e3799. doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 12.Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]
- 13.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–158. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(Pt 8):1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 15.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 16.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 17.IHC. The International Classification of Headache Disorders. Cephalalgia. (2) 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 18.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 19.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 20.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 21.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 22.Worsley KJ, Chen JI, Lerch J, Evans AC. Comparing functional connectivity via thresholding correlations and singular value decomposition. Philos Trans R Soc Lond B Biol Sci. 2005;360:913–920. doi: 10.1098/rstb.2005.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siniatchkin M, Reich AL, Shepherd AJ, et al. Peri-ictal changes of cortical excitability in children suffering from migraine without aura. Pain. 2009;147:132–140. doi: 10.1016/j.pain.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 24.May A. New insights into headache: an update on functional and structural imaging findings. Nat Rev Neurol. 2009;5:199–209. doi: 10.1038/nrneurol.2009.28. [DOI] [PubMed] [Google Scholar]
- 25.Bahra A, Matharu MS, Buchel C, et al. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–1017. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- 26.Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- 27.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Aurora SK, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia. 2007;27:1442–1453. doi: 10.1111/j.1468-2982.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- 29.Bowyer SM, Mason KM, Moran JE, et al. Cortical hyperexcitability in migraine patients before and after sodium valproate treatment. J Clin Neurophysiol. 2005;22:65–67. doi: 10.1097/01.wnp.0000150928.23523.a9. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Xiang J, Wang Y, et al. Identification of abnormal neuromagnetic signatures in the motor cortex of adolescent migraine. Headache. 2010;50:1005–1016. doi: 10.1111/j.1526-4610.2010.01674.x. [DOI] [PubMed] [Google Scholar]
- 31.van der Kamp W, Maassen VanDenBrink A, Ferrari MD, van Dijk JG. Interictal cortical hyperexcitability in migraine patients demonstrated with transcranial magnetic stimulation. J Neurol Sci. 1996;139:106–110. doi: 10.1016/s0022-510x(96)00044-5. [DOI] [PubMed] [Google Scholar]
- 32.van der Kamp W, MaassenVanDenBrink A, Ferrari MD, van Dijk JG. Interictal cortical excitability to magnetic stimulation in familial hemiplegic migraine. Neurology. 1997;48:1462–1464. doi: 10.1212/wnl.48.5.1462. [DOI] [PubMed] [Google Scholar]
- 33.Hoffken O, Stude P, Lenz M, et al. Visual paired-pulse stimulation reveals enhanced visual cortex excitability in migraineurs. Eur J Neurosci. 2009;30:714–720. doi: 10.1111/j.1460-9568.2009.06859.x. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-de-las-Penas C, Arendt-Nielsen L, Cuadrado ML, Pareja JA. Generalized mechanical pain sensitivity over nerve tissues in patients with strictly unilateral migraine. Clin J Pain. 2009;25:401–406. doi: 10.1097/AJP.0b013e31819655b3. [DOI] [PubMed] [Google Scholar]
- 35.Uthaikhup S, Sterling M, Jull G. Widespread sensory hypersensitivity is not a feature of chronic headache in elders. Clin J Pain. 2009;25:699–704. doi: 10.1097/AJP.0b013e3181a38f88. [DOI] [PubMed] [Google Scholar]
- 36.Kong J, Tu PC, Zyloney C, Su TP. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav Brain Res. 2010;211:215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denuelle M, Fabre N, Payoux P, et al. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47:1418–1426. doi: 10.1111/j.1526-4610.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 38.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- 39.Aurora SK, Barrodale PM, Tipton RL, Khodavirdi A. Brainstem dysfunction in chronic migraine as evidenced by neurophysiological and positron emission tomography studies. Headache. 2007;47:996–1003. doi: 10.1111/j.1526-4610.2007.00853.x. discussion 1004-1007. [DOI] [PubMed] [Google Scholar]
- 40.Napadow V, LaCount L, Park K, et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cauda F, D’Agata F, Sacco K, et al. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 81:806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A. Sagittal sections of the MNI brain illustrating brain regions of significantly increased functional resting-state connectivity with the periaqueductal gray in 17 subjects with migraine, interictally, relative to 17 age- and gender-matched healthy controls. Statistical threshold corresponded to Z>2.3 and a corrected cluster significance of p=0.05, including clusters with at least 20 contiguous voxels.


