By retaining NKG2D ligands within tumor cells, carcinoembryonic antigen–related cell adhesion molecule 1 (CEACAM1) facilitates tumor cell escape from NK cell–mediated cytolysis in vitro and in vivo.
Abstract
Although carcinoembryonic antigen (CEA)–related cell adhesion molecule 1 (CEACAM1) has been viewed as a tumor suppressor, increasing clinical evidence shows that high levels of CEACAM1 expression on tumors correlates with poor prognosis and high risk of metastasis. Here, we examined the consequences of CEACAM1 expression on tumor cells. We show that tumor cell–associated CEACAM1 causes intracellular retention of various NKG2D ligands in mouse and human tumor cells. CEACAM1-silenced tumor cells expressed more cell surface NKG2D ligands and exhibited greater sensitivity to natural killer cell–mediated cytolysis in vitro and rejection in vivo. Our studies reveal a novel mechanism through which CEACAM1-bearing tumor cells may escape immune-surveillance.
Carcinoembryonic antigen (CEA)–related cell adhesion molecule 1 (CEACAM1) is a member of the CEA-family of Ig-like transmembrane proteins (Gray-Owen and Blumberg, 2006). CEACAM1 is expressed in a wide range of normal tissues and tumors. It is characterized by significant alternate splicing, which generates isoforms that differ in cytoplasmic tail (CT) length and the number of extracellular Ig-like domains; these isoforms are named accordingly. The majority of CEACAM1 isoforms possess either a long (CEACAM1-L) CT or a short (CEACAM1-S) CT. CEACAM1-L isoforms predominate in most cell types, and contain two immunoreceptor tyrosine-based inhibitory motifs (ITIMs; Gray-Owen and Blumberg, 2006). Early studies have shown that CEACAM1 expression is often lost in sporadic colorectal and prostate cancers in humans and, consistent with this, tumor size and number are increased in Ceacam1−/− mice exposed to azoxymethane administration (Leung et al., 2006). Therefore, CEACAM1-L has been labeled a tumor suppressor. However, more recent clinical studies in a wide variety of human tumors, including melanoma (Gambichler et al., 2009; Markel et al., 2010) and cancers of the lung (Obrink, 2008), pancreas (Simeone et al., 2007), bladder (Tilki et al., 2009), colon (Kang et al., 2007), and thyroid (Liu et al., 2007), have observed that high levels of CEACAM1 expression on tumor cells directly correlates with poor prognosis and tumor metastasis.
To seek a biological basis for these clinical observations, we investigated the consequences of tumor cell expression of CEACAM1 on immune-surveillance. We focused our attention on the interactions between CEACAM1 and a receptor–ligand system that is expressed on both tumor-infiltrating lymphocytes (TILs) and tumors; namely, NK gene complex group 2 (NKG2) member D (NKG2D, gene name KLRK1) and its ligands (NKG2DL). NKG2D is an activating NK receptor expressed on all NK cells and CD8+ T cells, as well as a set of CD4+ T cells (Lanier, 2008). NKG2D plays a pivotal role in NK cell– and T cell–mediated immunity against cancers and infections, as well as in the development of autoimmune diseases (Lanier, 2008). NKG2D exerts its effects through recognition of its cognate ligands on target cells, resulting in their cytolytic destruction.
The ligands that bind to human NKG2D are MHC class I–related molecule A and B (MICA and MICB) and UL-16–binding proteins (ULBPs). In mouse, NKG2D ligands (NKG2DL) include retinoic acid early inducible cDNA clone 1 (Rae-1), murine UL16-binding protein 1 (MULT1), and the H60 family of glycoproteins. Expression of NKG2DL on the cell surface is tightly regulated at transcriptional and posttranscriptional levels (Spies, 2008). Moreover, tumors or virally infected cells have evolved other mechanisms to escape NKG2D- and NKG2DL-mediated immune surveillance (Wu et al., 2003; Stern-Ginossar et al., 2007; Champsaur and Lanier, 2010). Tumor cells shed autonomous NKG2DL, leading to insensitivity of tumor cells to cytolysis mediated by NK cells and CD8+ T cells (Groh et al., 2002). Tumor cells also retain NKG2DL intracellularly (Fuertes et al., 2008). However, the mechanism is unknown.
Here, we show that, like cytomegalovirus (CMV)-derived proteins which retain and inhibit maturation of certain NKG2DL (Wu et al., 2003; Champsaur and Lanier, 2010), CEACAM1 can mediate a similar process in tumor cells, resulting in diminished sensitivity to NKG2D-dependent cytolysis by NK cells. In so doing, this study demonstrates that CEACAM1 attenuates antitumor immunity by disabling NKG2DL function on tumor cells.
RESULTS AND DISCUSSION
Tumor cell–autonomous CEACAM1 differentially regulates tumor cell sensitivity to NK cell–mediated cytolysis and tumor rejection
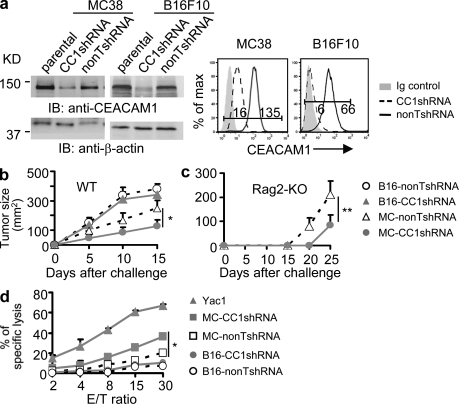
High expression of CEACAM1 on a variety of tumor cell types has been shown to directly correlate with a worse prognosis and risk of tumor metastasis (Sienel et al., 2003; Kang et al., 2007; Simeone et al., 2007; Dango et al., 2008; Xi et al., 2008; Tilki et al., 2009). We therefore sought to define a biological basis for this observation and examined the functional consequences of CEACAM1 expression on tumor cells. To this end, we silenced CEACAM1 expression on mouse tumor cell lines MC38 (colon cancer) and B16F10 (melanoma) derived from C57BL/6 (B6) mice. As shown (Fig. 1 a), both total and cell surface expression of CEACAM1 was decreased to a similar extent in MC38 and B16F10 cells that expressed CEACAM1-target short hairpin RNA (CC1shRNA), but not a control nontargeted shRNA (nonTshRNA). We then tested the effect of silencing tumor cell CEACAM1 on in vivo tumor growth. Interestingly, when subcutaneously inoculated into WT B6 mice, the growth of CEACAM1-silenced MC38 tumors (CC1shRNA) was significantly diminished in comparison to the growth of CEACAM1 nonsilenced (nonTshRNA) MC38 tumor cells, whereas silencing CEACAM1 on B16F10 tumor cells did not affect in vivo tumor growth (Fig. 1 b). Because T cells and NK cells play an important role in antitumor immunity, we conducted studies in RAG2-deficient mice, which lack T and B cells. As shown in Fig. 1 c, the tumor growth of CEACAM1-silenced MC38 cells in RAG2-deficient mice was retarded in comparison to the growth observed with nonsilenced MC38 cells. These studies in RAG2-deficient mice are consistent with an important role for NK cells in the enhanced immune protection against MC38 tumor cells.
Figure 1.
Silencing CEACAM1 differentially sensitizes tumor cells to NK cell–mediated in vitro cytolysis and in vivo tumor rejection. (a) Silencing CEACAM1 expression in mouse MC38 and B16F10 cells. (left) Immunoblot (IB) results. Parental, nontransduced cells; CC1shRNA, mouse CEACAM1 shRNA; nonTshRNA, control nontargeting shRNA. (right) Flow cytometric analysis of CEACAM1 surface expression. Ig, isotype-matched control Ig. The numbers in flow cytometric data are mean fluorescence intensity (MFI; applied to all flow cytometric data). (b and c) Tumor growth in wild type (WT; b) and Rag2−/− B6 (c) mice. Tumor size (two dimensions; n ≥ 6; mean ± SEM; *, P < 0.05; **, P < 0.01) measured by calipers. (d) Mouse NK cell–mediated cytotoxicity at various E/T (effector/target cells) ratios. Standard 51Cr release 4-h cytotoxicity assay and calculation of specific cytolysis percentages (triplicate; mean ± SEM; *, P < 0.05) was performed. Yac1 is a positive control for mouse NK cell–mediated cytolysis. All results are representative of three or four independent experiments.
To further confirm that down-regulation of CEACAM1 expression on MC38 cells results in increased sensitivity to NK cell–mediated immunity, we conducted in vitro cytotoxicity assays. Consistent with observations in vivo, CEACAM1-silenced MC38 cells exhibited increased sensitivity to primary naive NK cell–mediated cytolysis in comparison to nonsilenced MC38 cells (Fig. 1 d). Consistent with in vivo observations (Fig. 1 b), B16F10 cells, which are MHC class I–negative (Lim et al., 1998), were not lysed by NK cells (Fig. 1 d), indicating an insufficiency or absence of MHC class I–independent activating ligands on the cell surface of these cells or the presence of ligands for other inhibitory receptors on NK cells.
Because NK cells are capable of expressing CEACAM1 upon activation, which in turn is capable of inhibiting NK cell cytolytic function (Markel et al., 2002), it was important to examine CEACAM1 expression on the NK cells during the 4 h cytotoxicity assay. We observed no CEACAM1 expression on primary Ceacam1−/− and WT mice NK cells or on these cells after incubation with target cells during the cytotoxicity assay (unpublished data). In addition, NK cells from Ceacam1−/− and WT mice exhibited an equivalent increase in their ability to lyse CEACAM1-silenced MC38 cells (unpublished data). These studies show that the augmentation of NK cell–mediated lysis of CEACAM1-silenced MC38 cells was not a result of decreased inhibition caused by homophilic interactions between CEACAM1 on tumor cells and NK cells. Thus, CEACAM1 expression on MC38 cells was mediating a cell-autonomous decrease in the sensitivity to NK cell–mediated killing.
Tumor cell–autonomous CEACAM1 down-regulates NKG2DL expression and sensitivity to NKG2D-mediated cytolysis
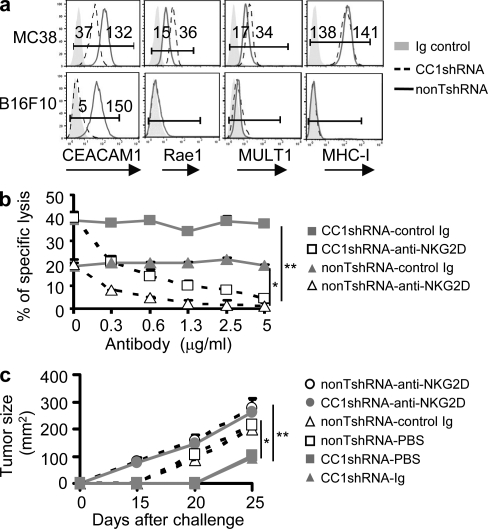
The aforementioned studies suggested that CEACAM1 expression on tumor cells caused decreased access to or expression of a tumor-associated ligand capable of activating NK cell cytolysis. Among such NK-activating receptors, NKG2D has well characterized ligands that are expressed on certain tumor cells and involved in antitumor immunity (Diefenbach et al., 2001; Lanier, 2008). Therefore, we investigated whether silencing CEACAM1 expression affected NKG2DL expression in MC38 and B16F10 cells and the effects that this had on NK cell–mediated cytolysis. Expression of Rae-1 and MULT1 was, indeed, observed on the cell surface of MC38 cells (Fig. 2 a). Moreover, silencing CEACAM1 expression in MC38 cells resulted in a further and significant increase in cell surface expression of Rae-1 and MULT1 (Fig. 2 a). Importantly, CEACAM1 silencing had no effect on the cell surface expression of MHC class I on MC38 cells (Fig. 2 a). In contrast, and consistent with the lack of sensitivity to NK cell–mediated cytolysis as a consequence of CEACAM1 silencing, no noteworthy expression of either NKG2DL (Rae-1 or MULT1) or MHC class I could be detected on the cell surface of CC1shRNA or nonTshRNA B16F10 cells (Fig. 2 a). These results show that CEACAM1 expression is associated with down-regulation of multiple NKG2DLs on the cell surface of M38 tumor cells.
Figure 2.
CEACAM1 regulates NKG2DL expression and NKG2D-mediated tumor cytolysis and rejection. (a) Flow cytometry analysis of CEACAM1, NKG2DL, and MHC-I expression on indicated tumor cells. CC1shRNA, mouse CEACAM1 shRNA; nonTshRNA, control nontargeting shRNA; Ig, isotype-matched control Ig. (b) Blocking assay for NKG2D-mediated in vitro cytolysis by indicated antibodies. 51Cr release assay results (triplicates; mean ± SEM; *, P < 0.05; **, P < 0.01). Effector cells were mouse primary NK cells. (c) Blocking assay for NKG2D-mediated in vivo tumor rejection was performed in Rag2−/− mice with the indicated antibodies (n = 6; mean ± SEM; *, P < 0.05; **, P < 0.01). All results are representative of three independent experiments
Therefore, we sought to confirm that the increased expression of NKG2DL as a consequence of CEACAM1 silencing directly contributed to the enhanced sensitivity of CEACAM1-silenced MC38 cells to NK cell–mediated cytolysis. To do so, we examined the in vitro lysis and in vivo rejection of CEACAM1-silenced MC38 cells in the presence and absence of anti-NKG2D blockade. As shown in Fig. 2 b, silencing CEACAM1 expression of MC38 cells resulted in increased sensitivity to NK cell–mediated cytolysis in comparison to nonsilenced MC38 cells. The enhanced lysis of silenced MC38 cells by NK cells was inhibited in a dose-dependent manner by a blocking anti–mouse NKG2D antibody, but not control antibody. In fact, the ability of NK cells to lyse MC38 cells in either the presence or absence of CEACAM1 silencing was totally dependent on NKG2D-mediated lysis, given the ability of 5 µg/ml of anti-NKG2D antibody to almost completely eliminate NK cell lysis of MC38 cells. This also indicates that MC38 cells either do not express or express insufficient quantities of other NK-activating receptor ligands.
We also confirmed that the increased NKG2DL expression on CEACAM1-silenced MC38 cells was the factor responsible for the increased immune protection. Specifically, we examined the ability of RAG2-deficient mice to reject CEACAM1-silenced MC38 cells in the presence and absence of NKG2D blockade. As shown in Fig. 2 c, blocking NKG2D function completely eliminated the increased immune protection provided by CEACAM1 silencing of MC38 cells such that the growth of MC38 cells bearing a targeting (CC1shRNA) or nontargeting (nonTshRNA) shRNA was similar in the presence of the blocking anti-NKG2D antibody (Fig. 2 c). Collectively, silencing CEACAM1 expression in tumor cells enhances NKG2D-mediated antitumor immunity via increased NKG2DL on the tumor cells.
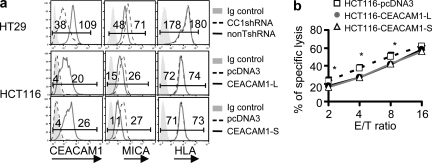
To determine whether human CEACAM1 expression also has an impact upon the expression of MICA or MICB, we examined the effects of silencing or overexpressing CEACAM1 in human colon cancer cell lines. In initial experiments, we observed that HT-29 cells express both CEACAM1 and MICA/B on the cell surface, and that transduction of HT-29 cells with human CC1shRNA resulted in decreased CEACAM1 expression and increased MICA/B expression compared with transfection of nonTshRNA (Fig. 3 a). Diminishing CEACAM1 expression by shRNA did not, however, have any effect on MHC class I expression. Conversely, another human colon cancer cell line, HCT116, which express endogenous MICA/B, but not endogenous CEACAM1, exhibited decreased MICA/B expression as a consequence of transfection with the human CEACAM1-3L isoform (Fig. 3 a). In contrast, forced expression of CEACAM1-3L did not result in alterations in the cell surface expression of MHC class I (HLA), indicating that CEACAM1 specifically targets the human NKG2DLs (Fig. 3 a). In another set of studies, we observed that HCT116 cells that were transfected with human CEACAM1-3S, which possesses the same set of extracellular and transmembrane domains as CEACAM1-3L but contains a short CT without ITIMs, also caused reduced MICA/B expression (Fig. 3 a). These studies show that the reduction in MICA/B expression caused by CEACAM1 expression is independent of ITIM domain-mediated inhibitory signaling. To further confirm these effects of CEACAM1 expression on MICA/B expression, we tested the effects of CEACAM1-3L transfection in two other human cell lines (HCT15 and HEK293) and observed that cell surface expression of MICA/B, but not MHC-I (HLA), was reduced in the presence of CEACAM1 expression (unpublished data). It was also observed that overexpression of either CEACAM1-L or CEACAM1-S in HCT116 cells did not affect ULBP expression (unpublished data). This is consistent with other observations in various human melanoma cell lines that CEACAM1 expression correlates inversely with MICA/B but not ULBP expression (Fig. 4). To further confirm that the reduction in MICA/B expression as a consequence of CEACAM1 expression was biologically significant, we analyzed the sensitivity of pcDNA3 (vector control), CEACAM1-3L, and CEACAM1-3S–transfected HCT-116 cells to primary NK cell–mediated cytolysis. These studies showed that forced expression of either CEACAM1 isoform resulted in decreased susceptibility to NK cell–mediated cytolysis in comparison to that observed with cells transfected with an empty vector (Fig. 3 b).
Figure 3.
Human CEACAM1 regulates human NKG2DL MICA/B expression and the sensitivity of tumor cells to NK cytolysis. (a) Flow cytometric analysis of CEACAM1, NKG2DL, and MHC-I (HLA) expression on indicated tumor cells. HT29 cells were engineered, where indicated, to express less CEACAM1. HCT116 cells were engineered, where indicated, to express vector encoding long or short isoforms of CEACAM1. CC1shRNA, mouse CEACAM1 shRNA; nonTshRNA, control nontargeting shRNA; Ig, isotype-matched control Ig. (b) 51Cr release cytotoxicity assay shows the sensitivity of indicated tumor cells to NK-mediated cytolysis (mean ± SEM; *, P < 0.05). All results are representative of two or three experiments.
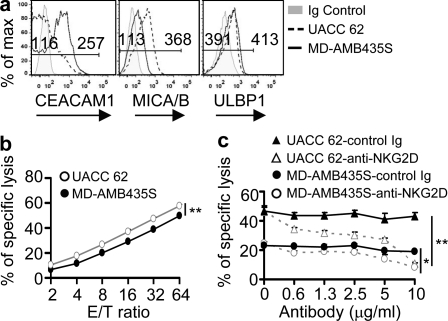
Figure 4.
Human tumor cell lines inversely express CEACAM1 and MICA/B. (a) Flow cytometry analysis of CEACAM1, MICA/B, and ULBP expression on representative human melanoma cell lines (UACC 62 and MD-AMB435S). Ig, isotype-matched control Ig. (b) 51Cr cytotoxicity assay show the sensitivity of indicated target cells to primary human NK cell–mediated cytolysis (mean ± SEM **, P < 0.01). (c) Blocking assay of NKG2D-mediated in vitro cytolysis of tumor cells by indicated antibodies (mean ± SEM *, P < 0.05; **, P < 0.01). All results are representative of two or three experiments.
Tumor cells that express more endogenous CEACAM1 exhibit increased resistance to NK cell–mediated cytolysis
To investigate whether the observations obtained from CEACAM1 loss-of-function (silencing) and gain-of-function (transfection) approaches is clinically relevant, we analyzed CEACAM1 and NKG2DL (MICA/B and ULBP) expression on several human melanoma cell lines. We chose to study human melanoma cells because melanocytes express CEACAM1 only after they are transformed, implicating melanoma-associated CEACAM1 expression in tumor progression by desensitization of the tumor cells to NKG2D-dependent cytolysis. The results presented in Fig. 4 a show that, indeed, tumor cells that express more CEACAM1 display less MICA/B, as witnessed with the MD-AMB435S cell line and vice-versa with the UACC 62 cell line. Interestingly, ULBP expression was observed to be indifferent to the relative levels of CEACAM1 on the tumor cells (Fig. 4 a). Moreover, consistent with the results obtained from transfection of CEACAM1 into HCT-116 cells (Fig. 3 b), the sensitivity of melanoma tumor cells to NK cell–mediated cytolysis was directly proportional to the cell surface expression of MICA/B levels and reciprocal to the cell surface display of CEACAM1 (Fig. 4 b). To further confirm that difference in MICA/B levels on the tumor cells determines their different sensitivities to NK cell–mediated cytolysis, we analyzed the effects of NKG2D blockade on the cytotoxicity of the melanoma cell lines. As shown in Fig. 4 c, the ability of primary NK cells to lyse the various tumor cells was nearly completely abolished by an anti–NKG2D-blocking antibody in a dose-dependent fashion. Collectively, these studies suggest that tumor cell–autonomous CEACAM1 expression is biologically important, in part through impairment of NKG2D-mediated antitumor immunity.
CEACAM1 regulates Rae-1 glycosylation and retains Rae-1 intracellularly
To understand the mechanism involved in the decreased cell surface expression of NKG2DL in the presence of CEACAM1, we undertook the following studies. We first observed that the transcriptional levels of mouse NKG2DL Rae-1 and MULT1 (unpublished data) were not regulated by silencing CEACAM1. In addition, we did not detect significant changes in the levels of possible human NKG2DL (MICA and MICB; Stern-Ginossar et al., 2007) and mouse NKG2DL (Rae-1) microRNAs (http://www.microrna.org) in CEACAM1-silenced and nonsilenced HT-29 cells and MC38 cells, further ruling out the possibility that the unchanged mRNA levels reflected a balance between an increased transcription rate and microRNA-mediated degradation (unpublished data). Collectively, these studies suggest that the regulation of NKG2DL by CEACAM1 occurs subsequent to transcription. Consistent with this, we observed that B16F10 cells that do not constitutively express NKG2DL are not affected by silencing CEACAM1 (Fig. 2 a).
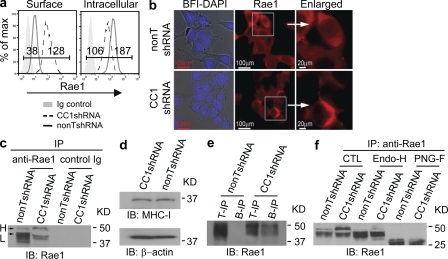
We therefore investigated a role for posttranslational regulation of NKG2DL by CEACAM1. Because tumor cells have evolved mechanisms to shed NKG2DLs to escape antitumor immunity, we first determined whether the supernatants of the tumor cells that expressed more CEACAM1 exhibit more NKG2DLs. Neither mouse tumor cells (MC38) nor human tumor cells that express more CEACAM1 (HT29) shed more NKG2DLs (Rae-1, MICA/B) than the tumor cells that express less CEACAM1 (HCT116; unpublished data). We therefore hypothesized that CEACAM1 may function in a manner similar to viral proteins that retain NKG2DLs intracellularly (Wu et al., 2003). Indeed, using flow cytometry analysis (Fig. 5 a) we observed that whereas cell surface expression of Rae-1 was increased in CEACAM1-silenced MC38 cells (CC1shRNA), intracellular Rae-1 protein was decreased in comparison to nonsilenced MC38 cells (nonTshRNA). These reciprocal changes in cell surface and intracellular expression of Rae-1 upon CEACAM1 silencing of MC38 cells were confirmed by fluorescence microscopy (Fig. 5 b). We previously demonstrated that CEACAM1 can associate with adaptor protein 1, which facilitates surface molecule expression (Nakajima et al., 2002). We determined whether CEACAM1 promotes Rae-1 internalization. We found that the internalization rate of Rae1 expressed on CEACAM1-silenced and nonsilenced MC38 cells was not significantly different (unpublished data). We therefore examined the biochemical properties of Rae-1 in CEACAM1-silenced MC38 cells.
Figure 5.
CEACAM1 mediates intracellular retention of NKG2DL. (a) Flow cytometric analysis shows cell surface and intracellular Rae-1 expression in indicated tumor cells. (b) Fluorescence microscopy analysis shows Rae-1(red) localization detected by goat anti-Rae1 followed by rhodamine-conjugated mouse anti–goat IgG in CEACAM1-silenced (CC1shRNA) and nonsilenced (nonTshRNA) MC38 tumor cells (bars: left two panels, 100 mm; right panel, 20 mm). (c) Immunoblot (IB) shows Rae-1 structure in indicated tumor cells (H, high molecular weight; L, low molecular weight). (d) Immunoblot analysis shows MHC class I expression in CEACAM1-silenced (CC1shRNA) and nonsilenced (nonTshRNA) MC38 tumor cells. (e) CEACAM1-silenced (CC1shRNA) and nonsilenced (nonTshRNA) cells were labeled with membrane impermeable biotin. Rae-1 was precipitated with Rae-1–specific antibody (T-IP) or streptavidin (B-IP) and detected with Rae-1–specific antibody. (f) The immunoblot (IB) shows the patterns of Rae-1 immunoprecipitated (IP) from CEACAM1-silenced and nonsilenced cell lysates followed by mock treatment (CTL: control) or treatment with either Endo-H or PNGase F (PNG-F). All results are representative of three or four independent experiments, respectively.
As shown in Fig. 5 c, the Rae-1 protein that was expressed when CEACAM1 was silenced was characterized by a significantly higher molecular weight (H, arrow) than that observed in nonsilenced MC38 cells (L, arrow). Notably, the molecular weight of MHC class I proteins in CEACAM1-silenced and nonsilenced MC38 cells was identical (Fig. 5 d), indicating CEACAM1 specifically targets structural modifications in Rae-1. To determine whether this high molecular weight isoform of Rae-1 was responsible for the increased cell surface expression of Rae-1 observed when CEACAM1 was silenced (Fig. 5 a), we separately labeled the cell surface proteins of the same numbers of CEACAM1-silenced and nonsilenced MC38 cells with cellular membrane impermeable biotin, precipitated the biotinylated cell surface proteins from cellular lysates with streptavidin beads or anti–Rae-1 antibody followed by protein G–conjugated Sepharose beads, and detected Rae-1 by immunoblotting with an anti–Rae-1–specific antibody. By this method, the high molecular weight form of Rae-1 was biochemically detectable on the cell surface of CEACAM1-silenced MC38 cells and undetectable on CEACAM1-nonsilenced cells (Fig. 5 e.) This suggested that silencing CEACAM1 expression in MC38 cells resulted in increased cell surface expression of Rae-1 that might possess an increased quantity of carbohydrate side-chain modifications in comparison to CEACAM1 nonsilenced cells, in which Rae-1 was observed to accumulate intracellularly as an incompletely glycosylated protein. This was indeed confirmed by performing Endoglycosidase-H (Endo-H) and N-glycanase (PNGase F) digestion of anti–Rae-1 immunoprecipitates obtained from CEACAM1-silenced and nonsilenced cells (Fig. 5 f). Whereas the high molecular band observed in CTL (control) samples was sensitive to Endo-H, the low molecular weight bands were resistant to Endo-H but sensitive to N-glycanase (Fig. 5 f). Collectively, these studies show that CEACAM1 retards Rae-1 intracellularly by disabling its full decoration with N-linked glycans, leading to insensitivity of tumor cells to NK cell–mediated immune responses.
We also examined the biochemical structure of MICA/B in HCT116 and HT29 cells in the context of different levels of CEACAM1 to determine whether the mechanism of CEACAM1-induced retention of MICA/B in human cells was the same as that observed in mouse cells. However, the molecular weight of MICA/B were the same in HCT116 and HT29 cell lines and not altered by the levels of CEACAM1 expression (unpublished data). These studies indicate that human CEACAM1 employs an alternative mechanism to down-regulate MICA/B surface expression consistent with the diversity and complexity of NKG2DL structure and transport (Coudert and Held, 2006; Arapovic et al., 2009; Champsaur and Lanier, 2010).
Collectively, we have demonstrated a role for CEACAM1 in the attenuation of immune surveillance by down-regulation of NKG2DL expression on tumor cells. These findings are consistent with accumulating clinical observations that elevated expression of CEACAM1 on tumor cells correlates with a poor prognosis. Moreover, in their totality, they advance the notion that CEACAM1 not only serves a role as a suppressor of tumor cell growth but also an important regulator of immune surveillance capability, with its attendant therapeutic implications.
MATERIALS AND METHODS
Mice and tumor assays.
WT C57BL/6 (B6) and RAG2-deficient (Rag2−/−, B6) mice were purchased from Charles River Laboratories or Taconic Farms Inc., respectively. Ceacam1−/− mice on the C57BL/6 background have been previously described (Leung et al., 2006). All mice were used between 8 and 12 wk of age. For in vivo NKG2D-blocking experiments, Rag2−/−, B6 mice were treated with anti–mouse NKG2D mAb (C7 clone; eBioscience) or control Ig mAb (200 µg i.p.) on days −1, 0, 1, 7, and 12 after tumor inoculation. Tumor cells 105 cells per injection were inoculated subcutaneously (for tumor size measurement) in the right and left flanks in 100 µl PBS. We measured the tumor size as indicated with a metric caliper. Mice were maintained under specific pathogen–free conditions at the Animal Facilities of Harvard Medical School. All animal experimentation was performed in accordance with institutional guidelines and Institutional Animal Care and Use Committee of Harvard Medical School, which granted permission for this study.
Cell culture.
MC38, B16F10, HT29, HEK293, HCT-15, and HCT116 cells were from the American Type Culture Collection. T. Kupper (Brigham and Women’s Hospital, Boston, MA) provided the UACC62 and MD-AMB435S melanoma cell lines. All cells were cultured in RPMI-1640 medium supplemented with 10% FBS, ampicillin, streptomycin, 10 mM Hepes, and 3 µM β-ME.
NK cells and cytotoxicity assays.
Mouse and human primary NK cells were isolated from mouse spleens and human leukopacks (Blood Donor Center, Brigham and Women’s Hospital), respectively, with corresponding NK cell isolation kits (Miltenyi Biotec) according to the manufacturer’s instruction. In some experiments, the effector cells were pretreated with anti-NKG2D-blocking antibodies (R&D Systems) as indicated, for 30 min at 4°C. A standard 4-h 51Cr (PerkinElmer) release cytotoxicity assay and calculation of percentage of specific cytolysis were performed as described previously (Chen et al., 2008).
Immunoprecipitation and Western blotting.
Immunoprecipitation and Western blotting were performed as previously described by using specific antibodies as indicated (Chen et al., 2008). For Rae-1 deglycosylation, the immunoprecipitates were denatured, treated with Endo-H or PNGase F according to the manufacturer’s instructions (New England Biolabs), and analyzed by Western blotting with anti–Rae-1–specific antibody (R&D Systems). For cell surface immunoprecipitation, cells were incubated with anti–Rae-1 on ice for 1 h and free antibodies were removed by extensive washing with PBS, lysed, and precipitated by adding protein G–conjugated Sepharose beads. For cell surface protein biotinylation, cells were labeled with EZ-Link sulfo-NHS-biotin (Thermo Fisher Scientific) according to the manufacturer’s instructions. The cell lysates were either immunoprecipitated with streptavidin beads or immunoblotted with an anti–Rae-1 antibody.
RNA silencing, detection, and protein expression.
Lentiviral particles of shRNA specific for mouse and human CEACAM1, as well as nontargeting shRNA, were purchased from Sigma-Aldrich. Transduction was performed according to the manufacturer’s instructions. Clones were selected with puromycin. Rae-1 RNA was detected by RT-PCR or quantitative PCR (qPCR) with primers as follows: forward, 5′-TCCGCAAAGCCAGGGCCAAA-3′, and reverse, 5′-GCTGGTAGGTGGAAGCGGGG-3′. MULT1 was detected by qPCR with primers as follows: forward, 5′-GCAGGCTGAGGTGTGTGGCC-3′, and reverse, 5′-CCAGGTCCTGCAGTCGCCCT-3′ on a LightCycler 480 qPCR machine (Roche).
Fluorescence microscopy.
CEACAM1 silenced and nonsilenced MC38 cells were seeded on coverslips. Rae1 (both cell surface and intracellular) was detected by goat anti-Rae1 (R&D Systems), followed by rhodamine-conjugated mouse anti–goat IgG using a Nikon Eclipse Ti-U fluorescence microscope and NIS Elements v3.1 image-processing software.
Flow cytometry.
For CEACAM1 expression analysis, cells were incubated with CC1 (mouse anti–mouse CEACAM1 monoclonal antibody (a gift from K. Holmes, University of Colorado, Denver, CO; Dveksler et al., 1993) or 5F4 (mouse anti–human CEACAM1 monoclonal antibody; Morales et al., 1999) for 20 min, followed by FITC-conjugated rat anti–mouse IgG1 (Jackson ImmunoResearch Laboratories). For detection of Rae-1, MULT1, H-60, ULBP, MICA/B surface expression cells were stained with FITC-conjugated anti–Rae-1 (R&D Systems), PE-conjugated anti-MULT1 (R&D Systems), PE-conjugated anti-H60 (eBioscience), PE-conjugated anti-ULBP (R&D Systems), and PE-conjugated anti-MICA/B (R&D Systems) antibodies, respectively. For intracellular Rae-1 staining, cells were stained with nonconjugated anti–Rae-1 (R&D Systems) for 30 min, fixed with Cytofix buffer (BD), permeabilized with Cytofix/perm buffer (BD), and then stained with the aforementioned FITC-conjugated anti-Rae1 antibody (R&D Systems). Stained cells were analyzed on a FACScan (BD). For Rae-1 internalization, cells are preincubated with nonconjugated rat anti-Rae-1 for 1 h at 4°C. Free antibodies were removed by washing cells twice with medium. Cells were then placed in 37°C for the indicated times and stained with FITC-conjugated goat anti–rat Ig. Percentage of mean fluorescence intensity (MFI) was calculated as MFI (t)/MFI (0 h) × 100. t indicates time point.
Statistics.
A Student’s t test (paired, two-tailed) was used to determine significance. A p-value of <0.05 was considered as significant.
Acknowledgments
The authors appreciate the technical assistance of Jessica Wagner for confocal microscopy analysis and Jennifer Cusick for technical support. We thank Dr. K. Holmes for providing the anti–mouse CEACAM1-specific antibody (CC1) and Dr. T. Kupper for providing human melanoma cell lines.
R.S. Blumberg was supported by National Institutes of Health (NIH) grants DK51362, DK44319, DK53056, and DK088199, the Harvard Digestive Diseases Center (NIH P30DK034854), and the High Pointe Foundation. R.S. Blumberg and O. Mandelboim were supported by the Israel-U.S. Binational Research Award. N. Beauchemin was supported by the Canadian Institutes of Health Research. L.L. Lanier is an American Cancer Society Professor and is supported by NIH grant AI066897. L. Chen is supported by a Research Fellowship Award from the Crohn’s & Colitis Foundation of America.
The authors declare they have no financial conflicts of interest.
Footnotes
Abbreviations used:
- CEA
- carcinoembryonic antigen
- CEACAM1
- CEA-related cell adhesion molecule 1
- CT
- cytoplasmic tail
- Endo-H
- endoglycosidase-H
- ITIM
- immunoreceptor tyrosine-based inhibitory motif
- MICA/B
- MHC class I–related molecule A/B
- MULT
- murine UL16-binding protein
- NKG2
- natural killer gene group 2
- NKG2D
- NKG2 member D
- NKG2DL
- NKG2D ligand
- Rae-1
- retinoic acid early inducible cDNA clone 1
- shRNA
- short hairpin RNA
- ULBP
- UL16-binding protein
References
- Arapovic J., Lenac T., Antulov R., Polic B., Ruzsics Z., Carayannopoulos L.N., Koszinowski U.H., Krmpotic A., Jonjic S. 2009. Differential susceptibility of RAE-1 isoforms to mouse cytomegalovirus. J. Virol. 83:8198–8207 10.1128/JVI.02549-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champsaur M.A., Lanier L.L. 2010. Effect of NKG2D ligand expression on host immune response. Immunol. Rev. 170:4196–4200 10.1111/j.0105-2896.2010.00893.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Chen L., Qiao S.W., Nagaishi T., Blumberg R.S. 2008. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J. Immunol. 180:6085–6093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert J.D., Held W. 2006. The role of the NKG2D receptor for tumor immunity. Semin. Cancer Biol. 16:333–343 10.1016/j.semcancer.2006.07.008 [DOI] [PubMed] [Google Scholar]
- Dango S., Sienel W., Schreiber M., Stremmel C., Kirschbaum A., Pantel K., Passlick B. 2008. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1) is associated with increased angiogenic potential in non-small-cell lung cancer. Lung Cancer. 60:426–433 10.1016/j.lungcan.2007.11.015 [DOI] [PubMed] [Google Scholar]
- Diefenbach A., Jensen E.R., Jamieson A.M., Raulet D.H. 2001. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 413:165–171 10.1038/35093109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dveksler G.S., Pensiero M.N., Dieffenbach C.W., Cardellichio C.B., Basile A.A., Elia P.E., Holmes K.V. 1993. Mouse hepatitis virus strain A59 and blocking antireceptor monoclonal antibody bind to the N-terminal domain of cellular receptor. Proc. Natl. Acad. Sci. USA. 90:1716–1720 10.1073/pnas.90.5.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes M.B., Girart M.V., Molinero L.L., Domaica C.I., Rossi L.E., Barrio M.M., Mordoh J., Rabinovich G.A., Zwirner N.W. 2008. Intracellular retention of the NKG2D ligand MHC class I chain-related gene A in human melanomas confers immune privilege and prevents NK cell–mediated cytotoxicity. J. Immunol. 180:4606–4614 [DOI] [PubMed] [Google Scholar]
- Gambichler T., Grothe S., Rotterdam S., Altmeyer P., Kreuter A. 2009. Protein expression of carcinoembryonic antigen cell adhesion molecules in benign and malignant melanocytic skin lesions. Am. J. Clin. Pathol. 131:782–787 10.1309/AJCP24KXJVBZXENS [DOI] [PubMed] [Google Scholar]
- Gray-Owen S.D., Blumberg R.S. 2006. CEACAM1: contact-dependent control of immunity. Nat. Rev. Immunol. 6:433–446 10.1038/nri1864 [DOI] [PubMed] [Google Scholar]
- Groh V., Wu J., Yee C., Spies T. 2002. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 419:734–738 10.1038/nature01112 [DOI] [PubMed] [Google Scholar]
- Kang W.Y., Chen W.T., Wu M.T., Chai C.Y. 2007. The expression of CD66a and possible roles in colorectal adenoma and adenocarcinoma. Int. J. Colorectal Dis. 22:869–874 10.1007/s00384-006-0247-x [DOI] [PubMed] [Google Scholar]
- Lanier L.L. 2008. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 9:495–502 10.1038/ni1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung N., Turbide C., Olson M., Marcus V., Jothy S., Beauchemin N. 2006. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene. 25:5527–5536 10.1038/sj.onc.1209541 [DOI] [PubMed] [Google Scholar]
- Lim Y.S., Kang B.Y., Kim E.J., Kim S.H., Hwang S.Y., Kim T.S. 1998. Augmentation of therapeutic antitumor immunity by B16F10 melanoma cells transfected by interferon-gamma and allogeneic MHC class I cDNAs. Mol. Cells. 8:629–636 [PubMed] [Google Scholar]
- Liu W., Wei W., Winer D., Bamberger A.M., Bamberger C., Wagener C., Ezzat S., Asa S.L. 2007. CEACAM1 impedes thyroid cancer growth but promotes invasiveness: a putative mechanism for early metastases. Oncogene. 26:2747–2758 10.1038/sj.onc.1210077 [DOI] [PubMed] [Google Scholar]
- Markel G., Lieberman N., Katz G., Arnon T.I., Lotem M., Drize O., Blumberg R.S., Bar-Haim E., Mader R., Eisenbach L., Mandelboim O. 2002. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J. Immunol. 168:2803–2810 [DOI] [PubMed] [Google Scholar]
- Markel G., Ortenberg R., Seidman R., Sapoznik S., Koren-Morag N., Besser M.J., Bar J., Shapira R., Kubi A., Nardini G., et al. 2010. Systemic dysregulation of CEACAM1 in melanoma patients. Cancer Immunol. Immunother. 59:215–230 10.1007/s00262-009-0740-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales V.M., Christ A., Watt S.M., Kim H.S., Johnson K.W., Utku N., Texieira A.M., Mizoguchi A., Mizoguchi E., Russell G.J., et al. 1999. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a). J. Immunol. 163:1363–1370 [PubMed] [Google Scholar]
- Nakajima A., Iijima H., Neurath M.F., Nagaishi T., Nieuwenhuis E.E., Raychowdhury R., Glickman J., Blau D.M., Russell S., Holmes K.V., Blumberg R.S. 2002. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J. Immunol. 168:1028–1035 [DOI] [PubMed] [Google Scholar]
- Obrink B. 2008. On the role of CEACAM1 in cancer. Lung Cancer. 60:309–312 10.1016/j.lungcan.2008.03.020 [DOI] [PubMed] [Google Scholar]
- Sienel W., Dango S., Woelfle U., Morresi-Hauf A., Wagener C., Brümmer J., Mutschler W., Passlick B., Pantel K. 2003. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 promotes progression of non-small cell lung cancer. Clin. Cancer Res. 9:2260–2266 [PubMed] [Google Scholar]
- Simeone D.M., Ji B., Banerjee M., Arumugam T., Li D., Anderson M.A., Bamberger A.M., Greenson J., Brand R.E., Ramachandran V., Logsdon C.D. 2007. CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas. 34:436–443 10.1097/MPA.0b013e3180333ae3 [DOI] [PubMed] [Google Scholar]
- Spies T. 2008. Regulation of NKG2D ligands: a purposeful but delicate affair. Nat. Immunol. 9:1013–1015 10.1038/ni0908-1013 [DOI] [PubMed] [Google Scholar]
- Stern-Ginossar N., Elefant N., Zimmermann A., Wolf D.G., Saleh N., Biton M., Horwitz E., Prokocimer Z., Prichard M., Hahn G., et al. 2007. Host immune system gene targeting by a viral miRNA. Science. 317:376–381 10.1126/science.1140956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilki D., Singer B.B., Shariat S.F., Behrend A., Fernando M., Irmak S., Buchner A., Hooper A.T., Stief C.G., Reich O., Ergun S. 2009. CEACAM1: A Novel Urinary Marker for Bladder Cancer Detection. Eur. Urol. 15:501–509 10.1016/j.eururo.2009.05.040 [DOI] [PubMed] [Google Scholar]
- Wu J., Chalupny N.J., Manley T.J., Riddell S.R., Cosman D., Spies T. 2003. Intracellular retention of the MHC class I-related chain B ligand of NKG2D by the human cytomegalovirus UL16 glycoprotein. J. Immunol. 170:4196–4200 [DOI] [PubMed] [Google Scholar]
- Xi L., Feber A., Gupta V., Wu M., Bergemann A.D., Landreneau R.J., Litle V.R., Pennathur A., Luketich J.D., Godfrey T.E. 2008. Whole genome exon arrays identify differential expression of alternatively spliced, cancer-related genes in lung cancer. Nucleic Acids Res. 36:6535–6547 10.1093/nar/gkn697 [DOI] [PMC free article] [PubMed] [Google Scholar]