Expression of the MAP kinase kinase kinase TAK1 in brain endothelial cells is needed for production of prostaglandin E2, and for induction of fever and sickness behavior, in response to peripheral inflammation.
Abstract
Systemic inflammation affects the brain, resulting in fever, anorexia, lethargy, and activation of the hypothalamus–pituitary–adrenal axis. How peripheral inflammatory signals reach the brain is still a matter of debate. One possibility is that, in response to inflammatory stimuli, brain endothelial cells in proximity to the thermoregulatory centers produce cyclooxygenase 2 (COX-2) and release prostaglandin E2, causing fever and sickness behavior. We show that expression of the MAP kinase kinase kinase TAK1 in brain endothelial cells is needed for interleukin 1β (IL-1β)–induced COX-2 production. Exploiting the selective expression of the thyroxine transporter Slco1c1 in brain endothelial cells, we generated a mouse line allowing inducible deletion of Tak1 specifically in brain endothelium. Mice lacking the Tak1 gene in brain endothelial cells showed a blunted fever response and reduced lethargy upon intravenous injection of the endogenous pyrogen IL-1β. In conclusion, we demonstrate that TAK1 in brain endothelial cells induces COX-2, most likely by activating p38 MAPK and c-Jun, and is necessary for fever and sickness behavior.
Infections, tissue injury, and autoimmune disorders all trigger a systemic inflammatory response. In patients, systemic inflammation often presents with sickness involving fever, anorexia, lethargy, and activation of the hypothalamus–pituitary–adrenal axis (HPA axis). Symptoms of sickness are thought to serve an adaptive function to combat infectious agents and they rely on specific pathways in the brain, mainly in the hypothalamus. Previous work has shown that various components of the sickness response involve distinct neural structures (Dantzer et al., 2008). Upon immune challenge, leukocytes and endothelial cells in the periphery release cytokines such as IL-1β, TNF, and IL-6, which subsequently induce the sickness response. Thus, administration of IL-1β or other cytokines has served as an experimental model to investigate the mechanisms underlying the sickness response. Previous work has shown that fever and other components of the CNS response to systemic inflammation require both cyclooxygenase 2 (COX-2; PTGS2) and the induction of prostaglandin E2 (PGE2; Pecchi et al., 2009).
How peripheral inflammatory signals reach the brain to elicit central reactions is still a subject of debate. Four potential routes have been discussed (Dantzer et al., 2008). One theory suggests that cytokines and endotoxins cross the blood–brain barrier within the circumventricular organs where the endothelium is fenestrated and act on microglia and other neural cells. A second hypothesis assumes that cytokines activate hypothalamic nuclei after being transported through brain endothelial cells. Third, peripheral inflammation could stimulate the vagus nerve that subsequently induces fever and sickness behavior. Finally, another hypothesis builds on the observation that cytokines or endotoxins induce Cox-2 in brain vascular cells of the preoptic area close to thermoregulatory centers and in other hypothalamic nuclei (Horai et al., 1998; Lacroix and Rivest, 1998; Gosselin and Rivest, 2008). By secreting PGE2 into the parenchyma, endothelial cells are thought to stimulate neurons to induce fever and other aspects of the sickness response. However, direct evidence to support the function of brain endothelial cells is still lacking. Moreover, other studies have localized IL-1β–induced COX-2 expression mainly to perivascular macrophages and thus question the role of endothelial cells in this process (e.g., Serrats et al., 2010).
The best evidence that endothelial cells play a role in sickness comes from a recent study in which an impaired fever response to IL-1β was found in a mouse line expressing siRNA against the IL-1β receptor IL-1RI under control of the pan-endothelial Tie2 promoter/enhancer (Ching et al., 2007). However, the diminished sickness response in this study may have been secondary to a mitigated systemic inflammatory reaction because the Tie2 promoter/enhancer is expressed in endothelial cells of all vascular beds. Indeed, endothelial cells play a key role in the systemic inflammatory reaction in vivo (Ye et al., 2008; Ding et al., 2009) and release the pyrogens PGE2 and IL-6 in response to IL-1β (Warner et al., 1987; Jirik et al., 1989). In addition, IL-1β induces its own expression in endothelial cells, providing a mechanism by which peripheral endothelial cells amplify the systemic inflammation (Warner et al., 1987).
To specifically test the role of brain endothelial cells in inducing fever and sickness behavior, we generated the Slco1c1-CreERT2 BAC transgenic mouse line that affords inducible and cell-specific recombination in brain endothelial cells. Using this line, we delete the MAP kinase kinase kinase Tak1 (Map3k7) in brain endothelial cells because it is an important component of IL-1β signaling upstream of p38 MAPK and of the transcription factors NF-κB and c-Jun that control Cox-2 gene transcription (Smith et al., 2000). Our data confirm that TAK1 is needed to activate p38 MAPK and c-Jun and to induce COX-2 in brain endothelial cells. Mice lacking Tak1 in brain endothelial cells (Slco1c1-CreERT2 × Tak1Fl/Fl mice) showed a blunted fever response and reduced lethargy upon i.v. stimulation with IL-1β, whereas anorexia and corticosterone release were not affected by deleting Tak1. These data provide the first direct evidence that brain endothelial cells mediate the induction of fever and sickness behavior.
RESULTS AND DISCUSSION
TAK1 mediates p38 MAPK and c-Jun activation by IL-1β in brain endothelial cells
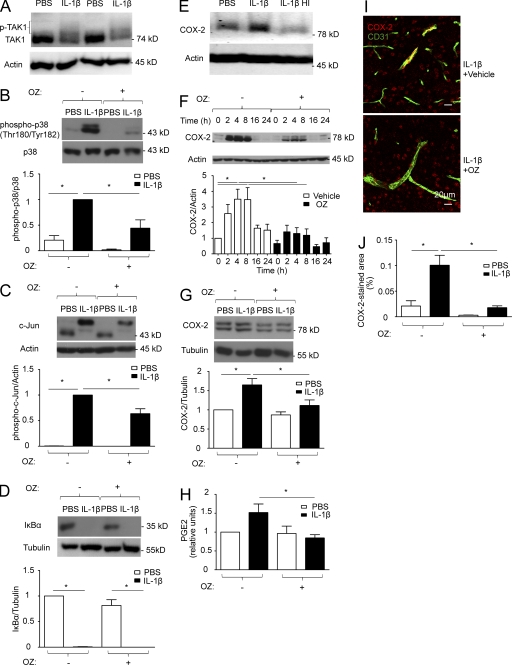
To confirm that IL-1β activates TAK1, we stimulated primary brain endothelial cells (PBECs) with IL-1β and pretreated cells with the protein phosphatase inhibitor okadaic acid to prevent rapid dephosphorylation of TAK1. IL-1β enhanced the phosphorylation of TAK1 as shown by the appearance of slower migrating forms of TAK1, which have been shown to correspond to TAK1 phosphorylation (Hanada et al., 2001; Fig. 1 A).
Figure 1.
In response to IL-1β, TAK1 activates p38 MAPK and c-Jun and induces COX-2 and PGE2 in brain endothelial cells. (A) PBECs were pretreated for 30 min with 250 nM okadaic acid to prevent rapid TAK1 dephosphorylation and then stimulated with 50 ng/ml PBS or IL-1β for 10 min. Protein lysates were analyzed by Western blotting. A representative blot out of three independent experiments showing two samples per treatment group is displayed. (B–D) PBECs were stimulated with 50 ng/ml PBS or IL-1β for 30 min with or without 30-min pretreatment with 600 nM of the TAK1 inhibitor OZ. Protein lysates were analyzed by Western blotting to determine the phosphorylation of p38 MAPK, the band shift of c-Jun indicating its phosphorylation, and the degradation of IκBα. Densitometric quantifications are shown below the blots (n = 4–6). (E) bEnd.3 cells were stimulated with 50 ng/ml PBS, IL-1β, or heat-inactivated IL-1β (IL-1β HI) for 2 h. COX-2 induction was analyzed by Western blotting. A representative example of two independent experiments is shown. IL-1β significantly (P < 0.05) induced COX-2 expression (densitometric COX-2/actin ratio 1.46 ± 0.14) compared with the PBS-group (1.00 ± 0.09) and the IL-1β HI–stimulated group (0.87 ± 0.11). IL-1β was heat inactivated at 90 °C for 60 min. (F) bEnd.3 cells were stimulated with 50 ng/ml PBS or IL-1β for the indicated time period with or without 600 nM OZ. COX-2 induction was monitored by Western blotting. Densitometric quantifications are shown below the blot (n = 4). (G) PBECs were stimulated with 50 ng/ml IL-1β for 2 h with or without 30-min pretreatment with 600 nM OZ. Protein lysates were analyzed by Western blotting to detect COX-2. Densitometric quantifications are shown below the blot (n = 4). (H) PGE2 release by bEnd.3 cells was measured by ELISA 4 h after stimulation with 50 ng/ml IL-1β, with or without 30-min pretreatment with 600 nM OZ (n = 5). PGE2 levels are expressed as units relative to the untreated control. (I and J) Wild-type mice received i.p. injections of 30 mg/kg OZ or vehicle 30 min before administration of 30 µg/kg IL-1β i.v. or PBS. 3 h later, the preoptic area was subject to immunostaining with antibodies against COX-2 and CD31. (I) Representative staining. (J) Quantification of the COX-2 and CD31 double-positive area (normalized to the total area). n = 3–5 mice per group. (B–D, F–H, and J) Pooled data of at least two independent experiments are shown. Values are means ± SEM. *, P < 0.05 (one-way ANOVA, Tukey’s post hoc test).
We also investigated signaling pathways downstream of TAK1 that have been implicated in regulating Cox-2 levels (Smith et al., 2000). IL-1β stimulation increased phosphorylation of p38 MAPK in brain endothelial bEnd.3 cells, peaking at 10 min (not depicted), and in PBECs (Fig. 1 B). Preincubating cells with the specific TAK1 inhibitor 5z-7-oxozeaenol (OZ; Ninomiya-Tsuji et al., 2003) reduced basal levels of p38 MAPK phosphorylation and blunted the IL-1β–dependent increase in phosphorylation in bEnd.3 cells (not depicted) and in primary endothelial cells (Fig. 1 B). Furthermore, IL-1β treatment induced phosphorylation of c-Jun, as indicated by the appearance of a c-Jun species with markedly reduced mobility in bEnd.3 cells (not depicted) and in primary endothelial cells (Fig. 1 C). Inhibiting TAK1 by OZ diminished c-Jun phosphorylation.
IL-1β also activated NF-κB as shown by the degradation of IκBα. Pretreatment of PBECs and bEnd.3 cells with OZ did not affect the activation of NF-kB (Fig. 1 D and not depicted). In addition, phosphorylation of IκBα as a further measure of NF-κB activation upon stimulation with IL-1β was not blocked by TAK1 inhibition either (unpublished data). These results are in accordance with previous studies showing that IL-1β is able to stimulate NF-κB independently of TAK1 (Yamazaki et al., 2009). In brain endothelial cells, TAK1-independent pathways of NF-κB activation seem to prevail. In summary, these data demonstrate that IL-1β activates p38 MAPK and c-Jun in brain endothelial cells in a TAK1-dependent manner.
TAK1 mediates the induction of COX-2 and PGE2 by IL-1β in brain endothelial cells
Stimulation of bEnd.3 cells with IL-1β transiently increased COX-2 protein levels after 2–8 h (Fig. 1, E and F). Heat-inactivated IL-1β lost its ability to induce COX-2 expression in bEnd.3 cells, excluding a contamination with endotoxin (Fig. 1 E). The IL-1β–induced increase of COX-2 in bEnd.3 cells was blocked by the TAK1 inhibitor OZ (Fig. 1 F). OZ also inhibited COX-2 induction by IL-1β in PBECs (Fig. 1 G). Similarly, PGE2 levels in the supernatant rose 4 h after stimulating bEnd.3 cells with IL-1β, whereas TAK1 inhibition blocked this increase (Fig. 1 H). To investigate TAK1 inhibition in vivo, we i.p. injected mice with OZ 30 min before i.v. administration of IL-1β. Inhibition of TAK1 with OZ blocked the IL-1β–stimulated increase in COX-2 expression in CD31-positive brain endothelial cells in the preoptic area (Fig. 1, I and J). These results indicate that in brain endothelial cells, TAK1 induces COX-2 and subsequently stimulates the secretion of PGE2, an important mediator of the sickness response. In accordance with our data that TAK1 has no significant effect on NF-κB signaling, Cox-2 induction by IL-1β in endothelial cells is known to be controlled by the MAP kinases p38 and JNK and only partially depends on NF-κB activity (Kuldo et al., 2005).
Slco1c1-CreERT2 mice allow brain endothelial-specific recombination
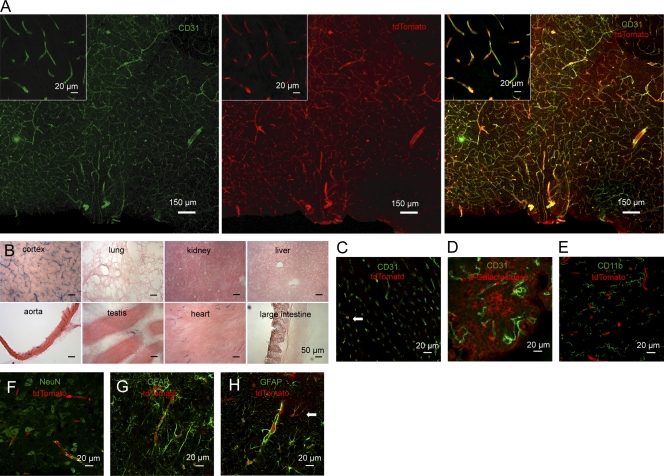
To investigate the role of Tak1 specifically in brain endothelial cells in the sickness response in vivo, we used a conditional approach. To our knowledge, mouse lines expressing Cre recombinase under the control of endothelial cell–specific promoters have not been used to address the role of brain endothelial cells in the sickness response, possibly because of poor recombination rates in the CNS. For example, mice expressing Cre recombinase under the control of the tie2 locus (Korhonen et al., 2009) show nearly no recombination in the brain (S. Offermanns, personal communication). The solute carrier organic anion transporter 1c1 (Slco1c1, Oatp14) has previously been reported to be selectively expressed in endothelial cells of the brain and in choroid plexus epithelial cells (Roberts et al., 2008). By immunofluorescent double staining of SLCO1C1 and CD31 as an endothelial marker, we verified that endothelial cells express SLCO1C1 in the brain of adult mice (unpublished data). Real-time RT-PCR confirmed the selective expression of Slco1c1 in the brain and spinal cord of adult mice (Lang et al., 2011). Therefore, we inserted the inducible CreERT2 in the Slco1c1 locus to generate a BAC-transgenic mouse line. CreERT2 was chosen to exclude effects of gene deletion during embryonic and postnatal development (Lang et al., 2011). Slco1c1-CreERT2 mice were crossed with the reporter mouse line Ai14 to investigate recombination (Madisen et al., 2010). After injecting tamoxifen, the reporter tdTomato was colocalized with CD31 in all blood vessels of the brain, showing widespread recombination in brain endothelial cells (Fig. 2 A). This finding was confirmed when Slco1c1-CreERT2 animals were crossed with the Rosa26 β-Galactosidase reporter line (Soriano, 1999; Fig. 2 B). In contrast, no signs of recombination were found in peripheral organs such as liver, kidney, lung, and intestine. In aorta, heart, and testis, only a small number of cells were positive for the reporter β-Galactosidase or tdTomato (Fig. 2, B and C). In accordance with SLCO1C1 expression in choroid plexus epithelial cells (Roberts et al., 2008), we also observed recombination in epithelial cells, but not in endothelial cells of the choroid plexus (Fig. 2 D).
Figure 2.
Brain endothelial-specific recombination in Slco1c1-CreERT2 mice. (A) The preoptic area of Slco1c1-CreERT2; Ai14 mice treated with tamoxifen was subjected to immunostaining with anti-CD31. tdTomato reporter is shown in the middle. Representative stainings from three independent experiments are shown. (B) Histochemical β-Galactosidase staining of several organs of Slco1c1-CreERT2; Rosa26 mice treated with tamoxifen. Representative stainings from three independent experiments are shown. (C) Heart cryosections of tamoxifen-treated Slco1c1-CreERT2; Ai14 reporter mice stained with anti-CD31. 3.2 ± 1.6% of all CD31-positive endothelial cells in the heart were expressing the reporter tdTomato (arrow). Representative stainings from two independent experiments are shown. (D) Immunostaining of brain cryosections of tamoxifen-treated Slco1c1-CreERT2; Rosa26 mice with anti–β-Galactosidase and anti-CD31 to analyze recombination in epithelial and in endothelial cells of the choroid plexus. Representative stainings from two independent experiments are shown. (E–G) Immunofluorescent staining for CD11b, NeuN, and GFAP to determine potential recombination in macrophages/microglia, neurons, and astrocytes in the preoptic area of Slco1c1-CreERT2; Ai14 reporter mice, treated with tamoxifen. Representative stainings from three independent experiments are shown. (H) The cortex of Slco1c1-CreERT2; Ai14 reporter mice treated with tamoxifen was subjected to immunostaining with anti-GFAP. tdTomato showed recombination in endothelial cells surrounded by GFAP-positive astrocytic end-feet. In addition, in the cortex a small number of astrocytes had undergone recombination (arrow). Representative stainings from three independent experiments are shown.
In Slco1c1-CreERT2; Ai14 mice, immunohistochemistry demonstrated that tdTomato-expressing cells were negative for CD11b, a marker for microglia and macrophages, for the neuronal marker NeuN, and for the astrocytic marker GFAP (Fig. 2, E–G), with the exception of granule cells in the dentate gyrus of the hippocampus (not depicted). Some tdTomato-expressing cells in the cortex did not show a vascular shape and were CD31 negative. We identified these cells as GFAP-positive astrocytes (Fig. 2 H). In primary cortical cultures from adult Slco1c1-CreERT2; Ai14 mice, 7% of S100β-positive astrocytes were tdTomato positive, indicating recombination. α-SMA–positive pericytes and IBA1-positive microglia/macrophages in culture never expressed tdTomato. In summary, the Slco1c1-CreERT2 mouse line allows inducible recombination in endothelial cells of the brain but not in other vascular territories.
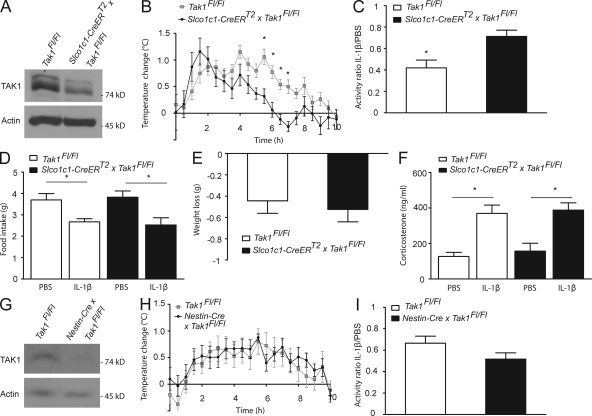
Brain endothelial-specific deletion of Tak1 interferes with the sickness response
We deleted Tak1 in brain endothelial cells (Slco1c1-CreERT2 × Tak1Fl/Fl) by crossing mice carrying Tak1 alleles flanked by loxP sites (Tak1Fl/Fl) with Slco1c1-CreERT2 mice (Sato et al., 2005). TAK1 expression in PBECs of Slco1c1-CreERT2 × Tak1Fl/Fl mice was markedly reduced at the protein (Fig. 3 A) and the mRNA level (Tak1Fl/Fl, 1.1 ± 0.3 relative units; Slco1c1-CreERT2 × Tak1Fl/Fl, 0.3 ± 0.1 relative units, P < 0.05, Student’s t test). Slco1c1-CreERT2 × Tak1Fl/Fl mice had a normal body temperature (unpublished data). However, IL-1β induced only a short-lasting fever response in Slco1c1-CreERT2 × Tak1Fl/Fl mice in comparison with Tak1Fl/Fl controls (Fig. 3 B). The fact that the early fever response to IL-1β remained intact in Slco1c1-CreERT2 × Tak1Fl/Fl mice is consistent with the finding that peripheral PGE2 release mediates the early temperature rise upon immune challenge (Steiner et al., 2006b). Injecting IL-1β at 10 a.m. reduced spontaneous locomotion the next night. Locomotion was reduced less in Slco1c1-CreERT2 × Tak1Fl/Fl mice than in Tak1Fl/Fl controls (Fig. 3 C), indicating that TAK1 in brain endothelial cells mediates fever and lethargy in response to IL-1β. In contrast, we found no change in IL-1β–induced anorexia (Fig. 3 D). Body weight also dropped to a similar extent in Tak1Fl/Fl and Slco1c1-CreERT2 × Tak1Fl/Fl mice (Fig. 3 E). In addition, corticosterone plasma levels, as a measure for HPA axis activation, rose to a similar extent in Tak1Fl/Fl and Slco1c1-CreERT2 × Tak1Fl/Fl mice (Fig. 3 F) at the time point 3 h after IL-1β stimulation that we have investigated.
Figure 3.
Slco1c1-CreERT2 x Tak1Fl/Fl mice show a blunted fever response and reduced sickness behavior upon stimulation with IL-1β. (A) Lysates of PBECs from indicated mice were analyzed by Western blotting. One out of three independent experiments is demonstrated. (B) The rise in body temperature in Slco1c1-CreERT2 × Tak1Fl/Fl mice in comparison to control Tak1Fl/Fl mice (n = 7–8 per genotype) upon administration of 30 µg/kg IL-1β i.v. Values are means ± SEM. *, P < 0.05 (repeated measures two-way ANOVA and Bonferroni’s post-test). Pooled data of two independent experiments are shown. (C) IL-1β (30 µg/kg) influence on locomotor activity of Slco1c1-CreERT2 × Tak1Fl/Fl mice and control Tak1Fl/Fl mice (n = 7–8 per group). The ratio of the activity of IL-1β– and PBS-injected animals in the dark phase is shown. Values are means ± SEM. *, P < 0.05 (Student’s t test). (D) IL-1β administration influence on food intake over 22 h in Slco1c1-CreERT2 × Tak1Fl/Fl mice and Tak1Fl/Fl mice. Values are means ± SEM. *, P < 0.05 (one-way ANOVA, Tukey’s post hoc test). (E) Weight loss of Slco1c1-CreERT2 × Tak1Fl/Fl mice and control Tak1Fl/Fl mice over 22 h after i.v. administration of IL-1β. Values are means ± SEM. (F) Plasma corticosterone levels 3 h after IL-1β and PBS administration in Slco1c1-CreERT2 × Tak1Fl/Fl mice and control Tak1Fl/Fl mice. Values are means ± SEM (n = 6–7 animals per group). *, P < 0.05 (one-way ANOVA, Tukey’s post hoc test). Pooled data of two independent experiments are shown. (G) Lysates from the hypothalamus of mice deficient for Tak1 in neurons, oligodendrocytes, and astrocytes (Nestin-Cre × Tak1Fl/Fl mice) and Tak1Fl/Fl mice were analyzed by Western blotting. One out of two independent experiments is shown. (H) The rise in body temperature in Nestin-Cre × Tak1Fl/Fl mice in comparison with Tak1Fl/Fl mice (n = 8 per genotype) upon administration of 30 µg/kg IL-1β i.v. Values are means ± SEM. Pooled data of two independent experiments are shown. (I) IL-1β (30 µg/kg) influence on locomotor activity of Nestin-Cre × Tak1Fl/Fl mice and control Tak1Fl/Fl mice (n = 8 per group). The ratio of the activity of IL-1β and PBS injected animals in the dark phase is shown. Values are means ± SEM.
Recently, astrocytes have been implicated in the induction of fever and sickness behavior (Hanada et al., 2009). We found recombination in a few cortical astrocytes of Slco1c1-CreERT2 mice (Fig. 2 H) and, therefore, we wanted to test the role of astrocytic TAK1 in the sickness response. Tak1 was deleted in astrocytes, oligodendrocytes, and neurons by crossing Tak1Fl/Fl mice with transgenic mice expressing the Cre recombinase under control of the Nestin promoter (Nestin-Cre × Tak1Fl/Fl; Tronche et al., 1999). Western blotting confirmed that TAK1 levels were markedly reduced in hypothalamic extracts of Nestin-Cre × Tak1Fl/Fl mice (Fig. 3 G). Nestin-Cre × Tak1Fl/Fl mice did not show an obvious phenotype. The IL-1β–induced fever response and the inhibition of locomotion were not impaired in Nestin-Cre × Tak1Fl/Fl mice (Fig. 3, H and I). This shows that TAK1 in astrocytes, oligodendrocytes, or neurons is not involved in fever or lethargy evoked by administering IL-1β. Additionally, IL-1β induced a similar fever response and reduction in locomotion in Slco1c1-CreERT2 mice without a floxed allele when compared with wild-type littermates (unpublished data). This excludes a confounding effect of the brain endothelial CreERT2 expression itself on the IL-1β–induced sickness response.
In summary, these data demonstrate that TAK1 in brain endothelial cells is an important mediator of immune-to-brain communication and is necessary for full development of the sickness response. In response to the endogenous pyrogen IL-1β, TAK1 activated p38 MAPK and c-Jun and increased COX-2 expression and PGE2 production in brain endothelial cells. Fever and lethargy, two main features of the sickness response, were reduced in Slco1c1-CreERT2 × Tak1Fl/Fl mice, demonstrating the importance of brain endothelial cells in the mutual communication between CNS and the immune system. A key role of brain endothelial cells in the sickness response is compatible with previous work using bone marrow transplantation to generate chimeric mice, in which the genotype of hematopoietic cells, including brain perivascular macrophages, differs from that of all other cells of the body (Chakravarty and Herkenham, 2005; Steiner et al., 2006a; Wisse et al., 2007; Gosselin and Rivest, 2008). These studies provided evidence that nonhematopoietic cells are involved in the sickness response but could not further define the cell types involved. Interestingly, we also found Tak1 mRNA in the choroid plexus. Tak1 mRNA levels in the plexus were reduced in Slco1c1-CreERT2 × Tak1Fl/Fl mice (0.5 ± 0.1 relative units) compared with Tak1Fl/Fl mice (1.1 ± 0.2 relative units; P < 0.05, Student’s t test). The choroid plexus has been shown to express COX-2 and other inflammatory mediators after stimulation of the peripheral immune system (Lacroix and Rivest, 1998; Gosselin and Rivest, 2008). Because COX-2 expression is mainly localized to vascular structures and macrophages in the choroid plexus (Lacroix and Rivest, 1998; Dantzer et al., 2008), it seems unlikely that recombination of the Tak1 gene in epithelial cells of the plexus (Fig. 2 D) could additionally contribute to the blunted sickness response in Slco1c1-CreERT2 × Tak1Fl/Fl mice.
The fact that IL-1β–induced anorexia, weight loss, and induction of plasma corticosterone did not differ between genotypes in our experiments illustrates that there are several ways of immune-to-brain interaction (Dantzer et al., 2008). Within brain endothelial cells, not all inflammatory pathways depend on TAK1. The TAK1-independent activation of NF-κB (Fig. 1 D) may induce IL-6, TNF, or other mediators that contribute to the sickness response. Thus, our data based on the selective deletion of Tak1 may underestimate the role of brain endothelial cells in the sickness response.
Another hypothesis would be that some facets of the sickness response, such as anorexia, weight loss, and HPA axis activation, use other cellular routes of immune-to-brain signaling independent of the endothelium. Inflammatory mediators induce anorexia by activating POMC-expressing neurons in the arcuate nucleus (Jang et al., 2010). This site of action is in close proximity to the median eminence, a circumventricular organ lacking the blood–brain barrier. Thus, IL-1β may directly act on POMC neurons to inhibit feeding. Alternatively, the vagal nerve has been proposed to mediate anorexia in response to IL-1β (Bret-Dibat et al., 1995), providing a possible mechanism underlying anorexia independent of endothelial function. Interestingly, a recent study has implicated perivascular macrophages in the neuroendocrine response to IL-1β (Serrats et al., 2010). By ablating brain perivascular macrophages with clodronate liposomes, Serrats et al. (2010) found that brain perivascular macrophages mediate the activation of the HPA axis and its neuronal circuitry evoked by administering IL-1β. However, macrophage depletion had no effect on IL-1β–induced lethargy and fever response to IL-1β administration (Serrats et al., 2010). Interestingly, according to our study, lethargy and fever, but not HPA axis activation, depend on endothelial cells, supporting the hypothesis that individual components of the sickness response depend differently on distinct cell types.
MATERIALS AND METHODS
Chemicals and antibodies.
For Western blotting and immunofluorescence, the following antibodies and dilutions were used: anti-TAK1 1:500 (Millipore), anti-p38 1:1,000, anti–phospho-p38 1:1,000, anti–c-Jun 1:1,000, anti–phospho-c-Jun 1:1,000, anti–phospho-TAK1 1:1,000, anti–phospho-IκBα 1:1,000 (Cell Signaling Technology), anti-IκBα 1:500, anti-actin 1:1,000 (Santa Cruz Biotechnology, Inc.), anti–COX-2 1:500/1:900 (Western blot/immunofluorescence; Cayman Chemical), anti-NeuN 1:100 (Millipore), anti-GFAP 1:500 (Dako), anti-CD31 1:300 (BD), anti-CD11b 1:100 (Serotec), anti-SLCO1C1 1:500 (provided by L. Roberts, Xenoport, Santa Clara, CA), and anti–β-Galactosidase 1:2,000 (Millipore). Tribromoethanol and tamoxifen were obtained from Sigma-Aldrich. OZ was purchased from AnalytiCon Discovery. Okadaic acid was obtained from EMD. Recombinant mouse IL-1β (endotoxin content <0.1 ng/µg IL-1β) was purchased from PeproTech.
Mice.
A bacterial artificial chromosome (BAC; RP24-85B20) was chosen that harbors the mouse Slco1c1 locus with an 80-kb 5′-upstream and an 81-kb 3′-downstream region. To modify the BAC, a similar strategy was used as described previously (Lang et al., 2011). In brief, the BAC was modified by homologous recombination to insert a cassette encoding a codon-improved Cre recombinase (iCre), a mutated ligand-binding domain of the human estrogen receptor (ERT2), and an ampicillin resistance cassette flanked by two FRT sites. The construct was linearized and electroporated into heat-induced EL250 bacteria harboring the BAC. Clones with the recombinant BAC were induced with l-arabinose to express FLP recombinase, which resulted in deletion of the ampicillin resistance cassette. The modified genomic fragment containing the iCreERT2 knockin at the ATG of the Slco1c1 gene was separated from the BAC backbone by NotI digestion and subsequent purification with Sepharose CL4b Column (GE Healthcare). The DNA was microinjected into the B6D2F1 hybrid mouse pronuclei. Transgenic offspring was identified by genotyping PCR from tail DNA. The following primers were used for genotyping: Rec1_UB5, 5′-CTCGAGGAAGTTCCTATTCTC-3′; and Rec1_DB3, 5′-TCTCTGTCTCCTCTGCTTATC-3′.
To verify activity and localization of the CreERT2 fusion protein, Slco1c1-CreERT2 mice were mated with animals of the Cre reporter lines Gt(ROSA)26Sortm1sor (ROSA26) (Soriano, 1999) and B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Ai14) (Madisen et al., 2010). For histological analysis of β-Galactosidase activity, histochemical stainings were performed on 40-µm cryosections, followed by eosin counterstaining as described previously (Korhonen et al., 2009). Tak1Fl/Fl mice (Sato et al., 2005) were provided by S. Akira (Laboratory of Host Defense, Osaka University, Osaka, Japan). All Slco1c1-CreERT2 animals used in this study were treated with 1 mg tamoxifen every 12 h for 5 d. All animal experiments were approved by the local animal ethics committee (Regierungspräsidium Karlsruhe; Ministerium für Landwirtschaft, Umwelt und ländliche Räume, Kiel, Germany).
Cell isolation and culture.
PBECs were prepared from 2–6-mo-old animals that had been injected with tamoxifen at the age of 5–6 wk in the case of genetically modified mice. Animals were sacrificed with CO2, and the cerebrum was isolated and dissected free of the meninges. The brain was homogenized in a Dounce homogenizer, and the resulting homogenate was centrifuged at 2,000 g for 7 min. The pellet was resuspended in 18% dextran solution (mol wt 60,000–90,000; Sigma-Aldrich) in DME-F12 (Invitrogen) and centrifuged at 7,840 g for 10 min. After removing the supernatant and myelin debris, the pellet was resuspended in DME (Invitrogen) containing 1 mg/ml collagenase/dispase (Roche), 40 µg/ml DNase 1 (Roche), and 0.147 µg/ml tosyllysine chloromethyl ketone (Sigma-Aldrich) and incubated at 37°C for 75 min with occasional agitation to free endothelial cells from pericytes, perivascular macrophages, and remains of the basement membrane. The cell suspension was centrifuged at 2,000 g for 7 min, the supernatant was discarded, and cells were washed and seeded in 6-well plates coated with mouse collagen IV (BD). Cells were grown in DME-F12 supplemented with 20% plasma-derived horse serum (First Link), 2 mM l-glutamine, 100 IU/ml penicillin, 100 µg/ml streptomycin, 0.25 µg/ml amphotericin B, 100 U/ml heparin (Braun), and 30 µg/ml of endothelial cell growth supplement (Sigma-Aldrich). 4 µg/ml puromycin (Sigma-Aldrich) was added for the first 48 h after preparation to deplete cells of nonendothelial origin. To prepare primary mixed cortical cultures, cortices of adult mice were dissected, homogenized, and digested with trypsin and DNase 1. Cells were grown in DME-F12 supplemented with 10% FCS, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 0.5 mM l-glutamine. The mouse brain endothelial cell line bEnd.3 (American Type Culture Collection) was grown in DME containing 4.5 g/liter glucose, FCS (10%), 100 IU/ml penicillin, 100 µg/ml streptomycin, and 2 mM l-glutamine.
Western blots.
PBECs and bEnd.3 cells were cultured on 6-well plates. A preincubation with 250 nM okadaic acid or with 600 nM OZ for 30 min was performed as indicated. Cells were stimulated with 50 ng/ml of mouse IL-1β for the indicated times and lysed in 2× hot Laemmli buffer, incubated at 95°C for 5 min, and then loaded on SDS-PAGE gels.
Mice were deeply anesthetized with 200 µl 2.5% tribromoethanol per 10 g of body weight and perfused with ice-cold Ringer’s solution. Hypothalamic tissue samples were homogenized in radioimmunoprecipitation (RIPA) buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.4, 10 mM EDTA, 0.1% SDS, 1% Triton X-100, 0.5% sodium deoxycholate, and 0.5 mM PMSF) supplemented with phosphatase inhibitor cocktails 1+2 (Sigma-Aldrich) and protease inhibitor tablets (Roche). Lysates were subjected to SDS-PAGE.
The membranes were then incubated with the indicated primary antibodies and, consequently, with HRP-conjugated secondary antibodies. For detection we applied enhanced chemiluminescence (ECL; Thermo Fisher Scientific).
PGE2-ELISA.
bEnd.3 cells were cultured on 6-well plates, preincubated with 600 nM OZ or DMSO for 30 min, and then treated with 50 ng/ml mouse IL-1β or PBS for 4 h. The supernatant was collected and the PGE2-ELISA (Cayman Chemical) was performed according to the manufacturer’s description.
Immunofluorescence.
Mice were deeply anesthetized by i.p. injection of 2.5% tribromoethanol (200 µl/10 g of body weight) and perfused with Ringer’s solution, followed by 4% PFA in PBS. Brains were postfixed for 4 h in 4% PFA in PBS and immersed in 30% sucrose in PBS overnight. Coronal cryosections were stained free floating overnight with the indicated primary antibodies. Alexa Fluor 488–labeled (Invitrogen) and Cy3-labeled (Jackson ImmunoResearch Laboratories) secondary antibodies were used for detection. For COX-2/CD31 immunofluorescence, we perfused mice only with Ringer’s solution, froze brains on dry ice, and stained 20-µm cryosections on slides. We quantified the COX-2/CD31 double-positive area with ImageJ software (National Institutes of Health).
Quantitative RT-PCR.
RNA from PBECs and choroid plexus from tamoxifen-treated mice were isolated using the RNeasy Mini kit (QIAGEN), according to the manufacturer’s protocol, and transcribed with Moloney Murine Leukemia Virus reverse transcription and random hexamer primers (Promega). The following primers were used: Cyclophilin A forward, 5′-AGGTCCTGGCATCTTGTCCAT-3′; Cyclophilin A reverse, 5′-GAACCGTTTGTGTTTGGTCCA-3′, PCR product 51 bp; Tak1 forward, 5′-AGAGGTTGTCGGAAGAGGAGCTT-3′; and Tak1 reverse, 5′-ACAACTGCCGGAGCTCCACAA-3′, PCR product 128 bp. Quantitative RT-PCR was performed according to the following protocol: 2 min at 50°C, 2 min at 95°C, 15 s at 95°C, and 1 min at 60°C (40 cycles). Amplification was quantified using Platinum SYBR Green qPCR SuperMix (Invitrogen). Quantified results for Tak1 cDNA was normalized to Cyclophilin A using the ΔΔCt method.
Mouse treatment and telemetric temperature and activity measurements.
OZ (30 mg/kg body weight) or vehicle (DMSO) was administered i.p. 30 min before IL-1β injection. Recombinant IL-1β was reconstituted in water to a concentration of 0.1 µg/µl and further diluted with PBS. Mice were injected retroorbitally under brief ether anesthesia (<60 s) with PBS or mouse IL-1β (30 µg/kg body weight). We used a radiotelemetry system (Data Sciences International) to monitor temperature and activity in conscious, unrestrained mice. Mice were kept at a room temperature of 25 ± 1°C and had ad libitum access to food and water. After i.p. implantation of the transmitters (TA-F20; DSI), mice were allowed to recover for at least 7 d. Mice were injected i.v. between 9:30 and 10:30am with PBS and, 2 d later, with mouse IL-1β. We collected data over a period of 10 s every minute and averaged over 30 min to measure temperature or over the whole dark phase to measure activity. Weight loss and food intake were determined 22 h after the injection.
Corticosterone measurement.
3 h after i.v. injection of IL-1β (30 µg/kg body weight), mice were anesthetized by i.p. injection of 150 µl 2.5% tribromoethanol/10 g of body weight, and blood samples were drawn from the vena cava. Plasma corticosterone levels were measured by RIA in the Steroid Laboratory of the Institute of Pharmacology, Heidelberg, Germany.
Statistical analysis.
Values are expressed as means ± SEM. Differences between two groups were analyzed by using the unpaired two-sided Student’s t test. The effect of IL-1β on body temperature was determined by subtracting the temperatures that were measured after PBS injection. To analyze temperature differences, repeated measures two-way ANOVA and Bonferroni’s post-test were used. For all other data, one-way ANOVA, followed by Tukey’s multiple comparison test, was used. Data were considered to be significant at P < 0.05.
Acknowledgments
We thank Nadine Gehrig, Beate Lembrich, and Christina Steinmeier-Stannek for expert technical assistance and Dr. Shizuo Akira (Osaka) for providing Tak1Fl/Fl mice. We are obliged to Dr. Roberts (Xenoport, Santa Clara, CA), for kindly providing anti-SLCO1C1 antibodies.
This study was supported by a grant of the Deutsche Forschungsgemeinschaft to M. Schwaninger (SCHW 416/5-1).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- COX-2
- cyclooxygenase 2
- HPA axis
- hypothalamus–pituitary–adrenal axis
- OZ
- 5z-7-oxozeaenol
- PBEC
- primary brain endothelial cell
- PGE2
- prostaglandin E2
References
- Bret-Dibat J.L., Bluthé R.M., Kent S., Kelley K.W., Dantzer R. 1995. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav. Immun. 9:242–246 10.1006/brbi.1995.1023 [DOI] [PubMed] [Google Scholar]
- Chakravarty S., Herkenham M. 2005. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 25:1788–1796 10.1523/JNEUROSCI.4268-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching S., Zhang H., Belevych N., He L., Lai W., Pu X.A., Jaeger L.B., Chen Q., Quan N. 2007. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J. Neurosci. 27:10476–10486 10.1523/JNEUROSCI.3357-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9:46–56 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Song D., Ye X., Liu S.F. 2009. A pivotal role of endothelial-specific NF-kappaB signaling in the pathogenesis of septic shock and septic vascular dysfunction. J. Immunol. 183:4031–4038 10.4049/jimmunol.0900105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin D., Rivest S. 2008. MyD88 signaling in brain endothelial cells is essential for the neuronal activity and glucocorticoid release during systemic inflammation. Mol. Psychiatry. 13:480–497 10.1038/sj.mp.4002122 [DOI] [PubMed] [Google Scholar]
- Hanada M., Ninomiya-Tsuji J., Komaki K., Ohnishi M., Katsura K., Kanamaru R., Matsumoto K., Tamura S. 2001. Regulation of the TAK1 signaling pathway by protein phosphatase 2C. J. Biol. Chem. 276:5753–5759 10.1074/jbc.M007773200 [DOI] [PubMed] [Google Scholar]
- Hanada R., Leibbrandt A., Hanada T., Kitaoka S., Furuyashiki T., Fujihara H., Trichereau J., Paolino M., Qadri F., Plehm R., et al. 2009. Central control of fever and female body temperature by RANKL/RANK. Nature. 462:505–509 10.1038/nature08596 [DOI] [PubMed] [Google Scholar]
- Horai R., Asano M., Sudo K., Kanuka H., Suzuki M., Nishihara M., Takahashi M., Iwakura Y. 1998. Production of mice deficient in genes for interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J. Exp. Med. 187:1463–1475 10.1084/jem.187.9.1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang P.-G., Namkoong C., Kang G.M., Hur M.-W., Kim S.-W., Kim G.H., Kang Y., Jeon M.-J., Kim E.H., Lee M.-S., et al. 2010. NF-kappaB activation in hypothalamic pro-opiomelanocortin neurons is essential in illness- and leptin-induced anorexia. J. Biol. Chem. 285:9706–9715 10.1074/jbc.M109.070706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirik F.R., Podor T.J., Hirano T., Kishimoto T., Loskutoff D.J., Carson D.A., Lotz M. 1989. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J. Immunol. 142:144–147 [PubMed] [Google Scholar]
- Korhonen H., Fisslthaler B., Moers A., Wirth A., Habermehl D., Wieland T., Schütz G., Wettschureck N., Fleming I., Offermanns S. 2009. Anaphylactic shock depends on endothelial Gq/G11. J. Exp. Med. 206:411–420 10.1084/jem.20082150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuldo J.M., Westra J., Asgeirsdóttir S.A., Kok R.J., Oosterhuis K., Rots M.G., Schouten J.P., Limburg P.C., Molema G. 2005. Differential effects of NF-kappaB and p38 MAPK inhibitors and combinations thereof on TNF-alpha- and IL-1beta-induced proinflammatory status of endothelial cells in vitro. Am. J. Physiol. Cell Physiol. 289:C1229–C1239 10.1152/ajpcell.00620.2004 [DOI] [PubMed] [Google Scholar]
- Lacroix S., Rivest S. 1998. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J. Neurochem. 70:452–466 10.1046/j.1471-4159.1998.70020452.x [DOI] [PubMed] [Google Scholar]
- Lang M.F., Salinin S., Ridder D.A., Kleesiek J., Hroudova J., Berger S., Schütz G., Schwaninger M. 2011. A transgenic approach to identify thyroxine transporter-expressing structures in brain development. J. Neuroendocrinol. In press 10.1111/j.1365-2826.2011.02216.x [DOI] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R., et al. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13:133–140 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J., Kajino T., Ono K., Ohtomo T., Matsumoto M., Shiina M., Mihara M., Tsuchiya M., Matsumoto K. 2003. A resorcylic acid lactone, 5Z-7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J. Biol. Chem. 278:18485–18490 10.1074/jbc.M207453200 [DOI] [PubMed] [Google Scholar]
- Pecchi E., Dallaporta M., Jean A., Thirion S., Troadec J.D. 2009. Prostaglandins and sickness behavior: old story, new insights. Physiol. Behav. 97:279–292 10.1016/j.physbeh.2009.02.040 [DOI] [PubMed] [Google Scholar]
- Roberts L.M., Woodford K., Zhou M., Black D.S., Haggerty J.E., Tate E.H., Grindstaff K.K., Mengesha W., Raman C., Zerangue N. 2008. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 149:6251–6261 10.1210/en.2008-0378 [DOI] [PubMed] [Google Scholar]
- Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O., Akira S. 2005. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6:1087–1095 10.1038/ni1255 [DOI] [PubMed] [Google Scholar]
- Serrats J., Schiltz J.C., García-Bueno B., van Rooijen N., Reyes T.M., Sawchenko P.E. 2010. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 65:94–106 10.1016/j.neuron.2009.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W.L., DeWitt D.L., Garavito R.M. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69:145–182 10.1146/annurev.biochem.69.1.145 [DOI] [PubMed] [Google Scholar]
- Soriano P. 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21:70–71 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Steiner A.A., Chakravarty S., Rudaya A.Y., Herkenham M., Romanovsky A.A. 2006a. Bacterial lipopolysaccharide fever is initiated via Toll-like receptor 4 on hematopoietic cells. Blood. 107:4000–4002 10.1182/blood-2005-11-4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A.A., Ivanov A.I., Serrats J., Hosokawa H., Phayre A.N., Robbins J.R., Roberts J.L., Kobayashi S., Matsumura K., Sawchenko P.E., Romanovsky A.A. 2006b. Cellular and molecular bases of the initiation of fever. PLoS Biol. 4:e284 10.1371/journal.pbio.0040284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F., Kellendonk C., Kretz O., Gass P., Anlag K., Orban P.C., Bock R., Klein R., Schütz G. 1999. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23:99–103 10.1038/12703 [DOI] [PubMed] [Google Scholar]
- Warner S.J., Auger K.R., Libby P. 1987. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J. Immunol. 139:1911–1917 [PubMed] [Google Scholar]
- Wisse B.E., Ogimoto K., Tang J., Harris M.K., Jr, Raines E.W., Schwartz M.W. 2007. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology. 148:5230–5237 10.1210/en.2007-0394 [DOI] [PubMed] [Google Scholar]
- Yamazaki K., Gohda J., Kanayama A., Miyamoto Y., Sakurai H., Yamamoto M., Akira S., Hayashi H., Su B., Inoue J. 2009. Two mechanistically and temporally distinct NF-kappaB activation pathways in IL-1 signaling. Sci. Signal. 2:ra66 10.1126/scisignal.2000387 [DOI] [PubMed] [Google Scholar]
- Ye X., Ding J., Zhou X., Chen G., Liu S.F. 2008. Divergent roles of endothelial NF-κB in multiple organ injury and bacterial clearance in mouse models of sepsis. J. Exp. Med. 205:1303–1315 10.1084/jem.20071393 [DOI] [PMC free article] [PubMed] [Google Scholar]