Griffin et al. respond:
In the January 2011 issue of the JEM, we identified a population of B cells in human umbilical cord and adult peripheral blood that manifests three key functional features of mouse B1 cells (spontaneous immunoglobulin secretion, tonic intracellular signaling, and efficient T cell stimulation). This population is marked by the phenotype CD20+CD27+CD43+CD70− (Griffin et al., 2011). We appreciate the interest shown in this work by Perez-Andres et al. and Descatoire et al., who have written letters to report CD3+ events detected within CD19+CD27+CD43+ and CD20+CD27+CD43+ gated populations, respectively, during their flow cytometry.
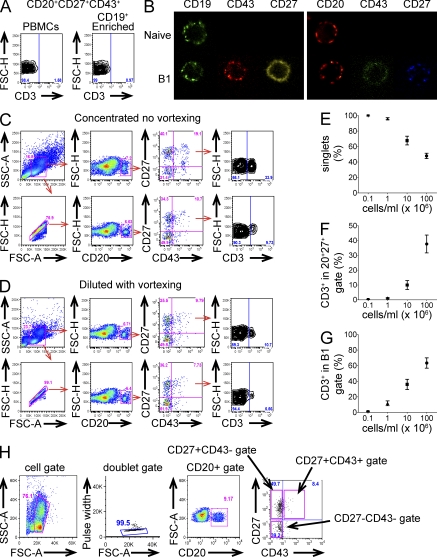
The problem of CD3 positivity among “a major fraction” (Perez-Andres et al., 2011) and “a large fraction” (Descatoire et al. 2011) of CD27+CD43+ B cells is not something we have experienced. In our hands, CD3+ events within CD20+CD27+CD43+ sort-purified populations are rare. In early work, we tested the function of B1 cells that were sort-purified from PBMCs without prior enrichment (Griffin et al., 2011); these CD20+CD27+CD43+ populations contain <3% CD3+ events (2.9 ± 0.66%; n = 3). In later work, we studied the function of B1 cells that were sort-purified from PBMCs after enrichment with anti-CD19 beads; these CD20+CD27+CD43+ populations contain <1% CD3+ events (0.57 ± 0.37%; n = 3; Fig. 1 a). The existence of CD27+CD43+ coexpressing B cells seems irrefutable; single cells expressing CD19, CD27, and CD43, and single cells expressing CD20, CD27, and CD43, can be readily visualized as confocal microscopic images (Fig. 1 b).
Figure 1.
Human CD20+CD27+CD43+ B1 cells do not express CD3. (A) Adult peripheral blood cells were immunofluorescently stained with specific antibodies. CD20+CD27+CD43+ B1 cells were sort-purified from nonenriched, as well as CD19-enriched PBMCs and sorted B1 cells were analyzed for CD3 expression. Gating was based on an isotype control. Results for one of three comparable experiments are shown. (B) Adult peripheral blood cells were immunofluorescently stained, sort-purified for naive (CD19+CD27−CD43−/CD20+CD27−CD43−) and B1 (CD19+CD27+CD43+/CD20+CD27+CD43+) cells, and examined by confocal microscopy. (C) Immunofluorescently stained PBMCs were analyzed at high concentration without vortexing without (top row) or with (bottom row) FSC-H/FSC-A doublet discrimination. The last panel shows CD3+ events among CD20+CD27+CD43+ cells. (D) The same sample as in C was diluted and vortexed, and then analyzed without (top row) or with (bottom row) FSC-H/FSC-A doublet discrimination. Note that many more cells appear as singlets (99.1%) as compared with C (75.9%). The last panel shows CD3+ events among CD20+CD27+CD43+ cells. Results for one of three comparable experiments are shown. (E–G) Adult peripheral blood cells were immunofluorescently stained, and then presented for flow cytometric analysis at various cell concentrations, as indicated (n = 12). The proportion of single cells determined by FSC-H/FSC-A gating is shown as a function of cell concentration in E. The proportion of CD3+ events among gated CD20+CD27+ cells is shown as a function of cell concentration in F. The proportion of CD3+ events among gated CD20+CD27+CD43+ cells is shown as a function of cell concentration in G. (H) Adult peripheral blood cells were immunofluorescently stained with specific antibodies, brought into a single cell suspension, and sort-purified on an Influx (BD) instrument. The gating strategy used for experiments reported in Griffin et al. (2011) is shown.
To discern the origin of the reported CD3+ events, we attempted to recreate the observations of Perez-Andres et al. (2011) and Descatoire et al. (2011). We reproduced the high frequency of double staining events observed by these investigators only by changing our usual protocol for cell preparation. When we reconstituted mononuclear cells at a relatively high concentration, we observed CD3+ events in the CD20+CD27+CD43+ gate. FSC-H/FSC-A discrimination decreased, but did not completely eliminate, CD3+ staining in that gate (Fig. 1 c). Diluting and vortexing the sample reduced the frequency of CD3+ events in the CD20+CD27+CD43+ gate, which was further reduced by applying FSC-H/FSC-A doublet discrimination (Fig. 1 d). Over a range of cell concentrations, we documented an inverse correlation between cell concentration and the proportion of singlets as determined by FSC-H/FSC-A, and a direct correlation between cell concentration and CD3+ events in the CD20+CD27+ gate and in the CD20+CD27+CD43+ B1 cell gate (Fig. 1, e and f). These results suggest that CD3+ events within CD20+ B cell populations result from the formation of B:T doublets.
To evaluate the possible existence of CD19+ T cells, as suggested by Perez-Andres et al. (2011), we examined CD27+CD43+ B cells by confocal microscopy and failed to identify single cells that express both CD3 and CD19 (unpublished data). We examined B cells by integrated flow cytometry/image microscopy and again failed to identify single cells marked by either CD19 or CD20 in combination with CD3. Although the possibility of rare B cells that express CD3 (Wang et al., 2009) or rare T cells that express CD20 (Yokose et al., 2001) cannot be entirely ruled out, these results indicate that such cells must be exceedingly rare. We conclude that CD19+CD3+ cells observed by Perez-Andres et al. (2011), like the CD20+CD3+ cells observed by Descatoire et al. (2011), represent B:T doublets.
In our results, CD20+CD27+CD43+ B1 cells are individual single cells by post-sort analysis. The gating strategy that we have used, and continue to use, is displayed in Fig. 1 h. Virtually all (99.9%) events fell within a tight FSC-H/FSC-A discrimination gate, and virtually all (99.9%) events corresponded to single nuclei after DNA staining with Hoechst33342 when analyzed post-sort (unpublished data). In addition, we showed by direct visualization of Hoechst33342-stained nuclei that single cell–sorted CD20+CD27+CD43+ B1 cells deposited onto glass slides are individual single cells, with not a single doublet observed in over 1,000 cells examined (unpublished data). Thus, the B1 cell functions delineated in our previous work correspond to the characteristics of single CD20+CD27+CD43+ B cells.
Descatoire et al. (2011) notes that in the human system allogeneic T cell stimulation has been ascribed to memory B cells (Good et al., 2009). However, CD20+CD27+ B cells, historically considered memory B cells, fail to allogeneically stimulate T cells after removal of CD43-expressing constituents (Griffin et al., 2011). Furthermore, doublets formed by naive B cells and T cells would not be expected to manifest T cell stimulatory capacity nor would their removal be expected to adversely affect any memory B cell function. This emphasizes in another way, through negative depletion, the existence of functionally distinct, CD43-expressing CD27+ B cells.
Descatoire et al. (2011) suggests that their CD3−CD20+CD27+CD43+ B cells were either IgD+ or IgD−; in our hands, CD20+CD27+CD43+ B1 cells predominantly expressed IgD, and gave rise to amplified and rearranged IgM sequences as shown in our earlier study (Griffin et al., 2011), although we identified IgD−CD20+CD27+CD43+ B1 cells that may be responsible for IgA produced by B1 cells, as in the mouse system (Baumgarth, 2011). Perez-Andres et al. (2011) noted that their CD3−CD19+CD27+CD43+ lymphocytes expressed elevated levels of CD38 and were most likely plasma cells. CD19 does not exclude plasmablasts and plasma cells, as does CD20 (Jego et al., 2001), which we used to identify B cells in our study. Descatoire et al. (2011) raised the possibility that CD20+CD27+CD43+ B cells might be activated cells on their way to plasma cell differentiation. We addressed this in our earlier work by showing that B1 cells do not express elevated levels of activation antigens, such as CD69 and CD70, and that culture of B1 cells with IL-6 for 5 d did not lead to loss of CD20 or acquisition of CD138 (Griffin et al., 2011).
Both Perez-Andres et al. (2011) and Descatoire et al. (2011) state that after excluding CD3+ events, they indeed identified the phenotypically unique CD27+CD43+ B cell population that we first reported in January (Griffin et al., 2011), amounting to 2–3% of all B cells in Weller’s hands. Furthermore, Perez-Andres et al. (2011) stated that their results confirm a decline with age among CD27+CD43+ B cells, recapitulating our published results (Griffin et al., 2011). It appears that the major issue raised by these two letters relates simply to different numbers for the frequency of CD27+CD43+ B1 cells. There are numerous reasons why different laboratories might arrive at different frequencies, including variations in sample size, donor age, cell preparation, antibody/fluorophore selection, and instrument and run characteristics, not to mention the efficacy of doublet discrimination. With respect to the first two reasons, in our January 2011 article (Griffin et al., 2011), we averaged noncord peripheral blood samples to obtain a mean CD27+CD43+ B cell frequency across 25 highly variable samples; however, many of these samples were obtained from children, whose B1 cell frequencies are elevated with respect to middle aged adults, and we included values that would now be considered outliers. In our current work with samples from individuals in the third through seventh decades of life, we obtain values over a wide range (1–9%) of CD20+CD27+CD43+ B1 cells among all circulating B cells, a range recapitulated by other groups, including the letter writers. Our recent identification of human B1 cells provides the means to explore the regulation and role of this unique population in human health and disease. Functional B1 cells in the human system phenotype as CD20+CD27+CD43+CD70− and do not express CD3.
References
- Baumgarth N. 2011. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 11:34–46. 10.1038/nri2901 [DOI] [PubMed] [Google Scholar]
- Descatoire M., Weill J.-C., Reynaud C.-A., Weller S.. 2011. A human equivalent of mouse B-1 cells? J. Exp. Med. 208:2563–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good K.L., Avery D.T., Tangye S.G.. 2009. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells. J. Immunol. 182:890–901. [DOI] [PubMed] [Google Scholar]
- Griffin D.O., Holodick N.E., Rothstein T.L.. 2011. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70Ú. J. Exp. Med. 208:67–80. 10.1084/jem.20101499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jego G., Bataille R., Pellat-Deceunynck C.. 2001. Interleukin-6 is a growth factor for nonmalignant human plasmablasts. Blood. 97:1817–1822. 10.1182/blood.V97.6.1817 [DOI] [PubMed] [Google Scholar]
- Perez-Andres M., Grosserichter-Wagener C., Teodosio C., van Dongen J.J.M., Orfao A., van Zelm M.C.. 2011. The nature of circulating CD27+CD43+ B cells. J. Exp. Med. 208:2565–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen C., Lau S., Raghavan R.I., Rowsell E.H., Said J., Weiss L.M., Huang Q.. 2009. CD3-positive large B cell lymphoma. Am. J. Surg. Pathol. 33:505–512. 10.1097/PAS.0b013e3181ad25d5 [DOI] [PubMed] [Google Scholar]
- Yokose N., Ogata K., Sugisaki Y., Mori S., Yamada T., An E., Dan K.. 2001. CD20-positive T cell leukemia/lymphoma: case report and review of the literature. Ann. Hematol. 80:372–375. 10.1007/s002770100297 [DOI] [PubMed] [Google Scholar]