To the Editor:
We recently read with great interest an article in The Journal of Experimental Medicine from the laboratory of Thomas Rothstein (Griffin et al., 2011). This paper described a new B cell population present in human cord blood and adult peripheral blood that displays functional characteristics shared with mouse B1 cells.
In contrast to B2 cells, B1 cells spontaneously produce IgM and are an important source of natural antibodies in the absence of antigen stimulation in mouse (Hayakawa et al., 1984; Sidman et al., 1986). Human B1 cells were defined based on coexpression of CD43 and CD27 (Griffin et al., 2011). In addition, human B1 cells were found to express CD20 and lack expression of CD69 and CD70. Notably, CD43 is also expressed on mouse B1 cells (Wells et al., 1994), as well as on human bone marrow B cell precursors and plasma cells (MacMichael et al., 1987; Wikén et al., 1988), whereas CD27 is generally regarded as a marker for memory B cells in humans (Agematsu et al., 1997; Tangye et al., 1998). Furthermore, CD27 and CD43 are coexpressed on the majority of blood T cells and plasma cells (MacMichael et al., 1987).
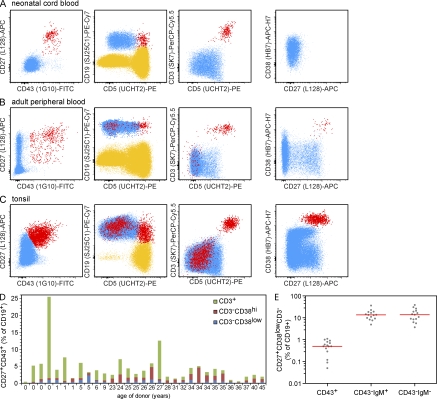
To gain further insight into the nature of CD27+CD43+ B1 cells in humans, we developed a flow cytometric strategy to discriminate between CD27+CD43+ B cells and CD27+ memory B cells in human blood (Berkowska et al., 2011). Like Griffin et al. (2011), we systematically identified a CD27+CD43+ subset within the CD19+ or CD20+ B cell population in neonatal cord blood (n = 4 samples), in the peripheral blood of children and adults (n = 27 samples), and in childhood tonsils (n = 7 samples; Fig. 1, A–C). Their frequencies ranged from 1 to 25.5% of total CD19+ or CD20+ cells in blood (Fig. 1 D), and from 0.3 to 3.7% of CD19+ cells in childhood tonsil (unpublished data).
Figure 1.
Flow cytometric analysis of CD19+CD27+CD43+ cells in human neonatal cord blood, peripheral blood, and tonsil samples. (A–C) CD27+CD43+ cells (red) were defined within CD19+ lymphocytes (blue; left plots). Where illustrative, the CD19− lymphocytes (T cells and NK cells) are shown in yellow. Samples were also stained with anti-CD3, anti-CD5, and anti-CD38 as indicated. The right plots only display CD19+CD3− events. Data are representative of 4 cord blood, 27 blood, and 7 tonsil samples. (D) Frequencies of CD27+CD43+ cells among CD19+ cells in neonatal cord blood and peripheral blood samples from individual children and adults of indicated ages. The relative contributions of CD3+ T cells, CD3−CD38hi plasma cells, and CD3−CD38low B lymphocytes are indicated for each individual. (E) Frequencies of CD43+, CD43−IgM+, and CD43-IgM− cells among CD19+CD27+CD3−CD38low cells within the blood of healthy adults. Individual frequencies are shown in gray and the median is indicated with a red bar. IgM expression was detected with a monoclonal IgM-HorizonV450 antibody (clone G20-127).
In contrast to most B lymphocytes, which are CD43−, most T lymphocytes and plasma cells are CD43+. To ensure that all identified CD43+CD27+ events constituted B lymphocytes, we further analyzed the CD27+CD43+ population. A substantial fraction (0.1–24%) of CD19+CD27+CD43+ events was highly positive for CD3 and dimly positive for CD19. Analysis of CD19 versus CD3 expression suggested that these cells were T lymphocytes that were incorrectly included in the CD19+ gate, and accounted for most CD5+ events in the gate (Fig. 1, A–C). Of the remaining CD3−CD27+CD43+ cells, the majority showed high expression of CD38 (0.1–4.6% of CD19+). Coexpression of CD27 and high levels of CD38 is typically seen on plasma cells, as well as on germinal center cells and plasma cells in tonsil (Klein et al., 2003; van Zelm et al., 2007; Perez-Andres et al., 2010).
Only a minority of the CD19+CD27+CD43+ events appeared to be true B lymphocytes (0.3–2.3% of CD19+CD3−CD38dim cells) and consisted of both IgM+ and IgM− cells (unpublished data). Their total frequency was >10-fold lower than the frequencies of both CD27+IgM+ and CD27+IgM− memory B cells in adult blood (Fig. 1 E). Therefore, we conclude that CD43+ B lymphocytes constitute a small minority of total CD27+ B lymphocytes.
In conclusion, our results confirm a decline with age of the overall population of CD27+CD43+ circulating human B cells, but indicate that caution should be taken in the definition of human B1 cells inside this heterogeneous cell population. Detailed flow cytometric analysis of CD19+CD27+CD43+ cells reveals that the majority may be CD38hi plasma cells or contaminating CD3+ T cells. The large proportion of T cells in our analysis might explain the high frequency of CD5+ cells reported by Griffin et al. (2011), as all T cells express CD5. Finally, we conclude that clearly defined CD3−CD38dimCD27+CD43+ B lymphocytes may be only a minor population of CD27+ memory B cells in adult blood. Understanding the functional similarities between these cells and mouse B1 cells will be important topics for additional studies in regard to infectious diseases and lymphoid malignancies. However, these studies will require the exclusion of T cells and circulating plasma cells from such analysis.
Acknowledgments
The authors are indebted to Dr. M. van der Burg and M.A. Berkowska (Department of Immunology, Erasmus MC) and Dr. J. Almeida (Department of Medicine, University of Salamanca) for fruitful discussions, and to Mrs. S. de Bruin-Versteeg (Department of Immunology, Erasmus MC) for assistance with preparing the Figure.
M.C. van Zelm is supported by fellowships from the Erasmus University Rotterdam (EUR-Fellowship) and the Erasmus MC, and by Veni grant 916.110.90 from ZonMW/NWO.
The authors have no conflicting financial interests.
References
- Agematsu K., Nagumo H., Yang F.C., Nakazawa T., Fukushima K., Ito S., Sugita K., Mori T., Kobata T., Morimoto C., Komiyama A.. 1997. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur. J. Immunol. 27:2073–2079. 10.1002/eji.1830270835 [DOI] [PubMed] [Google Scholar]
- Berkowska M.A., Driessen G.J., Bikos V., Grosserichter-Wagener C., Stamatopoulos K., Cerutti A., He B., Biermann K., Lange J.F., van der Burg M., et al. 2011. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 118:2150–2158. 10.1182/blood-2011-04-345579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D.O., Holodick N.E., Rothstein T.L.. 2011. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70-. J. Exp. Med. 208:67–80. 10.1084/jem.20101499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Honda M., Herzenberg L.A., Steinberg A.D., Herzenberg L.A.. 1984. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc. Natl. Acad. Sci. USA. 81:2494–2498. 10.1073/pnas.81.8.2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein U., Tu Y., Stolovitzky G.A., Keller J.L., Haddad J., Jr, Miljkovic V., Cattoretti G., Califano A., Dalla-Favera R.. 2003. Transcriptional analysis of the B cell germinal center reaction. Proc. Natl. Acad. Sci. USA. 100:2639–2644. 10.1073/pnas.0437996100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMichael A.J., Beverley P.C.L., Cobbold S., Crumpton M.J., Gilks W., Gotch F.M., Hogg N., Horton M., Ling N., MacLennan I.C.M., et al. 1987. Leukocyte Typing III. Oxford University Press, Oxford. 1050 pp. [Google Scholar]
- Perez-Andres M., Paiva B., Nieto W.G., Caraux A., Schmitz A., Almeida J., Vogt R.F., Jr, Marti G.E., Rawstron A.C., Van Zelm M.C., et al. ; Primary Health Care Group of Salamanca for the Study of MBL. 2010. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin. Cytom. 78:S47–S60. [DOI] [PubMed] [Google Scholar]
- Sidman C.L., Shultz L.D., Hardy R.R., Hayakawa K., Herzenberg L.A.. 1986. Production of immunoglobulin isotypes by Ly-1+ B cells in viable motheaten and normal mice. Science. 232:1423–1425. 10.1126/science.3487115 [DOI] [PubMed] [Google Scholar]
- Tangye S.G., Liu Y.J., Aversa G., Phillips J.H., de Vries J.E.. 1998. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J. Exp. Med. 188:1691–1703. 10.1084/jem.188.9.1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zelm M.C., Szczepanski T., van der Burg M., van Dongen J.J.. 2007. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. J. Exp. Med. 204:645–655. 10.1084/jem.20060964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells S.M., Kantor A.B., Stall A.M.. 1994. CD43 (S7) expression identifies peripheral B cell subsets. J. Immunol. 153:5503–5515. [PubMed] [Google Scholar]
- Wikén M., Björck P., Axelsson B., Perlmann P.. 1988. Induction of CD43 expression during activation and terminal differentiation of human B cells. Scand. J. Immunol. 28:457–464. 10.1111/j.1365-3083.1988.tb01476.x [DOI] [PubMed] [Google Scholar]