TRIF signaling triggers the amplification of macrophage bactericidal activity sufficient to eliminate invading intestinal pathogens through the sequential induction of IFN-β and IFN-γ from macrophages and NK cells, respectively.
Abstract
Toll-like receptor 4 (TLR4), which signals through the adapter molecules myeloid differentiation factor 88 (MyD88) and toll/interleukin 1 receptor domain-containing adapter inducing IFN-β (TRIF), is required for protection against Gram-negative bacteria. TRIF is known to be important in TLR3-mediated antiviral signaling, but the role of TRIF signaling against Gram-negative enteropathogens is currently unknown. We show that TRIF signaling is indispensable for establishing innate protective immunity against Gram-negative Yersinia enterocolitica. Infection of wild-type mice rapidly induced both IFN-β and IFN-γ in the mesenteric lymph nodes. In contrast, TRIF-deficient mice were defective in these IFN responses and showed impaired phagocytosis in regional macrophages, resulting in greater bacterial dissemination and mortality. TRIF signaling may be universally important for protection against Gram-negative pathogens, as TRIF-deficient macrophages were also impaired in killing both Salmonella and Escherichia coli in vitro. The mechanism of TRIF-mediated protective immunity appears to be orchestrated by macrophage-induced IFN-β and NK cell production of IFN-γ. Sequential induction of IFN-β and IFN-γ leads to amplification of macrophage bactericidal activity sufficient to eliminate the invading pathogens at the intestinal interface. Our results demonstrate a previously unknown role of TRIF in host resistance to Gram-negative enteropathogens, which may lead to effective strategies for combating enteric infections.
Gastrointestinal bacterial infections are a common health problem and have serious implications because of their epidemic potential (Tassios and Kerr, 2010). Enteric pathogens raise additional concerns because recent globalization of the food supply increases the chance of outbreaks and the potential for bioterrorism. Most clinically significant enteric pathogens, such as Yersinia, Salmonella, Vibrio, or Shigella, are Gram-negative bacteria which have evolutionarily acquired an ability to evade host immune defenses (Miao and Miller, 1999; Brodsky and Medzhitov, 2008; Arnold et al., 2010). The strategies used for immune evasion mainly target innate immunity, highlighting the importance of the innate immune system in intestinal host defense mechanisms. Although extensive studies have revealed the virulence factors of these pathogens, it is still unclear how the host innate immune cells formulate effective intestinal defense to these pathogens.
Pathogens are recognized by the host innate immune system through toll-like receptors (TLRs), which induce intracellular signaling mainly through two adapter molecules, myeloid differentiation factor 88 (MyD88) or toll/interleukin 1 receptor domain-containing adapter inducing IFN-β (TRIF). Although MyD88 is used by most TLRs, the TRIF pathway can only be recruited by TLR4 and TLR3. The MyD88 pathway strongly induces NF-κB activation and proinflammatory cytokine secretion associated with pathogen clearance (Lebeis et al., 2009). The TRIF pathway, in contrast, induces type I IFNs and slower NF-κB activation and has largely been associated with antiviral responses (Yamamoto et al., 2003; Guo and Cheng, 2007). Although TLR4 can signal through either pathway, the majority of LPS-induced genes in macrophages appear to use a MyD88-independent pathway, suggesting that the TRIF pathway may play a role in responses to Gram-negative pathogens (Björkbacka et al., 2004). These observations led us to question whether TRIF signaling has been overlooked as a mediator of host defense against enteric pathogens. Emerging evidence has demonstrated that TRIF is required for host resistance against pulmonary infection with multiple Gram-negative pathogens (Jeyaseelan et al., 2007; Cai et al., 2009), whereas intestinal protective immunity mediated through the TRIF pathway has not been studied.
In the current study, we used a well established animal model of oral Yersinia enterocolitica infection, which recapitulates many features of the human disease (Trülzsch et al., 2007). We found that TRIF-deficient (TrifLPS2) mice, which carry a frameshift mutation in the Trif gene, have impaired resistance to oral Y. enterocolitica infection. The TrifLPS2 mice showed defective inflammatory responses in Peyer’s patches (PPs) and rapid systemic dissemination of Y. enterocolitica. Mechanistically, we found an unexpectedly important role for TRIF in the bactericidal function of macrophages against Gram-negative pathogens. During Y. enterocolitica infection, TRIF-dependent protective immunity is mediated through an induction of IFN-β from infected macrophages in the mesenteric LN (MLN). This induction of IFN-β accelerates bactericidal activity of macrophages directly as well as indirectly via inducing IFN-γ from adjacent NK cells. Exogenous stimulation of the TRIF pathway through polyinosinic:polycytidylic acid (poly I:C) evoked protective immunity against Y. enterocolitica infection in WT mice. The protective effect of poly I:C was also observed in an oral infection with another Gram-negative enteric pathogen Salmonella typhimurium. Thus, TRIF-mediated protective immunity against enteric bacterial infection is mainly mediated by macrophages, which act as regional filters of infectious enteropathogens. Elucidation of TRIF-dependent intestinal immune regulation could lead to more effective immune-based prevention and/or treatments for enteric infectious diseases.
RESULTS
TRIF-deficient mice have increased susceptibility to oral infection with Y. enterocolitica
To examine the role of the TRIF pathway in the host response to Y. enterocolitica infection, WT and TrifLps2 mice were orally inoculated with 2 × 107 CFU Y. enterocolitica. TrifLps2 mice showed greater body weight loss than WT mice (Fig. 1 A). Furthermore, 10 out of 15 (66.7%) TrifLps2 mice died, whereas 10 out of 11 (90.9%) of WT mice survived throughout the experiment (Fig. 1 B). Almost half of the TrifLps2 mice died within 3 d of infection, indicating a specific role for TRIF in the early stage of infection.
Figure 1.
TRIF deficiency results in greater mortality and higher susceptibility to bacterial dissemination in the Y. enterocolitica infection model. WT and TrifLps2 mice were orally infected with 2 × 107 CFU Y. enterocolitica. (A) Weight loss in WT and TrifLps2 mice during Y. enterocolitica infection (n = 8 each; *, P < 0.05). Combined data from four independent experiments are shown. Error bars, SEM. (B) Kaplan Meier survival curves for infected WT (n = 11) and TrifLps2 mice (n = 15). Combined data from four independent experiments are shown (*, P < 0.01). (C) H&E staining of the spleen and liver taken 48 h after infection. Arrowheads indicate abscesses. Representative pictures from six mice of each genotype. Bars, 100 µm. (D) Y. enterocolitica titers in the spleens, MLNs, and PPs of individual infected WT and TrifLps2 mice taken 48 h after infection. Pooled data from three independent experiments are shown (n = 6 each; *, P < 0.05). Horizontal bars indicate mean.
To explore the cause of early mortality in TrifLps2 mice, histology of the infected mice was evaluated daily during the first 3 d of infection. Multiple abscesses were found in the spleens and livers of TrifLps2 mice starting 48 h after infection, which were not observed in WT mice (Fig. 1 C). The number of colonies detected in the spleens of TrifLps2 mice was strikingly higher than in WT mice (P = 0.0002), supporting bacterial dissemination and Yersinia sepsis as the cause of death (Fig. 1 D). Consistently, there were significantly higher numbers of Y. enterocolitica in the MLNs and PPs of TrifLps2 mice compared with WT mice, indicating that there is a greater regional burden of Y. enterocolitica in TrifLps2 mice leading to more efficient escape from the intestine and dissemination to distant organs. TRIF may be particularly important after oral infection. This is a result of the fact that intravenous Y. enterocolitica infection of WT and TrifLPS2 mice showed similar Y. enterocolitica titers in the spleen 5 d after infection (WT 3.9 ± 0.1 vs. TrifLPS2 4.1 ± 0.2 Log10CFU/g, n = 8 each, P = 0.39). Therefore, the TRIF pathway may play a significant role in establishing a regional defense mechanism in the intestine during the first 48 h of infection.
TRIF is required for initiation of protective immune responses to Y. enterocolitica in PPs
Given the striking differences in Y. enterocolitica dissemination between WT and TrifLps2 mice at 48 h after infection, we examined local events in the intestine after oral infection. Y. enterocolitica initially colonizes PPs (Autenrieth and Firsching, 1996). Histology of PPs showed an acute inflammatory infiltrate, occasional ulcerations, and small abscesses in both WT and TrifLps2 mice (Fig. 2 A). When we analyzed cellular populations in PPs, significantly fewer Gr1+ cells and CD161+ (NK1.1) cells were observed in TrifLps2 mice compared with WT mice (Fig. 2 B). As a result, histological severity of inflammation was significantly less in TrifLps2 mice than in WT mice, suggesting an impaired local immune response in TrifLps2 mice (Fig. 2 C). It seems unlikely that these differences are a result of developmental differences of PPs between WT and TrifLps2 mice because baseline PPs in TrifLps2 mice showed normal cell populations (Table S1).
Figure 2.
TrifLps2 mice exhibit a defective immune response in PPs in response to Y.enterocolitica infection. (A) H&E sections of PPs of WT and TrifLps2 mice 48 h after infection. Representative pictures from six mice of each genotype. A PP from an uninfected WT mouse is shown for comparison (left). Bars, 200 µm. Bacterial foci are indicated by circled area (right). Bars, 50 µm. (B) Cell population analysis in PPs of Y. enterocolitica–infected WT and TrifLps2 mice. Single cell suspensions of PPs were analyzed by FCM 48 h after infection (gathered data from two independent experiments, n = 4 each; *, P < 0.05; error bars, SEM). (C) Histological scoring in WT and TrifLps2 mice 48 h after infection (combined data from three independent experiments, n = 6 each; *, P < 0.05; error bars, SEM). (D) Interaction of macrophages in PPs (F4/80: FITC, green) with infected Y. enterocolitica (SYTO83, red) 48 h after infection. Representative pictures are of PPs from WT and TrifLps2 mice taken from one of the two independent experiments (n = 3 each). Macrophages positive for Y. enterocolitica are indicated by arrowheads. Extracellular Y. enterocolitica foci in TrifLps2 PPs are indicated by the arrow. Bars, 50 µm. (E) Real-time PCR analysis of IP-10, MIP-2, and KC expression in PPs taken 48 h after infection (n = 6 each, averages of samples from three independent experiments; *, P < 0.05). Control mice did not receive infection (n = 6 each). NS, not significant. Error bars, SEM. (F) FCM analysis of Gr1+ cells in PPs 48 h after infection. Data from WT and TrifLps2 mice that were treated with anti–IP-10 antibody and isotype control antibody are shown.
Because macrophages are a primary target of Y. enterocolitica and play a crucial role in establishing local immune responses (Bergsbaken and Cookson, 2007), we addressed whether macrophages from TrifLps2 mice normally interacted with Y. enterocolitica that invade PPs. The interaction between Y. enterocolitica and macrophages in PPs were examined at 48 h after infection with red fluorescent–labeled Y. enterocolitica (Fig. 2 D). Although the majority of the macrophages in PPs of WT mice were red fluorescent (Y. enterocolitica) positive, few macrophages in TrifLps2 mice were positive for Y. enterocolitica and there appeared to be more extracellular Y. enterocolitica foci in PPs of TrifLps2 mice than WT mice. In addition, real-time PCR for the chemokines IP-10 (CXCL10), KC (CXCL1), and MIP-2 (CXCL2), which can be induced by TRIF-mediated TLR signaling in innate immune cells (Jeyaseelan et al., 2007; Power et al., 2007; De Filippo et al., 2008; Kelly-Scumpia et al., 2010), demonstrated impaired expression of IP-10 but similar expression of MIP-2 and KC mRNA in PPs from TrifLps2 mice compared with WT mice (Fig. 2 E). IP-10 has been shown to be a macrophage early response gene and a potent chemoattractant for activated T cells, NK cells, and neutrophils in the face of bacterial pathogens (Jeyaseelan et al., 2007; Power et al., 2007; De Filippo et al., 2008; Kelly-Scumpia et al., 2010). In our system, TRIF-mediated IP-10 would be important for neutrophil recruitment to PPs because treatment of WT mice with a blocking anti–IP-10 antibody decreased Gr1+ cell population in PPs to the levels in TrifLps2 mice at 48 h after infection (Fig. 2 F). This treatment did not alter Gr1+ cell number in PPs of TrifLps2 mice, indicating the effect was TRIF dependent. These results indicate that TrifLps2 mice have defects in regional macrophage response to invading Y. enterocolitica and chemokine IP-10 expression in PPs, which may be responsible for the impaired local immune responses at the primary infection site.
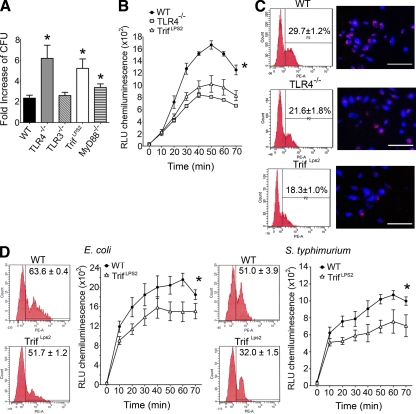
TRIF-deficient macrophages are defective in phagocytosis and intracellular killing of Gram-negative pathogens
To examine macrophage pathways involved in the elimination of Y. enterocolitica, we isolated peritoneal macrophages from mice deficient in TRIF, MyD88, TLR3, and TLR4 and infected them with Y. enterocolitica in vitro at a multiplicity of infection (MOI) of 1 (Wiley et al., 2006). A striking increase of Y. enterocolitica proliferation was found in TrifLps2 macrophages compared with WT macrophages, indicating impaired bactericidal function of TrifLps2 macrophages (Fig. 3 A). TLR4−/− but not TLR3−/− macrophages also had impaired bactericidal function, suggesting that TLR4 was the dominant TLR upstream of the TRIF pathway in this process (Fig. 3 A). MyD88−/− macrophages had a partial bactericidal defect against Y. enterocolitica (P = 0.0482). Exclusion of nonphagocytosed bacteria with gentamicin did not alter the bactericidal pattern of these macrophages, indicating that TrifLps2 macrophages had defective intracellular killing of Y. enterocolitica (Fig. S1). We further examined the endolysosomal function by oxidative burst in response to Y. enterocolitica infection. There were significant defects in the oxidative burst in TrifLps2 and TLR4−/− macrophages compared with WT macrophages (Fig. 3 B). Internalization of fluorescent-labeled Y. enterocolitica was also defective in both TLR4−/− and TrifLps2 macrophages compared with WT macrophages (Fig. 3 C). These phagocytic defects in TrifLPS2 macrophages were also seen in response to E. coli and heat-inactivated S. typhimurium, suggesting that the TRIF pathway is required for multiple steps of phagocytosis against Gram-negative pathogens (Fig. 3 D). However, the defects are pathogen specific because TrifLPS2 macrophages showed similar ability to uptake polystyrene microspheres as WT macrophages (WT, 78.9 ± 9.1% vs. TrifLps2, 78.7 ± 6.5%). Y. enterocolitica can induce apoptosis in eukaryotic cells (Ruckdeschel et al., 2004), which may influence the result of macrophage bactericidal function. However, we did not see differences in caspase-3 activities in these macrophages during infection (Fig. S2). These data demonstrate that multiple aspects of normal macrophage function in the context of a Gram-negative pathogen require TRIF signaling, which is originated through TLR4 engagement.
Figure 3.
TRIF is required for phagocytosis and intracellular killing of Gram-negative pathogens. (A) Bactericidal assay of infected peritoneal macrophages (MOI: 1). Y. enterocolitica growth during 6 h of incubation is shown as fold increase of CFU (*, P < 0.05; error bars, SEM). Representative data from five repeated experiments are shown (n = 3 each). (B) Oxidative burst of infected macrophages (MOI: 50) from WT, TLR4−/−, and TrifLps2 mice. Combined data from three independent experiments is shown (n = 9 each; *, P < 0.01). Data are expressed as relative light units (RLU). Error bars, SEM. (C) Phagocytic internalization of fluorescent-labeled Y. enterocolitica by macrophages. Macrophages were infected with Y. enterocolitica for 30 min (MOI: 50). FCM (left) and microscopic (right) analysis are shown. Data are representative from three repeated experiments (n = 3 each). Bars, 50 µm. (D) Phagocytosis and oxidative burst of macrophages in response to E. coli and S. typhimurium (MOI: 50). Representative data from three repeated experiments are shown (n = 9; *, P < 0.01 each). Error bars, SEM.
TRIF-mediated IFN-β is critical for the bactericidal function of Y. enterocolitica–infected macrophages
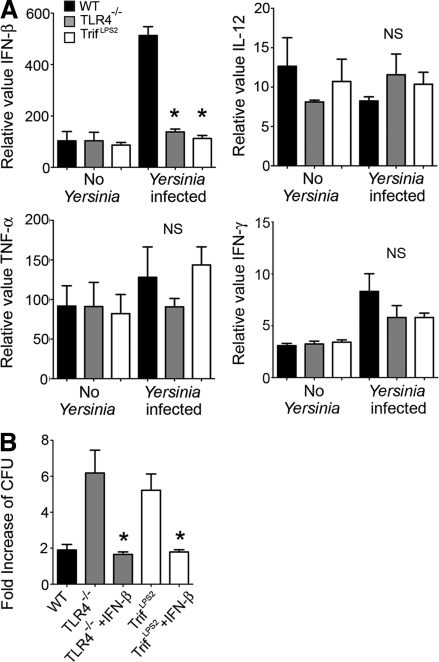
Next, we addressed whether the impaired bactericidal ability of TrifLps2 macrophages is associated with defective cytokine expression in response to Y. enterocolitica. IL-12p35, TNF, and IFN-γ have been implicated in host defense against Y. enterocolitica infection, and IFN-β is a primary inducible cytokine by the TRIF pathway (Autenrieth and Heesemann, 1992; Autenrieth et al., 1994; Bohn and Autenrieth, 1996). In vitro infected WT macrophages exhibited striking induction of IFN-β, but this induction of IFN-β was completely absent in TLR4−/− and TrifLps2 macrophages (Fig. 4 A). In contrast, induction of IL-12p35, TNF, and IFN-γ in response to Y. enterocolitica infection was not observed in those macrophages (Fig. 4 A).
Figure 4.
TRIF-induced IFN-β is crucial for elimination of Y. enterocolitica by macrophages. (A) Real-time PCR analysis of cytokine expression in control and infected macrophages. Peritoneal macrophages were infected with Y. enterocolitica for 5 h (MOI: 20). Combined data from three independent experiments (n = 9 each; *, P < 0.0001 by ANOVA; NS, not significant). Error bars, SEM. (B) Bactericidal assay of infected macrophages with and without 100 U/ml IFN-β (MOI: 1). Data are obtained from three independent experiments (n = 9 each; *, P < 0.0001, ANOVA). Error bars, SEM.
IFN-β can enhance phagocytic activity in paracrine and autocrine ways (Vogel et al., 1983; Ellermann-Eriksen, 1993). We tested the hypothesis that the defect in bactericidal function in TrifLps2 macrophages was a result of the impaired IFN-β production. Addition of recombinant IFN-β improved Y. enterocolitica bactericidal activity of TLR4−/− and TrifLps2 macrophages to WT level (Fig. 4 B). These results highlight an important role of IFN-β induction by TLR4-mediated TRIF activation to elicit macrophage bactericidal activity during Y. enterocolitica infection.
TRIF promotes early induction of IFNs in the MLN during Y. enterocolitica infection
Although PPs are the main portal of entry for many enteric pathogens, the MLN has been implicated in prevention of systemic bacterial dissemination in murine models of enteric infection (Macpherson and Uhr, 2004; Westphal et al., 2008; Voedisch et al., 2009). Because there was a striking Y. enterocolitica dissemination in TrifLPS2 mice but not in WT mice, we examined in vivo cytokine gene expression in the MLN, taken 48 h after infection. TrifLps2 mice exhibited significantly lower mRNA levels of IFN-γ and IFN-β in the MLN than WT mice, whereas the mRNA levels of IL-12p35 and TNF was similar between WT and TrifLps2 mice (Fig. 5 A). We confirmed these differential inductions of IFNs between WT and TrifLPS2 mice by examining protein levels in MLN cell suspensions harvested 48 h after infection (Fig. 5 B). The mRNA expression of these cytokines in PPs was very low and there were no differences between WT and TrifLps2 mice (Fig. S3).
Figure 5.
Defective induction of IFNs in the MLNs of TrifLps2 mice after Y. enterocolitica infection. (A) Real-time PCR analysis of IFN-γ, IFN-β, Il-12p35, and TNF expression in the MLNs taken from control and 48 h after infection of WT and TrifLps2 mice. Averages of samples from three independent experiments (n = 6 each; *, P < 0.0001). Error bars, SEM. NS, not significant. (B) IFN production by MLN cell suspensions harvested 48 h after infection. The protein levels of IFN-β and IFN-γ in the supernatants were measured by ELISA assays after an additional 48 h of incubation in vitro. Combined data from three independent experiments (n = 6 each; *, P < 0.001). Error bars, SEM. (C) Immunofluorescent and FCM analyses of IFN-β– and IFN-γ–expressing cells in the MLN taken from infected WT mice 48 h after infection. IFN-β–expressing cells positive for F4/80 are indicated by the arrowhead. IFN-β–expressing cells positive for CD11c are indicated by the arrow. Bars: (top and middle) 10 µm; (bottom) 5 µm. Representative pictures from infected WT mice (n = 4 from two independent experiments). FCM analysis shows that 76.2 ± 1.1 and 23.3 ± 1.0% of IFN-β–expressing cells are macrophages and myeloid DCs, respectively. Isotype control data (biotin rat IgG with PE streptavidin and APC-hamster IgG within IFN-β–positive cells) used for flow setting is shown (right). In addition, 71.5 ± 2.8% of IFN-γ–expressing cells are NK cells. 4.1 ± 0.3% of IFN-γ–expressing cells are CD3+ NKT cells. Isotype control data (FITC–mouse IgG and APC–hamster IgG within IFN-γ–positive cells) used for flow setting is shown (right). Representative data from infected WT mice (n = 3 each from two independent experiments). (D) Serum IFN-β measured by ELISA 48 h after infection. Combined data from three independent experiments (n = 6 each; *, P < 0.05). Error bars, SEM. (E) Kinetic analysis of cytokine gene expression represents regional (MLN) and systemic (spleen) cytokine responses during Y. enterocolitica infection (real-time PCR: averages of samples from three independent experiments, n = 6 each; *, P < 0.05; error bars, SEM). (F) FCM analysis of splenocytes expressing IFN-β in infected WT mice (n = 3 each from two independent experiments).
Immunofluorescent labeling of IFN-β demonstrated that F4/80+ cells, a phenotype indicative of macrophages, were the main source of IFN-β in the MLN of infected WT mice (Fig. 5 C). A small portion of these IFN-β–expressing F4/80+ cells was also CD11c+, a phenotype attributed to myeloid DCs (Fig. 5 C). Flow cytometric (FCM) analysis demonstrated that 23.3 ± 1.0% of total IFN-β–expressing cells were F4/80+CD11c+ DCs (Fig. 5 C). In contrast, the majority of IFN-γ–expressing cells in the MLN were CD3−CD161+ (NK1.1) NK cells (Fig. 5 C).
The decrease of IFN-β production in TrifLps2 mice was reflected even in the serum levels of IFN-β, suggesting a possible contribution of IFN-β to the systemic immune responses during enteric Y. enterocolitica infection (Fig. 5 D). In contrast, serum IFN-γ was not detectable in infected TrifLps2 or WT mice (unpublished data). Because innate cytokine responses may differ between regional and systemic compartments, mRNA expression of IL-12p35, TNF, IFN-γ, and IFN-β in the MLN and spleen was kinetically compared after infection. We could only follow up to day 5 after infection as a result of the high mortality of TrifLps2 mice during the infection (Fig. 1 B). The expression of these cytokines showed a peak at 72 h of infection and was greater in the MLN than the spleen. Striking induction of IFN-β was seen in the MLN and spleen of WT mice during 48 to 72 h after infection, but this induction was defective in TrifLps2 mice (Fig. 5 E). Again, IFN-β–expressing cells in the spleen were F4/80+ cells, but this time the population did not contain the CD11c+ subset (Fig. 5 F). In contrast, major induction of IFN-γ was seen typically in the MLN of WT mice from 48 h after infection and continued afterward (Fig. 5 E). This induction of IFN-γ was defective in TrifLps2 mice. IFN-γ expression in the spleen was about half of its expression in the MLN and there were no differences between WT and TrifLps2 mice at any time points (Fig. 5 E). IL-12p35 induction in the MLN was similar between WT and TrifLps2 mice throughout this time course. The expression pattern of TNF in both MLN and spleen was similar to the IL-12p35 expression in WT mice, but significantly higher expression of splenic TNF was found in surviving TrifLps2 mice compared with WT mice after 72 h of infection (Fig. 5 E). These results suggest that regional TRIF signaling in macrophages robustly induces both local and systemic IFN-β responses during the initial 72 h of Y. enterocolitica infection, and that it is associated with sustained IFN-γ expression by NK cells within the MLNs to establish a TRIF-dependent intestinal protective immunity.
TRIF-induced IFN-β elicits IFN-γ production from NK cells, an additive effect on macrophage elimination of the pathogens
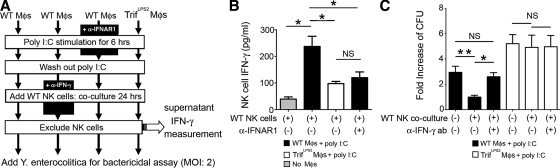
We demonstrated earlier that the impaired bactericidal activity of TrifLPS2 macrophages was a result of the defective induction of IFN-β in response to Y. enterocolitica infection (Fig. 4 B). We also found a significant difference in IFN-γ expression between WT and TrifLPS2 mice in the MLN during Y. enterocolitica infection in vivo. IFN-γ is known to be a potent activator of macrophages to enhance their bactericidal function (Gordon et al., 2005; Matteoli et al., 2008). Because NK cells are the major source of IFN-γ in the MLN, we addressed whether the reduction of NK cell–induced IFN-γ is associated with the impaired ability of TrifLPS2 mice to eliminate enteric infections, especially in the context of macrophage bactericidal function. To approach this issue, we tested whether macrophages from WT and TrifLPS2 mice could prime NK cells to enhance the bactericidal activity of macrophages (Fig. 6 A).
Figure 6.
TRIF-mediated IFN-β induction by macrophages elicits IFN-γ production from NK cells which further enhances the bactericidal function of the macrophages. (A) Peritoneal macrophages isolated from WT and TrifLPS2 mice were stimulated with poly I:C for 6 h in the presence or absence of anti-IFNAR1 antibody. After washing out poly I:C, macrophages were co-cultured with WT splenic NK cells for 24 h in the presence or absence of neutralizing anti–IFN-γ antibody. After excluding NK cells, the remaining macrophages were subjected to bactericidal assay. (B) ELISA measurement of IFN-γ production from WT NK cells after co-culturing with poly I:C–stimulated macrophages in the presence or absence of anti-IFNAR1 antibody. Combined data from three independent experiments (n = 6 each; *, P < 0.05). Error bars, SEM. (C) Effect of NK cell–induced IFN-γ on macrophage bactericidal activity during Y. enterocolitica infection. Combined data are from three independent experiments (n = 6 each; **, P < 0.01; *, P < 0.05). NS, not significant. Error bars, SEM.
First, we examined whether TRIF signaling in macrophages promotes IFN-γ production from NK cells in an IFN-β–dependent fashion. WT NK cells produced a substantial amount of IFN-γ after co-culturing with poly I:C–stimulated WT macrophages (Fig. 6, A and B). This IFN-γ production was significantly reduced when NK cells were co-cultured with poly I:C-stimulated TrifLPS2 macrophages or WT macrophages with anti-IFNAR1 antibody (Fig. 6, A and B). Therefore, TRIF signaling induces IFN-β production by macrophages, which then promotes IFN-γ production by NK cells. Next, we tried to determine if the NK cell–mediated IFN-γ further enhances the bactericidal function of TRIF-activated macrophages. WT and TrifLPS2 macrophages were preincubated with poly I:C, followed by co-culturing with WT NK cells in the presence or absence of neutralizing anti–IFN-γ antibody, and bactericidal activity was analyzed after removal of NK cells (Fig. 6, A and C). Co-culture of NK cells with poly I:C–stimulated WT macrophages showed a significant increase in bactericidal activity, but this increase was abolished by neutralization of IFN-γ (Fig. 6 C). We did not see these changes of the bactericidal activity in TrifLPS2 macrophages, indicating that TRIF signaling in macrophages induces NK cells to produce IFN-γ, which in turn amplifies bactericidal activity of macrophages. Collectively, TRIF-mediated IFN-β induction in macrophages is important to eliminate pathogens, not only to maintain homeostatic bactericidal function but also to summon an additive enhancement by NK cells through IFN-γ induction.
Early IFN-β induction is required for expression of IFN-γ in the MLN and for preventing dissemination of Y. enterocolitica
To examine the importance of the rapid IFN-β induction in host defense against Y. enterocolitica dissemination in vivo, we injected TrifLps2 mice with recombinant mouse IFN-β (10,000 U i.p./d) during the first 3 d of infection. This IFN-β treatment significantly protected TrifLps2 mice from infection, as all IFN-β–treated mice survived throughout the experiment (Fig. 7 A). None of the IFN-β–treated mice had detectable Y. enterocolitica colonies in the spleen at day 7 of infection. The bacterial titer in the MLN, but not in PPs, was significantly reduced in these IFN-β–treated mice compared with PBS treated control TrifLps2 mice (Fig. 7 B). At 48 h after infection, significant induction of IFN-γ gene expression in the MLN was found in the IFN-β–treated TrifLps2 mice compared with nontreated TrifLps2 mice (Fig. 7 C). We also did the complementary experiment and found that blocking IFN-β signaling with an anti-IFNAR1 antibody partially blocked IFN-γ expression in Y. enterocolitica–infected WT mice (Fig. 7 D). Accordingly, mice that received anti-IFNAR1 antibody showed a significant increase of Y. enterocolitica colonization in the MLNs 48 h after infection (Fig. 7 E). These results indicate that TRIF-mediated induction of IFN-β regulates expression of IFN-γ in the MLN during early stage of infection, which is important for blocking systemic Y. enterocolitica dissemination.
Figure 7.
IFN-β protects TrifLps2 mice against Y. enterocolitica infection by stimulating IFN-γ expression in the MLN. (A) Kaplan Meier survival curve for infected TrifLps2 mice (TrifLps2 mice, n = 9; IFN-β–treated mice, n = 7; 10,000 U i.p/d for the first 3 d). Combined data are from three independent experiments. (B) Y. enterocolitica titers in PPs and MLNs taken after 7 d of infection. Pooled data are from three independent experiments (*, P < 0.0001). NS, not significant. (C) Real-time PCR analysis of IFN-γ expression in the MLNs taken 48 h after infection. Combined data from three independent experiments (n = 6 each; *, P < 0.005). Error bars, SEM. (D) Real-time PCR analysis of IFN-γ in the MLNs taken 48 h after infection (100 µg i.p, the day before infection). Combined data from three independent experiments (n = 6 each; *, P < 0.005). Error bars, SEM. (E) Y. enterocolitica titers in the MLNs taken 48 h after infection. Pooled data from two independent experiments (n = 6 each; *, P < 0.005). Horizontal bars in B and E indicate mean.
Activation of TRIF protects WT mice from Y. enterocolitica infection
Given that the TRIF pathway is critical for protection against Y. enterocolitica dissemination, we wished to test whether stimulation of the TRIF pathway protects host against Y. enterocolitica infection. We used poly I:C to induce TRIF activation via TLR3. Single injection of poly I:C subcutaneously at a 50-µg dose did not induce any histologically detectable mucosal changes in the intestine (not depicted) but was physiologically active, as it induced transient IRF-3 activation in the MLNs (Fig. 8 A). WT mice were given subcutaneous injection of 50 µg poly I:C the day before, the day of, or one day after oral infection of Y. enterocolitica. Control mice only received infection. 7 d after infection, there were reduced numbers of Y. enterocolitica in PPs from the mice that received poly I:C either the day before or the same day of infection (Fig. 8 B). Examination of Y. enterocolitica dissemination to the MLN on day 7 of infection showed significant reductions in the colony numbers of Y. enterocolitica in all poly I:C–treated mice regardless of the timing of injection (Fig. 8 C). None of the mice that received poly I:C had detectable Y. enterocolitica colonization in the spleen.
Figure 8.
Activation of TRIF by poly I:C induces intestinal protective immunity in WT mice by enhancing IFN-β expression in the MLN and macrophage bactericidal activity. (A) Immunoblot analysis of IRF-3 activation (phosphorylation) in the MLNs. Poly I:C–treated (24 and 48 h after injection) and nontreated WT mice are shown. Representative pictures are from three repeated experiments (n = 3). (B) Y. enterocolitica titers in the PPs and MLNs taken 7 d after infection. Pooled data are from three independent experiments (*, P < 0.001 for PPs; *, P < 0.0001 for MLN). Horizontal bars indicate mean. (C) Real-time PCR analysis of IFN-β expression in the MLNs taken 48 h after infection. Combined data from three independent experiments (n = 6; *, P < 0.05). Error bars, SEM. (D) Kaplan Meier survival curve for Trif Lps2 mice that received poly I:C (50 µg/mouse s.c) the day before (d-1) Y. enterocolitica infection. Combined data from two independent experiments (n = 6; NS, not significant). (E) Spleen titer results 7 d after orogastrical inoculation with 108 CFU S. typhimurium. Pooled data from two independent experiments (n = 6 each; *, P < 0.001). Error bars, SEM. Horizontal bars indicate mean. (F) Kaplan Meier survival curve for poly I:C–treated mice (n = 10 for control, n = 9 for d-1, n = 7 each for d0 and d+1; *, P < 0.05, combined three independent experiments). (G) Kaplan Meier survival curve for poly I:C–treated (d-1) mice with or without anti-IFNAR antibody. Combined three independent experiments (n = 7 for control, n = 9 each for anti-IFNAR–treated and nontreated groups, n = 5 no-infection; *, P < 0.05). (H) Bactericidal assay of peritoneal macrophages (MOI: 1) with or without 5 µg/ml poly I:C. Combined data from three independent experiments (n = 6 each; *, P < 0.001). Error bars, SEM.
To determine whether poly I:C treatment altered IFN-β expression during the early stage of the infection, we examined the expression of IFN-β in the MLN 48 h after infection (Fig. 8 C). All poly I:C–treated mice showed a significant increase in IFN-β expression in the MLN compared with control mice. The protective effect of poly I:C (Fig. 8 D, d-1, poly I:C given 1 d before infection) was not observed in TrifLPS2 mice, confirming that the effect of poly I:C is mediated through the TRIF pathway. The protective effect of poly I:C is not restricted to Y. enterocolitica infection, because poly I:C treatment (Fig. 8 E, poly d0, poly I:C treatment same day as infection) also reduced dissemination of S. typhimurium to the spleen at the 7 d of oral infection.
To further examine whether poly I:C treatment alters the natural history of Y. enterocolitica infection, we orally infected WT mice with a higher dose (3 × 108 CFU/mouse) of Y. enterocolitica and examined the effect of poly I:C on mortality at 28 d. The mortality rates of the mice that received poly I:C (Fig. 8 F, poly d-1 and poly d0) were significantly lower than the mortality in control mice. The mice that received poly I:C 1 d after infection (Fig. 8 F, poly d+1) demonstrated reduced mortality, but the difference between control mice did not reach statistical significance. Blocking IFN-β by the IFNAR1 antibody administered 1 d before infection abolished the protective effect of poly I:C (d-1) on the mortality (Fig. 8 G). In vitro poly I:C treatment significantly enhanced bactericidal activity of WT peritoneal macrophages against Y. enterocolitica as well as S. typhimurium (Fig. 8 H). Collectively, systemic activation of the TRIF pathway protects the host against enteric Y. enterocolitica infection by enhancing IFN-β expression in the MLN and macrophage bactericidal activity.
DISCUSSION
The development of effective strategies to protect the host against multiple types of pathogens is challenging. Because innate immune responses are antigen nonspecific, we became interested in how the innate immune response in the intestine naturally protects against pathogens and whether strategies could be developed to enhance innate immunity against enteric pathogens. Although TRIF is known as an antiviral signaling pathway downstream of TLR3, its role for antibacterial immunity has not been well studied. Using Y. enterocolitica and S. typhimurium as model enteric pathogens, this study demonstrates an unexpected role for the TRIF pathway in enteric infection by Gram-negative bacterial pathogens. Our results highlight an immunological model in which TLR4-mediated TRIF activation leads to the rapid induction of IFN-β by macrophages, and IFN-β in turn promotes IFN-γ production by NK cells that then feed back on the macrophages, enhancing their bactericidal activity. We chose to study these pathogens because they are enteric bacterial species that naturally infect both man and mouse (House et al., 2001; Trülzsch et al., 2007). Enteric pathogens invade the body mainly through microfold (M) cells and colonize within the PPs, and then invade through draining MLNs and subsequently spread systemically (Jepson and Clark, 2001; Oellerich et al., 2007; Westphal et al., 2008). Our results show a previously unappreciated property of regional macrophages as a crucial part of the innate protection against enteric pathogens and one that depends on TRIF signaling.
TRIF-deficient mice have multiple defects in the ability to mount an effective inflammatory response and, as a result, animals succumbed to systemic dissemination of a Gram-negative pathogen. First, Trif LPS2 mice were limited in the capacity to establish an immune response in PPs. The defective local immune response was accompanied by decreased neutrophil infiltration as the result of an impaired production of the CXC chemokine IP-10. IP-10 has been shown to be a potent neutrophil chemoattractant (Zeng et al., 2005) and plays an important role in preventing sepsis in the cecal ligation model (Kelly-Scumpia et al., 2010). Therefore, defective IP-10 induction in PPs may be involved in rapid Y. enterocolitica dissemination in TrifLPS2 mice after oral infection. Second, TRIF is required for proper function of phagocytosis in uptake, as well as intracellular killing, during Gram-negative bacterial infection. Although MyD88 has been implicated in phagocytic function through activation of p38 mitogen-activated protein kinase (Blander and Medzhitov, 2004), involvement of TRIF signaling in phagocytic function has not previously been described. Others have suggested that TLR-mediated phagocytosis depends on MyD88-independent p38 activation (Kong and Ge, 2008). TLR2xTLR4 double-deficient macrophages seem to have a more profound phagocytic defect than MyD88-deficient macrophages (Blander and Medzhitov, 2004). Because the TRIF pathway can also activate p38, these results strongly support our observation that the phagocytosis of Gram-negative pathogens by macrophages involves the TRIF pathway.
At the center of the defect seen in TrifLPS2 mice is impaired IFN-β production by infected macrophages. The hallmark of IFN-β is its rapid production as a result of fast transcriptional turnover and mRNA accumulation mediated through a feed-forward mechanism (Levy et al., 2003; Hall and Rosen, 2010). We found that the rapid induction of IFN-β is important for inducing NK cells to produce IFN-γ, a potent antimicrobial cytokine which has previously been implicated in macrophage activation and host resistance against a variety of pathogens including Y. enterocolitica (Autenrieth et al., 1996). We show that, in spite of normal TNF and IL-12p35 levels in the MLN of TrifLPS2 mice during the first 48 h after infection, TrifLPS2 mice do not produce optimal levels of IFN-γ. The major cell sources of IFN-β and IFN-γ in the MLNs during Y. enterocolitica infection were macrophages and NK cells, respectively. These data indicate that macrophage TRIF signaling, and not just MyD88 signaling, is required for optimal induction of IFN-γ by NK cells to acquire the regional defense mechanism in the MLN. Kinetic analyses of regional versus systemic cytokine responses to enteric Y. enterocolitica infection show robust IFN-β induction both regionally and systemically. This induction of IFN-β was transient, as it was only observed between 48 and 72 h after infection. Yet, this induction of IFN-β is necessary and sufficient to establish protective immunity downstream of TRIF. To substantiate this statement, we show that exogenous IFN-β during the first 72 h of infection can restore TrifLPS2 mice to a nearly normal ability to survive infection with Y. enterocolitica. Interestingly, a late increase of regional and systemic TNF expression was observed in the minority of TrifLPS2 mice that survived Y. enterocolitica infection. No systemic induction of TNF was found in WT mice, presumably because IFN-β was protective. TNF is a potent antimicrobial mediator but can have a systemic toxicity (Autenrieth and Heesemann, 1992; Bhatia et al., 2009). These results suggest that TRIF-mediated IFN-β orchestrates effective immunity against enteric Y. enterocolitica infection, which regionally facilitates macrophage elimination of the pathogen and minimizes the needs for a systemic inflammatory response.
We show in this study a novel role of the TRIF pathway in induction of intestinal protective immunity against Gram-negative bacterial infection. Our results are particularly exciting because the pathway can be manipulated, and we show that it applies to more than one Gram-negative pathogen. Our results provide an important way to activate the host innate immune response without causing systemic toxicity. We envision that targeting this pathway can be operationalized to treat patients within a window of infection.
MATERIALS AND METHODS
Mice and interventions.
TLR4−/−, MyD88−/− mice and C57BL/6J, TLR3−/− mice were obtained from Oriental Bio Service, Inc. and The Jackson Laboratory, respectively. TrifLps2 mice were gifts from B. Buetler (Scripps Research Institute, La Jolla, CA). Mice were kept in specific pathogen-free conditions and fed by free access to diet and water. WT Y. enterocolitica (WA-314 serotype O:8) was used. S. typhimurium (SL1344) was a gift from A.T. Gewirtz and M. Vijay-Kumar (Emory University, Atlanta, GA). Mice were orogastrically inoculated with Y. enterocolitica or S. typhimurium (Echeverry et al., 2007). In some infections, Y. enterocolitica was labeled with red fluorescent dye (SYTO83) according to the manufacturer’s instructions (Invitrogen). Systemic infection was performed by a single injection of a sublethal dose of Y. enterocolitica (2 × 105 CFU) through the retro-orbital vein (Handley et al., 2004). The IFNAR1 antibody was administered 1 d prior infection (100 µg i.p./mouse; Leinco Technologies), and CHO cell–derived recombinant IFN-β was administered daily for the first 3 d of infection (10,000 U i.p/mouse; Hycult Biotech). This dose was based on serum IFN-β levels measured in WT mice during infection. Poly I:C was s.c injected (50 µg/mouse; InvivoGen) as indicated. Rabbit anti–murine IP-10 antibody was administered at the same time as infection (100 µg i.p/mouse; PeproTech; Liu et al., 2001; Michalec et al., 2002). Protocols for animal experiments were approved by and performed according to the University of Miami Miller School of Medicine Animal Care and Use Committees’ guidelines.
Infected mice were monitored daily for body weight. Histological assessment was performed by two veterinary pathologists. The scoring system used was: PPs and MLNs, 0 = normal, 1 = hyperplastic follicles, 2 = diffuse inflammatory infiltrate, 3 = necrosis and lymphocytic depletion; surrounding mucosa, 0 = normal, 1 = mild inflammatory infiltrate, 2 = moderate to marked infiltration, 3 = ulceration; area (percentage), 0 = <5, 1 = ∼5–10, 2 = ∼11–14, 3 = >15. The full score is 9. Isolated MLN, PPs, and spleens were homogenized in Ca2+ Mg2+ free HBSS (Cellgro) and cultured in Yersinia selective agar (YSA; BD) plates at 28°C.
Macrophage bactericidal assay.
Bactericidal assay was performed using our previously described method (Wiley et al., 2006). Peritoneal macrophages were isolated from mouse peritoneal lavage using sterile PBS. 2.5 × 105 macrophages were incubated for 48 h with DME containing 10% (vol/vol) FBS and 1% (vol/vol) PenStrep in a 24-well plate. PenStrep was removed before infection. Macrophages were then infected with Y. enterocolitica (MOI: 1) for 30 min. Unattached Y. enterocolitica were washed out, and Y. enterocolitica associated with the macrophages were collected by lysing macrophages with 500 µl of distilled water (0-h samples). Other wells were incubated for 6 h after washing out the unattached Y. enterocolitica, and samples were collected by lysing macrophages with distilled water (6-h samples). The same procedure was done for S. typhimurium infection. 100 U/ml of recombinant mouse IFN-β (Hycult Biotech) or 5 µg/ml poly I:C (InvivoGen) were added to some wells. Those 0- and 6-h samples were plated on YSA plates. Data were expressed as fold increase relative to the mean CFU of 0 h samples.
NK cell isolation and macrophage co-culture.
Splenic NK cells were isolated from WT mice using PE anti–mouse DX5 (eBioscience) and anti-PE Multi-Sort kit (Miltenyi Biotec). For the IFN-γ production assay, peritoneal macrophages isolated from WT and TrifLPS2 mice were stimulated with 10 µg/ml poly I:C for 6 h and then washed to exclude poly I:C (Fig. 6 A). Isolated WT NK cells were co-cultured with the prestimulated macrophages for 24 h in the presence or absence of 10 µg/ml anti-IFNAR1 antibody. Supernatants were analyzed for IFN-γ concentration using ELISA.
A set of the co-culture was performed in the presence or absence of 10 µg/ml of neutralizing anti-IFN-γ antibody (XMG1.2; eBioscience; Fig. 6 A). After 24 h of co-culture, NK cells were removed and remaining macrophages were infected with Y. enterocolitica at an MOI of 2. Bactericidal activity was analyzed as previously described.
Phagocytosis and flow cytometry.
Y. enterocolitica, E. coli (K12 strain), or S. typhimurium were labeled with SYTO83 (Invitrogen) and opsonized with FBS (Sigma-Aldrich). Freshly isolated macrophages were infected with SYTO83-labeled bacteria (MOI: 50) for 30 min. Red fluorescent–contained microspheres (2 µm diameter) were used as control (Cospheric LLC). Subsequently, cells were washed with DME containing 1% (vol/vol) PenStrep and were analyzed by FACSDiva (BD).
The following antibody sets were used to identify IFN-expressing cell populations in the MLN: FITC rat anti–mouse IFN-β (PBL Interferon source), biotinylated rat anti–mouse F4/80 (Serotec) with PE streptavidin (BD), allophycocyanin (APC) hamster anti–mouse CD11c (eBioscience) and PE rat anti–mouse IFN-γ (eBioscience), FITC mouse anti–mouse NK1.1 (CD161: eBioscience), and APC hamster anti–mouse CD3 (eBioscience). 6 h before the isolation of the MLN and spleen, mice were i.p injected with 250 µg Brefeldin A (eBioscience; Liu and Whitton, 2005). Intracellular IFN staining was performed using a Fixation and Permeabilization kit (eBioscience). IFN-positive cells were gated for surface marker analysis.
Real-time PCR.
Total RNA was isolated from the MLN, spleen and PPs, and Y. enterocolitica–infected macrophages (MOI: 20) using RNA Bee (Tel-Test Inc). 1 µg RNA was reverse transcribed using the Transcriptor First Strand cDNA synthesis kit (Roche). All primers used are listed in Table S2. Primers for KC (CXCL1) were obtained from Applied Biosystems (Mm00433859_m1). Real-time PCR was performed using SYBR Premix Ex Taq (Takara Bio) with a LightCycler 480 (Roche). Relative expression levels were calculated with the comparative 2−ΔΔCt method using β-actin as the endogenous control.
ELISA.
Protein levels of IFN-β and IFN-γ were determined in mouse serum or primary cell culture media by using DuoSet ELISA Development System (R&D Systems) according to the manufacturer’s instructions. The supernatant of MLN cell suspensions (2 × 106/ml) harvested 48 h after infection was measured for concentrations of IFN-β and IFN-γ after 48 h of incubation.
Western blot analysis.
10 µg of lysate was subjected to 10% SDS–PAGE and transferred to Immobilon-PDVF membranes (Millipore). After blocking, the membrane was blotted with antibodies against phospho–IRF-3 and IRF-3 (Cell Signaling Technology), followed by horseradish peroxidase–conjugated goat anti–mouse (Sigma-Aldrich) and goat anti–rabbit IgG (Invitrogen), respectively. The membrane was exposed on radiographical film using SuperSignal West Dura kit (Thermo Fisher Scientific).
Immunohistochemistry.
The 5-µm cryosections were incubated with 5% milk for 1 h and stained with rabbit anti–IFN-β (1:100; Sigma-Aldrich) in combination with Alexa Fluor 488 anti–rabbit IgG (1:400; Invitrogen), or rat anti–mouse IFN-γ (1:80; BD) in combination with Alexa Fluor 568 anti–rat IgG (1:400; Invitrogen) by overnight incubation at 4°C. To identify macrophages and DCs, sections were serially incubated with rat anti–mouse F4/80 (1:100; Serotec) and biotin anti–mouse CD11c (1:80; eBioscience) overnight at 4°C, followed by Alexa Fluor 568 anti–rat IgG and Alexa Fluor 647 streptavidin (1:400; Invitrogen), respectively. To identify NK cells and NKT cells, sections were serially incubated with FITC anti-CD161 (1:80; eBioscience) and APC anti–mouse CD3 (1:80; eBioscience) overnight at 4°C. Sections were incubated with 5% milk for 1 h before the incubation with each primary antibody. The specificity of staining was confirmed using isotype-matched control antibodies. Slides were examined using a confocal microscope (TCS-SP5; Leica).
Oxidative burst assay.
106 peritoneal macrophages in 100 µl were infected with opsonized Y. enterocolitica, E. coli (K12 strain), or S. typhimurium (MOI: 50) and subsequently reacted with 10 µl Luminol (10 mM; Sigma-Aldrich) in a 96-well flat-bottom lumitrac plate (Greiner Bio One). Chemiluminescence was measured by a GloMax-96 Luminometer every 10 min with a 10-s integration per reading.
Statistics.
Kaplan Meier survival curve was generated for infected mice. Student’s t test was used for two independent groups. One-way ANOVA was used for more than two independent groups. Fisher’s exact test was used to determine probability. All tests were performed with Prism (GraphPad Software), and a p-value of <0.05 was considered statistically significant.
Online supplemental material.
Fig. S1 shows the results of intracellular killing assay. Fig. S2 shows caspase 3 activity in infected macrophages. Fig. S3 shows cytokine mRNA expression in PPs 48 h after Y. enterocolitica infection. Table S1 shows cell population analysis in PPs of uninfected mice. Table S2 shows primer sequences that were used in this study. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20110547/DC1.
Acknowledgments
We thank Dr. Salas Pedro for help with the confocal analysis.
This study was supported by National Institute of Allergy and Infectious Diseases (R56 AI095255) and Senior Research Award from Crohn’s Colitis Foundation of America for M. Fukata.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- FCM
- flow cytometric
- MLN
- mesenteric LN
- MOI
- multiplicity of infection
- MyD88
- myeloid differentiation factor 88
- poly I:C
- polyinosinic:polycytidylic acid
- PP
- Peyer’s patch
- TLR
- Toll-like receptor
- TRIF
- toll/interleukin 1 receptor domain-containing adapter inducing IFN-β
References
- Arnold R., Jehl A., Rattei T. 2010. Targeting effectors: the molecular recognition of Type III secreted proteins. Microbes Infect. 12:346–358 10.1016/j.micinf.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Autenrieth I.B., Heesemann J. 1992. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med. Microbiol. Immunol. (Berl.). 181:333–338 10.1007/BF00191545 [DOI] [PubMed] [Google Scholar]
- Autenrieth I.B., Firsching R. 1996. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44:285–294 10.1099/00222615-44-4-285 [DOI] [PubMed] [Google Scholar]
- Autenrieth I.B., Beer M., Bohn E., Kaufmann S.H., Heesemann J. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenrieth I.B., Kempf V., Sprinz T., Preger S., Schnell A. 1996. Defense mechanisms in Peyer’s patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T., Cookson B.T. 2007. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 3:e161 10.1371/journal.ppat.0030161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M., He M., Zhang H., Moochhala S. 2009. Sepsis as a model of SIRS. Front. Biosci. 14:4703–4711 10.2741/3561 [DOI] [PubMed] [Google Scholar]
- Björkbacka H., Fitzgerald K.A., Huet F., Li X., Gregory J.A., Lee M.A., Ordija C.M., Dowley N.E., Golenbock D.T., Freeman M.W. 2004. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol. Genomics. 19:319–330 10.1152/physiolgenomics.00128.2004 [DOI] [PubMed] [Google Scholar]
- Blander J.M., Medzhitov R. 2004. Regulation of phagosome maturation by signals from toll-like receptors. Science. 304:1014–1018 10.1126/science.1096158 [DOI] [PubMed] [Google Scholar]
- Bohn E., Autenrieth I.B. 1996. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J. Immunol. 156:1458–1468 [PubMed] [Google Scholar]
- Brodsky I.E., Medzhitov R. 2008. Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence. PLoS Pathog. 4:e1000067 10.1371/journal.ppat.1000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., Batra S., Shen L., Wakamatsu N., Jeyaseelan S. 2009. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J. Immunol. 183:6629–6638 10.4049/jimmunol.0901033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo K., Henderson R.B., Laschinger M., Hogg N. 2008. Neutrophil chemokines KC and macrophage-inflammatory protein-2 are newly synthesized by tissue macrophages using distinct TLR signaling pathways. J. Immunol. 180:4308–4315 [DOI] [PubMed] [Google Scholar]
- Echeverry A., Schesser K., Adkins B. 2007. Murine neonates are highly resistant to Yersinia enterocolitica following orogastric exposure. Infect. Immun. 75:2234–2243 10.1128/IAI.01681-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermann-Eriksen S. 1993. Autocrine secretion of interferon-alpha/beta and tumour necrosis factor-alpha synergistically activates mouse macrophages after infection with herpes simplex virus type 2. J. Gen. Virol. 74:2191–2199 10.1099/0022-1317-74-10-2191 [DOI] [PubMed] [Google Scholar]
- Gordon M.A., Jack D.L., Dockrell D.H., Lee M.E., Read R.C. 2005. Gamma interferon enhances internalization and early nonoxidative killing of Salmonella enterica serovar Typhimurium by human macrophages and modifies cytokine responses. Infect. Immun. 73:3445–3452 10.1128/IAI.73.6.3445-3452.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Cheng G. 2007. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J. Biol. Chem. 282:11817–11826 10.1074/jbc.M700017200 [DOI] [PubMed] [Google Scholar]
- Hall J.C., Rosen A. 2010. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol. 6:40–49 10.1038/nrrheum.2009.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley S.A., Dube P.H., Revell P.A., Miller V.L. 2004. Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect. Immun. 72:1645–1656 10.1128/IAI.72.3.1645-1656.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House D., Bishop A., Parry C., Dougan G., Wain J. 2001. Typhoid fever: pathogenesis and disease. Curr. Opin. Infect. Dis. 14:573–578 10.1097/00001432-200110000-00011 [DOI] [PubMed] [Google Scholar]
- Jepson M.A., Clark M.A. 2001. The role of M cells in Salmonella infection. Microbes Infect. 3:1183–1190 10.1016/S1286-4579(01)01478-2 [DOI] [PubMed] [Google Scholar]
- Jeyaseelan S., Young S.K., Fessler M.B., Liu Y., Malcolm K.C., Yamamoto M., Akira S., Worthen G.S. 2007. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J. Immunol. 178:3153–3160 [DOI] [PubMed] [Google Scholar]
- Kelly-Scumpia K.M., Scumpia P.O., Delano M.J., Weinstein J.S., Cuenca A.G., Wynn J.L., Moldawer L.L. 2010. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J. Exp. Med. 207:319–326 10.1084/jem.20091959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Ge B.X. 2008. MyD88-independent activation of a novel actin-Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell Res. 18:745–755 10.1038/cr.2008.65 [DOI] [PubMed] [Google Scholar]
- Lebeis S.L., Powell K.R., Merlin D., Sherman M.A., Kalman D. 2009. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 77:604–614 10.1128/IAI.00907-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D.E., Marié I., Prakash A. 2003. Ringing the interferon alarm: differential regulation of gene expression at the interface between innate and adaptive immunity. Curr. Opin. Immunol. 15:52–58 10.1016/S0952-7915(02)00011-0 [DOI] [PubMed] [Google Scholar]
- Liu F., Whitton J.L. 2005. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J. Immunol. 174:5936–5940 [DOI] [PubMed] [Google Scholar]
- Liu M.T., Keirstead H.S., Lane T.E. 2001. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J. Immunol. 167:4091–4097 [DOI] [PubMed] [Google Scholar]
- Macpherson A.J., Uhr T. 2004. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 303:1662–1665 10.1126/science.1091334 [DOI] [PubMed] [Google Scholar]
- Matteoli G., Fahl E., Warnke P., Müller S., Bonin M., Autenrieth I.B., Bohn E. 2008. Role of IFN-gamma and IL-6 in a protective immune response to Yersinia enterocolitica in mice. BMC Microbiol. 8:153 10.1186/1471-2180-8-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao E.A., Miller S.I. 1999. Bacteriophages in the evolution of pathogen-host interactions. Proc. Natl. Acad. Sci. USA. 96:9452–9454 10.1073/pnas.96.17.9452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalec L., Choudhury B.K., Postlethwait E., Wild J.S., Alam R., Lett-Brown M., Sur S. 2002. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J. Immunol. 168:846–852 [DOI] [PubMed] [Google Scholar]
- Oellerich M.F., Jacobi C.A., Freund S., Niedung K., Bach A., Heesemann J., Trülzsch K. 2007. Yersinia enterocolitica infection of mice reveals clonal invasion and abscess formation. Infect. Immun. 75:3802–3811 10.1128/IAI.00419-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M.R., Li B., Yamamoto M., Akira S., Lin T.J. 2007. A role of Toll-IL-1 receptor domain-containing adaptor-inducing IFN-beta in the host response to Pseudomonas aeruginosa lung infection in mice. J. Immunol. 178:3170–3176 [DOI] [PubMed] [Google Scholar]
- Ruckdeschel K., Pfaffinger G., Haase R., Sing A., Weighardt H., Häcker G., Holzmann B., Heesemann J. 2004. Signaling of apoptosis through TLRs critically involves toll/IL-1 receptor domain-containing adapter inducing IFN-beta, but not MyD88, in bacteria-infected murine macrophages. J. Immunol. 173:3320–3328 [DOI] [PubMed] [Google Scholar]
- Tassios P.T., Kerr K.G. 2010. Hard to swallow—emerging and re-emerging issues in foodborne infection. Clin. Microbiol. Infect. 16:1–2 10.1111/j.1469-0691.2009.03112.x [DOI] [PubMed] [Google Scholar]
- Trülzsch K., Oellerich M.F., Heesemann J. 2007. Invasion and dissemination of Yersinia enterocolitica in the mouse infection model. Adv. Exp. Med. Biol. 603:279–285 10.1007/978-0-387-72124-8_25 [DOI] [PubMed] [Google Scholar]
- Voedisch S., Koenecke C., David S., Herbrand H., Förster R., Rhen M., Pabst O. 2009. Mesenteric lymph nodes confine dendritic cell-mediated dissemination of Salmonella enterica serovar Typhimurium and limit systemic disease in mice. Infect. Immun. 77:3170–3180 10.1128/IAI.00272-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S.N., Finbloom D.S., English K.E., Rosenstreich D.L., Langreth S.G. 1983. Interferon-induced enhancement of macrophage Fc receptor expression: beta-interferon treatment of C3H/HeJ macrophages results in increased numbers and density of Fc receptors. J. Immunol. 130:1210–1214 [PubMed] [Google Scholar]
- Westphal S., Lügering A., von Wedel J., von Eiff C., Maaser C., Spahn T., Heusipp G., Schmidt M.A., Herbst H., Williams I.R., et al. 2008. Resistance of chemokine receptor 6-deficient mice to Yersinia enterocolitica infection: evidence of defective M-cell formation in vivo. Am. J. Pathol. 172:671–680 10.2353/ajpath.2008.070393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D.J., Nordfeldth R., Rosenzweig J., DaFonseca C.J., Gustin R., Wolf-Watz H., Schesser K. 2006. The Ser/Thr kinase activity of the Yersinia protein kinase A (YpkA) is necessary for full virulence in the mouse, mollifying phagocytes, and disrupting the eukaryotic cytoskeleton. Microb. Pathog. 40:234–243 10.1016/j.micpath.2006.02.001 [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Sato S., Hemmi H., Hoshino K., Kaisho T., Sanjo H., Takeuchi O., Sugiyama M., Okabe M., Takeda K., Akira S. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 301:640–643 10.1126/science.1087262 [DOI] [PubMed] [Google Scholar]
- Zeng X., Moore T.A., Newstead M.W., Deng J.C., Kunkel S.L., Luster A.D., Standiford T.J. 2005. Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect. Immun. 73:8226–8236 10.1128/IAI.73.12.8226-8236.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]